Abstract
Purpose:
Recurrent meningiomas remain therapeutically challenging, often progressive despite multimodality salvage. There are limited data guiding reirradiation (reRT), and proton beam radiation therapy (PBRT) offers a potential advantage owing to lower integral brain dose.
Patients and Methods:
We retrospectively conducted a review of 16 patients who received PBRT reRT for recurrent meningiomas. Kaplan-Meier and proportional hazards were used to determine post-PBRT progression-free survival (PFS) and overall survival (OS) and to evaluate clinical predictors.
Results:
At diagnosis, 7 (44%), 8 (50%), and 1 (6%) patient had World Health Organization (WHO) grade I, II and III tumors, respectively. All received prior radiation therapy (RT) to a median of 54 Gy (range 13-65.5). Median time to PBRT reRT after prior RT was 5.8 years (range 0.7-18.7). Median PBRT dose was 60 Gy(RBE) (range 30-66.6), and median planning tumor volume (PTV) was 76 cm3 (range 8-249). Median follow-up was 18.8 months. At last follow-up, 7 intracranial recurrences (44%) and 3 disease-related deaths (19%) were found. Median cohort PFS was 22.6 months, with 1- and 2-year PFS of 80% and 43%, respectively. Median OS was not achieved, with 1- and 2-year OS of 94% and 73%; all deaths were felt to be related to meningioma. Patients with initially grade I tumors had improved PFS versus higher grade (Hazard Ratio, HR = 0.23, P = .03) with 1- and 2-year PFS estimates of 100% versus 71% and 75% versus 29%, respectively. Longer interval between prior RT and PBRT also predicted improved PFS (P = .03) and OS (P = .049). Overall late grade 3+ toxicity rate was 31%. Two patients (13%) developed radionecrosis at 6 and 16 months after PBRT; only 1 was symptomatic.
Conclusions:
This is the first series specifically analyzing PBRT alone as a reRT strategy for recurrent meningioma. We report fair intracranial control with low rates of radionecrosis at 1 year after reRT. However, strategies to achieve durable outcomes are needed, particularly for high-grade tumors.
Keywords: proton therapy, meningioma, reirradiation, salvage therapy, radiation therapy
Introduction
Meningiomas are the most common primary central nervous system tumor [1]. Active surveillance is generally accepted for small, incidentally discovered or asymptomatic lesions and maximal safe surgical resection remains the mainstay of treatment for large or symptomatic tumors. Radiation therapy (RT) also serves a key role in the upfront treatment of inoperable meningiomas [2] or adjuvant treatment for most partially resected and some grossly resected higher-grade (atypical or anaplastic) variants to improve local control [3] and possibly overall survival (OS) [4].
Recurrent meningioma particularly when high grade, can pose significant treatment challenges. Management options are more variable but salvage options include additional surgery with or without brachytherapy [5, 6], conventional fractionated external beam radiation therapy (EBRT), stereotactic radiosurgery (SRS), hypofractionated RT, or systemic therapy [7].
Rates of meningioma recurrence after RT vary and are dependent on clinical factors such as extent of resection, timing of RT, tumor grade, and anatomic location. Multiple studies have retrospectively investigated recurrence risks after RT and reported rates of 0% to 23% (typically <10% for modern series) for World Health Organization (WHO) grade I lesions, using either EBRT or SRS [8]. A large retrospective series from Korea reported a 7.3% total recurrence rate for patients with a single meningioma who underwent Gamma Knife radiosurgery [9]. Rates of failure for higher-grade lesions are significantly increased, though immediate adjuvant RT, to higher doses, does appear to improve outcomes [10]. This was confirmed by the recently reported prospective RTOG 0539 trial, which reported a benefit for adjuvant RT for intermediate-risk meningiomas [11].
Pattern-of-failure studies show that most recurrences tend to occur either within the prior RT field or marginally [12–16]; therefore, salvage reirradiation (reRT) has the potential for significant anatomic overlap. Nevertheless, the safety and efficacy of reRT remains unclear. Proton beam radiation therapy (PBRT) offers a dosimetric advantage for reRT, especially for patients with multiple prior courses of RT, owing to improved ability to spare toxicity to nearby normal structures. To our knowledge, the utilization of PBRT for reRT has not been explicitly studied and we sought to report a modern series of patients to better understand the utility and risks of this approach.
Materials and Methods
Patient Cohort
A retrospective analysis was performed of 16 consecutive patients who received PBRT reRT between April 2013 and May 2017 at a single proton therapy facility for intracranial meningioma due to local recurrence after initial course of RT. This study was approved by the institutional review boards at both the academic center leading this work, and the proton therapy center.
Proton Beam Reirradiation
Patients were simulated by using computed tomography (CT)–based planning in either the supine or upright chair positioning with an Aquaplast mask. The CT slice thickness was 1.25 mm and intravenous contrast was administered. The planning CT scan was fused to a gadolinium contrast-enhanced magnetic resonance image. The gross tumor volume (GTV) included macroscopic tumor if gross total resection had not been achieved before PBRT reRT. The clinical target volume (CTV) included the GTV plus areas concerning for microscopic disease, including the surgical cavity for patients who had complete resections. In general, the planning tumor volume (PTV) reflected the GTV or CTV with a 3-mm uniform expansion to account for setup error and motion.
For 5 patients, previous RT DICOM treatment plans were digitally fused with rigid registration to the PBRT plan to estimate cumulative total dose. For 11 patients, no digital plan was available, and a best attempt was made at reconstruction of prior dosing from the paper records, including isodose diagrams, reported organ at risk (OAR) dose statistics, and port films. Dose constraints for OARs were set on the basis of published data and clinical expertise. In most cases, cumulative doses of mean dose to the optic chiasm <58 Gy(RBE), point dose to the optic nerves <60 Gy(RBE), core maximum dose to the brainstem <53Gy (RBE), and surface maximum dose to the brainstem <64 Gy(RBE), with <76 Gy(RBE) maximum dose to the temporal lobe and <72 Gy(RBE) to 2 cm3 to the temporal lobe were achieved. A relative biologic effectiveness (RBE) for protons of 1.1 was assumed and proton doses are presented in Gy(RBE), which equals the proton physical dose multiplied by 1.1. Setup accuracy was confirmed with daily orthogonal imaging centered at the isocenter and verified on bony anatomy.
All patients received conventionally fractionated PBRT reRT with either 1.8 or 2.0 Gy(RBE) daily fractionation and either uniform scanning or pencil beam scanning (PBS). At the average PBS cranial treatment energy (150 MeV), the spot size in air is 5 mm [17]. Total dose was selected on the basis of tumor histology and previous treatment history. In general, our treatment recommendations are 50 to 54 Gy(RBE) for low-grade tumors and at least 59.4 Gy(RBE) for higher-grade lesions. In our series, 1 patient with a large, grade II lesion of the right frontal lobe dura with previous RT to 60 Gy received only 54 Gy(RBE) owing to concerns about composite dose to the nearby optic nerve and brainstem. One patient with a recurrent grade I lesion was treated more aggressively to 60 Gy(RBE). Specifically, the range of reRT doses for grade I tumors was 50.4 to 60 Gy(RBE). The range of reRT doses for grade II tumors was 54 to 66.6 Gy(RBE) and the range of reRT doses for grade III tumors was 59.4 to 64.8 Gy(RBE). The beam arrangement was selected from PTV geometry and size as well as dose limits to neighboring OARs.
Follow-up
In general, follow-up serial magnetic resonance imaging (MRI) and clinical examination were performed at 3- to 6-month intervals for the first year depending on the grade of tumor and clinical symptoms and discretion of the treating physician. Local failure was defined radiographically as any evidence of progressive tumor within the PBRT field observed on post-treatment MRI.
Toxicities and Treatment-Related Effects
Acute and late toxicities were retrospectively assessed and scored by using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4. Radionecrosis was diagnosed radiographically from MRI with perfusion sequences and clinically with treating physicians' consensus opinion.
Statistical Analysis
Outcomes of interest included OS and progression-free survival (PFS) defined as earliest date of either progressive meningioma or death. All outcomes were measured from the first day of the PBRT reRT and estimated using the Kaplan-Meier approach. Outcome differences between various demographic and therapeutic subgroups were assessed using the log-rank test. Univariate Cox proportional hazards were also used to assess predictors of PFS and OS. Two-sided tests were used and a P value of ≤0.05 was considered significant. All analysis was performed by using either GraphPad Prism v7 (GraphPad, La Jolla, California) or SPSS v24 (IBM, Armonk, New York).
Results
Patient Cohort
Patient demographic and treatment characteristics are described in Table 1. All patients' conditions were initially diagnosed pathologically. Six patients (38%) had repeated resection or biopsy immediately before PBRT reRT, which informed pre-PBRT pathologic grade. For the remainder of the patients, the histologic grade from the most current previous surgery was assumed at the time of PBRT. Most patients had high-grade lesions at the time of PBRT reRT, and 31% of patients had transformation to a higher grade from the initially diagnosed tumor grade. Patients with newly diagnosed RT-induced meningiomas were excluded from the analysis.
Table 1.
Cohort characteristics.
|
Characteristics |
No. (%) |
| No. of patients | 16 |
| Median age at time of PBRT reRT (range), y | 61.6 (29.0–84.9) |
| Female gender | 10 (63%) |
| Anatomic location | |
| Skull base including cavernous sinuses and sphenoid wings | 11 (69%) |
| Frontal or parietal lobe dura | 3 (19%) |
| Vertex | 1 (6%) |
| Ethmoid sinus | 1 (6%) |
| Initial pathologic grade (WHO) | |
| Grade I | 7 (44%) |
| Grade II | 8 (50%) |
| Grade III | 1 (6%) |
| Pathologic grade at time of PBRT reRT | |
| Grade I | 4 (25%) |
| Grade II | 8 (50%) |
| Grade III | 4 (25%) |
| Meningioma that underwent transformation to a higher grade from the initially diagnosed tumor grade at the time of PBRT reRT | 5 (31%) |
| No. of prior surgeries | |
| 1 | 4 (25%) |
| 2 | 7 (44%) |
| 3+ | 5 (31%) |
| Extent of most recent surgery | |
| Gross total resection | 2 (13%) |
| Subtotal resection | 14 (87%) |
| Total No. of prior courses of RT | |
| 1 | 14 (87%) |
| 2 | 1 (6%) |
| 4 | 1 (6%) |
| Prior RT modality | |
| Conventional fractionation (IMRT or 3D conformal) | 9 (56%) |
| SRS | 5 (31%) |
| Multiple modalities | 2 (13%) |
| Estimated median dose of prior photon RT in PBRT field (range), Gy | 54 (13–66) |
| Median duration of time between prior RT and PBRT (range), y | 5.8 (0.7–18.7) |
| Median received dose of PBRT (range), Gy(RBE) | 60 (30–66.6) |
| Median PBRT PTV (range), cm3 | 76.1 (7.7–249.1) |
| Median PBRT OAR dosimetry (range), Gy(RBE) | |
| Brainstem | 19.6 (0–55.6) |
| Optic nerve(s) | 27.1 (0–59.5) |
| Optic chiasm | 7.2 (0–55.1) |
| Cochleae | 6.5 (0–62.7) |
Abbreviations: PBRT, proton beam radiation therapy; reRT, reirradiation; WHO, World Health Organization; RT, radiation therapy; IMRT, intensity-modulated radiation therapy; SRS, stereotactic radiosurgery; PTV, planning tumor volume; OAR, organ at risk; RBE, relative biological effectiveness.
Most patients had 1 prior course of RT before PBRT reRT. Two patients, one with grade II and one with grade III disease, had 2 and 4 courses of RT before PBRT reRT, respectively. The first patient received 54 Gy with photon intensity-modulated radiation therapy (IMRT) followed by Gamma Knife SRS (11.5 Gy to the 50% isodose line) for recurrent disease after 39.6 months. Review of the plans suggests that only the SRS plan was fully overlapping with the PBRT.
The second patient received 59.4 Gy with 3D conformal RT followed by Linac-based SRS (20 Gy to the 50% isodose line) for recurrence after 17.6 months, followed by Gamma Knife treatment (18 Gy to the 60% isodose line) for second recurrence after 41.6 months, followed by hypofractionated SRS (35 Gy in 5 fractions) for third recurrence after 71.8 months. For this patient, estimated prior dose to a significant portion of the brain in 2-Gy equivalents (EQD2) using an alpha-beta ratio of 2 Gy exceeded 100 Gy.
Characteristics of Proton Beam Radiation Therapy
Six patients underwent PBRT reRT with uniform scanning system using brass apertures and wax compensators; 9 patients underwent PBS PBRT reRT without apertures, and 1 patient received 2 courses of PBRT reRT—the first course with uniform scanning followed 16 months later by a separate course of PBS PBRT reRT with minimal overlap. Total number of planned fields ranged from 2 to 6, typically with 2 to 3 fields delivered per day.
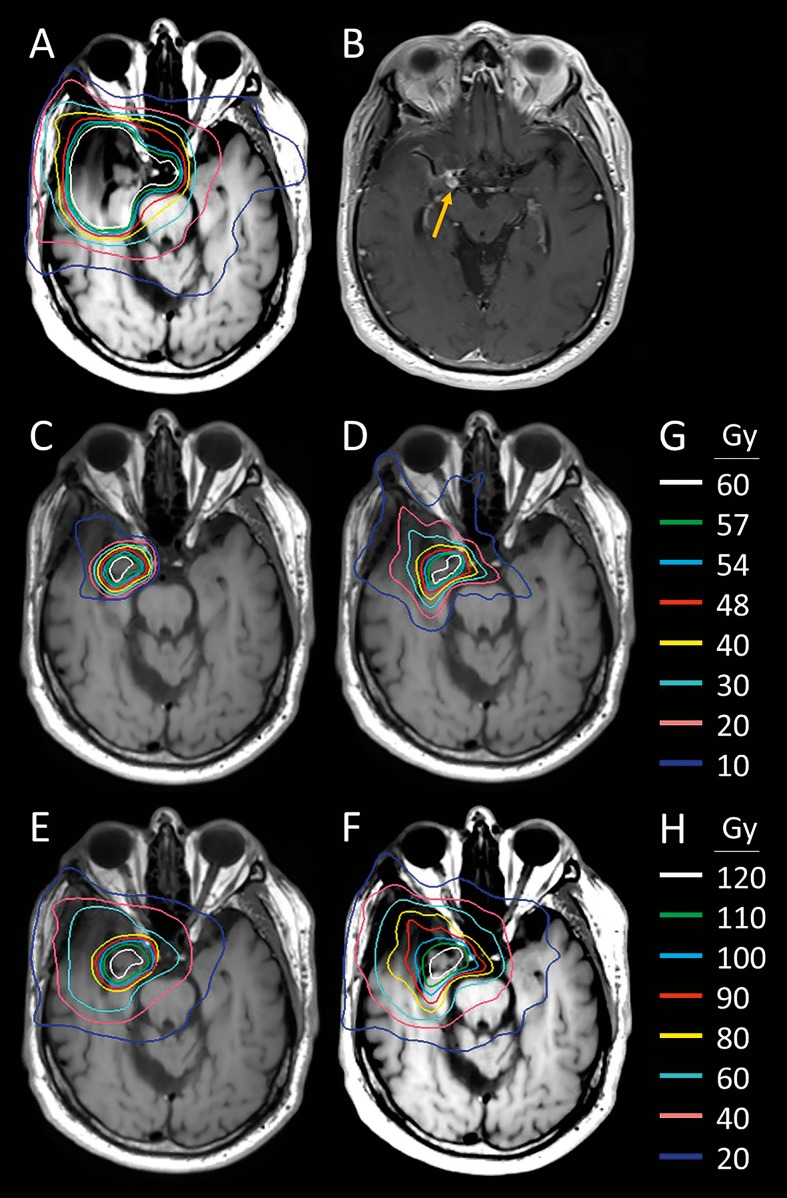
Median percentage of the volume of the PTV that received at least 95% of the prescription dose (V95) for the cohort was 92.3% (range 58.4%-100%). Reflecting the fact that this was a mix of postoperative and unresected patients, most of the cohort was planned by using either a GTV or a CTV but not both. For cases where there was no explicit CTV, we assumed GTV equals CTV. With this assumption, median V95 of the CTV was 96.9% (range 65.5%-100%). Figure 1 shows a representative patient with anaplastic meningioma who underwent PBRT reRT with comparative photon plan. The dosimetric advantage of proton reRT can be seen in cumulative dose distributions.
Figure 1.
Proton and photon comparative dosimetry for a 72-year-old man with an anaplastic meningioma of the right cavernous sinus. (A) Original photon IMRT plan, which delivered 59.4 Gy in conventional fractionation. (B) Nodular area of T1 post contrast enhancement posterior to the right carotid artery suggestive of in-field recurrence (yellow arrow). (C) Proton reirradiation plan delivered to 60 Gy(RBE) in 2 Gy(RBE) fractions with 2 beams and uniform scanning. (D) Hypothetical comparison reirradiation plan using photon VMAT (not delivered). (E) Cumulative dose delivered by using proton beam radiation therapy. (F) Hypothetical cumulative dose delivered had photon VMAT plan been delivered. (G) Isodose lines in Gy for plans in (A), (C), and (D). (H) Cumulative isodose lines in Gy(RBE) for plans in (E) and (F). Abbreviations: IMRT, intensity-modulated radiation therapy; VMAT, Volumetric Modulated Arc Therapy.
In general, our institutional PBRT planning practice is to respect pre-established OAR constraints, sacrificing target coverage if necessary. However, particularly in the reRT setting, some flexibility with OAR dose may be allowed by the treating physician. As this was a retrospective effort, some of this nuanced discussion between the planners and the radiation oncologists is difficult to capture. When re-reviewing all of the plans, there were 6 cases where target coverage (in terms of PTV V95) was sacrificed as a compromise to respect the institutional OAR guidelines.
Treatment Outcomes
The median follow-up for the cohort after PBRT reRT was 18.8 months (range 1.2-41.5 months). At last follow-up, 7 patients experienced local recurrence (44%), and 3 meningioma-related deaths (19%) were observed. Patients who experienced meningioma-related death all had high-grade histology at the time of PBRT reRT (2 atypical, 1 anaplastic). Median PFS for the full cohort was 22.6 months (95% CI 15.2-29.9), with a 1- and 2-year PFS of 80% and 43%, respectively. Median OS post PBRT was not reached, with a 1- and 2-year freedom from death of 94% and 73%.
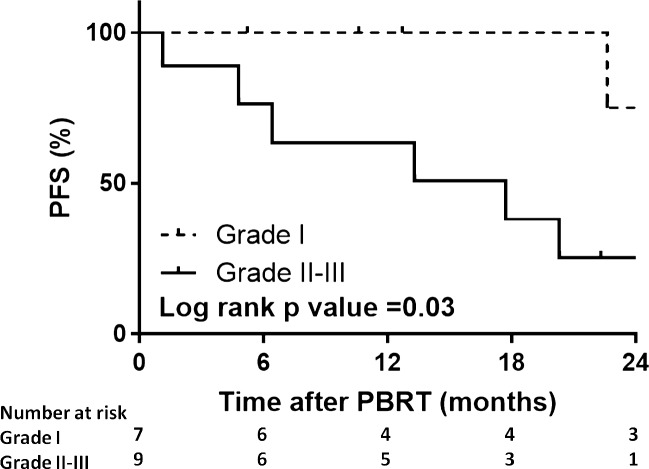
We next analyzed the impact of several factors on PFS after PBRT including age, gender, histologic grade, number of prior surgeries, time interval between previous RT and current course of PBRT, and size of PTV. We found that histologic grade is a significant predictor of local failure (Hazard Ratio, HR 4.4, 95% CI 1.07-18.0, P = .03) with higher-grade tumors at greater risk for progression after PBRT reRT. At 12 months, the local control rate for patients with grade I versus grade II-III tumors who underwent PBRT reRT was 100% versus 64% (P = .03, Figure 2). Patients with grade II-III tumors at the time of PBRT reRT demonstrated a trend for worse PFS (HR 4.5, 95% CI 0.96-20.7, P = .056).
Figure 2.
PFS after PBRT by initial histologic grade showing the subcohort with WHO grade I meningioma (dashed line) compared to those with WHO grade II or grade III tumors. Abbreviations: PBRT, proton beam radiation therapy; PFS, progression-free survival; WHO, World Health Organization.
Table 2 shows the univariate Cox proportional hazards for predictors of PFS. Greater interval between the prior course of RT and PBRT (treated as a continuous variable) was associated with improved PFS following reRT, likely due to more indolent tumor biology (P = .031). Patients with shorter time between prior RT and PBRT also had significantly poorer OS post PBRT (P = .049).
Table 2.
Univariate Cox proportional hazards modeling of PFS.
|
Covariate |
HR of PFS |
P
Value |
| Age at PBRT | 1.08 | .095 |
| Male gender | 1.72 | .48 |
| Initially WHO grade II-III pathology | 7.89 | .063 |
| WHO grade II-III pathology at the time of PBRT | 38.9 | .26 |
| Transformation to higher grade from grade at initial diagnosis | 1.26 | .75 |
| More than 1 prior surgery | 0.33 | .18 |
| Interval between PBRT and last RT course | 0.71 | .031 |
| PBRT PTV (cm3) | 1.00 | .69 |
Abbreviations: HR, hazard ratio; PFS, progression-free survival; PBRT, proton beam radiation therapy; WHO, World Health Organization; RT, radiation therapy; PTV, planning tumor volume.
Three patients received additional salvage treatment after PBRT, 2 of whom ultimately succumbed to meningioma. One received surgery and adjuvant SRS to a separate progressive lesion (deceased at 16 months following PBRT); 1 patient received an additional course of PBRT for a separate site of intracranial progression (deceased at 22 months following initial PBRT). The final patient received a single dose of bevacizumab for mixed tumor progression and radionecrosis and was alive with ongoing progression and clinical decline at last imaging nearly 40 months after PBRT.
Treatment-Related Toxicities
Five patients in our cohort (31%) experienced toxicities during or shortly after PBRT reRT, including 1 patient who experienced grade 1 muffled hearing and 1 patient who experienced mild visual disturbance felt to be RT-related irritation to the optic apparatus, which resolved with a course of dexamethasone. One patient with an ethmoid sinus–based lesion and 4 total prior courses of RT developed intolerable grade 3 nasal mucositis and sinus pressure and requested to stop treatment after 30 Gy(RBE) of a planned dose of 60 Gy(RBE). Two patients experienced toxicities that were felt not to be related to PBRT reRT. One patient with an anaplastic lesion of the cavernous sinus and prior seizures suffered a grade 4 seizure 5 days after completion of his 63 Gy(RBE) PBRT reRT due to noncompliance of antiepileptic medication. Another patient who was receiving treatment to a symptomatic progressive lesion involving the bilateral cavernous sinuses suffered a grand mal seizure during the PBRT reRT course after 2 fractions with cumulative dose of 3.6 Gy(RBE), which progressed into status epilepticus and ultimately fatal cardiopulmonary arrest. Imaging revealed coincident progression of convexity meningiomas, which was felt to be the source of her seizure and not the cavernous sinus lesion that received PBRT reRT. The remainder of the patients did not have any reported acute side effects outside of expected fatigue and dermatitis.
Eight patients (50%) had longer-term toxicities that were possibly attributable to underlying PBRT reRT. Table 3 details these side effects. While most were mild, there were 5 more significant toxicities. Patient 4 with right parietal ventriculostomy catheter at the time of PBRT reRT developed symptomatic exacerbation of hydrocephalus requiring shunt revision. Patient 7 developed new symptomatic hydrocephalus and underwent ventriculoperitoneal shunting 6 months after completion of PBRT reRT. Patient 5 developed progressive gait instability felt to be due to exacerbation of baseline hydrocephalus but declined further intervention outside of physical therapy. Of note, this patient did have baseline gait instability and mild radiographic ventriculomegaly that preceded PBRT; however, this had been attributed to age-related diffuse volume loss. Patient 6 with skull base disease abutting the right internal carotid artery was found to have carotid stenosis and subsequently symptomatic cerebrovascular ischemia. The stenosis was felt to be due to post-PRBT reRT inflammation and possibly concomitant tumor progression. Patient 8 developed worsening seizures and fatigue, which was felt to be attributable to radionecrosis. No secondary malignancies of the brain or head and neck were noted in our series.
Table 3.
Detailed post-PBRT toxicities.
|
Patient |
Age at PBRT (y) |
PBRT treatment area |
WHO grade at PBRT |
Total PBRT dose Gy(RBE) |
Prior RT dose |
Prior RT modality |
Post-PBRT toxicity |
Retrospective toxicity grade |
Interval between PBRT and toxicity (mo) |
Management |
| 1 | 60.3 | R sphenoid wing | Grade II | 64 | 25 | SRS | Persistent R retro-orbital pressure | Grade 1 | 11 | Supportively managed |
| 2 | 53.8 | L skull base | Grade II | 60 | 22.5 | SRS | Bilateral tinnitus | Grade 1 | 15 | Expectant management |
| 3 | 53.8 | R frontal convexity | Grade II | 54 | 60 | IMRT | Asymptomatic radionecrosis | Grade 1 | 16 | Expectant management |
| 4 | 66.4 | L cavernous sinus | Grade II | 54 | 59.4 | IMRT | Hydrocephalus | Grade 3 | 1 | VP shunt revision |
| 5 | 75.1 | R frontoparietal vertex | Grade I | 60 | 54 | IMRT | Hydrocephalus | Grade 3 | 3 | VP shunt recommended but patient declined, prolonged physical therapy |
| 6 | 72.9 | R skull base | Grade III | 59.4 | 60 | IMRT | Carotid stenosis and subsequent cerebrovascular ischemia | Grade 3 | 3 | Evolving stroke symptoms in context of possible tumor progression |
| 7 | 74.8 | R skull base | Grade II | 60 | 25 | SRS | Hydrocephalus | Grade 3 | 6 | VP shunt placement |
| 8 | 58.4 | R frontal lobe | Grade II | 60 | 54 | IMRT | Seizures, possible TIA, fatigue | Grade 3 | 6 | Surgical resection for symptomatic radionecrosis |
Abbreviations: PBRT, proton beam radiation therapy; WHO, World Health Organization; RT, radiation therapy; R, Right; SRS, stereotactic radiosurgery; L, Left; IMRT, intensity-modulated radiation therapy; VP, Ventriculoperitoneal; TIA, Transient ischemic attack; RBE - relative biological effectiveness.
Radionecrosis Risk
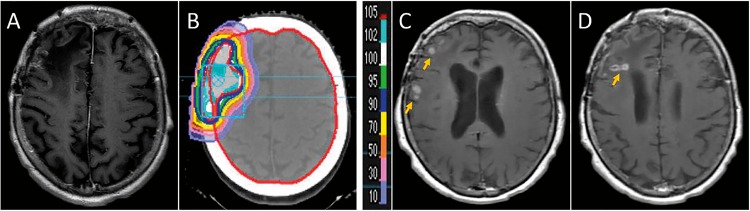
Two patients (13%) in the series developed radionecrosis. Patient 3 (Table 3), with a right frontal convexity atypical meningioma treated with 60 Gy of IMRT 6.5 years before treatment with 54 Gy(RBE) of PBRT reRT, was discovered to have radiographic evidence of radionecrosis 16.3 months after completing proton therapy (Figure 3). She has remained clinically asymptomatic. The second patient had recurrent atypical meningioma of the right frontal region and underwent 3 prior resections and 54 Gy of IMRT 10 years before PBRT reRT. She received 60 Gy(RBE) and shortly after completion she experienced several months of fatigue and increased seizure frequency. Interval MRI at 4 months was concerning for radionecrosis. At 6 months after PBRT reRT, she presented with acute onset left-sided weakness and was found to have significant worsening of vasogenic edema and underlying radionecrosis. After minimal improvement with high-dose dexamethasone, she underwent re-resection of tumor with pathology demonstrating viable meningioma with focal necrotizing treatment effect as well as more prominent radionecrosis of the adjacent cerebral white matter. She ultimately received bevacizumab 1.5 years after the last surgery followed by continued symptomatic progression of her radionecrosis.
Figure 3.
(A) T1 post contrast MRI before PBRT reRT. (B) Cumulative isodose lines after PBRT reRT. (C, D) Areas of asymptomatic radionecrosis denoted on post-treatment MRI (yellow arrows). Abbreviations: MRI, magnetic resonance imaging; PBRT, proton beam radiation therapy; reRT, reirradiation.
Discussion
We present a contemporary series of patients who underwent reRT using proton beam radiation for recurrent intracranial meningioma and report fair local control and OS with acceptable toxicity. To our knowledge, this is the largest case series that explicitly studied the use of PBRT reRT. The value added of proton therapy in reRT is its unique physical property (Bragg peak), which enables delivery of defined radiation doses at a prescribed depth with low exit doses beyond that depth [18]. This is of particular utility in the brain as it maximizes full-dose delivery to the tumor with minimization of integral dose to the surrounding normal structures, theoretically reducing the medium to long-term toxicity from reRT [19]. In our series, the advantage of PBRT fell into 2 categories. For all patients, there was a generalized reduction of dose to overlapping targeted normal brain tissues. Given that 38% of patients had large treatment areas with PTV >100 cm3, this benefit is not insignificant. There was also a subset of patients where the utilization of PBRT enabled a constraint of a critical OAR. For example, in Figure 1, the comparative dosimetry shows that utilization of IMRT for this patient would have resulted in unacceptable dose to the right optic nerve.
In our series, we reported 1-year PFS rate of 80% with good treatment tolerance for most patients given a heavily pretreated cohort. Many of the patients in our cohort had few other treatment options at the time of PBRT. For example, most patients had skull base lesions where reoperation carries significant risk of complications [20]. The Heidelberg Ion Therapy Center recently published its experience using particle therapy for reRT of 42 patients with recurrent meningioma [21]. In that analysis, 81% of patients received carbon ion therapy and only 8 patients received PBRT and the outcomes are pooled across particles. Nevertheless, the authors reported similar outcomes to our study, including 1-year PFS rate of 71%.
Photon-based SRS reRT has also been retrospectively studied as a strategy to limit dose to previously treated brain tissue. Kim and colleagues [9] analyzed 33 patients (48% grade II-III) who had previously received Gamma Knife for a single meningioma and then underwent repeated SRS for local recurrence; 76% of patients had received microsurgical resection before reRT. With mean follow-up of about 11 years, 1- and 2-year PFS were 85% and 60%, respectively, comparable to our findings. Crude tumor control rate was nearly 50%. The only factor that was found to be prognostic of improved PFS for reRT was grade I histology. Wojcieszynski and colleagues [22] reported on a mixed cohort of 19 patients who received photon-based SRS or EBRT reRT and report a similar 1-year PFS of 66% with a median time to post-reRT recurrence of 10 months.
More recently, Lin and colleagues [23] reported their single-institutional experience reirradiating 43 patients with locally recurrent meningioma with a mix of stereotactic radiosurgery (67%) or conventionally fractionated RT with protons or photons (33%). After median follow-up of 19 months, they report similar efficacy, with 1-year PFS of 73% overall and 60% for grade II/III histology. They also found that high-grade histology was associated with poorer PFS, as was lower Karnofsky score.
Outside of re-resection and reRT, the therapeutic landscape for recurrent meningiomas is limited. Systemic treatments have generally shown poor efficacy for the treatment of recurrent meningiomas. Hydroxyurea has been shown to have limited efficacy with risk of bone marrow suppression in the relapsed setting [24]. A phase II trial of sunitinib in a heavily pretreated cohort of high-grade meningiomas noted median PFS of around 5 months and a 6-month PFS of 42% [25]. Outcomes of bevacizumab have been more promising, particularly for grade II-III histology, with a recent systematic review estimating 6-month PFS to be 73% (95% CI 44%-93%) [26]. A recent review of medical therapy (hormonal therapies, tyrosine kinase inhibitors, and systemic chemotherapies) estimates an overall 6-month PFS of <30% for all meningioma grades [27].
We also reported limited toxicities associated with PBRT reRT. The most common post-treatment complication was new or worsened hydrocephalus in 3 patients. Review of the pre-PBRT reRT imaging suggested that all 3 patients had some degree of radiographic ventriculomegaly before PBRT. In 2 cases, the enlargement of the ventricles was felt to be postsurgical ex vacuo dilatation. The third patient's ventriculomegaly was initially attributed to age-related diffuse volume loss; however, given coexistent gait difficulties, it is possible that he had some degree of hydrocephalus preexistent to PBRT reRT. In a cohort of heavily pretreated patients, it is challenging to say which particular therapy was responsible; however, it is plausible that patients with preexistent radiographic ventriculomegaly are more susceptible to the local inflammatory changes induced by reRT.
Despite increasing availability of PBRT, it remains a relatively scarce and expensive treatment option. One potential justification for PBRT would be theoretical reduction in the risk of complications like radionecrosis. In our series, we discovered a low risk of radionecrosis; with a median follow-up of 18.8 months, 13% of patients developed radionecrosis with only 1 patient with symptoms requiring surgical intervention.
Based on the limited published data, the composite rates of symptomatic radionecrosis for photon-based reRT may be higher than the rates we have observed with PBRT reRT. Lin et al [23] reported 13% asymptomatic and 15% symptomatic radionecrosis in a pooled cohort of 39 patients who received a mixture of photon radiosurgery, conventionally fractionated photon radiation, and conventionally fractionated PBRT. Reported rate of serious grade 3-4 radionecrosis was 10%. The authors did not break down the risks by radiation modality; however, the cases of symptomatic necrosis all occurred with a composite biological equivalent dose of greater than 120 Gy. Only one grade 1 case was observed in a patient who received less than 100 Gy cumulative dose, which has previously been shown to be an important threshold of brain tolerance [28]. The interpretation of this 120-Gy threshold as a meaningful endpoint is unclear in the context of PBRT, since this dose is most likely to fall within the target reRT region. Planning with IMRT or PBRT is similarly likely to be able to deliver high-dose target coverage; therefore, this threshold may serve as a general planning limit for either modality. The most significant benefit of PBRT, particularly in an reRT context, is its ability to reduce the volume receiving low or intermediate doses to maximize function of remaining brain and to potentially avoid critical OARs.
Other smaller studies with photon reRT have reported rates of symptomatic radionecrosis of 16% to 21%, greater than the rate we have observed with PBRT reRT [29–31]. These smaller series had slightly longer follow-up (22.4-28.1 months) so it is possible that surveillance duration accounts for the differential rates of reported radionecrosis; however, we think this is less likely given that the follow-up period of 19.4 months in the largest study (Lin et al [23]) was nearly identical to ours at 18.8 months. More of the patients in the series of Lin and colleagues [23] originally received single fraction or multifraction SRS, compared to our cohort, which might contribute to the different degree of radionecrosis. Two other small series of photon-treated patients do not explicitly discuss necrosis [9, 22].
The lower rate of radiographic radiation injury observed in the current study is consistent with the prevalence reported by the Heidelberg group for reRT with particle therapy [21] and the prevalence reported by colleagues in our institution who studied risk of temporal lobe necrosis for PBRT reRT of skull base head and neck cancers [32].
Our series is hypothesis generating for prognosticating the patients who might benefit most from PBRT: those with longer interval between prior RT and reRT and those with initially grade I tumors. The finding that grade I histology portends improved prognosis following reRT has been recapitulated in other small series [9, 21–23]. This consistent finding suggests that full-dose reRT is unlikely to be sufficient for durable long-term response in patients with grade II-III disease, and future prospective efforts may need to consider novel combinations of RT with systemic agents to overcome a relatively radioresistant histology.
There were several limitations to our study, including its retrospective nature and small size with relatively few events. Our proton center is referral based and outpatient, therefore there may be a selection bias for relatively healthier and better performing patients at the time of PBRT. The anatomic and histologic diversity of the population is reflective of the inherent heterogeneity of recurrent meningiomas. While the median duration of follow-up is short, many of our patients had atypical or anaplastic disease at the time of PBRT. One series with roughly 11 years of average follow-up found that post-RT failures for indolent meningiomas continue for about 5 years, whereas most failures for grade II/III lesions occur in the first 2 years post RT [9]. Therefore, in our own series, with longer follow-up we anticipate more recurrences and deaths, particularly in the grade I subcohort. Given the small size of the sample, we chose to evaluate all patients, even those with short post-PBRT follow-up to assess for short-term toxicities. Despite the limitations, there are relatively sparse clinical data guiding reRT for meningiomas and we feel there is utility in our analysis.
PBRT reRT may be a relatively efficacious strategy for recurrent meningiomas, a patient population lacking durable therapeutic options. Even with significant prior radiation exposure, radionecrosis rates appear low. We feel that prospective investigation of the modality is warranted to validate incremental improvement over traditional photon RT.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Dr Yamada serves as a speaker for Varian Medical Systems, BrainLab, and Vision RT Institute for Medical Education. He also sits on the medical advisory board of the Chordoma Foundation. All other authors declare that there are no relevant conflicts of interest, financial or personal, that might bias or be seen to bias their work for this submission. All authors contributed substantially to the study and approved the final version of the manuscript. This manuscript, or any part of it, has not been previously published nor submitted concurrently to any other journal.
Funding: This work was supported by NIH grant P30 CA 008748.
Acknowledgments: This work was presented at the American Society for Radiation Oncology (ASTRO) 2018 Annual Meeting in San Antonio, Texas.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19:v1–88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korah MP, Nowlan AW, Johnstone PAS, Crocker IR. Radiation therapy alone for imaging-defined meningiomas. Int J Radiat Oncol Biol Phys. 2010;76:181–6. doi: 10.1016/j.ijrobp.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 3.Kaur G, Sayegh ET, Larson A, Bloch O, Madden M, Sun MZ, Barani IJ, James CD, Parsa AT. Adjuvant radiotherapy for atypical and malignant meningiomas: a systematic review. Neuro Oncol. 2014;16:628–36. doi: 10.1093/neuonc/nou025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Kaprealian TB, Suh JH, Kubicky CD, Ciporen JN, Chen Y, Jaboin JJ. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol. 2017;19:1263–70. doi: 10.1093/neuonc/nox007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutin PH, Leibel SA, Hosobuchi Y, Crumley RL, Edwards MS, Wilson CB, Lamb S, Weaver KA. Brachytherapy of recurrent tumors of the skull base and spine with iodine-125 sources. Neurosurgery. 1987;20:938–45. doi: 10.1227/00006123-198706000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Ware ML, Larson DA, Sneed PK, Wara WW, McDermott MW. Surgical resection and permanent brachytherapy for recurrent atypical and malignant meningioma. Neurosurgery. 2004;54:55–63. doi: 10.1227/01.neu.0000097199.26412.2a. [discussion in Neurosurgery 2004;54:63–4] [DOI] [PubMed] [Google Scholar]
- 7.Karsy M, Guan J, Cohen A, Colman H, Jensen RL. Medical management of meningiomas: current status, failed treatments, and promising horizons. Neurosurg Clin N Am. 2016;27:249–60. doi: 10.1016/j.nec.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Rogers L, Barani I, Chamberlain M, Kaley TJ, McDermott M, Raizer J, Schiff D, Weber DC, Wen PY, Vogelbaum MA. Meningiomas: knowledge base, treatment outcomes, and uncertainties: a RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M, Lee DH, Kim Rn HJ, Cho YH, Kim JH, Kwon DH. Analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg. 2017;153:93–101. doi: 10.1016/j.clineuro.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Hug EB, Devries A, Thornton AF, Munzenride JE, Pardo FS, Hedley-Whyte ET, Bussiere MR, Ojemann R. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151–60. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 11.Rogers L, Zhang P, Vogelbaum MA, Perry A, Ashby LS, Modi JM, Alleman AM, Galvin J, Brachman D, Jenrette JM, De Groot J, Bovi JA, Werner-Wasik M, Knisely JPS, Mehta MP. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129:35–47. doi: 10.3171/2016.11.JNS161170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn EN, Chan MD, Tatter SB, Ellis TL. Gamma knife stereotactic radiosurgery for radiation-induced meningiomas. Stereotact Funct Neurosurg. 2012;90:365–9. doi: 10.1159/000339636. [DOI] [PubMed] [Google Scholar]
- 13.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(suppl):283–6. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 14.Choi CYH, Soltys SG, Gibbs IC, Harsh GR, Jackson PS, Lieberson RE, Chang SD, Adler JR. Cyberknife stereotactic radiosurgery for treatment of atypical (WHO grade II) cranial meningiomas. Neurosurgery. 2010;67:1180–8. doi: 10.1227/NEU.0b013e3181f2f427. [DOI] [PubMed] [Google Scholar]
- 15.Attia A, Chan MD, Mott RT, Russell GB, Serif D, Daniel Bourland J, Deguzman AF, Ellis TL, McMullen KP, Munley MT, Tatter SB, Shaw EG. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol. 2012;108:179–85. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Press RH, Prabhu RS, Appin CL, Brat DJ, Shu HK, Hadjipanayis C, Olson JJ, Oyesiku NM, Curran WJ, Crocker I. Outcomes and patterns of failure for grade 2 meningioma treated with reduced-margin intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:1004–10. doi: 10.1016/j.ijrobp.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Chen C-C, Chang C, Moyers MF, Gao M, Mah D. Technical Note: spot characteristic stability for proton pencil beam scanning. Med Phys. 2016;43:777–82. doi: 10.1118/1.4939663. [DOI] [PubMed] [Google Scholar]
- 18.DeLaney TF. Proton therapy in the clinic. Front Radiat Ther Oncol. 2011;43:465–85. doi: 10.1159/000322511. [DOI] [PubMed] [Google Scholar]
- 19.Combs SE, Laperriere N, Brada M. Clinical controversies: proton radiation therapy for brain and skull base tumors. Semin Radiat Oncol. 2013;23:120–6. doi: 10.1016/j.semradonc.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Magill ST, Lee DS, Yen AJ, Lucas C-HG, McDermott MW. Surgical outcomes and complications after reoperation for recurrent skull base meningiomas. J Neurol Surg B. 2016;77:A025. [Google Scholar]
- 21.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, Bougatf N, Jäkel O, Debus J, Combs SE. Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol. 2018;13:86. doi: 10.1186/s13014-018-1026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcieszynski AP, Ohri N, Andrews DW, Evans JJ, Dicker AP, Werner-Wasik M. Reirradiation of recurrent meningioma. J Clin Neurosci. 2012;19:1261–4. doi: 10.1016/j.jocn.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin AJ, Hui C, Dahiya S, Lu HC, Kim AH, Campian JL, Tsien C, Zipfel GJ, Rich KM, Chicoine M, Huang J. Radiologic response and disease control of recurrent intracranial meningiomas treated with reirradiation. Int J Radiat Oncol Biol Phys. 2018;102:194–203. doi: 10.1016/j.ijrobp.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlain MC. Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol. 2012;107:315–21. doi: 10.1007/s11060-011-0741-z. [DOI] [PubMed] [Google Scholar]
- 25.Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, Lassman AB, Nolan CP, DeAngelis LM, Gavrilovic I, Norden A, Drappatz J, Lee EQ, Purow B, Plotkin SR, Batchelor T, Abrey LE, Omuro A. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol. 2015;17:116–21. doi: 10.1093/neuonc/nou148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franke AJ, Skelton WP IV, Woody LE, Bregy A, Shah AH, Vakharia K, Komotar RJ. Role of bevacizumab for treatment-refractory meningiomas: a systematic analysis and literature review. Surg Neurol Int. 2018;9:133. doi: 10.4103/sni.sni_264_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, Wen P. Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol. 2014;16:829–40. doi: 10.1093/neuonc/not330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70:1350–60. doi: 10.1016/j.ijrobp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Laperriere NJ, Bahl G, Ménard C, Millar B, Mason W, Hodaie M, Schwartz M, Bernstein M. Reirradiation for recurrent meningiomas: outcomes and complications. Int J Radiat Oncol Biol Phys. 2008;72(suppl):S220. [Google Scholar]
- 30.Lanning RM, Chohan MO, Ryan C, Singh R, Kohutek ZA, Ogilvie SQ, Cambridge L, Yamada Y, Tabar V, Beal K, Gutin PH. Outcomes after salvage reirradiation for recurrent meningioma. Int J Radiat Oncol Biol Phys. 2015;93:E86–7. [Google Scholar]
- 31.Pritchard AG, Nguyen TK, Bauman GS. Lessons learned from reirradiation of recurrent skull base meningioma: a case report and review of the literature. Adv Radiat Oncol. 2017;2:1–5. doi: 10.1016/j.adro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A, Pitter KL, Chow CHJ, Sine K, Cahlon O, McBride S, Tsai CJ, Leeman JE, Riaz N, Higginson DS, Waldenberg T, Cohen M, Ganly I, Boyle JO, Wong RJ, Brennan CW, Baxi SS, Sherman E, Michel L, Lee N. Temporal lobe radiation necrosis after re-irradiation involving the skull base with proton therapy: a single-institutional experience. Int J Radiat Oncol Biol Phys. 2018;100:1397–8. [Google Scholar]