Abstract
We have developed a robust optimization approach for intensity modulated proton therapy treatment plans with multi-isocenter large fields. The method creates a low-gradient field dose in the junction regions to mitigate the impact caused by misalignment errors and is more efficient than the conventional junction shifting technique.
Keywords: craniospinal irradiation, mesothelioma, optimization, intensity modulated proton therapy
Introduction
Proton therapy is being used for an increasing range of disease sites as a result of the development of patient-specific planning and delivery techniques that improve the therapeutic ratio by taking advantage of finite proton ranges in patients [1–6]. For large and irregularly shaped tumors, such as those requiring craniospinal irradiation (CSI) [4, 5, 7] and mesothelioma irradiation [3], techniques are being developed at our center. In those cases, the size of the target volume normally exceeds the mechanical limitations of the treatment field size, and multiple fields with different isocenters must be matched together to cover the target [8, 9]. Normally, the field dose in the junction area has a steep gradient, which makes the treatment plan sensitive to misalignment errors, and even small uncertainties can significantly affect dose uniformity. Traditionally, preventing the risk of dose deviation in junction regions usually requires a manual shift of the field junctions, which can be technically challenging.
In conjunction with the development of the ability to apply intensity-modulated proton therapy (IMPT) to more disease sites, there has been major progress in the robust optimization techniques [10–13]. Robust optimization methods have been developed for mitigating the effects of proton range, setup, and anatomical motion uncertainties on dose delivered to a patient. However, none of the robust optimization methods reported in the literature are dealing with junction mismatch, which is special for large and irregular targets.
Here, we introduce a general robust optimization approach for IMPT plans with multi-isocenter large fields. This approach incorporates field misalignment uncertainties during the optimization process and generates a low-gradient field dose in junction regions.
Materials and Methods
We selected one CSI case and one mesothelioma case to demonstrate the use of the proposed approach. Both patients underwent the simulation in the supine position. Images were obtained from patients in the treatment position with a multi-slice computed tomography scanner at a 2.5-mm slice thickness. Target structures and organs at risk were outlined by experienced dosimetrists or radiation oncologists. The clinical target volume (CTV) in the CSI patient comprised the brain and spinal canal and was extended caudally to just beyond the thecal sac. In the mesothelioma patient, the gross tumor volume encompassed gross disease on the postsurgical positron emission computer tomography scan, the CTV was contoured by radiation oncologist, and the planning target volume (PTV) was consist with a 0.5-cm margin expansion around the gross tumor volume plus a 6-mm internal margin and a 1-cm external margin expansion on CTV.
For the CSI patient, a radiobiological equivalent dose of 36 Gy in 1.8-Gy fractions was prescribed for CTV. For the mesothelioma patient, the prescription dose was 45 Gy in 1.8-Gy fractions to PTV. For contouring, spot arrangement, and dose we used the Eclipse version 13.0 system (Varian Medical Systems, Palo Alto, California). The robust optimization was performed using an in-house proton treatment planning system [10]. All plans were normalized to 95% of target volume (ie, CTV for the CSI patient and PTV for the mesothelioma patient) received 100% prescribed dose. The homogeneity index (homogeneity index = D5/D95) was used to evaluate the target dose uniformity. The beam has a spot size with a diameter of approximately 1.6–2.2 cm (full width half maximum).
Field Setup and Spot Arrangement
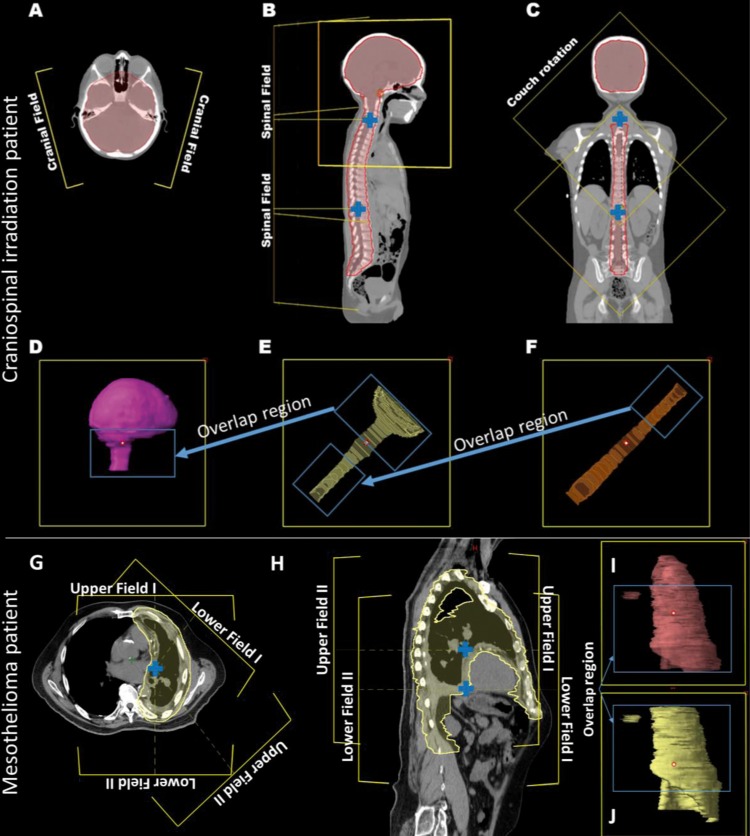
Figure 1A–C shows representative axial, sagittal, and coronal views with marked field projections for the CSI patient. Two brain fields with the same isocenter are typically angled 15° posteriorly from the horizontal plane to reduce the dose to the lens (Figure 1A). For each field, the corresponding CTV included the brain contour and a portion of the upper spine contour that extended approximately 1 to 2 cm superior to the shoulders (Figure 1D). The spinal fields were equally spaced along the spine axis, and the isocenters were designed to minimize the total number of spinal fields and maximize the field overlap region for junctions (Figure 1B and C). The target covered by the spinal field immediately inferior to the brain fields may include the upper spine as well as portions of the brain target (Figure 1E). The maximum field size of our system is 30 cm × 30 cm; to maximize junction size, we applied a 45° couch rotation for spinal fields (Figure 1C–F).
Figure 1.
Field arrangement for the craniospinal irradiation patient (A–F) and mesothelioma irradiation patient (G–J). (A) Two brain fields. (B) Upper spine field. (C) Lower spine field. (D–F) Corresponding target volumes. (G) Axial view. (H) Sagittal view. (I, J) Target volumes corresponding to the 2 upper and 2 lower fields, respectively. Field isocenters are indicated (blue cross).
Figure 1G and H shows representative axial and sagittal views with marked field projections for the mesothelioma patient. The PTV was covered by 4 fields (Figure 1G): 2 upper fields with 1 isocenter matched with 2 lower fields with another isocenter (Figure 1H). The corresponding targets for the upper and lower fields are shown in Figure 1I and J.
For both patients, the spot arrangement volume of each field was expanded by 8 mm uniformly in all directions from the corresponding target contour.
Robust Optimization and Uncertainty Setup
In our in-house proton treatment planning system, the worst-case dose algorithm is adopted for robust optimization. In this algorithm, the dose distributions from different scenarios, including the nominal dose (ie, without uncertainties) and different uncertainty setups, are computed. The worst-case dose distribution is represented by the maximum (for overdosage) or minimum (for underdosage) dose from all computed dose distributions in each voxel corresponding to specific structures. The formulation can be described as follows:
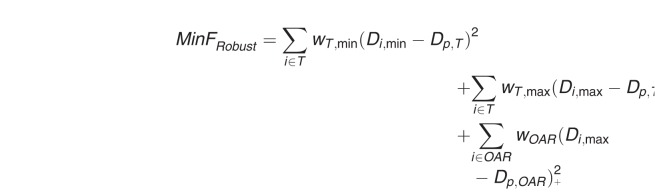 |
Where Di is the worst-case dose at voxel i, Dp is the prescription dose for the target or OAR, w is the penalty weight of the specific structure; the step function (Di,max – Dp,OAR)+ equals (Di,max – Dp,OAR) if Di,max > Dp,OAR but zero if Di,max ≤ Dp,OAR.
We designed 2 uncertainty scenarios for robust optimization to simulate misalignment errors that may occur at all field junctions. In these scenarios, field isocenters shift ±3 mm in the superior–inferior direction alternately. For example, for the CSI patient, 2 brain fields are shifted by −3 mm, and the first and second spinal fields are shifted by +3 and −3 mm in scenario I, respectively. In scenario II, the fields are shifted by 3 mm in the opposite direction with respect to scenario I.
Plan Robustness Evaluation
Robust optimized and conventional nonrobust IMPT plans were generated for both patients. Alternating isocenter shifts of 3 mm per field (6-mm total error) were performed to simulate the longitudinal mismatching error for robustness analysis. The dose profiles in the junction regions were used to demonstrate the deviation caused by misalignment uncertainty. For the CSI patient, robust IMPT plans with different junction sizes (8, 12, 16, and 26 cm) were generated to illustrate the relationship between junction size and dose deviation, and the robustness of a robust optimized IMPT plan with a large junction size was compared with that of a robust optimized treatment plan with a small junction and conventional junction shifting.
Results
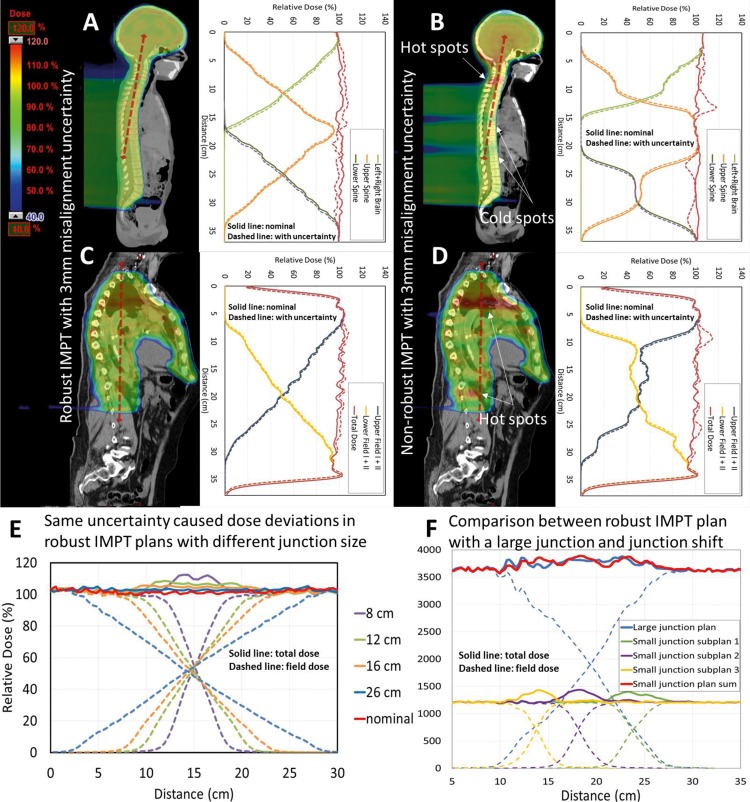
First, we evaluated the robustness of the dose distribution in field junctions for the robust and conventional IMPT plans for the CSI patient. As shown in Figure 2A, the field dose in the junction region has a low smooth gradient in the robust IMPT plan but is irregular (not smooth) in the conventional IMPT plan (Figure 2B).The hot and cold doses were evenly distributed in the junction region in the robust plan, and the deviation for the simulated error was around 5% (Figure 2A), which is significantly smaller than the 20% deviation in the conventional plan (Figure 2B). Similar results were observed for the mesothelioma patient (Figure 2C and D).
Figure 2.
Dose color wash and corresponding dose profiles of the robust and conventional intensity modulated proton therapy (IMPT) plans for the craniospinal irradiation patient (A, B) and the mesothelioma patient (C, D). The dose color wash and dashed lines represent the dosimetric deviations resulting from a 3-mm alternating misalignment error (brain fields, −3 mm; upper spine field, +3 mm; lower spine field, −3 mm). (E) Dose profiles in junctions for the craniospinal irradiation IMPT plans with junction sizes of 8, 12, 16, and 26 cm and a longitudinal misalignment error of 3 mm per field (total, 6 mm). (F) Robustness comparison between a robust IMPT plan with a large dose junction (18 cm) and a robust IMPT plan with a small dose junction (7 cm) and junction shifting for the craniospinal irradiation patient.
Figure 2E shows the dose profiles in robust IMPT plans for the CSI patient with different junction sizes (8, 12, 16, and 26 cm) and a 3-mm misalignment error. And the uncertainty yield was 9.9%, 5.4%, 4.5%, and 2.6% dose deviation in the junction region for the IMPT plans with 8, 12, 16, and 26 cm junction size, respectively. For a given uncertainty level, the dose deviation decreased as junction size increased. This result is also consistent with the results reported in previous studies [4, 5]. The relationship between dose deviation, uncertainty, and junction size can be roughly simplified as follows:
 . .
|
Figure 2F demonstrates 2 strategies to increase the robustness of the misalignment errors: a robust IMPT plan with an 18-cm dose junction and a robust IMPT plan with a 7-cm dose junction and junction shifting. The second plan included 3 subplans, each delivering 1/3 of the total dose. The total lateral dose profiles for the 2 plans are quite similar. Each subplan in the second plan has large dose deviations, but shifting the junction helps to spread the uncertainty. Thus, in general, the dose deviations of the 2 plans are similar. This result suggests that if the overlapping region is sufficiently enlarged, the shifting of junctions will not be necessary for the robust IMPT plan.
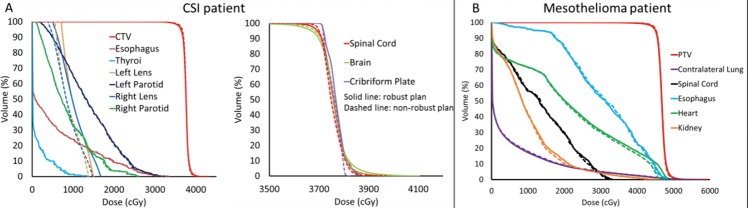
The dose-volume histograms of robust and nonrobust IMPT plans are illustrated in Figure 3. The tradeoff between target uniformity and robustness between robust and nonrobust IMPT plans was within 1.5% for the 2 patients. For the CSI patient, the homogeneity index scores of the spinal cord, brain, and cribriform plans were 1.041, 1.051, and 1.030, respectively, in the robust IMPT plan compared with 1.036, 1.045, and 1.025, respectively, in the nonrobust IMPT plan. And the mean doses of the left lens and right lens were increased from 8.9 and 8.7 Gy to 10.3 to 10.1 Gy from the nonrobust plan to the robust plan, respectively. For the mesothelioma patient, the robust IMPT plan achieved a plan of similar quality to the nonrobust plan in nominal scenario.
Figure 3.
Dose volume histograms of robust and nonrobust intensity modulated proton therapy (IMPT) plans for the craniospinal irradiation patient (A) and the mesothelioma patient (B). Solid lines: robust IMPT plan; Dashed lines: nonrobust IMPT plan.
Discussion
Robust optimization is aimed at reducing uncertainty in IMPT. Whereas previous studies only investigated setup errors in single-isocenter treatment plans [10], the current study provides, to our knowledge, the first demonstration of efficient integration of intrafractional setup errors for multi-isocenter fields into a general robust planning algorithm. Such robust optimization is especially important for treatment planning for large, complex, and irregularly shaped targets.
Many strategies have been proposed to handle field misalignment errors during treatment. For CSI treatment planning, a volumetric gradient dose optimization (GDO) methodology [14] was recently introduced for IMPT technology [4, 5]. The GDO method, which was initially introduced for volume-modulated arc therapy (VMAT) planning [14], is a 2-step manual planning approach. In this method, gradient volumes are generated in the overlap regions as 4 equally spaced sections. The first step is to optimize the first volume field so that the 4 gradient volumes receive 80%, 60%, 40%, and 20% of the prescribed volume. The second step is to optimize the second field separately so that the 4 gradient volumes receive 20%, 40%, 60%, and 80% of the prescribed volume. This method, which produces a tapered dose distribution in the junction regions, has several limitations: (1) in both VMAT and IMPT planning, the GDO method increases the optimization time significantly, since the manual GDO requires delineation of structures for optimizing the dose in the junction and running extra optimizations; so, an automatic process is desired; (2) in the GDO method, the assigned field dose in gradient volumes was not continuous, so it is hard to produce a more general tapered dose distribution for large junction sizes; and (3) the GDO method applies single-field optimization. This process cannot be used for mesothelioma cases since it often requires at least 2 fields for each isocenter. A nonoptimal GDO solution for a large overlap region has been described for a VMAT optimization [14].
An important finding of the current study is that dose gradients that are low and tapered in field junctions can be achieved through a robust optimization that is simpler and much more general than manual single-field optimization [4, 5]. Our approach overcomes the limitations of the GDO method in that it (1) is automated, (2) can be used for any junction size, and (3) uses multi-field optimization and can be used for large and complex targets. In addition, as the use of scanning beam proton therapy is increasing, the robust optimization planning method is being implemented in commercially available treatment planning systems, such as Eclipse V13.7 system (Varian Medical Systems). So, our general robust optimization method for multi-isocenter large field treatment plan can be easily applied in other proton therapy center. Our work is the first to report the utilization of this automatic process for 2 distinct disease sites.
As shown earlier, robust IMPT greatly improves the efficiency of treatment over conventional IMPT. For the CSI treatment, one of the important results is that junction shifting was not necessary. For the mesothelioma treatment, the second isocenter was set up simply by shifting the couch during treatment, since the plan is robust enough to accommodate intrafractional junction shifting. Currently, our center uses the robust optimization planning approach for complex-target treatments and can perform CSI or mesothelioma IMPT in 45-minute sessions.
Although robust optimization tools have been well developed, planners still lack experience in clinical application. The setup of uncertainty scenarios is crucial for the use of robust optimization in clinical practice. The inclusion of too many scenarios will increase the computation burden and thereby prevent optimization in an acceptable time frame, whereas the inclusion of too few scenarios may not guarantee robustness. More experience is also needed in determining how to balance the plan's robustness and quality in nominal scenarios. For example, in determining how to increase the dose conformality in brain target and keep taped dose in junction in a CSI case. The uncertainty scenarios can change to 2 brain fields, which are kept still, and the first and second spinal fields are shifted by ±3 mm. The dose-volume histograms of this uncertainty setting are demonstrated in Figure 4. It shows that in the brain a target robust IMPT plan achieved the same plan quality of a nonrobust IMPT plan in a nominal case. The selective robust optimization strategies [15] can also apply to increasing the dose uniformity in nominal cases. In this study, we only discussed uncertainty scenarios to generate a robust field junction. The conventional interfractional patient setup uncertainties and system range uncertainties can also be integrated into treatment plan optimization.
Figure 4.
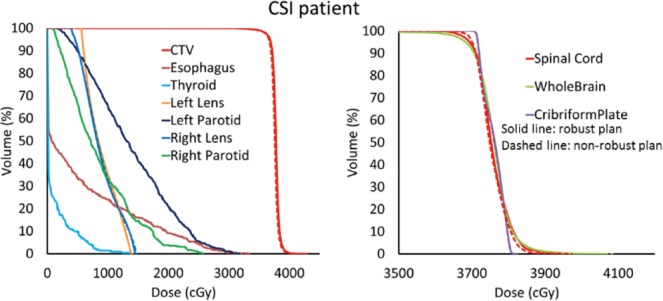
Dose volume histograms of robust intensity modulated proton therapy (IMPT) plan with fixed brain fields uncertainty setup and nonrobust IMPT plan for the craniospinal irradiation patient. Solid lines: robust IMPT plan; Dashed lines: nonrobust IMPT plan.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have none to disclose.
Acknowledgments: This work was supported in part by NIH NCI Cancer Center Support (Core) Grant CA016672 to The University of Texas MD Anderson Cancer Center. We would also like to show our gratitude to Arthur Gelmis and Christine F Wogan for their comments and editing of the manuscript.
References
- 1.Chang JY, Li H, Zhu XR, Liao Z, Zhao L, Liu A, Li Y, Sahoo N, Poenisch F, Gomez DR, Wu R, Gillin M, Zhang X. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2014;90:809–18. doi: 10.1016/j.ijrobp.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan HY, Jiang S, Sutton J, Liao Z, Chance WW, Frank SJ, Zhu XR, Li H, Fontanilla HP, Zhang X, Gomez DR. Early experience with intensity modulated proton therapy for lung-intact mesothelioma: a case series. Pract Radiat Oncol. 2015;5:e345–53. doi: 10.1016/j.prro.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Stoker JB, Grant J, Zhu XR, Pidikiti R, Mahajan A, Grosshans DR. Intensity modulated proton therapy for craniospinal irradiation: organ-at-risk exposure and a low-gradient junctioning technique. Int J Radiat Oncol Biol Phys. 2014;90:637–44. doi: 10.1016/j.ijrobp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Lin HB, Ding XF, Kirk M, Liu HY, Zhai HF, Hill-Kayser CE, Lustig RA, Tochner Z, Both S, McDonough J. Supine craniospinal irradiation using a proton pencil beam scanning technique without match line changes for field junctions. Int J Radiat Oncol Biol Phys. 2014;90:71–8. doi: 10.1016/j.ijrobp.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 6.St Clair WH, Adams JA, Bues M, Fullerton BC, La Shell S, Kooy HM, Loeffler JS, Tarbell NJ. Advantage of protons compared to conventional x-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–34. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 7.Liao L, Jiang S, Li Y, Wang X, Li H, Zhu X, Sahoo N, Gillin M, Mahajan A, Grosshans DR. TH-C-BRD-12: robust intensity modulated proton therapy plan can eliminate junction Shifts for craniospinal irradiation. Med Phys. 2014;41:553. [Google Scholar]
- 8.Giebeler A, Newhauser WD, Amos RA, Mahajan A, Homann K, Howell RM. Standardized treatment planning methodology for passively scattered proton craniospinal irradiation. Radiat Oncol. 2013;8:32. doi: 10.1186/1748-717X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalski JM, Klein EE, Gerber R. Method to plan, administer, and verify supine craniospinal irradiation. J Appl Clin Med Phys. 2002;3:310–6. doi: 10.1120/jacmp.v3i4.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–91. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unkelbach J, Bortfeld T, Martin BC, Soukup M. Reducing the sensitivity of IMPT treatment plans to setup errors and range uncertainties via probabilistic treatment planning. Med Phys. 2009;36:149–63. doi: 10.1118/1.3021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredriksson A, Forsgren A, Hardemark B. Minimax optimization for handling range and setup uncertainties in proton therapy. Med Phys. 2011;38:1672–84. doi: 10.1118/1.3556559. [DOI] [PubMed] [Google Scholar]
- 13.Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2008;53:1689–700. doi: 10.1088/0031-9155/53/6/013. [DOI] [PubMed] [Google Scholar]
- 14.Myers P, Stathakis S, Mavroidis P, Esquivel C, Papanikolaou N. Evaluation of localization errors for craniospinal axis irradiation delivery using volume modulated arc therapy and proposal of a technique to minimize such errors. Radiother Oncol. 2013;108:107–13. doi: 10.1016/j.radonc.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Niemela P, Liao L, Jiang S, Li H, Poenisch F, Zhu XR, Siljamaki S, Vanderstraeten R, Sahoo N, Gillin M, Zhang X. Selective robust optimization: a new intensity-modulated proton therapy optimization strategy. Med Phys. 2015;42:4840–7. doi: 10.1118/1.4923171. [DOI] [PubMed] [Google Scholar]