Abstract
Purpose:
The purpose of this study was to analyze national trends and disparities in proton therapy use among patients with head and neck cancer receiving radiotherapy to primary disease sites.
Patients and Methods:
Using the National Cancer Database, we identified patients diagnosed with any nonmetastatic head and neck primary malignancy between 2005 and 2014 who were treated with radiation therapy or proton therapy directed specifically at the primary disease site. Distributions of patient and clinical factors between the two groups were evaluated. Multivariable logistic regression was used to correlate factors associated with proton therapy use compared with other modalities of radiation therapy.
Results:
There were 220 491 patients who received any radiation therapy as part of their initial treatment course, only 417 (0.2%) of whom received proton therapy. The use of protons underwent a small increase from 0.13% in 2005-06 to 0.41% by 2013-14 (P < .001). The most common primary sites treated with proton therapy were the nasal cavity/nasopharynx (n = 151, 36.2%) and the oral cavity (n = 98, 23.5%). Most patients had T4 disease (n = 94, 31.0%). On multivariable logistic regression, all primary sites compared with hypopharynx/larynx sites (odds ratio [OR], 2.53-10.53; P < .001), treatment at an academic facility (OR, 2.54; P < .001), ≥ 13-mile distance from the treating facility (OR, 1.94; P < .001), and highest median household income quartile (> $63 000; OR, 2.52; P = .002) were associated with an increased likelihood of receiving proton therapy.
Conclusion:
Proton use has undergone an incremental increase in the United States but remains an uncommon modality for the treatment of primary head and neck cancer.
Keywords: head and neck cancer, proton therapy, NCDB
Introduction
Radiation therapy (RT) is an important component in the treatment of head and neck cancers. Given the proximity of critical structures to key target areas, an emphasis on delivering adequate dose while sparing healthy structures has been an ongoing focus of local management.
Intensity-modulated radiation therapy (IMRT) is widely accepted as the predominant RT technique to optimize the therapeutic ratio for head and neck cancers. The implementation of IMRT has been shown to decrease both acute and chronic toxicities. However, adjacent healthy structures can still receive considerable dose with IMRT because of the physical properties of photon beams.
To improve the therapeutic window with external beam RT, the use of particles has gained much interest, and proton beam therapy (PBT) specifically has been shown to be both safe and effective. The characteristic sharp falloff in exit dose after the high-dose proton therapy has been deposited in the target has been shown to decrease toxicities in head and neck cancers. A study by van der Laan et al [1] found that patients treated with intensity-modulated proton therapy had decreased dose to the pharyngeal constrictors, resulting in decreased rates of dysphagia compared with IMRT. Furthermore, several comparison studies [2–5] have shown lower energy delivered to regions outside the target and, thus, lower integral dose with proton therapy.
Although the dosimetric benefits of proton therapy are well-documented and evidence for long-term clinical benefits are emerging, only a small proportion of patients in the United States have access to this technology. To better understand the current landscape of proton use in head and neck cancers, we sought to analyze national trends and disparities for PBT use among patients with head and neck cancer in the primary setting from a large, hospital-based database.
Materials and Methods
The National Cancer Database (NCDB) is a hospital-based registry that is the joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. It is estimated that 70% of all diagnosed malignancies in the United States are captured by facilities participating in this registry and reported to the NCDB. The NCDB and the hospitals participating in the NCDB are the source of the deidentified data used in this study. However, the Commission on Cancer has not verified and is not responsible for the statistical validity or conclusions derived by the authors of this study. A waiver was obtained from the Memorial Sloan Kettering institutional review board before initiation of the study.
Using the NCDB, we identified patients diagnosed with any head and neck primary malignancy (International Classification of Disease for Oncology [ICD-0-3] codes C00-C14 and C30-C32) of all stages between 2005 and 2014. Those who had distant metastatic disease were excluded from analysis. Furthermore, patients with missing information regarding dose and those who received RT doses ≤ 5000 centigrays (cGy) were excluded. Patients treated with any RT (including 3-dimensional conformal [3D-CRT], IMRT, brachytherapy, and proton therapy) directed to the primary site were selected. The treatment variable was defined as proton therapy versus all other modalities of RT (Figure 1).
Figure 1.
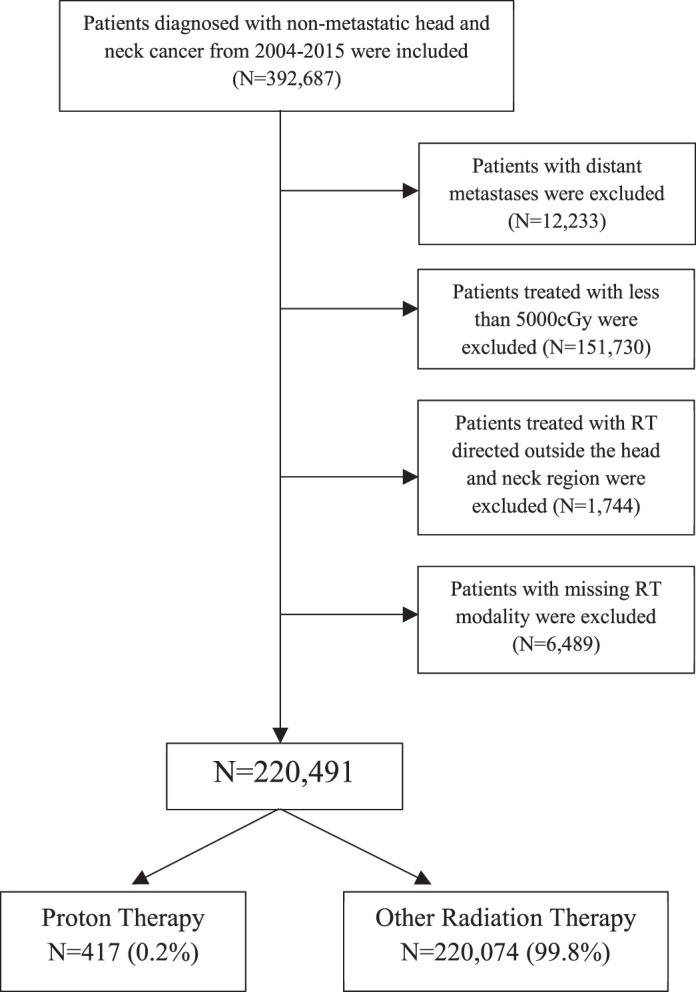
Consort diagram.
To obtain descriptive statistics, distribution of patient/clinical factors, socioeconomic status, demographic factors, and treatment-facility information were compared via the Pearson χ2 or Fisher exact tests. The variables included were primary site (hypopharynx/larynx, nasal cavity/nasopharynx, oral cavity, oropharynx, and salivary), age (65 years and younger or older than 65 years), Charlson/Deyo score (0, 1, or ≥ 2), chemotherapy (no or yes), secondary primary (no or yes), race/ethnicity (white non-Hispanic, Hispanic, black, or other), facility type (nonacademic or academic), insurance (uninsured, private insurance, Medicaid/Medicare, or other), clinical T category (T1, T2, T3, or T4), geographic region (Northeast, Midwest, South, or West), region of patient's residence (metropolitan, urban, or rural), distance from the hospital categorized by the median (< 13 miles or ≥ 13 miles), median household income (< $38 000, $38 000-$47 999, $48 000-62 999, or ≥ $63 000), percentage with no high-school education (≥ 21%, 13%-20.9%, 7%-12.9%, or < 7%), and year of diagnosis (2005-06, 2007-08, 2009-10, 2011-12, or 2013-14).
Median household income and percentage of patients with no high-school education were aggregated variables, estimated by matching the zip code of the patient recorded at the time of diagnosis. For the geographic-region variable, the Northeast included Connecticut, Massachusetts, Maine, New Hampshire, Rhode Island, Vermont, New Jersey, New York, and Pennsylvania; the Midwest included Illinois, Indiana, Michigan, Ohio, Wisconsin, Iowa, Kansas, Minnesota, Missouri, North Dakota, Nebraska, and South Dakota; the South included the District of Columbia, Delaware, Florida, Georgia, Maryland, North Carolina, South Carolina, Virginia, West Virginia, Alabama, Kentucky, Mississippi, and Tennessee; and the West included Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, Wyoming, Alaska, California, Hawaii, Oregon, and Washington.
Multivariable logistic regression was performed to correlate socioeconomic factors associated with proton therapy use compared with other modalities of RT. The variables included were primary site (hypopharynx/larynx, nasal cavity/nasopharynx, oral cavity, oropharynx, or salivary), age (65 years and younger or older than 65 years), Charlson/Deyo score (0, 1, or ≥ 2), chemotherapy (no or yes), race/ethnicity (white non-Hispanic, Hispanic, black, or other), facility type (nonacademic or academic), insurance (uninsured, private insurance, Medicaid/Medicare, or other), clinical T category (T1, T2, T3, or T4), geographic region (Northeast, Midwest, South, or West), region of patient's residence (metropolitan, urban, or rural), distance from the hospital (< 13 miles or ≥ 13 miles), median household income (< $38 000, $38 000-$47 999, $48 000-62 999, or ≥ $63 000), percentage with no high-school education (≥ 21%, 13%-20.9%, 7%-12.9%, or < 7%), and year of diagnosis (2005-06, 2007-08, 2009-10, 2011-12, or 2013-14).
A second logistic-regression model was performed to assess interactions between the dichotomous distance variable to treatment facility and the median household income quartile. All analyses were conducted with SPSS software (version 23.0, IBM Inc, Armonk, New York). All tests were 2 sided with a value of P < .05 as the threshold for significance.
Results
Clinical and Patient Demographics
There were 220 491 who received any RT as part of their initial treatment course and 417 (0.2%) who received proton therapy. The use of protons underwent a small increase from 0.13% in 2005-06 to 0.41% by 2013-14 (OR, 2.90; P < .001; Figure 2). Among those who received all other forms of radiation, 106 320 (48.2%) received IMRT, 8443 (3.8%) received 3D-CRT, 559 (0.3%) received brachytherapy, 214 (0.1%) received radiosurgery, and the rest received forms of external beam radiation for which the specific modality was not specified. The most common primary sites treated with proton therapy were the nasal cavity/nasopharynx (n = 151; 36.2%) and the oral cavity (n = 98; 23.5%). There were 213 454 patients who received other RT modalities and 399 patients who received PBT with known chemotherapy status. A larger proportion of patients who received other RT modalities received chemotherapy (n = 130 974; 61.4%) compared to those who received PBT (n = 171, 42.9%). Further details on patient- and clinical-related characteristics can be found in Table 1.
Figure 2.
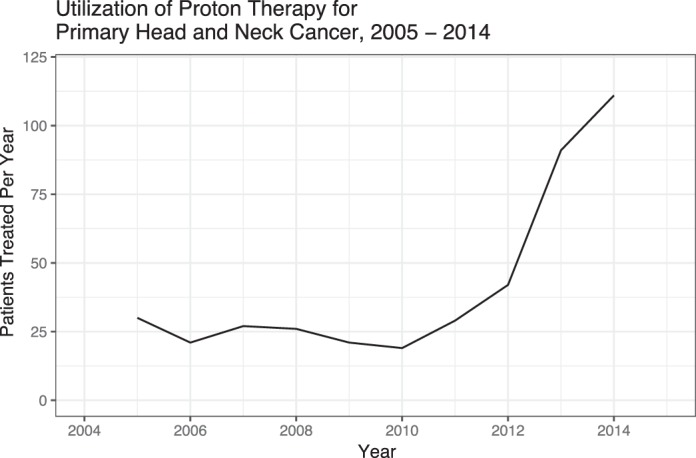
Use of proton beam therapy for primary head and neck cancer, 2005-14.
Table 1.
Comparison among patients with head and neck cancer receiving protons versus other modalities of radiation therapy.
|
Variable |
No. (%) |
P
value |
|
| Other radiation therapy | Proton therapy | ||
|
n = 220 074 (99.8%) |
n = 417 (0.2%) |
||
| Primary site | < .001 | ||
| Hypopharynx/larynx | 73 386 (33.3) | 36 (8.6) | |
| Nasal cavity/nasopharynx | 17 170 (7.8) | 151 (36.2) | |
| Oral cavity | 68 674 (31.2) | 98 (23.5) | |
| Oropharynx | 46 959 (21.3) | 65 (15.6) | |
| Salivary | 13 885 (6.3) | 67 (16.1) | |
| Age, y | .259 | ||
| ≤ 65 | 142 894 (64.9) | 282 (67.6) | |
| > 65 | 77 180 (35.1) | 135 (32.4) | |
| Charlson-Deyo comorbidity index | < .001 | ||
| 0 | 176 583 (80.2) | 366 (87.8) | |
| 1 | 33 853 (15.4) | 42 (10.1) | |
| ≥ 2 | 9638 (4.4) | 9 (2.2) | |
| Chemotherapy | n = 213 454 | n = 399 | < .001 |
| No | 82 480 (38.6) | 228 (57.1) | |
| Yes | 130 974 (61.4) | 171 (42.9) | |
| Second primary | n = 220 058 | n = 417 | .901 |
| No | 171 722 (78.0) | 327 (78.4) | |
| Yes | 48 336 (22.0) | 90 (21.6) | |
| Race/ethnicity | n = 208 391 | n = 410 | .002 |
| White, non-Hispanic | 169 821 (81.5) | 342 (83.4) | |
| Hispanic | 9257 (4.4) | 19 (4.6) | |
| Black | 22 158 (10.6) | 25 (6.1) | |
| Other | 7155 (3.4) | 24 (5.9) | |
| Facility type | n = 219 974 | n = 417 | < .001 |
| Nonacademic | 130 852 (59.5) | 158 (37.9) | |
| Academic | 89 122 (40.5) | 259 (62.1) | |
| Insurance | n = 220 074 | < .001 | |
| Uninsured | 11 366 (5.2) | 10 (2.4) | |
| Private insurance | 93 556 (42.5) | 225 (54.0) | |
| Medicaid | 21 509 (9.8) | 23 (5.5) | |
| Medicare | 81 714 (37.1) | 146 (35.0) | |
| Other government/unknown | 11 929 (5.4) | 13 (3.1) | |
| Clinical T stage | n = 184 158 | n = 303 | < .001 |
| T1 | 48 515 (26.3) | 72 (23.8) | |
| T2 | 63 261 (34.4) | 91 (30.0) | |
| T3 | 38 031 (20.7) | 46 (15.2) | |
| T4 | 34 351 (18.7) | 94 (31.0) | |
| Geographic region | n = 213 723 | n = 367 | < .001 |
| Northeast | 43 346 (20.3) | 132 (36.0) | |
| Midwest | 56 491 (26.4) | 65 (17.7) | |
| South | 82 142 (38.4) | 64 (17.4) | |
| West | 31 744 (14.9) | 106 (28.9) | |
| Region of patient's residence | n = 220 074 | n = 417 | < .001 |
| Metropolitan | 175 724 (79.8) | 370 (88.7) | |
| Urban | 33 337 (15.1) | 29 (7.0) | |
| Rural | 11 013 (5.0) | 18 (4.3) | |
| Distance from the hospital | n = 212 686 | n = 408 | < .001 |
| < 13 miles | 117 114 (55.1) | 152 (37.3) | |
| ≥ 13 miles | 95 572 (44.9) | 256 (62.7) | |
| Median household income, US$ | n = 217 381 | n = 412 | < .001 |
| < $38 000 | 43 276 (19.9) | 41 (10.0) | |
| $38 000-$47 999 | 53 577 (24.6) | 82 (19.9) | |
| $48 000-62 999 | 57 417 (26.4) | 87 (21.1) | |
| > $63 000 | 63 111 (29.0) | 202 (49.0) | |
| Percentage with no high-school education | n = 217 523 | n = 412 | < .001 |
| ≥ 21 | 40 109 (18.4) | 61 (14.8) | |
| 13-20.9 | 60 010 (27.6) | 95 (23.1) | |
| 7-12.9 | 70 069 (32.2) | 123 (29.9) | |
| < 7 | 47 335 (21.8) | 133 (32.3) | |
| Year of diagnosis | n = 220 074 | n = 417 | < .001 |
| 2005-06 | 38 908 (17.7) | 51 (12.2) | |
| 2007-08 | 41 590 (18.9) | 53 (12.7) | |
| 2009-10 | 43 836 (19.9) | 40 (9.6) | |
| 2011-12 | 46 357 (21.1) | 71 (17.0) | |
| 2013-14 | 49 393 (22.4) | 202 (48.4) | |
The use of PBT over time by subsite showed significant differences, with a decline in the use for hypopharynx/larynx primaries from 23.5% (12 of 51) in 2005-06 to 5.4% (11 of 202) in 2013-14 and an increase in use for oropharynx primaries from 9.8% (5 of 51) in 2005-06 to 22.8% (46 of 202) in 2013-14 (P < .001; Table 2).
Table 2.
Distribution of proton use for primary subsite over time (P < 0.001).
|
Primary Subsite |
No. (%) |
|||||
|
2005-06 |
2007-08 |
2009-10 |
2011-12 |
2013-14 |
Total |
|
| Hypopharynx/larynx | 12 (23.5) | 7 (13.2) | 3 (7.5) | 3 (4.2) | 11 (5.4) | 36 (8.6) |
| Nasal cavity/nasopharynx | 13 (25.5) | 15 (28.3) | 19 (47.5) | 36 (50.7) | 68 (33.7) | 151 (36.2) |
| Oral cavity | 14 (27.5) | 16 (30.2) | 10 (25.0) | 9 (12.7) | 49 (24.3) | 98 (23.5) |
| Oropharynx | 5 (9.8) | 5 (9.4) | 5 (12.5) | 4 (5.6) | 46 (22.8) | 65 (15.6) |
| Salivary | 7 (13.7) | 10 (18.9) | 3 (7.5) | 19 (26.8) | 28 (13.9) | 67 (16.1) |
| Totals | 51 (12.2) | 53 (12.7) | 40 (9.6) | 71 (17.0) | 202 (48.4) | 417 (100) |
Multivariable Logistic Regression
On multivariate logistic regression, age older than 65 years (OR, 0.97; 95% confidence interval [95% CI], 0.68-1.39; P < .001), Hispanic ethnicity (OR, 0.43; 95% CI 0.20-0.94; P = .035), “other” race (OR, 0.38; 95% CI, 0.19-0.75; P = .006), location in the South (OR, 0.29; 95% CI, 0.19-0.43; P < .001) or Midwest (OR, 0.40; 95% CI, 0.27-0.58; P < .001), higher percentage with no high-school education (OR, 0.33-0.60; P < .05), receipt of chemotherapy (OR, 0.45; 95% CI, 0.35-0.60; P < .001), and urban site of a patient's residence (OR, 0.41; 95% CI, 0.23-0.71; P = .002) were associated with decreased likelihood of receiving PBT. All primary sites compared with the hypopharynx/larynx (OR, 2.53-10.53; P < .001), treatment at an academic facility (OR, 2.54; 95% CI, 1.89-3.42; P < .001), ≥ 13 mile distance from the treating facility (OR, 1.94; 95% CI, 1.47-2.57; P < .001), and highest median household income quartile (> $63 000; OR, 2.52; 95% CI, 1.42-4.50; P = .002) were associated with an increased likelihood of receiving proton therapy. There were no differences in use of the Charlson-Deyo comorbidity index, insurance status, or clinical T-stage. Further details can be found in Table 3.
Table 3.
Multivariable logistic regression for receipt of proton therapy.
|
Variable |
Multivariable |
|
|
OR (95% CI) |
P
value |
|
| Primary site | ||
| Hypopharynx/larynx | 1 (Ref) | |
| Nasal cavity/nasopharynx | 10.53 (6.53-16.99) | < .001 |
| Oral cavity | 2.53 (1.60-4.00) | < .001 |
| Oropharynx | 3.37 (2.07-5.47) | < .001 |
| Salivary | 5.91 (3.50-9.98) | < .001 |
| Age, y | ||
| ≤ 65 | 1 (Ref) | |
| > 65 | 0.97 (0.68-1.39) | < .001 |
| Charlson-Deyo comorbidity index | ||
| 0 | 1 (Ref) | |
| 1 | 0.95 (0.66-1.37) | .779 |
| ≥ 2 | 0.57 (0.25-1.30) | .182 |
| Chemotherapy | ||
| No | 1 (Ref) | |
| Yes | 0.45 (0.35-0.60) | < .001 |
| Race/ethnicity | ||
| White, non-Hispanic | 1 (Ref) | |
| Hispanic | 0.43 (0.20-0.94) | .035 |
| Black | 0.72 (0.40-1.30) | .275 |
| Other | 0.38 (0.19-0.75) | .006 |
| Facility type | ||
| Nonacademic | 1 (Ref) | |
| Academic | 2.54 (1.89-3.42) | < .001 |
| Insurance | ||
| Uninsured | 1 (Ref) | |
| Private insurance | 1.47 (0.64-3.37) | .368 |
| Medicaid | 0.73 (0.27-2.00) | .544 |
| Medicare | 1.61 (0.67-3.84) | .286 |
| Other government/unknown | 0.78 (0.26-2.34) | .656 |
| Clinical T stage | ||
| T1 | 1 (Ref) | |
| T2 | 1.07 (0.77-1.50) | .682 |
| T3 | 0.84 (0.55-1.28) | .409 |
| T4 | 1.38 (0.96-2.00) | .082 |
| Geographic region | ||
| Northeast | 1 (Ref) | |
| Midwest | 0.40 (0.27-0.58) | < .001 |
| South | 0.29 (0.19-0.43) | < .001 |
| West | 1.46 (1.06-2.01) | .020 |
| Region of patient's residence | ||
| Metropolitan | 1 (Ref) | |
| Urban | 0.41 (0.23-0.71) | .002 |
| Rural | 0.96 (0.50-1.82) | .893 |
| Distance from the hospital | ||
| < 13 miles | 1 (Ref) | |
| ≥ 13 miles | 1.94 (1.47-2.57) | < .001 |
| Median household income, US$ | ||
| < $38 000 | 1 (Ref) | |
| $38 000-$47 999 | 1.82 (1.08-3.08) | .024 |
| $48 000-62 999 | 1.23 (0.70-2.15) | .475 |
| > $63 000 | 2.52 (1.42-4.50) | .002 |
| Percentage with no high-school education | ||
| ≥ 21% | 1 (Ref) | |
| 13%-20.9% | 0.60 (0.39-0.93) | .023 |
| 7%-12.9% | 0.44 (0.28-0.70) | < .001 |
| < 7% | 0.50 (0.31-0.82) | .006 |
| Year of diagnosis | ||
| 2005-06 | 1 (Ref) | |
| 2007-08 | 0.96 (0.59-1.56) | .862 |
| 2009-10 | 0.51 (0.30-0.88) | .016 |
| 2011-12 | 0.63 (0.38-1.04) | .068 |
| 2013-14 | 2.21 (1.49-3.29) | < .001 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; Ref, referent group.
An additional logistic regression model found there was significant interaction between median household income quartile and distance from treating facility, and for patients treated at a facility that was ≥ 13 miles from their home, they were more likely to receive proton therapy if in the highest median household income quartile ($63 000; OR, 2.42; 95% CI, 1.23-4.78; P = .011).
Discussion
In this large hospital-based analysis, there was a significant increase in the utilization of PBT for head and neck malignancies from 51 cases treated in 2005-2006 to 202 cases treated in 2013-2014 (P < .001). The most common primary site was the nasal cavity and nasopharynx (36.2%) followed by the oral cavity (23.5%), and the majority of patients treated had T4 disease (31.0%). Proton therapy use was associated with academic facilities (OR, 2.54; P < .001) and least utilized in the South (OR, 0.29; P < .001) and Midwest (OR, 0.40; P < .001).
Most studies reporting on the efficacy of proton therapy are related to treatment of cancers of the base of skull, nasopharynx, nasal cavity, and paranasal sinuses, where the tenuous balance of tumoricidal dose and the risk for neural toxicity are particularly recognizable. Although IMRT can provide conformality in these clinical situations, reports have shown suboptimal outcomes for patients with T4 tumors in nasopharyngeal cancer, likely because of the advanced nature of the disease in close proximity to critical structures, such as the temporal lobes and cranial nerves [6, 7]. A retrospective study [8] of 14 patients with unresectable adenoid cystic carcinoma of the nasopharynx treated with proton therapy found 93% had T4 disease. At median follow-up of 69 months, there were 3 local failures (21%) and 3 patients (21%) with grade 3 or higher late toxicity, thus highlighting the acceptable rates in an otherwise difficult disease to control [8] Most patients in the current study had T4 (94 of 303; 31.0%) underscoring the utility of proton therapy in advanced disease.
A systematic review and meta-analysis of patients with nasal cavity and paranasal sinus cancer from 41 observational studies compared 1186 photon cases to 286 charged-particle cases [9]. The authors found 5-year overall survival and disease-free survival was significantly higher with charged-particle use, despite treating to similar median treatment doses. Toxicity between the groups was similar, except for higher neurologic toxicity in the charged-particle group. This study suggested improved target coverage and higher radiobiologic effect as possible explanations for the improved outcomes, which may be important because these tumors are difficult to resect completely and historically do not fare well.
Although dosimetrically PBT is better for decreasing dose to healthy structures, there has not been sufficient evidence of improved outcomes to warrant its widespread adoption yet, and even a decline in use was seen in certain subsites, such as the hypopharynx/larynx. This may be a combination of the efficacy of 3D-CRT for early stage laryngeal cancers, as well as the benefit of a low-dose bath with IMRT, for more-advanced cases in which there may be a greater concern for marginal misses. Furthermore, PBT is not without toxicities that may be observed more frequently than photon therapy. These include dermatitis because there is less skin-sparing effect [10], neurological toxicity [9], and temporal lobe necrosis [11]. There is also uncertainty in the physical properties of protons for changes in density and volumes that could affect dose delivery [12]. Lateral scatter is also a concern, although the integral dose is lower [2]. Taken together, it is worth considering the possibility of reporting bias with toxicity rates tracked more carefully for newer technologies.
In the present study, PBT use in the primary setting was stable at 0.1%-0.2% from 2005 to 2012 but doubled with an incremental increase to 0.4% in 2013-14. Numerous studies have shown the benefit of PBT in the recurrent setting [10, 13–16], but the evidence is not as robust in the primary setting, which may explain the absolute low numbers of PBT use. In these studies, PBT allowed definitive doses to be given in the recurrent setting, which translated into local control rates that simply could not have been achieved with photons, without the cost of excessive toxicity.
The challenges that may explain the slow adoption of PBT are well known. Primarily, there is the high cost associated with building and operating proton therapy facilities, which directly affects cost of treatment. A course of IMRT for head and neck cancer typically costs $49 656, whereas a course of intensity-modulated proton therapy costs $61 697 [17]. With no level I evidence supporting PBT over photon therapy, patients have difficulty obtaining full coverage for their treatments, especially in the primary setting [18]. The American Society for Radiation Oncology (Arlington, Virginia) considers PBT for head and neck cancer suitable if the patient is enrolled in an institutional review board–approved clinical trial or multi-institutional patient registry [19]. On multivariate logistic regression, patients in the highest median household income quartile ($63 000; OR, 2.52; 95% CI, 1.42-4.50, P = .002) and those treated at an academic facility (OR, 2.54; 95% CI, 11.89-3.42; P < .001) were significantly more likely to receive PBT. Concordantly, insurance status did not affect the likelihood of receiving proton therapy over other forms of RT. Our data thus suggest that access to PBT is associated with income and access to an academic facility.
A single-institutional study from the University of Pennsylvania (Philadelphia) similarly found that patients living in poverty were less likely to receive PBT over IMRT for prostate cancer (OR, 0.97; P < .001) but found no significant difference among private versus nonprivate insurers (OR, 1.16; P = .475) [18]. An NCDB analysis of proton therapy use in prostate cancer found rates of PBT were highest among private insurance and Medicare holders [20], but noted that insurance companies significantly scaled down on reimbursements when it was reported around 2008. This may partially explain the findings in the present study. However, because of the few PBT facilities compared with photon therapy centers, the out-of-pocket costs associated with driving long distances may also contribute to those disparities [21].
Several studies using the NCDB have found an overall increase in use of PBT in other malignancies, with similar observations for disparities in the receipt of this modality. Among 4637 pediatric patients from 2004-12, only 267 patients (5.8%) received PBT, with increased use among those in the highest income bracket, were younger age, had private insurance holder, and who lived > 200 miles from a treatment facility [22]. A significant increase in use was seen over time from < 1% in 2004 to 15% in 2012. The low number of PBT uses in this population is notable, given this population would benefit the most from decreased late effects. Another NCDB study of patients with localized prostate cancer found the use of PBT doubled from 334 reported cases in 2004 to 663 cases by 2013 and found increased development of PBT centers in the southern region [23]. Although our study showed greatest usage in the Northeast and West, that is likely tied to clinical investigation among academic centers in those regions lead by highly specialized physicians who may be more willing to adopt new technologies.
There are limitations associated with analyzing a large hospital-based database. Namely, we were unable to ascertain the true clinical reasons for which certain patients received proton beam over photon therapy. Data regarding toxicity and cost were not available to us and thus a direct comparison between their effectiveness could not be performed. Furthermore, the current analysis only included patients treated in the primary setting because NCDB does not capture salvage treatment. This is the largest limitation of the study because the low absolute number of PBT use in the primary setting was so small. There are several explanations for this—namely, this could be due to coding errors as is a potential issue when using any population-based data. Patients who received a proton boost were likely documented as having received IMRT. Perhaps, most important, standalone proton treatment centers have less traditional relationships with academic institutions and community hospitals; thus, they may not be as well equipped to share the deidentified data with the American College of Surgeons. With the growing number of proton facilities tied to academic centers, a future study of more-recent years of diagnosis is likely to yield larger numbers. Nevertheless, this is the first study of its kind, to our knowledge, describing trends in proton therapy use for primary head and neck cancers on a national level and highlights underrepresented populations that may be important to consider in future clinical trial design as the availability of, and indications for, PBT increase.
Conclusions
Protons have undergone an incremental increase in use in the United States but remain an uncommon modality for treatment of head and neck cancer, particularly in the primary setting. As the indications and benefits of proton therapy in head and neck cancer continue to emerge, it will be important to increase access to protons, not only by decreasing the cost of their use but also by recognizing important disparities in their provision to date.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Funding: Funding was provided by the Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748-50).
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.van der Laan HP, van de Water TA, van Herpt HE, Christianen ME, Bijl HP, Korevaar EW, Rasch CR, van 't Veld AA, van der Schaaf A, Schilstra C, Langendijk JA. Rococo Cooperative Group. The potential of intensity-modulated proton radiotherapy to reduce swallowing dysfunction in the treatment of head and neck cancer: a planning comparative study. Acta Oncol. 2013;52:561–9. doi: 10.3109/0284186X.2012.692885. [DOI] [PubMed] [Google Scholar]
- 2.Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomax AJ, Bortfeld T, Goitein G, Debus J, Dykstra C, Tercier PA, Coucke PA, Mirimanoff RO. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiother Oncol. 1999;51:257–71. doi: 10.1016/s0167-8140(99)00036-5. [DOI] [PubMed] [Google Scholar]
- 4.Stromberger C, Cozzi L, Budach V, Fogliata A, Ghadjar P, Wlodarczyk W, Jamil B, Raguse JD, Böttcher A, Marnitz S. Unilateral and bilateral neck SIB for head and neck cancer patients: intensity-modulated proton therapy, tomotherapy, and RapidArc. Strahlenther Onkol. 2016;192:232–9. doi: 10.1007/s00066-016-0945-4. [DOI] [PubMed] [Google Scholar]
- 5.Kandula S, Zhu X, Garden AS, Gillin M, Rosenthal DI, Ang KK, Mohan R, Amin MV, Garcia JA, Wu R, Sahoo N, Frank SJ. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim. 2013;38:390–4. doi: 10.1016/j.meddos.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Han F, Zhao C, Huang SM, Lu LX, Huang Y, Deng XW, Mai WY, Teh BS, Butler EB, Lu TX. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2012;24:569–76. doi: 10.1016/j.clon.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Huang HI, Chan KT, Shu CH, Ho CY. T4-locally advanced nasopharyngeal carcinoma: prognostic influence of cranial nerve involvement in different radiotherapy techniques. ScientificWorldJournal. 2013. p. 439073. [DOI] [PMC free article] [PubMed]
- 8.Gentile MS, Yip D, Liebsch NJ, Adams JA, Busse PM, Chan AW. Definitive proton beam therapy for adenoid cystic carcinoma of the nasopharynx involving the base of skull. Oral Oncol. 2017;65:38–44. doi: 10.1016/j.oraloncology.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027–38. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 10.Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, Fox JL, Mah D, Garg MK, Han-Chih Chang J, Lee NY. Proton beam reirradiation for recurrent head and neck cancer: multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys. 2016;95:386–95. doi: 10.1016/j.ijrobp.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016;11:32. doi: 10.1186/s13014-016-0600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez LC, Moraes FY, Poon I, Marta GN. The management of head and neck tumors with high technology radiation therapy. Expert Rev Anticancer Ther. 2016;16:99–110. doi: 10.1586/14737140.2016.1121111. [DOI] [PubMed] [Google Scholar]
- 13.Riaz N, Hong JC, Sherman EJ, Morris L, Fury M, Ganly I, Wang TJ, Shi W, Wolden SL, Jackson A, Wong RJ, Zhang Z, Rao SD, Lee NY. A nomogram to predict loco-regional control after re-irradiation for head and neck cancer. Radiother Oncol. 2014;111:382–7. doi: 10.1016/j.radonc.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Suresh K, Nguyen R, Sapir E, Dow JS, Arnould GS, Worden FP, Spector ME, Prince ME, McLean SA, Shuman AG, Malloy KM, Casper K, Bradford CR, Schipper MJ, Eisbruch A. Predictors of severe long-term toxicity after re-irradiation for head and neck cancer. Oral Oncol. 2016;60:32–40. doi: 10.1016/j.oraloncology.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Lin R, Slater JD, Yonemoto LT, Grove RI, Teichman SL, Watt DK, Slater JM. Nasopharyngeal carcinoma: repeat treatment with conformal proton therapy—dose-volume histogram analysis. Radiology. 1999;213:489–94. doi: 10.1148/radiology.213.2.r99nv29489. [DOI] [PubMed] [Google Scholar]
- 16.Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Beadle B, Ma D, Zafereo ME, Hutcheson KA, Kupferman ME, William WN, Jr, Frank SJ. Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys. 2016;96:30–41. doi: 10.1016/j.ijrobp.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 17.Ramaekers BL, Grutters JP, Pijls-Johannesma M, Lambin P, Joore MA, Langendijk JA. Protons in head-and-neck cancer: bridging the gap of evidence. Int J Radiat Oncol Biol Phys. 2013;85:1282–8. doi: 10.1016/j.ijrobp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Woodhouse KD, Hwang WT, Vapiwala N, Jain A, Wang X, Both S, Shah M, Frazier M, Gabriel P, Christodouleas JP, Tochner Z, Deville C. Sociodemographic disparities in the utilization of proton therapy for prostate cancer at an urban academic center. Adv Radiat Oncol. 2017;2:132–9. doi: 10.1016/j.adro.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen AM, Pawlicki T, Dong L, Fourkal E, Buyyounouski M, Cengel K, Plastaras J, Bucci MK, Yock TI, Bonilla L, Price R, Harris EE, Konski AA. An evidence based review of proton beam therapy: the report of ASTRO's emerging technology committee. Radiother Oncol. 2012;103:8–11. doi: 10.1016/j.radonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Mahal BA, Chen YW, Efstathiou JA, Muralidhar V, Hoffman KE, Yu JB, Feng FY, Beard CJ, Martin NE, Orio PF, III, Nguyen PL. National trends and determinants of proton therapy use for prostate cancer: a National Cancer Data Base study. Cancer. 2016;122:1505–12. doi: 10.1002/cncr.29960. [DOI] [PubMed] [Google Scholar]
- 21.Jung OS, Guzzo T, Lee D, Mehler M, Christodouleas J, Deville C, Hollis G, Shah A, Vapiwala N, Wein A, Pauly M, Bekelman JE. Out-of-pocket expenses and treatment choice for men with prostate cancer. Urology. 2012;80:1252–7. doi: 10.1016/j.urology.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2017;97:60–63. doi: 10.1016/j.ijrobp.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Amini A, Raben D, Crawford ED, Flaig TW, Kessler ER, Lam ET, Maroni P, Pugh TJ. Patient characterization and usage trends of proton beam therapy for localized prostate cancer in the United States: a study of the National Cancer Database. Urol Oncol. 2017;35:438–446. doi: 10.1016/j.urolonc.2017.01.013. [DOI] [PubMed] [Google Scholar]


