Abstract
Purpose:
Radiosurgery treatment of liver metastases with photon beams has been an established method for more than a decade. One method commonly used is the stereotactic body radiation therapy (SBRT) technique. The aim of this study was to investigate the potential sparing of the organs at risk (OARs) that the use of intensity-modulated proton therapy (IMPT), instead of SBRT, could enable.
Patients and Methods:
A comparative treatment-planning study of photon-beam and proton-beam based liver-cancer radiosurgery was performed. Ten patients diagnosed with liver metastasis and previously treated with SBRT at the Karolinska University Hospital were included in the study. New IMPT plans were prepared for all patients, while the original plans were set as reference plans. The IMPT planning was performed with the objective of achieving the same target dose coverage as with the SBRT plans. Pairwise dosimetric comparisons of the treatment plans were then performed for the OARs. A 2-sided Wilcoxon signed-rank test with significance level of 5% was carried out.
Results:
Improved sparing of the OARs was made possible with the IMPT plans. There was a significant decrease of the mean doses delivered to the following risk organs: the nontargeted part of the liver (P = .002), the esophagus (P = .002), the right kidney (P = .008), the spinal cord (P = .004), and the lungs (P = .002). The volume of the liver receiving less than 15 Gy was significantly increased with the IMPT plans (P = .004).
Conclusion:
The IMPT-based radiosurgery plans provided similar target coverage and significant dose reductions for the OARs compared with the photon-beam based SBRT plans. Further studies including detailed information about varying tissue heterogeneities in the beam path, due to organ motion, are required to evaluate more accurately whether IMPT is preferable for the radiosurgical treatment of liver metastases.
Keywords: liver metastases, treatment planning, stereotactic body radiation therapy, intensity-modulated proton therapy
Introduction
The liver is the most common site of metastatic spread for several malignancies, mainly primary gastrointestinal tumors [1]. Surgical resection is the treatment of choice for both primary liver tumors and oligo-liver metastases. Nevertheless, approximately 70% to 90% of liver metastases are not resectable [2]. A number of noninvasive modalities for treatment of liver malignancies are currently being used in clinics, for example, radiofrequency ablation and radiation therapy, with promising local control rates [3–5]. Several reports have described the feasibility of dose escalation in radiation therapy of focal lesions if only a limited part of the healthy liver volume is treated [6, 7]. However, a nonnegligible risk of radiation-induced side effects after radiation therapy remains, increasing with the volume of the treated tumor. Long-term migration to more advanced Child-Pugh scores have been reported to cause radiation-induced liver disease, resulting in liver failure foremost in patients with scores of B and C [8].
Photon-based stereotactic body radiation therapy (SBRT) has emerged as a radiation surgery technique with which high fraction doses can be delivered to targets of moderate sizes in different parts of the body [7], for example, targets in the liver. Treatment planning studies of SBRT implemented with proton beams have also been reported [9, 10]. Radiation therapy of lesions located in the liver is influenced by anatomical changes due to respiratory motion. Thus, high-precision radiation surgery of liver cancer remains challenging, despite the recent advances made in methods used for patient setup and image-guided radiation therapy [11]. In order to reduce the treatment delivery uncertainties, methods such as 4-dimensional (4D)-planning, respiratory gating and abdominal pressure can be used.
In this study, we compared photon-based SBRT with intensity-modulated proton therapy (IMPT)–based radiosurgery, on a treatment-planning basis, for the treatment of liver metastases of different sizes and locations. The main objective of the IMPT planning was to create similar dose coverage of the planning target volume (PTV) as obtained with the SBRT plans. The aim was thereafter to estimate quantitatively the potential dosimetric advantages of IMPT over SBRT in terms of sparing of the organs at risk (OARs). Of primary concern was the dose delivered to the healthy part of the liver, which is the main limiting factor in SBRT of liver malignancies.
Materials and Methods
Patient Selection and Treatment Planning
For this study, 10 patients previously treated for metastases in the liver with SBRT at the Department of Oncology and Pathology at Karolinska University Hospital were included. These patients were diagnosed with tumors of different volumes (range, 18.6 to 332.3 cm3) and locations (Table 1).
Table 1.
Patient and treatment characteristics.
|
Patient no. |
Modality (photon RT) |
Fractionation |
Abdominal pressure |
Target volume (cm3) |
Target location |
| 1 | Static fields | 15 Gy × 3 | Yes | 59.6 | Central-peripheral |
| 2 | Static fields | 17 Gy × 3 | Yes | 73.1 | Superior |
| 3 | VMAT | 8 Gy × 7 | No | 332.3 | Posterior/whole liver extent axially |
| 4 | Static fields | 8 Gy × 5 | Yes | 302.6 | Central/whole liver extent axially |
| 5 | Static fields | 7 Gy × 8 | No | 66.4 | Central-periphery |
| 6 | VMAT | 7 Gy × 8 | No | 294.1 | Central-superior |
| 7 | Static fields | 15 Gy × 3 | No | 18.6 | Central-periphery |
| 8 | VMAT | 7 Gy × 8 | Yes | 78.6 | Superior |
| 9 | Static fields | 17 Gy × 3 | No | 30.2 | Central |
| 10 | Static fields | 15 Gy × 3 | No | 72.3 | Central |
Abbreviations: RT, radiation therapy; VMAT, volumetric-modulated arc therapy.
The static computed tomography (CT) examinations used for the treatment planning contained 3.0-mm-thick slices. The internal target volume (ITV) was delineated by contouring the gross tumor volume in different breathing phases in the 4D-CT image study. Expansion margins of 5 mm in the transversal direction and 10 mm in the craniocaudal direction were added to the ITV to create the PTV.
The SBRT plans were prepared with the Eclipse treatment planning system, version 11.0.42 (Varian Medical Systems, Palo Alto, California). Beam data obtained for 6 MV photon beams, produced by Varian linear accelerators (Varian Medical Systems), were used in the planning. Two types of SBRT were used for the patient treatments, that is, a multiple static-field technique or volumetric-modulated arc therapy (VMAT). The VMAT was mostly used for treatments of large targets or when critical structures were located in the proximity of the target. The stereotactic body frame was used for all patients. Furthermore, abdominal pressure was used in those cases when the target motion amplitude was larger than the expansion margins of the PTV. The dose prescription in the SBRT plans was made at the periphery of the PTV. The central parts of the PTV received maximum doses in the interval of 145% to 160% of the prescribed dose. The analytical anisotropic algorithm was used for the photon-beam dose calculation and the progressive resolution optimizer algorithm was used for optimization of the VMAT plans. Descriptions of the patient setup, the photon-beam SBRT technique used, the prescribed doses and the sizes of the PTV volumes for each patient are shown in Table 1.
The SBRT plans were set as reference plans, and all patients were then retrospectively planned with IMPT. The Eclipse treatment planning system was also used for the IMPT treatment planning. To eliminate possible uncertainties specific for the proton dose calculation, the stereotactic frame was assigned the Hounsfield unit value of air in the planning CT studies before the treatment planning. A 2-field beam-setup configuration was used to prepare the IMPT plans. The choice of beam angles varied depending on the target location, the size of the PTV, and the proximity of the OARs. Direct irradiation of the spinal cord and right kidney was avoided. The proton beam data used in the treatment planning were obtained from the Skandion clinic, a facility with an IBA (Ion Beam Applications S.A., Louvain-La-Neuve, Belgium) proton cyclotron (the range of incident proton energies was 60 to 230 MeV). The spot-scanning technique combined with multifield optimization, that is, IMPT, was used in the planning. The proton dose calculation was made using the proton convolution superposition algorithm (version 13.0.28). The IMPT treatment planning was performed with the objective of making the dose-volume histogram (DVH) for the PTV coincide with that obtained with the SBRT plan. The optimization objectives were set as follows: 100% of the PTV should receive 100% of the prescribed dose, and the maximum dose given to the PTV should be in the range from 145% to 160% of the prescribed dose. In addition, 3 distinct isodose levels (between 70% and 97% of the maximum dose) in the photon plans were converted into structures and used in the optimization of the proton plans.
Dosimetric Analysis of the Treatment Plans
To quantify the obtained dose conformity around the PTV for the 2 alternative modalities, the conformity index (CI) was calculated using the definition in Report No. 62 of the International Commission on Radiation Units and Measurements [12], that is, the quotient of the treated volume (in this study it was the volume enclosed by the prescription [100%] isodose surface) and the volume of the PTV. A relative biological effectiveness of 1.1 was assumed for the proton beams, and the produced doses were reported in isoeffective equivalent dose, Gy (IsoE).
The OARs considered relevant for this study were the normal liver tissue (main risk organ), the esophagus, the right kidney, the lungs, and the spinal cord. The normal liver was considered to be the part of the liver that is not included in the PTV. The Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) guidelines define risk levels for the whole liver, excluding the gross tumor volume [13]. The dosimetric evaluation was based on pairwise comparisons of the dose-volume values for the different OARs obtained from the treatment plans prepared with either photon or proton beams. A comparison of the dosimetric values obtained with those recommended by QUANTEC (Table 2) was also performed. The dose-volume metrics used for the evaluation were the mean dose in the liver and the volume of the normal liver that received a dose <15 Gy, the mean doses given to the right kidney and the lungs, V35 and the mean dose for the esophagus, and the maximum and mean doses given to the spinal cord.
Table 2.
QUANTEC recommendations for the threshold values of the dose-volume metrics for the organs at risk and the corresponding clinical endpoints.
|
Organ |
Volume segmented |
Endpoint |
Dose-volume metric |
Rate (%) |
| Liver | Whole liver, GTV | Classic RILD | Dmean <15 Gy (3 fx SRS) | <5 |
| Dmean <20 Gy (6 fx SRS) | ||||
| >700 cm3 of normal liver | Classic RILD | VD<15 >700 cm3 | <5 | |
| Right kidney | Bilateral whole kidney | Clinically relevant renal dysfunction | Dmean <28 Gy | <50 |
| Lungs | Whole organ | Symptomatic pneumonitis | Dmean <13 Gy | 10 |
| Spinal cord | Partial organ | Myelopathy | Dmax <20 Gy (3 fx SRS) | 1 |
| Esophagus | Whole organ | Grade ≥2 acute esophagitis | V35 <50% | <30 |
| Grade ≥3 acute esophagitis | Dmean <34 Gy | 5–20 |
Abbreviations: QUANTEC, Quantitative Analyses of Normal Tissue Effects in the Clinic; GTV, gross tumor volume; RILD, radiation-induced liver disease; fx, fraction; SRS, stereotactic radiosurgery.
Note: Data from Marks LB et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-9.
A 2-sided Wilcoxon signed-rank test with a significance level of P < .05 was used for the statistical comparisons of the dosimetric values extracted from the treatment plans.
Results
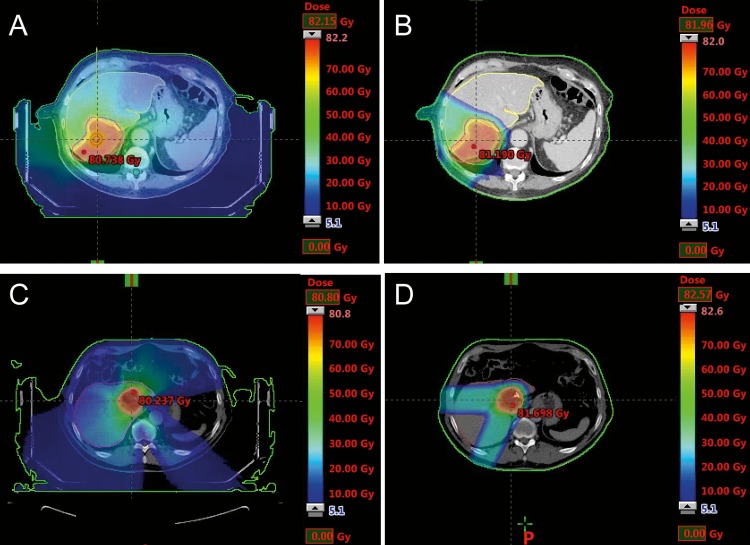
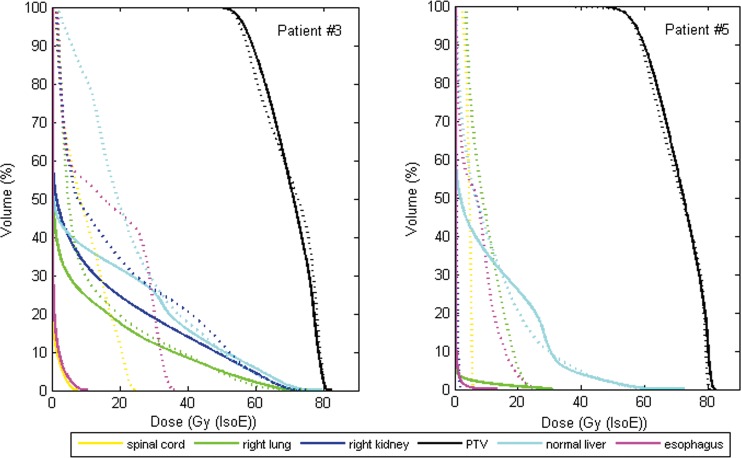
A visual comparison of the calculated dose distributions in the photon-beam and proton-beam based plans indicates that larger volumes of healthy tissue were irradiated in the VMAT and the static-field SBRT plans compared with the corresponding IMPT plans. Planned dose distributions for 2 representative patient cases (patient No. 3 and patient No. 5) are presented in Figure 1. Patient No. 3 had a large target volume (332.3 cm3) and was planned for VMAT-based SBRT or IMPT, as shown in Figure 1A and 1B. Patient No. 5 had a small target volume (66.4 cm3) and was planned for static-field SBRT or IMPT (Figure 1C and 1D). The DVHs obtained for the PTVs and the OARs for these 2 patients are displayed in Figure 2. The objective to produce a similar DVH for the PTV with the IMPT plan as with the SBRT plan was reached, as illustrated in Figure 2. However, it should be emphasized that the DVHs do not provide spatial information, that is, they do not indicate whether the dose distributions are matching pairwise from voxel to voxel. The same value of the CI was obtained with either the SBRT or the IMPT plans for all patients (0.99), except for patient No. 3, for whom the CI was 0.93 with the VMAT plan and 0.97 with the IMPT plan. The important differences between the plans, obtained with the 2 different beam types, could be observed in the DVHs obtained for the OARs. There was a clear reduction of the irradiated volumes of most OARs in all patients with IMPT.
Figure 1.
Calculated dose distributions at the position of the planning isocenter for (A–B) patient No. 3 and (C-D) patient No. 5. (A) A volumetric-modulated arc therapy based stereotactic body radiation therapy (SBRT) plan. (B) A 2-field intensity-modulated proton therapy (IMPT) plan. (C) A static-field SBRT plan. (D) A 2-field IMPT plan.
Figure 2.
The dose-volume histograms obtained for the planning target volumes and for the organs at risk with the stereotactic body radiation therapy plans (dashed curves) or the intensity-modulated proton therapy plans (solid curves) for 2 patients (No. 3 and No. 5).
Clinically relevant dose-volume values for the OARs are presented in Tables 3 and 4. The mean dose given to the normal liver tissue decreased from an average value of 11.3 Gy in the SBRT plans to 6.9 Gy (IsoE) in the IMPT plans. This decrease was mainly due to a reduction of the volumes irradiated with low and intermediate doses (Figure 2). The volume of the liver receiving a physical dose of less than 15 Gy increased from an average value of 1181.5 cm3 in the SBRT plans to 1354.7 cm3 in the IMPT plans. The threshold value for the mean dose given to the normal liver, for observing the classic radiation-induced liver disease, was exceeded for 1 of the 10 patients in the SBRT plan (with 3.8 Gy for patient No. 3). The mean dose given to the healthy part of the liver was below this threshold value in the IMPT plans for all patients. Patient No. 3 also did not pass the volume constraint for normal liver (VD<15 > 700 cm3) with the SBRT plan. However, this constraint was passed for all patients with the IMPT plans.
Table 3.
Dosimetric values for the liver, right kidney and spinal cord. Proton doses are given in Gy (IsoE).
| Patient # |
Normal liver |
Right kidney |
Spinal cord |
||||||||
| Mean dose (Gy) |
VD<15 (cm3) |
Mean dose (Gy) |
Maximum dose (Gy) |
Mean dose (Gy) |
|||||||
| Photon |
Proton |
Photon |
Proton |
% increase |
Photon |
Proton |
Photon |
Proton |
Photon |
Proton |
|
| 1 | 8.1 | 3.5 | 1550.6 | 1787.6 | 13.3 | 0.5 | 0.0 | 21.0 | 1.4 | 9.3 | 0.0 |
| 2 | 8.9 | 4.0 | 1535.0 | 1718.3 | 10.7 | 1.2 | 0.0 | 4.0 | 0.0 | 0.3 | 0.0 |
| 3 | 23.8 | 14.9 | 397.6 | 726.0 | 45.2 | 18.6 | 12.8 | 24.7 | 7.8 | 9.7 | 0.4 |
| 4 | 16.6 | 10.9 | 740.6 | 928.8 | 20.3 | 8.6 | 1.7 | 21.0 | 0.1 | 9.3 | 0.0 |
| 5 | 11.5 | 10.4 | 740.6 | 733.1 | -1.0 | 0.5 | 0.0 | 5.6 | 0.0 | 4.4 | 0.0 |
| 6 | 14.0 | 6.8 | 1054.0 | 1352.7 | 22.1 | 1.0 | 0.0 | 10.5 | 3.6 | 3.4 | 0.1 |
| 7 | 5.5 | 3.6 | 1017.9 | 1059.2 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 8 | 6.3 | 3.3 | 1658.2 | 1730.8 | 4.2 | 0.7 | 0.0 | 18.9 | 0.0 | 7.8 | 0.0 |
| 9 | 6.6 | 3.3 | 2239.4 | 2474.6 | 9.5 | 0.5 | 0.0 | 6.1 | 0.0 | 1.3 | 0.0 |
| 10 | 11.6 | 8.1 | 881.4 | 1036.0 | 14.9 | 0.0 | 0.0 | 7.5 | 0.3 | 3.2 | 0.0 |
| Mean | 11.3 | 6.9 | 1181.5 | 1354.7 | 14.3 | 3.2 | 1.5 | 11.9 | 1.3 | 4.9 | 0.0 |
| P-value | 0.002 | 0.004 | 0.008 | 0.004 | 0.004 | ||||||
Table 4.
Dosimetric values for the lungs and esophagus; proton doses are given in Gy (IsoE).
|
Patient no. |
Both lungs, Mean dose (Gy) |
Right lung, Mean dose (Gy) |
Esophagus, Mean dose (Gy) |
|||
|
Photon |
Proton |
Photon |
Proton |
Photon |
Proton |
|
| 1 | 2.2 | 1.2 | 3.3 | 2.2 | 7.0 | 1.7 |
| 2 | 0.5 | 0.0 | 0.6 | 0.0 | 1.8 | 0.0 |
| 3 | 9.6 | 5.5 | 11.9 | 8.9 | 16.3 | 0.6 |
| 4 | 2.2 | 0.7 | 3.3 | 0.7 | 5.4 | 0.0 |
| 5 | 1.1 | 0.2 | 1.1 | 0.4 | 7.1 | 0.2 |
| 6 | 5.3 | 3.6 | 8.1 | 6.7 | 7.1 | 0.5 |
| 7 | 0.1 | 0.0 | 0.2 | 0.0 | 0.3 | 0.0 |
| 8 | 4.4 | 0.2 | 5.0 | 0.4 | 12.7 | 0.0 |
| 9 | 0.6 | 0.0 | 0.6 | 0.0 | 0.4 | 0.0 |
| 10 | 2.3 | 0.5 | 3.2 | 0.9 | 2.2 | 0.8 |
| Mean | 2.8 | 1.2 | 3.7 | 2.1 | 6.0 | 0.4 |
| P value | .002 | .002 | .002 | |||
The average mean dose given to the right kidney decreased from 3.2 Gy in the photon plans to 1.5 Gy (IsoE) in the proton plans. For patient No. 3, however, for whom the right kidney was adjacent to the target, higher mean doses were given to the right kidney in both the photon and proton plans. For all patients, the mean doses given to the right kidney were below the limit of 28 Gy for which clinically relevant renal dysfunction may appear. However, the latter threshold value has been determined for bilateral kidney irradiation. In our study, this value was used for comparison with the dose given to the right kidney only.
For the spinal cord, the maximum doses decreased from an average value of 11.9 Gy in the photon plans to 1.3 Gy (IsoE) in the proton plans. The mean doses given to the spinal cord also decreased, from 4.9 Gy in the photon plans to 0.0 Gy (IsoE) in the proton plans. The threshold value above which radiation myelopathy may be observed was exceeded with 1.0 Gy for patient No. 1 with the SBRT plan. For 2 other patients (patient No. 3 and patient No. 4), the maximum doses given to the spinal cord were 21.0 Gy and 24.7 Gy, respectively, in the SBRT plans. Both of these values are above the 20.0 Gy threshold. However, this threshold value for the maximum dose has been determined for hypofractionated treatments given in 3 fractions. For these 2 patients (No. 3 and No. 4), the doses were given in 7 and 5 fractions, respectively. For all patients, the maximum doses given to the spinal cord were reduced to below the threshold value in the IMPT plans.
The mean dose given to both lungs, counted as 1 organ, may be considerably lower than the dose given to the right lung, which is adjacent to the liver. Therefore, the mean doses given to the right lung and to both lungs (counted as 1 organ) were separately determined in this study. There was a reduction of the mean doses given to both lungs, from an average value of 2.8 Gy in the photon plans to 1.2 Gy (IsoE) in the proton plans. The average mean dose, given to the right lung only, decreased from 3.7 Gy in the photon plans to 2.1 Gy (IsoE) in the proton plans. The threshold value for observing symptomatic pneumonitis was not exceeded in any of the SBRT or IMPT plans.
The mean doses given to the esophagus decreased from an average value of 6.0 Gy in the photon plans to 0.4 Gy (IsoE) in the proton plans. The V35 of the esophagus was 0% in all treatment plans. The threshold values for observing acute esophagitis of grades ≥2 (V35 <50 %) or 3 (mean dose <34 Gy) were not exceeded in any of the treatment plans.
For all patients, the median of the mean doses given to the OARs were compared (Table 5). The median values of the percentage decrease of the mean doses given to the OARs with the IMPT—compared with the SBRT—plans are also presented. There was a significant reduction of the mean doses given to the OARs in the IMPT plans compared with the SBRT plans (P < .05).
Table 5.
Comparison of the median of the mean doses given to the organs at risk for all patients.
|
Organs at risk |
Median of mean dose (range), Gy |
Median of the percentage decrease in mean doses (range) |
P
value |
|
|
Photon plan |
Proton plan |
|||
| Healthy liver | 10.2 (5.5 to 23.8) | 5.4 (3.3 to 18.3) | 42.5 (9.6 to 56.8) | .002 |
| Esophagus | 6.2 (0.3 to 16.3) | 0.1 (0.0 to 1.7) | 98.6 (63.6 to 100) | .002 |
| Spinal cord | 3.9 (0.0 to 9.7) | 0.0 (0.0 to 0.4) | 100 (0.0 to 100) | .004 |
| Whole lung | 1.7 (0.1 to 9.6) | 0.4 (0.0 to 5.5) | 75.0 (32.1 to 100) | .002 |
| Right lung | 3.3 (0.2 to 11.9) | 0.7 (0.0 to 8.9) | 67.8 (32.1 to 100) | .002 |
| Right kidney | 0.6 (0.0 to 18.6) | 0.0 (0.0 to 12.8) | 100 (0.0 to 100) | .008 |
Discussion
The results of this study indicate significant dosimetric advantages, in terms of sparing of the OARs, of IMPT compared with photon-beam based SBRT for the treatment of liver metastases. The potential for an improved normal-tissue sparing was also indicated in the comparisons of the obtained dose-volume values with the QUANTEC recommendations. Previously published reports comparing the use of proton and photon beams for conventionally fractionated [14–16] and hypofractionated [9] radiation therapy of liver malignancies have obtained similar results. Furthermore, clinical trials with proton and carbon-ion beams for the treatment of hepatocellular carcinoma (HCC) obtained improved local control rates, compared to photon-beam based radiation therapy, with the same or lower high-grade toxicity [17]. In a previous comparison of IMRT and IMPT plans for radiosurgery of large metastases in the liver, Petersen et al [9] reported an improved sparing of the normal liver tissue with IMPT. In the present study, an improved sparing of normal liver tissue with IMPT was observed for both small and large PTVs. The mean doses given to the normal liver decreased with a median value of 42.5% (range, 9.6% to 56.8%), and the volume of the normal liver tissue receiving <15 Gy increased with a median value of 11% (range, −1.0% to 45.2%) with IMPT. Furthermore, for patients with larger targets, we noticed a relative increase in the sparing of the normal liver tissue with IMPT compared with results obtained for patients with smaller targets.
The choice of beam angles is an important aspect when planning proton-beam treatments of liver cancer. The increased accuracy of the IMPT technique improves the possibility to spare the functional part of a liver with underlying liver disease, that is, cirrhosis [18]. Information about the functional parts of the liver can be obtained with, for example, positron emission tomography–magnetic resonance imaging studies, which then could be used in the IMPT planning.
Radiation therapy of malignancies in the liver is, to a certain degree, influenced by anatomical variations, mainly due to the breathing motion. In this work, the effects of organ motion have been considered by using 4D-CT image studies and the ITV concept for the PTV delineation. However, further studies with 4D treatment planning are required to quantify the uncertainties in the dose delivery and to evaluate the robustness of both the SBRT and IMPT treatments. Petersen et al [9] suggested that the organ motion uncertainties during liver cancer treatments could be handled with increased PTV margins. However, we believe that the interplay effect also [19, 20], which is of importance for proton-beam spot scanning of moving targets, must be considered. In the worst-case scenario, this effect could produce cold spots in the target, which could not be remedied with increased PTV margins. The treatment delivery uncertainties can be handled by different means, for example, abdominal pressure, increased spot size, gating, and/or multiple repainting. The development of image-guided radiation therapy [11, 21–23] may further improve the robustness of proton therapy of tumors located in regions affected by involuntary motion and fluctuating tissue heterogeneities. Issues related to anatomical variations during proton radiation therapy of liver malignancies will be investigated in a future project.
In addition, in this work the IMPT treatment planning was performed on CT studies with structures delineated for photon therapy, maintaining the same target volumes and risk structures without considering the specific range uncertainties for proton beams. For the clinical implementation of proton therapy, all elements of the treatment planning should be adapted to the characteristics of proton beams.
Conclusion
In this study, a treatment-planning–based comparison of photon-beam SBRT and IMPT for radiosurgery of liver metastases was performed. The IMPT treatment was shown to provide significant dosimetric advantages, in terms of sparing of the OARs, compared with what was achievable with the SBRT technique. This can be expected to lead to a reduction of the number of observed side effects after radiosurgery of liver metastases. However, further studies with the aim of quantifying the uncertainties in the treatment delivery due to fluctuating tissue heterogeneities and involuntary organ motion should be carried out in order to accurately evaluate the advantages of using IMPT instead of SBRT for treating malignancies in the liver.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of interest: The authors have no conflicts to disclose.
Acknowledgments: This study was financially supported by the Swedish International Development Cooperation Agency (SIDA) through the International Science Programme (ISP). Financial support from the Cancer Research Funds of Radiumhemmet at Karolinska Institute, Stockholm, Sweden, is also gratefully acknowledged. The authors would like to acknowledge Bo Nilsson and Maja Malmberg for their valuable comments and critical suggestions, which strongly contributed to this report. Jan-Olov Persson at the Department of Mathematical Statistics is also acknowledged for statistics support.
References
- 1.Brodt P. Introduction. In: Brodt P, editor. Liver Metastasis: Biology and Clinical Management. Dordrecht, The Netherlands: Springer Netherlands; 2011. pp. 1–5. [Google Scholar]
- 2.Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol. 2014;5:190–7. doi: 10.3978/j.issn.2078-6891.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 4.Hung H. Treatment modalities for hepatocellular carcinoma. Curr Cancer Drug Targets. 2005;5:131–8. doi: 10.2174/1568009053202063. [DOI] [PubMed] [Google Scholar]
- 5.Wu KT, Wang CC, Lu LG, Zhang WD, Zhang FJ, Shi F, Li CX. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities. World J Gastroenterol. 2013;19:3649–57. doi: 10.3748/wjg.v19.i23.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–21. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 7.Lax I, Blomgren H, Naslund I, Svanstrom R. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677–83. doi: 10.3109/02841869409121782. [DOI] [PubMed] [Google Scholar]
- 8.Schulz RA, Huntzinger C, Blacksburg S, Rosenzweig K. Stereotactic body radiation therapy (SBRT) for early-stage primary liver cancer (HCC) Appl Radiat Oncol. 2013;4:12–8. [Google Scholar]
- 9.Petersen JB, Lassen Y, Hansen AT, Muren LP, Grau C, Hoyer M. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol. 2011;50:823–8. doi: 10.3109/0284186X.2011.590526. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi SJ, Liang X, Ding X, Zhu TC, Ben-Josef E, Plastaras JP, Metz JM, Both S, Apisarnthanarax S. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pract Radiat Oncol. 2015;5:209–18. doi: 10.1016/j.prro.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Mundt AJ, Roeske JC. Intensity Modulated Radiation Therapy: A Clinical Perspective. Shelton, CT: People's Medical Publishing House - USA; 2005. [Google Scholar]
- 12.International Commission on Radiation Units and Measurements (ICRU) Prescribing, Recording and Reporting Photon Beam Therapy. (Supplement to ICRU Report 50) ICRU Report 62. Bethesda, USA: International Commission on Radiation Units and Measurements; 1999. [Google Scholar]
- 13.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–9. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Krishnan S, Zhang X, Dong L, Briere T, Crane CH, Martel M, Gillin M, Mohan R, Beddar S. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim. 2008;33:259–67. doi: 10.1016/j.meddos.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Sugahara S, Oshiro Y, Nakayama H, Fukuda K, Mizumoto M, Abei M, Shoda J, Matsuzaki Y, Thono E, Tokita M, Tsuboi K, Tokuuye K. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2010;76:460–6. doi: 10.1016/j.ijrobp.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Kim JY, Lim YK, Kim TH, Cho KH, Choi SH, Jeong H, Kim DW, Park JH, Shin DH, Lee SB, Kim SS, Kim JY, Kim DY, Park JW. Normal liver sparing by proton beam therapy for hepatocellular carcinoma: Comparison with helical intensity modulated radiotherapy and volumetric modulated arc therapy. Acta Oncol. 2015;54:1827–32. doi: 10.3109/0284186X.2015.1009637. [DOI] [PubMed] [Google Scholar]
- 17.Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, Hori Y, Hishikawa Y, Ku Y, Murakami M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890–904. doi: 10.1002/cncr.26134. [DOI] [PubMed] [Google Scholar]
- 18.Meyer JJ, Czito BG, Willett CG. Particle radiation therapy for gastrointestinal malignancies. Gastrointest Cancer Res. 2007;1:S50–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips MH, Pedroni E, Blattmann H, Boehringer T, Coray A, Scheib S. Effects of respiratory motion on dose uniformity with a charged particle scanning method. Phys Med Biol. 1992;37:223–34. doi: 10.1088/0031-9155/37/1/016. [DOI] [PubMed] [Google Scholar]
- 20.Knopf AC, Hong TS, Lomax A. Scanned proton radiotherapy for mobile targets—the effectiveness of re-scanning in the context of different treatment planning approaches and for different motion characteristics. Phys Med Biol. 2011;56:7257–71. doi: 10.1088/0031-9155/56/22/016. [DOI] [PubMed] [Google Scholar]
- 21.Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–99. doi: 10.1038/nrclinonc.2012.194. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu S, Miyamoto N, Matsuura T, Fujii Y, Umezawa M, Umegaki K, Hiramoto K, Shirato H. A proton beam therapy system dedicated to spot-scanning increases accuracy with moving tumors by real–time imaging and gating and reduces equipment size. PLoS One. 2014;9:e94971. doi: 10.1371/journal.pone.0094971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori S, Karube M, Shirai T, Tajiri M, Takekoshi T, Miki K, Shiraishi Y, Tanimoto K, Shibayama K, Yasuda S, Yamamoto N, Yamada S, Tsuji H, Noda K, Kamada T. Carbon-ion pencil beam scanning treatment with gated markerless tumor tracking: an analysis of positional accuracy. Int J Radiat Oncol Biol Phys. 2016;95:258–66. doi: 10.1016/j.ijrobp.2016.01.014. [DOI] [PubMed] [Google Scholar]