Abstract
Purpose:
Unresectable soft tissue sarcomas (STSs) do not usually exhibit significant tumor downstaging with preoperative radiotherapy and/or chemotherapy due to their limited radiosensitivity/chemosensitivity. Limb amputations for tumors of the extremities inevitably lead to considerable loss of function and impairment in quality of life. Local hyperthermia at 39°C to 43°C and proton irradiation combine thermoradiobiological and physical dose distribution advantages, possibly mimicking those of a 12C ion therapy. We report the first 2 patients treated with this unique approach of proton thermoradiotherapy.
Materials and Methods:
Both patients had an unresectable STS of the left lower leg (1 grade 2 myxoid fibrosarcoma, 1 grade 3 undifferentiated pleomorphic sarcoma). Both patients had declined the above-knee amputation that had been advised due to their involvement of the neurovascular bundles. They were, therefore recruited to the Hyperthermia and Proton Therapy in Unresectable Soft Tissue Sarcoma (HYPROSAR) study protocol (ClinicalTrials.gov NCT01904565). Local hyperthermia was delivered using radiofrequency waves at 100 Mhz once a week after proton therapy. Proton irradiation was undertaken to a dose of 70 to 72 Gy (relative biological effectiveness) delivered at 2.0 Gy (relative biological effectiveness)/ fraction daily for 7 weeks.
Results:
Patients tolerated the treatment well with no significant acute or late morbidity. Both primary tumors showed a near complete response on serial magnetic resonance imaging. At a follow-up of 5 and 14 months, the patients were able to carry out indoor and outdoor activities with normal limb function.
Conclusion:
This is the first report of proton beam irradiation combined with hyperthermia for cancer therapy. Our first experience in 2 consecutive patients with unresectable STSs shows that the approach is safe, feasible, and effective, achieving functional limb preservation with near total tumor control.
Keywords: soft tissue sarcoma, hyperthermia, proton therapy, radiation therapy, unresectable
Introduction
Soft tissue sarcomas (STSs) constitute a diverse and heterogeneous group of tumors with a wide spectrum of extraskeletal origins, including muscle, fat, nerves and fibrous tissue. For their optimum management, shared decision making by a multidisciplinary sarcoma team of key specialists is essential. Complete surgical excision of the tumor and its normal tissue barrier remains the mainstay of treatment of STS. Attaining a microscopically clear resection margin with limb preservative surgery minimizes the risk of local tumor recurrence and offers a longer disease-free quality of life [1, 2].
However, patients with unresectable STS tumors pose therapeutic challenges, especially when attempting limb preservation. Various strategies, including preoperative radiation therapy (RT), preoperative chemotherapy (CT), or chemoradiotherapy have been tried to downstage these tumors [2–4]. Postoperative wound complications are an important consideration following preoperative RT. Even with the use of image-guided intensity-modulated RT, nearly one-third of patients are reported to develop significant wound complications [4]. Postoperative RT could result in a higher incidence of postoperative long-term edema, fibrosis, and joint stiffness [5, 6]. On the other hand, preoperative CT has not been shown to yield consistent results [7]. In one retrospective study, preoperative CT benefited only high-grade tumors more than 10 cm long [8], while another cohort analysis of 674 patients reported that the advantage of preoperative or postoperative CT was not sustained beyond a year [9].
Issels et al [10] treated patients with STSs in a phase III randomized multicentric study with neoadjuvant CT (etoposide, ifosfamide, and doxorubicin) followed by surgery, postoperative RT (if indicated), and adjuvant CT (etoposide, ifosfamide, and doxorubicin). Patients were randomized to receive CT with or without local hyperthermia (HT). It was shown that CT with HT improved both progression-free survival and disease-free survival compared with those who received CT only [10]. Thus, HT, through its synergistic action with CT, has been shown to improve outcomes in resectable or potentially resectable STSs.
Due its selective cytotoxicity against radio-resistant hypoxic cells and S-phase cells, HT at 39°C to 43°C is known to be one of the most potent radiosensitizers [11]. In addition, it inhibits repair of the RT-induced damage [11, 12]. As these thermoradiobiological properties are similar to those of high linear-energy transfer (LET) radiation, such as 12C ions, HT is also described as a “poor man's high-LET radiation” [13]. The superior physical dose distribution of protons over photons is comparable to that of 12C ions, and thus, a combination of protons and HT could mimic therapy with 12C ions, which is known to have superior outcomes in STSs [14–16].
Based on this presumption and the knowledge that STSs are relatively radioresistant to conventional photon RT, the phase I/II Hyperthermia and Proton Therapy in Unresectable Soft Tissue Sarcoma (HYPROSAR) study of HT combined with proton therapy was recently initiated (Figure 1). The study aims to explore the safety and efficacy of this strategy in unresectable STSs (ClinicalTrials.gov NCT01904565) [17]. We present here the first 2 patients treated with this unique approach of proton thermoradiation therapy.
Figure 1.
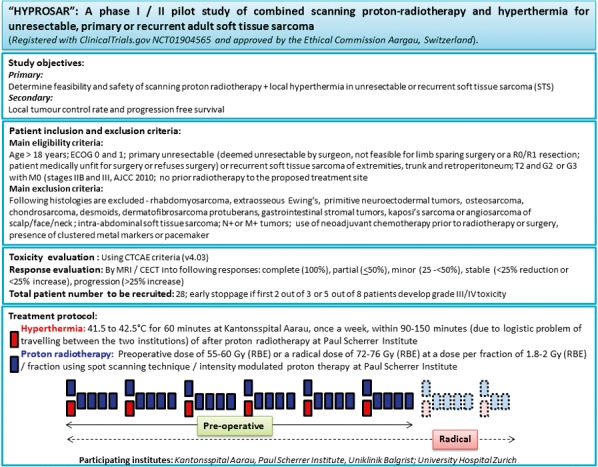
Summary of the Hyperthermia and Proton Therapy in Unresectable Soft Tissue Sarcoma (HYPROSAR) study protocol.
Case Report
Both patients were treated according to the HYPROSAR study protocol, a brief outline of which is presented in Figure 1. This is a phase I/II study using protons and HT in unresectable and recurrent STSs. Briefly, patients receive local HT to a temperature of 39°C to 43°C once a week following proton treatment. Proton therapy is delivered using a scanning beam and a dose of 55 to 60 Gy (relative biological effectiveness [RBE]) at 1.8 to 2 Gy (RBE)/fraction 5 days a week preoperatively. If the tumor remains ineligible for limb preservation surgery after 5 weeks of combined treatment, the patient continues on treatment to a radical dose of 72 to 76 Gy (RBE) along with local HT. Both the patients presented in this report were treated with the radical dose as described in the sections that follow. A well-informed consent was obtained from both these patients before starting the treatment.
Case 1
An 80-year-old man presented with pain, swelling, and stiffness in the left calf. On examination, there was diffuse, tender, swelling, and both knee flexion and extension were limited by pain. The patient avoided weight bearing on his left leg while walking. Magnetic resonance imaging (MRI) revealed a soft tissue mass in the left posterior compartment of the calf with encasement of the left popliteal artery and left peroneal nerve (Figure 2). A partial excision of the tumor was carried out, and the histology showed a grade 2 myxoid fibrosarcoma. The tumor was staged as T2bN0M0. An 18F-FDG-PET scan showed 2 lesions (standardized uptake value maximum 12 and 4.2), and an MRI scan confirmed residual tumor along with disease progression. In view of the neurovascular involvement, the Sarcoma Tumor Board recommended an above-knee amputation as limb preservation was deemed unfeasible. However, the patient refused amputation and was offered treatment according to the HYPROSAR study protocol.
Figure 2.
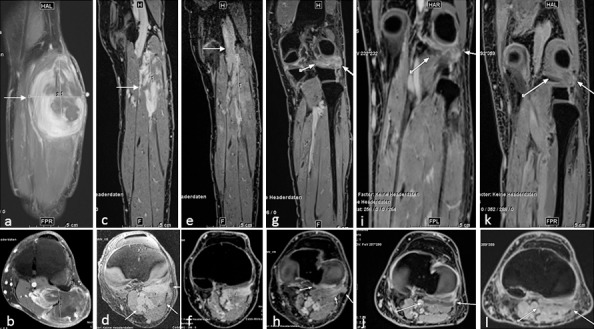
Patient 1. Myxofibrosarcoma of the left lower leg (indicated by white arrows) at presentation before surgery, measuring 11.1 × 7.4 × 4.3 cm3 (maximum diameter) in the flexor musculature of the left lower leg on coronal and (A) axial (B) contrast-enhanced fat-suppressed T1-weighted magnetic resonance imaging. The periarticular tumor extended to the knee joint and showed inhomogeneous contrast medium enhancement with encasement of the popliteal artery and peroneal nerve. (C, D) Post-biopsy scans taken 3 months after partial tumor resection and before proton thermoradiotherapy demonstrated tumor debulking (8.5 × 3 × 4.4 cm3) with homogeneous enhancement and tumor extension to the knee. (E, F) Magnetic resonance imaging after 4 weeks of proton and hyperthermia therapy revealed a slight increase in tumor size (9.8 × 2.9 × 4 cm3) but visible reduction of contrast enhancement. (G, H) Magnetic resonance imaging 7 weeks after completion of proton thermoradiotherapy shows a small residual tumor with minimal periarticular contrast enhancement dorsal to the knee joint. (I, J) Four months after completion of treatment, liquefaction with very subtle residual rim enhancement without new nodular tumor enhancement could be observed. (K, L) Ten months later, no further significant changes were observed.
The patient was evaluated for local HT at Kantonsspital Aarau (KSA), Aarau, Switzerland. He underwent a HT treatment planning CT scan using the hammock couch as used in the deep HT unit treating with radiofrequency waves at 100 MHz (BSD 2000 unit, M/s Pyrexar Medical, Salt Lake City, Utah, USA). The gross tumor volume was outlined on consecutive slices. The HT treatment planning was carried out using SigmaHyperPlan software (M/s Dr. Sennewald Medizintechnik GmbH, Munich, Germany) by segmentation and creation of a grid model of the various body tissues according to their dielectric properties (eg, tumor, muscle, bone, fat) followed by simulation of the electric fields. The SigmaHyperplan, version 2.0, has specific perfusion factors for each tissue type (fat, muscle, bone, tumor) that take into account the blood circulation in the tissue. The above structures were defined individually during the contouring and thermal treatment planning. Using appropriate power and steering parameters, a specific absorption rate distribution in the target volume was generated using finite element modeling. A warm-up heating phase of 30 minutes was followed by 60 minutes of HT treatment. The resultant specific absorption rate distribution and the tumor temperature were evaluated throughout the target volume. A thermal dose-volume histogram was generated to evaluate the temperature distribution in the tumor and the other adjacent normal tissues. The cumulative thermal temperature-volume histogram showed that 95% of the tumor volume received 39°C, 70% received 41.5°C, and 56% of the volume had a temperature of at least 43°C (Figure 3). The maximum intratumoral temperature was estimated as 52°C for an average applied power of 350W. No invasive thermometry was used; however, 7 Bowman temperature sensors (BSD Bowman temperature probe, M/s Pyrexar Medical, Salt Lake City, Utah, USA) were placed on the skin to monitor skin temperature within the entire heated region.
Figure 3.
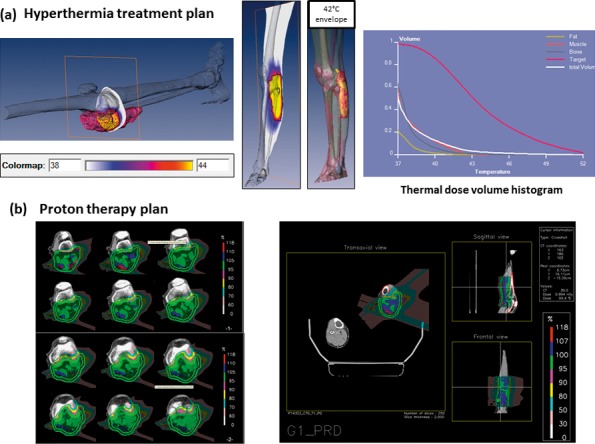
Patient 1 with myxoid fibrosarcoma of the left lower leg treated with hyperthermia and proton therapy. (A) Hyperthermia treatment plan: 95% of the tumor volume received a temperature of 39°C, 70% received a temperature of 41.5°C while 56% of the volume had a temperature of at least 43°C as evident in the thermal dose-volume histogram. The maximum intratumoral temperature was estimated as 52°C. (B) Proton therapy plan for planning target volume 1 (clinical target volume 1 plus 7 mm) including tumor and muscle compartment with safety margins: 64 Gy (RBE), 2 Gy (RBE) per fraction, 2 lateral fields per treatment plan. Not shown: boost plan with additional 6 Gy (RBE). Abbreviation: RBE, relative biological effectiveness
The patient was assessed for proton beam therapy with a 250 MeV cyclotron using a pencil beam scanning technique at the Centre for Proton Therapy, Paul Scherrer Institute (PSI), Villigen, Switzerland. The technique enables the proton pencil beams to be scanned within the tumor volume in 3 dimensions using a magnetic sweep of the beam, mechanical automated table movement, and sequential polycarbonate sheet absorbers or range shifter plates. The patient was fixed in an individualized vacuum mold in a supine position, and a high-resolution planning CT scan with slices of 2- to 4-mm thickness was performed. The relevant MRI series (pre- and post-excision, T1 and T2 sequences with and without gadolinium contrast agent) were fused with the planning CT scan to define the tumor volume and organs at risk. The proton dose distribution was computed using the 3-dimensional proton treatment planning system developed at PSI (PSI-Plan). A multiplanar isodose distribution was generated, and dose-volume histograms were used to optimize the treatment plans.
The patient received a total dose of 70 Gy (RBE) in 35 fractions at 2.0 Gy (RBE) per fraction, 5 days a week. The target delineation was carried out in consultation with the radiologist. It included visible tumor plus a margin of 2 cm (high-risk area) modified according to the anatomical structures. Several MRI sequences were used: pre- and post-excision, with or without contrast using all the sequences, T1, and T2. The initial clinical target volume (CTV1) included the tumor and its muscle compartment and received 64 Gy (RBE). The boost clinical target volume (CTV2) consisted of the tumor plus a 2-cm margin to include microscopic spread and was modified according to the adjacent structures. The CTV2 received an additional 6 Gy (RBE), resulting in a cumulative dose of 70 Gy (RBE) (Figure 3). The planning target volumes (PTV1 and PTV2) were created using an isotropic margin of 7 mm all around CTV1 and CTV2, respectively, to compensate for possible uncertainties, including CT calibration, stopping power, errors in the beam delivery system, patient setup, and intrafraction motion. The aim of treatment was to apply a homogenous dose to the CTVs as well as to spare the knee joint and avoid high doses to the entire circumference of the lower leg.
The patient received weekly HT treatments (7 in total), after the fraction of proton therapy on that day (Figure 4). Due to the logistics of traveling between PSI and KSA, an effort was made to initiate HT at KSA within 90 to 120 minutes of completion of proton treatment at PSI. During the actual HT treatment, 350W was typically applied with a Sigma eye applicator (M/s Pyrexar Medical, Salt Lake City, Utah, USA). Online skin temperatures were monitored using 7 Bowman temperature sensors along the length of the catheter (typical mapping length 16 to 30 cm) with a mapping interval of 5 to 10 minutes. The power was adjusted according to patient tolerance with a maximum limit of 40.5°C. The maximum skin temperature recorded during all 7 sessions was 40.2°C.
Figure 4.
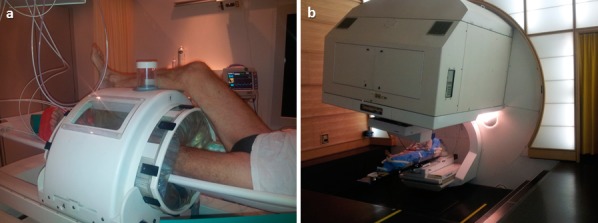
Patient 1 with myxoid fibrosarcoma of the left lower leg treated with (A) hyperthermia at Kantonsspital, Aarau, Switzerland, and (B) proton beam therapy at Paul Scherrer Institute, Villigen, Switzerland.
An MRI scan after 44 Gy (RBE) proton therapy and 4 HT treatments showed a good tumor response, but amputation remained the only surgical option. The tumor board consensus was therefore to continue proton thermoradiotherapy. At 70 Gy (RBE), the patient developed a small patch of radiodermatitis grade 2 in the left popliteal fold. To avoid further toxicity, the total proton dose was limited to 70 Gy (RBE).
In general, proton therapy and HT were very well tolerated. After completing treatment, the patient remained under regular follow-up. No other tumor therapy was administered. The skin reaction healed with conservative management within a week, and no analgesics were required. The patient was able to carry out his normal activities without any limitations. Serial MRI scans showed a steady and gradual tumor regression. The last MRI 12 months after treatment showed a promising radiologic response and no further entrapment of the neurovascular bundle. The patient is now 14 months posttreatment and continues to lead a normal life with a fully functional left leg. There is no evidence of any late toxicity.
Case 2
A 39-year-old woman presented with complaints of pain, swelling, and restriction of her left leg movements. There was a diffuse tender swelling extending from the left popliteal fossa to the calf. An MRI scan revealed a tumor measuring 4 × 2.3 × 5.2 cm3 in the posterior compartment of the left lower leg (Figure 5). On biopsy, it was reported to be an undifferentiated grade 3 pleomorphic sarcoma. The tumor was staged as T2bN0M0. In view of the involvement of the neurovascular bundle, the Sarcoma Tumor Board advised an above-knee amputation. The patient refused amputation and was therefore offered treatment in the HYPROSAR study.
Figure 5.
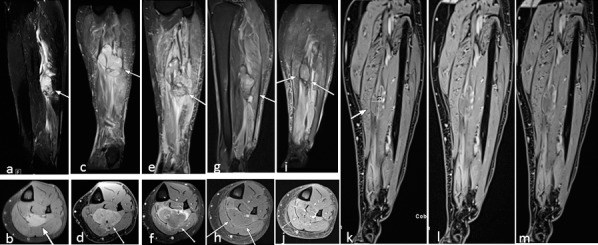
Patient 2 with undifferentiated pleomorphic sarcoma of the left lower leg (indicated by white arrows) at presentation, measuring 4 × 2.3 × 5.2 cm3 in the flexor musculature of the left lower leg on (A) sagittal short T1 inversion recovery magnetic resonance image and (B) axial contrast-enhanced fat-suppressed T1-weighted magnetic resonance imaging. (C, D) Post-biopsy magnetic resonance image scans at the time of proton beam and hyperthermia planning demonstrated tumor progression (5.1 × 3.9 × 7.5 cm3) with inhomogeneous enhancement and increasing edema of adjacent musculature. (E, F) Magnetic resonance image after 4 weeks of proton and hyperthermia therapy revealed a slight increase in tumor size with reduction of contrast media uptake. (G, H) The progressive perifocal edema might be a consequence of irradiation. Follow-up magnetic resonance image at 6 weeks after therapy shows both reduction in tumor size and perifocal edema. The tumor shows post-therapy changes with liquefaction and only very subtle residual rim enhancement without nodular tumor enhancement. (I) Tumor regression continued at 20 weeks after completion of therapy with no significant differences concerning signal intensity on coronal short T1 inversion recovery technique. No new nodular enhancement was seen on dynamic contrast enhanced images acquired after contrast administration at (J, K) 5 minutes, (L) 10 minutes, and (M) 15 minutes.
The patient was reviewed at KSA for HT treatment and at PSI for proton therapy. She underwent pretreatment scans and treatment planning as described previously. The cumulative thermal temperature-volume histogram showed that 100% of the tumor volume attained 39°C, 58% received 41.5°C, and 11% of the volume acquired 43°C or more (Figure 6). Maximum intratumoral temperature was estimated as 45°C. A typical power of 150W and a Sigma eye applicator was used for the deep HT sessions. She was treated with 8 weekly sessions of HT following proton therapy. The maximum online skin temperature recorded using the 7 multiple Bowman temperature sensors during the HT sessions was 40.8°C.
Figure 6.
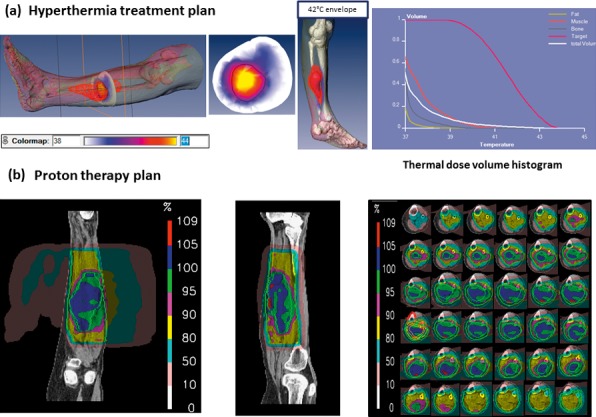
Patient 2 with undifferentiated pleomorphic sarcoma of the left lower leg treated with hyperthermia and proton therapy. (A) Hyperthermia treatment plan with the color map of the temperature distributions along the target volume (upper row, inserts 1 to 3). The cumulative temperature-volume histogram (last insert in the upper panel) shows that 95% of the tumor volume received a temperature of 39°C, 70% received a temperature of 41.5%, and 56% of the volume had a temperature of at least 43°C with a maximum intratumoral temperature of 45°C. (B) Proton therapy summary plan with 2 lateral fields per treatment plan prescribed for 2 Gy (RBE) per fraction. Planning target volume 1 (clinical target volume 1 plus 7 mm; outer green line) including tumor and muscle compartment with safety margins: total dose 60 Gy (RBE). Boost planning target volume 2 (clinical target volume 2 plus 7 mm; inner green line): total dose 12 Gy (RBE). The distribution of the proton dose profiles along the target volume is depicted (last insert in the lower panel). Abbreviation: RBE, relative biological effectiveness
The patient received a total dose of 72 Gy (RBE) in 36 fractions at 2.0 Gy (RBE) per fraction, delivered 5 days a week. The initial target volume CTV1 (tumor and adjacent muscle compartment) received 60 Gy(RBE) while the CTV2 (tumor plus area at high risk of microscopic spread) received an additional 12 Gy (RBE) (Figure 6). The PTVs were created as described for the previous patient.
At the start of treatment, the patient had severe pain in the leg and was unable to walk without crutches. Hospital admission was necessary for pain management with analgesics and steroids. At the end of the first week of therapy, the local pain increased, and she required increased analgesia and steroids resulting in moderate cushingoid facies. As the treatment progressed, a steady reduction in pain permitted a gradual tapering of her medication. By the end of 4 weeks of treatment, she was able to walk a few steps without crutches. The symptomatic improvement was sustained, and by the end of treatment she no longer required analgesics or steroids and could walk indoors without crutches. Treatment was completed without any significant problems, and she developed only grade I/II acute skin toxicity.
After completing treatment, multiple pulmonary metastases were diagnosed and she was been started on CT, which has been well tolerated to date. An MRI of the left leg 5 months after completion of proton thermoradiotherapy showed continued tumor regression, significant reduction in entrapment of the neurovascular bundle, and no new nodular enhancements on dynamic contrast images. Presently, she can walk normally without any crutches, has near complete functional recovery, and does not need any analgesics. There is no evidence of any late toxicity.
Discussion
HYPROSAR is a phase I/II clinical trial of local HT and proton therapy in unresectable STSs (Figure 1) [14, 17]. The trial was based on the hypothesis that protons share similar a physical dose distribution to 12C ions, characterized by a low-dose entrance region and a steep fall-off to an almost negligible dose beyond the target. The 12C ions exhibit a sharper Bragg peak with less scatter, resulting in a better penumbra than protons but a more gradual fall-off in the fragment tail behind the peak [15, 18]. As the mean LET of protons is slightly higher than 250 kVp X-rays, protons could be considered to be low-LET radiation similar to photons [19]. The RBE would therefore be just slightly higher than photons. Thus, the average RBE for protons at the middle of the spread out Bragg peak is generally accepted to be 1.1 compared with 150 to 250 MeV photon beams, but it could substantially increase in the distal end of the Bragg peak (RBE: 1.35 to 1.70) [19–21]. On the contrary, 12C ions have a higher RBE ranging from 1.5 at the proximal edge to 6.7 at the distal edge (average RBE of 12C ions considered as 2.3 to 3), and are classified as high-LET radiation.
Hyperthermia at 39°C to 43°C displays selective cytotoxicity toward radioresistant hypoxic cells and S-phase cells and reduces radiation-induced DNA repair. It also exhibits an intrinsic thermosensitivity towards sarcoma cell lines [12]. Thus, a combination of protons and HT could result in effects similar to those of 12C ions [14]. Since 12C has been shown to improve outcomes in unresectable STSs, it is compelling to explore the efficacy of proton thermoradiotherapy in these tumors [15, 22–24].
Apart from the thermoradiobiological and physical dose distribution advantages of proton thermoradiotherapy, it has now been demonstrated that HT can modulate RT-induced adaptive antitumor immunity through the induction of heat shock proteins, especially heat shock protein-70 [25, 26]. The heat shock proteins and the tumor antigen-containing exosomes released from apoptotic and necrotic cells form complexes to attract dendritic cells and activate CD8+ T cells. This could result in the acceleration of RT-induced immunomodulation and thus achieve an enhanced tumor response with the addition of HT [2–7–29]. Use of HT and proton therapy could therefore, not only increase the efficacy of local cell kill by mimicking 12C ion, but also potentiate the immune response. This could further contribute toward an enhanced tumor response.
Both the patients described in this report have shown remarkable clinical and radiologic responses to proton thermoradiotherapy. The treatment was well tolerated, and there was no additional acute or late morbidity. Even with large, unresectable tumors, the patients were spared an amputation and continue to have fully functional limbs with local tumor control. The first patient, who is now more than 1 year after completion of treatment, continues to enjoy a normal life with no evidence of disease. The second patient, although well controlled locally with no functional disability, is receiving CT for distant metastases.
One of the aims in HT treatment has been to minimize the time interval between radiotherapy and HT. In the present study, the 2 treatments were available at 2 different institutions, and every effort was made to minimize the interval between proton therapy and HT. Because 60 to 75 minutes were necessary to travel between the sites, the protocol therefore had a requirement to initiate the HT within 90 to 150 minutes after proton therapy. This was successfully achieved by active communication and coordination by the technical staff at both institutions.
The outcomes demonstrated by these patients after proton thermoradiotherapy are encouraging. Both patients showed gradual tumor regression during the course of RT, as expected with STSs. The MRI after 4 to 5 weeks of treatment did not show sufficient tumor shrinkage to permit limb preservation surgery (Figures 2 and 5). Both patients continued to receive radical doses without any appreciable acute or late toxicity. However, as evident in follow-up MRIs, the tumor showed progressive shrinkage that was corroborated with gradual complete restoration of limb function.
Conclusion
This first insight into the outcomes demonstrated by the first 2 patients treated with proton thermoradiotherapy is very promising. If the outcomes of this phase I/II trial indicate therapeutic efficacy, proton thermoradiotherapy could be a novel therapeutic option in the management of these unresectable STSs and could pave the way for a future randomized trial comparing alternative approaches, such as 12C ions.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of interest: None of the authors has any conflict of interest to be declared.
Acknowledgments: The study is supported by the Research Council, Kantonsspital Aarau, Aarau Switzerland, and Günter und Regine Kelm Stiftung, Switzerland. The authors acknowledge Dr Susanne Rogers for reviewing the manuscript.
References
- 1.EMSO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii102–12. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 2.Casali PG, Blay JY. EMSO/CONTICANET/EUROBONET Consensus Panel of experts. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v198–203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 3.Haas RL, Delaney TF, O'Sullivan B, Keus RB, Le Pechoux C, Olmi P, Poulsen JP, Seddon B, Wang D. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84:572–80. doi: 10.1016/j.ijrobp.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan B, Griffin AM, Dickie CI, Sharpe MB, Chung PW, Catton CN, Ferguson PC, Wunder JS, Deheshi BM, White LM, Kandel RA, Jaffray DA, Bell RS. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119:1878–84. doi: 10.1002/cncr.27951. [DOI] [PubMed] [Google Scholar]
- 5.Davis AM, O'Sullivan B, Bell RS, Turcotte R, Catton CN, Wunder JS, Chabot P, Hammond A, Benk V, Isler M, Freeman C, Goddard K, Bezjak A, Kandel RA, Sadura A, Day A, James K, Tu D, Pater J, Zee B. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–7. doi: 10.1200/JCO.2002.03.084. [DOI] [PubMed] [Google Scholar]
- 6.Davis AM, O'Sullivan B, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, Goddard K, Freeman C, Sadura A, Zee B, Day A, Tu D, Pater J, Canadian Sarcoma Group NCI Canada Clinical Trial Group Randomized Trial. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol. 2005;75:48–53. doi: 10.1016/j.radonc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Gortzak E, Azzarelli A, Buesa J, Bramwell VH, van Coevorden F, van Geel AN, Ezzat A, Santoro A, Oosterhuis JW, van Glabbeke M, Kirkpatrick A, Verweij J. EORTC Soft Tissue Bone Sarcoma Group and the National Cancer Institute of Canada Clinical Trials Group/Canadian Sarcoma Group. A randomised phase II study on neo-adjuvant chemotherapy for “high-risk” adult soft-tissue sarcoma. Eur J Cancer. 2001;37:1096–103. doi: 10.1016/s0959-8049(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 8.Grobmyer SR, Maki RG, Demetri GD, Mazumdar M, Riedel E, Brennan MF, Singer S. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15:1667–72. doi: 10.1093/annonc/mdh431. [DOI] [PubMed] [Google Scholar]
- 9.Tierney JF, Mosseri V, Stewart LA, Souhami RL, Parmar MK. Adjuvant chemotherapy for soft-tissue sarcoma: review and meta-analysis of the published results of randomised clinical trials. Br J Cancer. 1995;72:469–75. doi: 10.1038/bjc.1995.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, Vujaskovic Z, Wessalowski R, Jauch KW, Durr HR, Ploner F, Baur-Melnyk A, Mansmann U, Hiddemann W, Blay JY, Hohenberger P. European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (EORTC-STBSG), European Society for Hyperthermic Oncology (ESHO). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11:561–70. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overgaard J. The heat is (still) on—the past and future of hyperthermic radiation oncology. Radiother Oncol. 2013;109:185–7. doi: 10.1016/j.radonc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E, Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41:742–53. doi: 10.1016/j.ctrv.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Corry PM, Armour EP. The heat shock response: role in radiation biology and cancer therapy. Int J Hyperthermia. 2005;21:769–78. doi: 10.1080/02656730500394197. [DOI] [PubMed] [Google Scholar]
- 14.Datta NR, Puric E, Schneider R, Weber DC, Rogers S, Bodis S. Could hyperthermia with proton therapy mimic carbon ion therapy? Exploring a thermo-radiobiological rationale. Int J Hyperthermia. 2014;30:524–30. doi: 10.3109/02656736.2014.963703. [DOI] [PubMed] [Google Scholar]
- 15.Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, Niemierko A, Hall E, Flanz J, Hallman J, Trofimov A. Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiother Oncol. 2010;95:3–22. doi: 10.1016/j.radonc.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Kamada T. Twenty years of carbon ion radiation therapy at the National Institute of Radiological Sciences: accomplishments and prospects. Int J Part Ther. 2016;2:459–63. doi: 10.14338/IJPT-15-00030.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta NR. Hyperthermia and Proton Therapy in Unresectable Soft Tissue Sarcoma (HYPROSAR) 2013 doi: 10.14338/IJPT-16-00016.1. https://clinicaltrials.gov/ct2/show/NCT01904565 Accessed March 26, 2016. [DOI] [PMC free article] [PubMed]
- 18.Wilkens JJ, Oelfke U. Direct comparison of biologically optimized spread-out bragg peaks for protons and carbon ions. Int J Radiat Oncol Biol Phys. 2008;70:262–6. doi: 10.1016/j.ijrobp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, Suit HD. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–21. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 20.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–72. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto Y, Matsuura T, Wada M, Egashira Y, Nishio T, Furusawa Y. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res. 2014;55:816–22. doi: 10.1093/jrr/rrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jingu K, Tsujii H, Mizoe JE, Hasegawa A, Bessho H, Takagi R, Morikawa T, Tonogi M, Tsuji H, Kamada T, Yamada S. Organizing Committee for the Working Group for Head-and-Neck Cancer. Carbon ion radiation therapy improves the prognosis of unresectable adult bone and soft-tissue sarcoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;82:2125–31. doi: 10.1016/j.ijrobp.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Imai R, Kamada T, Tsuji H, Sugawara S, Serizawa I, Tsujii H, Tatezaki S. Working Group for Bone and Soft Tissue Sarcomas. Effect of carbon ion radiotherapy for sacral chordoma: results of Phase I-II and Phase II clinical trials. Int J Radiat Oncol Biol Phys. 2010;77:1470–6. doi: 10.1016/j.ijrobp.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Timmermann B, Schuck A, Niggli F, Weiss M, Lomax AJ, Pedroni E, Coray A, Jermann M, Rutz HP, Goitein G. Spot-scanning proton therapy for malignant soft tissue tumors in childhood: first experiences at the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys. 2007;67:497–504. doi: 10.1016/j.ijrobp.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 25.Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, Fietkau R, Gaipl US. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528–42. doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 26.Repasky EA. Progress in development of biomedical applications of heat shock proteins and thermal stress. Int J Hyperthermia. 2013;29:359–61. doi: 10.3109/02656736.2013.825015. [DOI] [PubMed] [Google Scholar]
- 27.Datta NR, Grobholz R, Puric E, Bode-Lesniewska B, Lomax N, Khan S, Gaipl US, Fuchs B, Bodis S. Enhanced tumour regression in a patient of liposarcoma treated with radiotherapy and hyperthermia: hint for dynamic immunomodulation by hyperthermia. Int J Hyperthermia. 2015;31:574–7. doi: 10.3109/02656736.2015.1033482. [DOI] [PubMed] [Google Scholar]
- 28.Rubner Y, Wunderlich R, Ruhle PF, Kulzer L, Werthmoller N, Frey B, Weiss EM, Keilholz L, Fietkau R, Gaipl US. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2012;2:75. doi: 10.3389/fonc.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, Lodermann B, Rodel F, Sauer R, Fietkau R, Gaipl US. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther Onkol. 2010;186:587–99. doi: 10.1007/s00066-010-2154-x. [DOI] [PubMed] [Google Scholar]


