Abstract
Purpose:
To describe volume changes following proton beam therapy (PBT) for juvenile pilocytic astrocytoma (JPA), we analyzed post-PBT magnetic resonance imaging (MRI) to clarify survivorship, response rate, and the concept of pseudoprogression.
Materials and Methods:
Pediatric patients with a histologic diagnosis of JPA after a biopsy or subtotal resection and at least 4 post-PBT MRIs were retrospectively reviewed. After PBT, tumors were contoured on follow-up T1-contrasted MRIs, and 3-dimensional volumes were plotted against time, with thresholds for progressive disease and partial response. Patterns of response, pseudoprogression, and progression were uncovered. Post-PBT clinical course was described by the need for further intervention and survivorship.
Results:
Fifteen patients with a median of 10 follow-up MRIs made up this report: 60% were heavily pretreated with multiple lines of chemotherapy, and 67% had undergone subtotal resection. With a median follow-up of 55.3 months after a median of 5400 centigray equivalents PBT, estimates of 5-year overall survival and intervention-free survival were 93% and 72%, respectively. The crude response rate of 73% included pseudoprogressing patients, who comprised 20% of the entire cohort; the phenomenon peaked between 3 and 8 months and resolved by 18 months. One nonresponder expired from progression. Post-PBT intervention was required in 53% of patients, with 1 patient resuming chemotherapy. There were no further resections or radiotherapy. One patient developed acute lymphoblastic leukemia, and another developed biopsy-proven radionecrosis.
Conclusion:
The PBT for inoperable/progressive JPA provided 72% 5-year intervention-free survival in heavily pretreated patients. Although most patients responded, 20% demonstrated pseudoprogression. The need for post-PBT surveillance for progression and treatment-induced sequelae should not be underestimated in this extended survivorship cohort.
Keywords: juvenile pilocytic astrocytoma, proton beam therapy, pseudoprogression
Introduction
Pediatric malignancies account for < 1% of all cancer diagnoses, with an expected 10 380 new cases in 2015 and a projected 1250 cancer-related deaths among children aged 0 to 14 years [1]. Mortality has declined approximately two thirds during the past 4 decades, which is largely attributed to modern treatment strategies, care coordination, and successful enrollment in clinical trials [1]. Second to hematologic malignancies, primary tumors of the central nervous system comprise 26% of new pediatric cancer diagnoses, with a 5-year overall survival (OS) rate for those diagnosed between 2000 and 2006 of 75% [2].
Pilocytic astrocytoma, or juvenile pilocytic astrocytoma (JPA), is the most common brain tumor in children under 20 years, accounting for approximately 20% of new central nervous system neoplasms [3]. Tumors arise in both infratentorial and supratentorial locations and may involve the optic apparatus, brainstem, and spinal cord. Radiographically, JPAs are well-circumscribed, polycystic-enhancing lesions, and this classic appearance in an often inaccessible location leads to many diagnoses confirmed at the time of surgery [4]. Recent studies have investigated the molecular pathways underlying JPAs, noting impressive heterogeneity and the mitogen-activating protein kinase pathway implicated in many instances [3].
Standards of care for JPA remain gross total resection, with 10-year OS rates approaching 96%. Approximately 20% of patients experience surgical failure or progressive disease [3]. For patients with inoperable disease or those in whom location does not permit safe, gross total resection, progression rates can be as high as 80%. In these cases, radiation therapy (RT) has been established as a viable local therapy with OS > 80% at 10 years [5, 6]. Chemotherapy is frequently used to delay radiation and its known side-effect profile on the developing brain for as long as possible [7, 8]. One study comparing chemotherapeutic regimens in this circumstance quoted a 5-year event-free survival of 39% to 52% [8]. Recent advances in RT include the disseminated use of proton beam therapy (PBT), which uses the physical properties of the beam's dose deposition (its Bragg peak) to ultimately deliver less integral dose to the developing child's organs at risk without compromising tumor control. Extended follow-up is warranted to assess for the long-term efficacy and benefit of PBT on late radiation side effects and incidence of RT-induced secondary malignancies.
Scattered reports after RT for subtotal resected and inoperable JPAs have expressed concern for volumetric changes seen during follow-up, which can prompt discussion of therapy reinitiation or supportive care [9–14]. Such volumetric changes can include tumor regression or progression, hemorrhage, cystic changes, or pseudoprogression—an apparent increase in radiographic volume followed by subsequent regression without further therapy—a not-uncommon effect after RT for low-grade glioma [15–17]. Although those reports focused on photon RT, we sought to quantify those volumetric changes on serial magnetic resonance images (MRIs) after PBT in a cohort of pediatric patients with subtotally resected or inoperable pilocytic astrocytomas.
Materials and Methods
Subjects of this retrospective report were younger than 18 years at diagnosis, with histologically confirmed pilocytic astrocytomas. Patients whose disease was deemed to be inoperable or who had subtotal resection were included if they had received PBT and had at least 4 available post-RT MRIs. Data on prior surgical, radiation, and chemotherapeutic therapies were collected, along with basic demographics. Complete data on corticosteroid therapy adherence and dosing was unavailable and is, thus, not reported.
All patients were treated with 206 MeV protons in a customized multiple-field arrangement to preserve organs at risk and to minimize the integral dose using a uniform scanning nozzle system. The magnetically scanned beam-spreading system with dose layer stacking has previously been described in detail [18, 19]. Patient-specific devices employed included alpha cradles for immobilization and custom-milled, brass apertures for beam shaping and range compensators for each field. Patients requiring sedation underwent general anesthesia on site with each fraction.
Patients' contrasted, T1 series MRIs used for PBT planning were imported into the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, California), and the entire lesion, including the resection cavity plus the solid, cystic, and enhancing components, was contoured by one clinical radiation oncologist (E.M.M.). This resultant clinical target volume (CTV) in cubic centimeters was established as the baseline value. Subsequent MRIs were collected for 3 years after completion of the PBT, imported into the Eclipse treatment planning system, and lesions were contoured, with attention to consistency across serial scans. The volume in cubic centimeters was compared with baseline, with all changes reported as a percentage of volume change plotted against time in days. Graphic thresholds for progressive disease were set at 20% and 25% increase in volume, and to evaluate partial response, as 30% and 50% decrease in volume. These thresholds were extrapolated from World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) criteria, although here, they were applied to a 3-dimensional volume.
Subsequent post-PBT interventions included further surgery, radiation or chemotherapy, cerebral spinal fluid shunt revision, stereotactic cyst aspiration, or hyperbaric oxygen. Responders were identified from the temporal plot of volume changes as patients whose successive tumor volumes were less than the pre-PBT volume. Among those responders, we sought to identify patients whose tumors displayed pseudoprogression, defined as an initial increase in CTV, followed by regression to a responding lesion without further intervention. Given the extensive prior treatment courses for these patients with WHO grade 1 JPA, a conservative approach of reserving interventions for symptomatic patients was employed. Patients whose tumors showed successive volumes greater than pre-PBT (ie, progressive disease) were denoted nonresponders.
SAS software (version 9.1, SAS Institute, Cary, North Carolina) was used to compute descriptive statistics. Exploratory, univariate analysis was undertaken to investigate the significance of demographic and treatment variables on the need for intervention, the likelihood of response, and intervention-free survival (IFS, alive without further intervention). The IFS variable was employed to describe patients were alive and whose extensive treatment course ended with PBT. The OS and IFS were calculated from the end of PBT.
Results
Patient demographics and treatment data are outlined in the Table. Fifteen pediatric patients with a median age of 10.9 years (range, 4–20 years) upon receiving PBT were the subject of this report. Median follow-up was 55.3 months (range, 41.6–70.9 months), calculated from the end of PBT, which was delivered between August 2005 and October 2007 at 180 centigray equivalents (cGyE)/d to a median dose of 5400 cGyE, with 1 patient receiving 5940 cGyE and another 5040 cGyE.
Table.
Demographic and treatment data for the entire cohort.
|
Patient no. |
Age, y |
Sex |
Prior chemotherapy |
Surgery |
Time to PBT, mo |
Initial volume, cm3 |
Response |
Intervention (time since PBT, mo) |
Notes |
| 1 | 10 | M | Carboplatin, vincristine, pyrrolidine dithiocarbamate | Yes | 6.7 | 40.7 | Pseudoprogression | ΔShunt (90.1) | 5940 cGyE |
| 2 | 8 | F | Carboplatin, vincristine, actinomycin D, temozolomide, O6-benzylguanine, lomustine, thioguanine, procarbazine | Yes | 46.0 | 50.72 | Nonresponder | ΔShunt (7.8) | Deceased, 9.6 mo after PBT |
| 3 | 6 | M | Carboplatin, vincristine, cisplatin, etoposide | Yes ×2 | 27.5 | 57.96 | Pseudoprogression | Chemotherapy for T-cell ALL (21.9) | Second malignancy |
| 4 | 6 | M | Carboplatin, vincristine, temozolomide, vinblastine | Yes ×2 | 6.6 | 7.55 | Nonresponder | Hyperbaric O2 (87.8) | |
| 5 | 5 | M | Carboplatin, vincristine | Yes ×2 | 7.3 | 9.26 | Pure responder | None | |
| 6 | 12 | F | Carboplatin, vincristine, lomustine, procarbazine, temozolomide | No | N/A | 14.75 | Nonresponder | Hyperbaric O2 (25.4) | Prior radiotherapy; biopsy-proven radionecrosis |
| 7 | 4 | M | Cisplatin, vincristine, temozolomide | Yes | 46.0 | 13.23 | Pure responder | ΔShunt (38.6) | |
| 8 | 8 | F | Carboplatin, vincristine, gefitinib | No | N/A | 46.56 | Pure responder | Cyst drainage (23.4) | 5040 cGyE |
| 9 | 17 | M | None | Yes | 3.1 | 15.15 | Pure responder | None | |
| 10 | 16 | F | None | No | N/A | 3.37 | Pure responder | None | |
| 11 | 14 | M | None | Yes | 3.1 | 1.60 | Pure responder | None | |
| 12 | 10 | F | None | No | N/A | 14.13 | Nonresponder | Chemotherapy (7.7) | |
| 13 | 20 | M | None | Yes | 17.3 | 3.15 | Pseudoprogression | None | |
| 14 | 11 | M | Carboplatin, vincristine | Yes | 93.9 | 20.93 | Pure responder | None | |
| 15 | 16 | F | None | No | N/A | 8.86 | Pure responder | None |
Abbreviations: PBT, proton beam therapy; F, female; M, male; ALL, acute lymphoblastic leukemia; O2, oxygen; N/A, not applicable; cGyE, centigray equivalents; .
All patients had histologically confirmed JPA: 10 of 15 patients (67%) underwent ≥ 1 subtotal resection, with 3 of 10 patients (30%) undergoing 2 subtotal resections before PBT, and 9 of 15 patients (60%), with an average age of 7.8 years, underwent chemotherapy before initiation of PBT. All received multiple cycles of combination regimens with a platinum-vinca alkaloid backbone, and 7 of 9 patients (78%) received ≥ 1 additional agent (most often temozolomide). One patient, 7 years earlier, had received prior radiation that included fractionated delivery of 5400 cGy using photons and intracystic P-32, followed by salvage stereotactic radiosurgery 4 years before PBT. Mean time for all patients from last surgery/biopsy to completion of PBT was 25.8 months (range, 3.1–93.9 months). A median of 10 (range, 4–12) post-RT T1 plus contrast MRIs were collected on each patient, with a mean length of time from completion of RT to final follow-up MRI of 34 months (range, 9.5–53 months).
Response
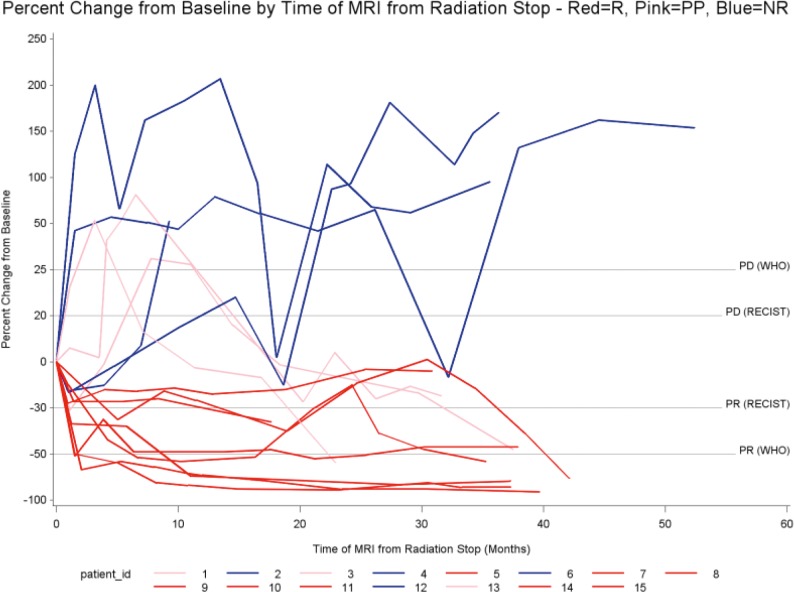
Volume changes ranged from 91% reduction to 207% increase when compared with pre-PBT MRI. Figure 1 reveals that 11 of 15 patients (73%; 95% confidence interval [CI], 41.6-70.9) displayed successive CTV volumes less than the pre-PBT volume and were classified as responders; 8 of these 11 patients (73%) did not display a CTV that could be considered progressive disease (> 20%-25% enlargement) throughout their radiographic follow-up. Of those 8 patients, 4 demonstrated successive CTVs with > 50% reduction in volume, and 6 consistently had CTVs with > 30% reduction in volume. Among these “pure responders” was 1 patient who received < 5400 cGyE (5040 cGyE). All pure responders declared within 3 months after PBT.
Figure 1.
Clinical target volume percentage change from baseline volume versus time (in days) since proton beam therapy. Color key: red, pure responders (R); pink, pseudoprogression (PP); blue, nonresponders (NR). Abbreviations: Δshunt, shunt revision; +chemo, chemotherapy; ALL, acute lymphoblastic leukemia; O2, hyperbaric oxygen; PD, progressive disease; PR, partial response.
The remaining 3 of the 11 responders (27%) all displayed multiple CTVs beyond both progressive disease thresholds before eventually being classified as responders; these patients were denoted as having pseudoprogression. Two of 3 patients (66%) had midline or optic chiasm tumors. Pre-PBT CTVs were 57.96, 40.7, and 3.15 cm3. One patient received 5940 cGyE. Another patient underwent no prior chemotherapy. Among the pseudoprogressing patients, the maximum volume was observed 3 to 8 months after PBT, and all 3 patients' CTVs regressed to at least baseline by approximately 18 months. During MRI follow-up, CTVs ranged from 81% increase to 59% regression. Figure 2 illustrates the initial, peak pseudoprogression and maximal response observed CTVs in those 3 patients. Overall incidence of pseudoprogression was 3 of 15 patients (20%).
Figure 2.
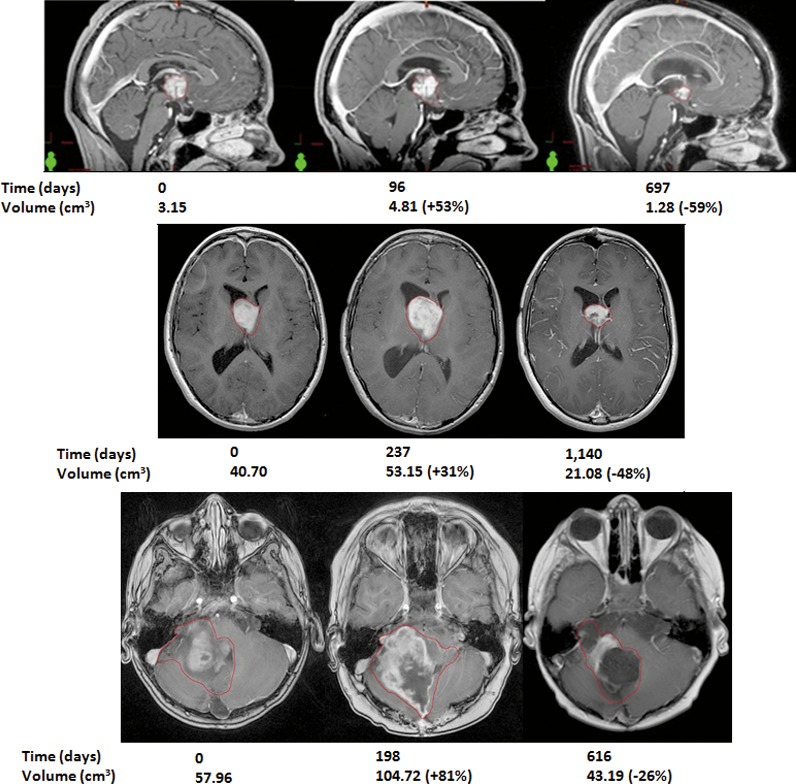
T1-contrasted magnetic resonance imaging with clinical target volume (in red) displaying initial volume, peak pseudoprogression, and maximal response volume with time.
Four of 15 patients (27%) displayed successive CTVs greater than baseline (nonresponders), with 1 patient died from progressive disease (CTV increased by 52% at last follow-up MRI) 9.6 months after completion of PBT. That patient was among the 2 of 4 nonresponders (50%) who had an approximately 20% CTV reduction at their 1-month follow-up, followed immediately by progressively enlarging CTVs. The remaining 2 of the 4 nonresponders (50%) were classified by their 1-month follow-up CTV enlargement, followed by successive growth. Three of 8 documented post-PBT interventions (38%) occurred among the nonresponders during the radiographic follow-up period, as denoted in Figure 1. Univariate analysis failed to uncover significant variables that were predictive of response.
Overall and Postproton Beam Therapy Intervention-Free Survival
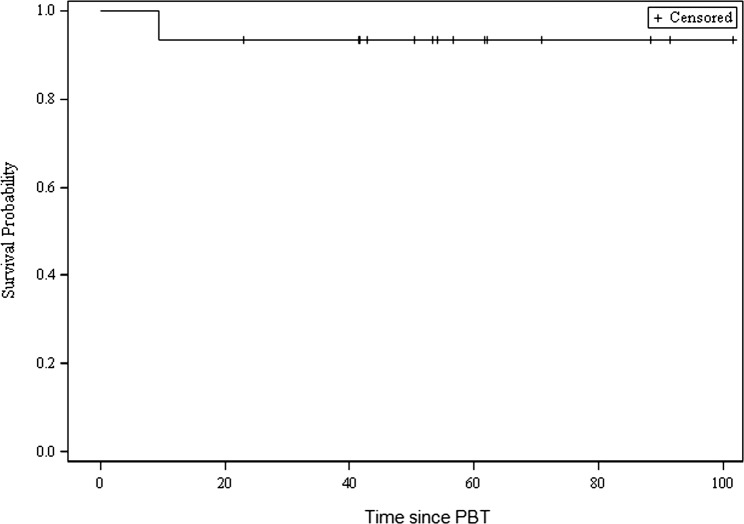
One patient died during the follow-up period from progressive disease at midline/optic chiasm 9.6 months after PBT, 4.5 years after diagnosis. A shunt revision was performed 55 days before her death. Notably, that patient received 8 different chemotherapy agents, no prior RT, and underwent a single, subtotal resection; her initial CTV was 50.7 cm3, the second largest of the cohort. Thus, for the entire cohort, 14 of 15 patients (93%) remained alive through the extent of follow-up. Median survival was not estimable. Kaplan-Meier OS estimates at 1, 2, 3, and 5 years was 93.3% (Figure 3).
Figure 3.
Overall survival since proton beam therapy (in months).
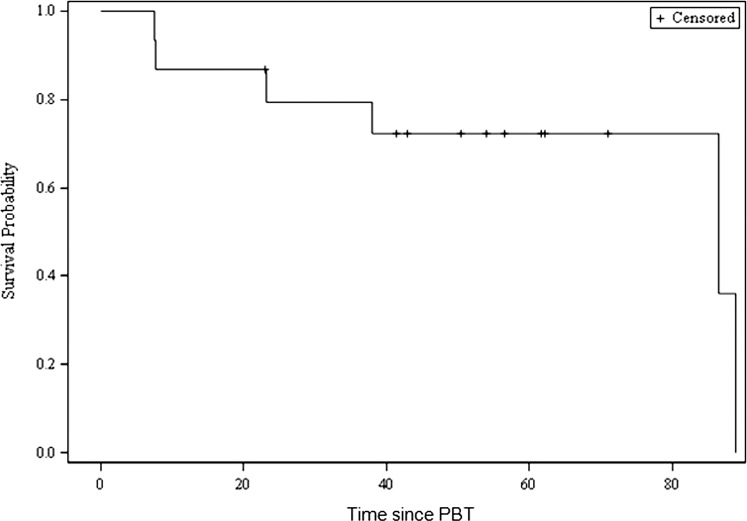
Median IFS calculated from the end of RT until intervention or death was 86.7% (95% CI, 23.1-88.9); 8 of 15 patients (53%) required no further therapy or interventions after PBT. Kaplan-Meier IFS estimates at 1, 2, 3, and 5 years was 86.7%, 79.4%, 79.4%, and 72.2%, respectively (Figure 4). Univariate exploratory analysis failed to uncover statistically significant predictors of IFS with the inclusion of temozolomide in pre-PBT regimens trending toward significance (P = .09).
Figure 4.
Intervention-free survival since proton beam therapy (in months).
Postproton Beam Therapy Interventions
Eight of 15 patients (53%) required intervention at a median time of 31 months (range, 7.7-90.1) after PBT. The most common intervention was shunt revision with 3 of 12 shunts (25%) needing revision, 1 at 7.8 months and the other 2 cases at 87.8 and 90.1 months, respectively. One responder underwent stereotactic cyst aspiration at 23.4 months. Two nonresponders required hyperbaric oxygen for presumed radionecrosis, with 1 patient, who had the radionecrosis confirmed by biopsy, had previously (7 years earlier) been irradiated using fractionated photons to 5400 cGy plus intracystic P-32 and had stereotactic radiosurgery 4 years before PBT.
Of the 8 interventions, 5 (63%) occurred during radiographic follow-up. Three of the 5 in nonresponders as shown in Figure 1. One nonresponder was restarted on multiagent chemotherapy per Children's Oncology Group A9952 protocol, just 7.7 months after PBT but was intolerant. One pseudoprogressing responder developed central nervous system 3 T-cell acute lymphoblastic leukemia at 21.9 months after PBT and was started on chemotherapy. This occurred 79.6 months and 6.6 years after original diagnosis in a patient who underwent 2 subtotal resections and had received 4 chemotherapeutic agents (carboplatin, cisplatin, vincristine, etoposide) before PBT. Univariate exploratory analysis failed to uncover statistically significant predictors of the need for post-PBT intervention.
Discussion
Our analysis is, to our knowledge, the first extended volumetric follow-up study of a cohort of children treated for pilocytic astrocytoma with PBT. As a WHO grade I neoplasm, gross total surgical resection remains the standard of care, with studies clearly showing reresection of residual disease leading to extended progression-free survival [20]. Unfortunately, not all lesions are amenable to resection or reresection, leaving RT as the local-control modality, with chemotherapy regimens used to delay the developmental consequences of neuroirradiation in children [7, 8]. In adults, the cost of such a delay was studied in the European Organisation for Research and Treatment of Cancer 22845 clinical trial [12], comprising mostly nonpilocytic astrocytomas, demonstrated extended progression-free survival (5.3 versus 3.4 years) and better seizure control at 1 year without an OS benefit in patients undergoing immediate RT. A 2006 retrospective study [21] also found improved progression-free survival with immediate RT. This risk of progression must be balanced against the risk of treatment sequelae on the developing brain in pediatric patients. Subgroup analysis of the Himtumorstudien–low-grade glioma 1996 study of 117 patients who underwent RT for inoperable or progressive pilocytic astrocytomas to a median dose of 5400 cGy revealed excellent 10-year OS of 97% and progression-free survival of 70%, leading the authors to conclude that RT can provide excellent control in these patients and to suggest a lower total dose of 5040 cGy to minimize side effects; children younger than 5 years received 4520 cGy, and iodine-125 brachytherapy was permitted [9, 10].
A 2006 American Society for Radiation Oncology abstract [22] analyzing MRI T1-contrasted volume changes after 5040 cGy photon therapy for pilocytic astrocytomas showed 70% of patients had a transient increase in CTV within 6 months of RT, 3 occurring rapidly. The authors concluded this pseudoprogression was most pronounced at 6 months and spontaneously resolved by 18 months, which is fairly consistent with our data. The concept of pseudoprogression proposed by Hoffman et al [23] in 1979 following RT for high-grade glioma has remained ill-defined, with a recent article [15] defining it as “transient increase in the size of the tumor following RT that resolved without further anti-tumor therapy.” A 2011 retrospective review of patients with high-grade glioma treated with chemoradiation (and temozolomide) found a 21% rate of pseudoprogression, defined in the first 3 months after CRT [24]. Bakardjiev et al [17] described this phenomenon by including radiographic changes of increased size, intensity/enhancement, edema, mass effect, or cysts/cavitations and uncovered an incidence of 43% with most occurring 9 to 12 months after RT and resolving by 15 to 21 months; only 1 patient developed new symptoms.
Chawla et al [16] investigated “spurious progression” and found an increased incidence in low-grade lesions, with a median time to development of 2.4 months and median time to stability or regression of 4 months, both time points inconsistent with this report and perhaps confounded by the inclusion of edematous changes. A recent article from Pittsburgh, Pennsylvania [15] retrospectively analyzed 24 pediatric patients after a median of 5220 cGy RT for inoperable or residual low-grade gliomas with 71% JPAs, with 2 of 24 patients receiving PBT. Pseudoprogression, defined as a transient increase in the size of the tumor after RT without further antitumor therapy, occurred in 54% of patients, beginning at approximately 6 months, peaking at 8 months, and lasting a median of 2.1 years, with larger target size trending to significance; 70% of patients required steroid therapy for symptoms [15].
The promise of integral dose reduction with particle therapy has led to the adoption of PBT for pediatric brain tumors [25, 26]. A report from Heidelberg, Germany, on 19 patients with low-grade glioma treated with 5400 cGyE for progressive disease describes 1 case of pseudoprogression occurring with clinical decline and edema at 1 to 2 months, with resolution at 6 months [25]. Progressive symptoms are commonly addressed with steroids but Foster et al [27] reviewed the cases of 5 pediatric patients with symptomatic radiographic enlargement of a low-grade glioma at a median 4.2 months after RT and found administration of bevacizumab at 5 to 10 mg/kg intravenously every 2 to 4 weeks to be associated with radiographic improvement and ability to wean steroid dosing to physiologic levels; none of the 5 patients received further antineoplastic therapies.
The strengths of this report begin with histologic confirmation of JPAs treated with PBT. Three-dimensional, contoured volumes by 1 doctor (E.M.M.) added significant quality assurance. A definition for pseudoprogression was abstracted from a graphic representation of retrospective, T1-contrasted volumes, allowing conclusions with incomplete steroid administration records while defining pseudoprogression independent of edematous changes. Limitations include those biases inherent to retrospective reviews, such as selection, misclassification, and information bias. Some critics may see fault with restricting the analysis to T1-contrasted series, but this was done purposely to rule out confounding by edematous changes and to provide comparisons to prior published work [15–17, 22]. Incomplete follow-up records from the particle therapy center—inherent to the international referral network—prohibited collection of doses and cycle numbers for chemotherapeutic agents.
Conclusion
This study demonstrates the benefit of 5400 cGyE PBT in a pediatric cohort of heavily pretreated, inoperable or progressive pilocytic astrocytomas, with a crude response rate of 73% and, among the responders, a 27% incidence of pseudoprogression, with peak volume observed at 3 to 8 months and resolving by 18 months, consistent with prior studies. However, 53% of patients required a post-PBT intervention, with only 1 patient reinitiated on chemotherapy for JPA. With extended OS, the actuarial 5-year IFS of 72.2% suggests these patients be followed vigilantly for the need for supportive therapy. Because these outcomes are similar to those observed with photon RT, long-term data to evaluate the expected reduction in late effects from using PBT is needed. Based on this cohort, by 3 months, responders had successive volume reductions, whereas nonresponders or pseudoprogressing patients were determined to have increasing volumes. Further delineation may best be reserved until the onset of symptoms to permit for volume regression (pseudoprogression volumes began regressing by 12 months), which, in this cohort, occurred nearly as often as a nonresponse to PBT. Although this report is the most robust volumetric analysis to date in JPA treated with PBT, to our knowledge, investigations of noninvasive imaging modalities, spectroscopy, variables predictive of volume changes, and possible therapies, such as bevacizumab are warranted to further define pseudoprogression versus failure to respond and the subsequent need for continued therapy.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2015 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/ Accessed March 23, 2016.
- 2.Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA, Edwards BK. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–36. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadighi Z, Slopis J. Pilocytic astrocytoma: a disease with evolving molecular heterogeneity. J Child Neurol. 2013;28:625–32. doi: 10.1177/0883073813476141. [DOI] [PubMed] [Google Scholar]
- 4.Halperin EC, Wazer DE, Perez CA, Brady LW. Perez & Brady's Principles and Practice of Radiation Oncology 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 5.Wallner KE, Gonzales MF, Edwards MS, Wara WM, Sheline GE. Treatment results of juvenile pilocytic astrocytoma. J Neurosurg. 1988;69:171–6. doi: 10.3171/jns.1988.69.2.0171. [DOI] [PubMed] [Google Scholar]
- 6.Shaw EG, Scheithauer BW, Gilbertson DT, Nichols DA, Laws ER, Earle JD, Daumas-Duport C, O'Fallon JR, Dinapoli RP. Postoperative radiotherapy of supratentorial low-grade gliomas. Int J Radiat Oncol Biol Phys. 1989;16:663–8. doi: 10.1016/0360-3016(89)90482-3. [DOI] [PubMed] [Google Scholar]
- 7.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–7. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30:2641–7. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller K, Gnekow A, Falkenstein F, Scheiderbauer J, Zwiener I, Pietsch T, Warmuth-Metz M, Voges J, Nikkhah G, Flentje M, Combs SE, Vordermark D, Kocher M, Kortmann RD. Radiotherapy in pediatric pilocytic astrocytomas: a subgroup analysis within the prospective multicenter study HIT-LGG 1996 by the German Society of Pediatric Oncology and Hematology (GPOH) Strahlenther Onkol. 2013;189:647–55. doi: 10.1007/s00066-013-0357-7. [DOI] [PubMed] [Google Scholar]
- 10.Gnekow AK, Falkenstein F, von Hornstein S, Zwiener I, Berkefeld S, Bison B, Warmuth-Metz M, Driever PH, Soerensen N, Kortmann RD, Pietsch T, Faldum A. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14:1265–84. doi: 10.1093/neuonc/nos202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lizarraga KJ, Gorgulho A, Lee SP, Rauscher G, Selch MT, DeSalles AA. Stereotactic radiation therapy for progressive residual pilocytic astrocytomas. J Neurooncol. 2012;109:129–35. doi: 10.1007/s11060-012-0877-5. [DOI] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström PO, Collette L, Piérart M, Mirimanoff R, Karim AB, EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–90. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 13.Mansur DB, Rubin JB, Kidd EA, King AA, Hollander AS, Smyth MD, Limbrick DD, Park TS, Leonard JR. Radiation therapy for pilocytic astrocytomas of childhood. Int J Radiat Oncol Biol Phys. 2011;79:829–34. doi: 10.1016/j.ijrobp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Kayama T, Tominaga T, Yoshimoto T. Management of pilocytic astrocytoma. Neurosurg Rev. 1996;19:217–20. doi: 10.1007/BF00314833. [DOI] [PubMed] [Google Scholar]
- 15.Naftel RP, Pollack IF, Zuccoli G, Deutsch M, Jakacki RI. Pseudoprogression of low-grade gliomas after radiotherapy. Pediatr Blood Cancer. 2015;62:35–9. doi: 10.1002/pbc.25179. [DOI] [PubMed] [Google Scholar]
- 16.Chawla S, Korones DN, Milano MT, Hussain A, Hussien AR, Muhs AG, Mangla M, Silberstein H, Ekholm S, Constine LS. Spurious progression in pediatric brain tumors. J Neurooncol. 2012;107:651–7. doi: 10.1007/s11060-011-0794-z. [DOI] [PubMed] [Google Scholar]
- 17.Bakardjiev AI, Barnes PD, Goumnerova LC, Black PM, Scott RM, Pomeroy SL, Billett A, Loeffler JS, Tarbell NJ. Magnetic resonance imaging changes after stereotactic radiation therapy for childhood low grade astrocytoma. Cancer. 1996;78:864–73. doi: 10.1002/(SICI)1097-0142(19960815)78:4<864::AID-CNCR25>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Farr JB, Mascia AE, Hsi WC, Allgower CE, Jesseph F, Schreuder AN, Wolanski M, Nichiporov DF, Anferov V. Clinical characterization of a proton beam continuous uniform scanning system with dose layer stacking. Med Phys. 2008;35:4945–54. doi: 10.1118/1.2982248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anferov VA. Scan pattern optimization for uniform proton beam scanning. Med Phys. 2009;36:3560–7. doi: 10.1118/1.3158731. [DOI] [PubMed] [Google Scholar]
- 20.Bowers DC, Krause TP, Aronson LJ, Barzi A, Burger PC, Carson BS, Weingart JD, Wharam MD, Melhem ER, Cohen KJ. Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg. 2001;34:229–34. doi: 10.1159/000056027. [DOI] [PubMed] [Google Scholar]
- 21.Kidd EA, Mansur DB, Leonard JR, Michalski JM, Simpson JR, Perry A. The efficacy of radiation therapy in the management of grade I astrocytomas. J Neurooncol. 2006;76:55–8. doi: 10.1007/s11060-005-2913-1. [DOI] [PubMed] [Google Scholar]
- 22.McMullen KP, Papagikos MA, Glazier SS, McLean TW, Maldjian JA, Shaw EG. Pseudo-progression based on MRI changes following radiation therapy for pilocytic astrocytoma [abstract] Int J Radiat Oncol Biol Phys. 2006;66:S243–4. [Google Scholar]
- 23.Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50:624–8. doi: 10.3171/jns.1979.50.5.0624. [DOI] [PubMed] [Google Scholar]
- 24.Gunjur A, Lau E, Taouk Y, Ryan G. Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: a retrospective analysis. J Med Imaging Radiat Oncol. 2011;55:603–10. doi: 10.1111/j.1754-9485.2011.02319.x. [DOI] [PubMed] [Google Scholar]
- 25.Hauswald H, Rieken S, Ecker S, Kessel KA, Herfarth K, Debus J, Combs SE. First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol. 2012;7:189. doi: 10.1186/1748-717X-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Combs SE, Kessel KA, Herfarth K, Jensen A, Oertel S, Blattmann C, Ecker S, Hoess A, Martin E, Witt O, Jakel O, Kulozik AE, Debus J. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol. 2012;7:170. doi: 10.1186/1748-717X-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster KA, Ares WJ, Pollack IF, Jakacki RI. Bevacizumab for symptomatic radiation-induced tumor enlargement in pediatric low grade gliomas. Pediatr Blood Cancer. 2014;62:240–5. doi: 10.1002/pbc.25277. [DOI] [PubMed] [Google Scholar]