Abstract
Purpose:
Proton therapy for prostate cancer may reduce bowel dose and risk of bowel symptoms relative to photon-based methods. Here, we determined the effect of using a biodegradable, injectable hydrogel spacer on rectal dose on plans for treating prostate cancer with intensity-modulated proton therapy (IMPT) or passive scattering proton therapy (PSPT).
Materials and Methods:
Pairs of IMPT and PSPT plans for 9 patients were created from fused computed tomography/magnetic resonance imaging scans obtained before and after spacer injection. Calculated values of rectal V40, V60, V70, V80, and maximum dose (Dmax) were compared with Wilcoxon signed rank tests. Displacements at the base (BP), midgland (MP), and apex (AP) of the prostate relative to the anterior rectal wall with the spacer in place were averaged for each patient and correlated with V70 by using linear regression models.
Results:
The presence of a spacer reduced all dosimetric parameters for both PSPT and IMPT, with the greatest difference in V70, which was 81.1% lower for PSPT-with-spacer than for IMPT-without-spacer. Median displacements at BP, MP, and AP were 12 mm (range 7-19), 2 mm (range 0-4), and 1 mm (range 0-5) without the spacer and 19 mm (range 12-23), 10 mm (range 8-16), and 7 mm (range 2-12) with the spacer. Modest linear trends were noted between rectal V70 and displacement for IMPT-with-spacer and PSPT-with-spacer. When displacement was ≥8 mm, V70 was ≤5.1% for IMPT-with-spacer and PSPT-with-spacer.
Conclusion:
Use of biodegradable hydrogel spacers for prostate cancer treatment provides a significant reduction of radiation dose to the rectum with proton therapy. Significant reductions in rectal dose occurred in both PSPT and IMPT plans, with the greatest reduction for IMPT-with-spacer relative to PSPT alone. Prospective studies are ongoing to assess the clinical impact of reducing rectal dose with hydrogel spacers.
Keywords: IMPT, PSPT, prostate cancer, proton therapy, hydrogel
Introduction
Patients receiving radiation therapy for prostate cancer are at risk of developing treatment-related rectal toxicity, particularly as hypofractionated and stereotactic ablative approaches have become more prominent. Toxicity can manifest as rectal bleeding or bowel urgency, and the risk correlates with dosimetric parameters such as overall dose and the volume of rectum receiving at least 70 Gy (rectal V70) [1–3]. The increase in fractional doses raises concerns regarding greater rectal toxicity, but longer-term results are needed to clarify this issue [4]. Approaches to minimize rectal radiation doses and thereby reduce treatment-related morbidity have become increasingly important in the management of prostate cancer.
Proton therapy has been used as a strategy to minimize radiation dose to adjacent structures including the rectum [5–7]. Prior reports indicate that rectal volumes receiving 10 to 80 Gy are significantly lower with proton therapy (eg, V70 = 7.9%) than with intensity-modulated (photon) radiation therapy (IMRT) (eg, V70 = 14%) [5], although others have questioned whether proton therapy alone is sufficient to reduce the rectal volume receiving high radiation doses [8, 9]. The 2 primary proton modalities, passive scattering proton therapy (PSPT) and intensity-modulated proton therapy (IMPT), have been compared for their relative ability to spare the rectum [10, 11]. An emerging approach aimed at further rectal sparing involves the use of biodegradable hydrogel spacers that physically displace the prostate from the rectal wall during treatment [11–16]. In one randomized trial, use of such a spacer led to a relative reduction in mean rectal V70 of 74% [17]. These studies suggest that significant dosimetric benefit requires at least 7- to 15-mm separation between the prostate and rectal wall [13, 18–22].
The purpose of this study is to determine the effect of a biodegradable, injectable hydrogel spacer on rectal dose in treatment plans for PSPT and IMPT for prostate cancer. We analyzed a variety of clinically relevant dosimetric parameters for both modalities in the presence and absence of these spacers, and we correlated the extent of displacement between the prostate and rectal wall (with the spacer in place) with rectal V70 to determine the optimal amount of displacement in terms of reducing rectal dose in both modalities.
Materials and Methods
For this dosimetric comparison study, we obtained de-identified diagnostic computed tomography (CT) and magnetic resonance imaging (MRI) scans from 12 patients with prostate cancer from a previous multicenter randomized trial [17] who had a hydrogel spacer injected to separate the prostate and seminal vesicles from anterior rectum (SpaceOAR System, Augmenix Inc, Waltham, Massachusetts). Each patient's preinjection CT and MR images were fused, and postinjection CT and MR images were fused, such that each patient had pairs of fused images. This study was conducted under institutional review board approval.
IMPT and PSPT plans were created at The University of Texas MD Anderson Cancer Center from the fused preinjection and fused postinjection images for each patient by using Eclipse treatment planning system (version 13.7, Varian Medical Systems, Palo Alto, California). Left lateral and right lateral beams were used for IMPT and PSPT plans. Each patient was assumed to have high-risk prostate cancer and thus the clinical target volume (CTV) included prostate and seminal vesicles and was to be treated to a prescribed dose of 78 Gy(RBE). The CTV to planning target volume (PTV) expansion was 1.2 cm laterally, 0.6 cm anteriorly, 0.4 cm posteriorly, and 0.5 cm superiorly/inferiorly. Rectal dose constraints were prespecified as follows: the volume of rectum receiving 40 Gy or more (V40) was to be no more than 55%; the V60, ≤35%; the V70, ≤15%; the V80, ≤5%; and the maximum dose to the rectum (Dmax) was ≤82 Gy.
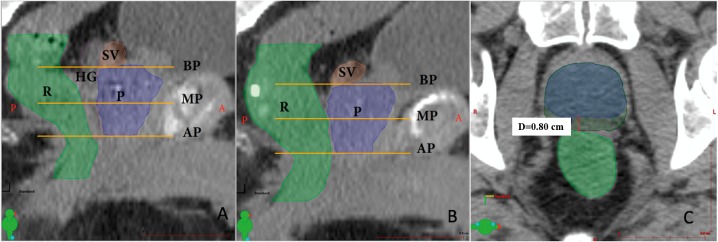
Rectal V40, V60, V70, V80, and Dmax were calculated for each plan and compared on the preinjection and postinjection IMPT and PSPT plans by using nonparametric Wilcoxon rank sum tests. The minimum displacements were measured (3 values) on axial scans between the anterior rectum and the prostate base (BP), anterior rectum to midgland (MP), and anterior rectum to apex (AP) for each patient before and after the spacer injection. These 3 values of displacements at BP, MP, and AP were averaged across patients and the means correlated with V70 by linear regression. Measurements were obtained by creating a new contour defined as CTV + posterior margin. The posterior margin was increased until the contour overlapped with the rectum to minimize potential errors by manual measurements (Figure 1C). Statistical analyses were performed with SPSS (version 24.0, IBM, Armonk, New York).
Figure 1.
Sagittal cross-sections of fused images from a patient with prostate cancer indicate the relative positions and sizes of the contoured structures with a hydrogel spacer (A) and without a hydrogel spacer in place (B). The method for calculating displacement from the anterior rectum (light green contour) to the midgland of the prostate (blue contour). (C) The minimum distance on the axial slice between the 2 structures is calculated. Measurements were obtained by creating a new contour defined as CTV (blue contour) + posterior margin (dark green contour). The posterior margin was increased until the contour overlapped with the rectum to minimize potential errors by manual measurements. This method was repeated at 2 more axial slices at the MG and AP. Abbreviations: AP, prostate apex; BP, prostate base; CTV, clinical target volume; HG, hydrogel spacer; MP, prostate midgland; P, prostate; R, rectum; SV, seminal vesicles.
Results
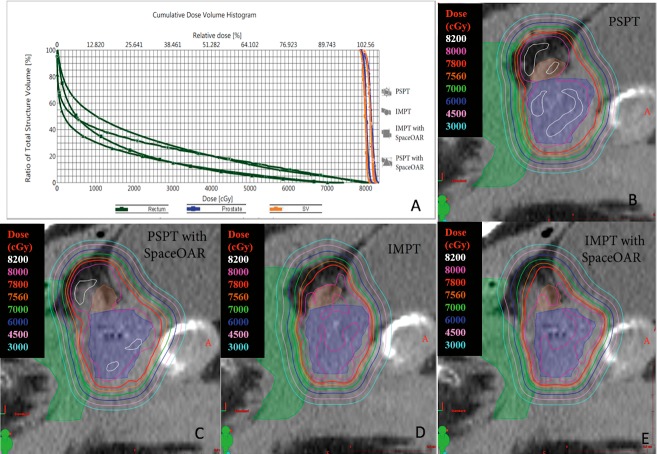
Patients (9 of 12) meeting the prespecified rectal dose constraints were included in this analysis. The volume of hydrogel spacer injected for each patient is shown is Table 1. Dosimetric data for excluded patients (3 of 12) who did not meet the prespecified rectal dose constraints are included in Table 2. For these 3 patients, rectal dose constraints were not met with PSPT-without-spacer plans. However, the addition of rectal spacers allowed these constraints to be achieved. At least 95% of the defined PTVs for all plans received the total prescription dose of 78 Gy. Representative contours of the rectum, prostate, seminal vesicles, and spacer, along with the planes of the BP, MP, and AP of a patient with and without a spacer in place, plus the respective isodose contours in an IMPT plan, are shown in Figure 1. An axial slice clarifying the method of calculating minimum displacement from MP to the anterior rectum is shown. Figure 2 shows the dose-volume histogram for a representative patient in our study for plans using PSPT and IMPT with and without spacer.
Table 1.
Injected volume of hydrogel spacer.
|
Patient |
Hydrogel volume (mL) |
| 1a | 10.1 |
| 2 | 9.7 |
| 3 | 10.5 |
| 4 | 9.6 |
| 5 | 10.3 |
| 6 | 12 |
| 7 | 11.6 |
| 8 | 12.8 |
| 9 | 12.4 |
| 10a | 11.7 |
| 11 | 10.5 |
| 12a | 11.7 |
| Mean ± SD | 10.9 ± 1.1 |
Patients not included in dosimetric analysis due to not meeting prespecified rectal dose constraints.
Table 2.
Dosimetric parameters for the 3 patients who did not originally meet rectal dose constraints.
|
Rectal dosimetric parameter |
PSPT−HG |
PSPT+HG |
IMPT−HG |
IMPT+HG |
| Patient 1 | ||||
| V40 (%) | 41.8 | 35.5 | 33.4 | 26.6 |
| V60 (%) | 26.1 | 18.5 | 14.1 | 6.2 |
| V70 (%) | 15.9 | 9.7 | 5 | 1 |
| V80 (%) | 0.61 | 1.2 | 0.1 | 0 |
| Dmax (%) | 81.67 | 82 | 80.64 | 77.33 |
| Patient 2 | ||||
| V40 (%) | 52.5 | 46.2 | 33.6 | 27.3 |
| V60 (%) | 36.3 | 27.6 | 16.2 | 8.6 |
| V70 (%) | 25.4 | 16.7 | 6.3 | 2.1 |
| V80 (%) | 4.8 | 0.7 | 0 | 0 |
| Dmax (%) | 82.88 | 80.41 | 80.85 | 77.1 |
| Patient 3 | ||||
| V40 (%) | 47.2 | 42.1 | 35.9 | 27.1 |
| V60 (%) | 31.2 | 23.7 | 15.6 | 6.2 |
| V70 (%) | 20.6 | 13.3 | 5.9 | 1 |
| V80 (%) | 1.2 | 1.7 | 0.7 | 0 |
| Dmax (%) | 81.08 | 81.95 | 81.66 | 74.95 |
Abbreviations: Dmax, maximum dose; HG, hydrogel spacer; IMPT, intensity-modulated proton therapy; PSPT, passive scattering proton therapy.
Figure 2.
Dose-volume histogram for prostate, rectum, and seminal vesicles for representative patient (A); isodose lines for PSPT without spacer (B), PSPT with spacer (C), IMPT without spacer (D), and IMPT with spacer (E). Normal tissues depicted with colorwash include prostate (blue), seminal vesicles (orange), and rectum (green). Abbreviations: IMPT, intensity-modulated proton therapy; PSPT, passive scattering proton therapy.
The dosimetric variables for the PSPT and IMPT plans, with and without the spacer in place, are shown in Figure 3. The V70 values showed the greatest differences among the 4 sets of plans, with V70 of 9.9% for the PSPT-without-spacer plan, 8.5% for the IMPT-without-spacer plan, 1.5% for the PSPT-with-spacer plan, and 1.4% for the IMPT-with-spacer plan. The V40, V60, V70, V80, and Dmax values were all significantly lower in the PSPT-with-spacer plans than in the PSPT-without-spacer plans (P = .008 for V40, V60, and V70; P = .028 for V80; and P = .015 for Dmax). Without the spacer, PSPT plans produced significantly lower V80 than IMPT plans (P = .043), while IMPT plans produced significantly lower V60 and V70 than the PSPT plans (P = .021). These variables were also significantly lower in the PSPT-with-spacer plans than in the IMPT-without-spacer plans (P = .008 for V60 and V70; P = .012 for V80). The IMPT-with-spacer plans produced lower values of V40, V60, V70, V80, and Dmax than the PSPT-without-spacer plans (P = .008 for V40, V60 and V70; P = .043 for V80; P = .021 for Dmax). The IMPT-with-spacer plans produced lower V40 and V60 than did the PSPT-with-spacer plans (P = .028 and P = .013), but produced comparable V70, V80, and Dmax (P = .155, P = .414, and P = .594).
Figure 3.
Rectal dosimetric values for PSPT and IMPT plans, each with or without spacer. Bars indicate means and error bars represent standard deviations. *P < .05 by the Wilcoxon rank sum test. Abbreviations: Dmax, maximum dose; HG, hydrogel spacer; IMPT, intensity-modulated proton therapy; PSPT, passive scattering proton therapy.
All images obtained with the spacers in place revealed increased displacement relative to the images without spacers, at the BP (median, 19 mm versus 12 mm), MP (median, 10 mm versus 2 mm), and AP (median, 7 mm versus 1 mm) (Table 3). Without the spacer in place, no linear trends were found for the PSPT plans (PSPT−HG [hydrogel spacer]) (R2 = 0.0002) or the IMPT plans (IMPT−HG) (R2 = 0.03) (Figure 4). With the spacers in place, modest linear trends were noted between V70 and average displacement for both the PSPT plans (PSPT+HG) (R2 = 0.54) and the IMPT plans (IMPT+HG) (R2 = 0.63). When the mean displacement at the BP was ≥8.0 mm, the V70 in both the PSPT and IMPT plans was ≤5.1%. When the mean displacement at the BP was ≥14.3 mm, the V70 in both the PSPT and IMPT plans was ≤0.2%.
Table 3.
Displacements at the prostate base, midgland, and apex for patients meeting rectal dose constraints.
|
Displacement without spacer |
Displacement with spacer |
|||||
|
Base |
Midgland |
Apex |
Base |
Midgland |
Apex |
|
| Median (range), mm | 12 (7–19) | 2 (0–4) | 1 (1–3) | 19 (12–23) | 10 (8–16) | 7 (2–12) |
| Mean (SD), mm | 13.4 (4.6) | 1.9 (1.3) | 1.9 (1.8) | 17.7 (3.6)a | 10.8 (2.6)a | 6.4 (3.5)a |
P < .05 with spacer versus without spacer.
Figure 4.
Correlation of V70 values with mean displacement for plans created with and without a spacer in place. This displacement was calculated by using the average of minimum displacements between anterior rectum to BP, anterior rectum to MP, and anterior rectum to AP. The plans shown include PSPT−HG (dark blue), IMPT−HG (light blue), PSPT+HG (orange), and IMPT+HG (brown). The dashed line represents the rectal dose constraint at V70 (15%). Abbreviations: AP, prostate apex; BP, prostate base; HG, hydrogel spacer; IMPT, intensity-modulated proton therapy; MP, prostate midgland; PSPT, passive scattering proton therapy.
Discussion
Strategies to reduce the rectal dose during radiation therapy for prostate cancer are needed, particularly with the increasing use of hypofractionated and stereotactic ablative techniques. Here we present a dosimetric analysis of rectal dose sparing when using biodegradable, injectable hydrogel spacers combined with either PSPT or IMPT. Our results suggest that a prostate-to-rectum displacement of at least 8 mm provides significant reductions in clinically relevant rectal dose parameters. Use of PSPT with a spacer also led to a greater reduction in rectal dose than IMPT without a spacer. Collectively, these findings strengthen our understanding of dosimetric considerations when delivering proton therapy in combination with biodegradable hydrogel spacers for prostate cancer treatment.
Our data are consistent with previous comparisons of IMRT with IMPT in demonstrating rectal dose reduction with biodegradable spacers. Absolute reductions of 8% to 10% were achieved for rectal V70 after spacer placement, with V70 values of 10% to 14% without a spacer versus 2% to 4% with a spacer [13–15, 17]. We found that the rectal V70 without a spacer was 8.5% to 10% depending on the proton modality used and 1.5% with a spacer. These results suggest that the use of biodegradable hydrogel spacers can improve dosimetry for both proton therapy (IMPT, PSPT) and photon therapy (IMRT).
IMPT can reduce the rectal dose relative to PSPT in the absence of spacers [10], as we observed for V60 and V70 in this study. These reductions may be attributed to more precise dose deposition achieved with IMPT, which allows avoidance of normal structures with complex, curved geometries such as the seminal vesicles; IMPT also uses a “layer-by-layer” approach to deliver radiation to a target volume [23]. In the present study, including the seminal vesicles in the CTV contributed to the rectal doses being higher in the PSPT-without-spacer plans. Nevertheless, the PSPT-with-spacer plans led to superior rectal dose reductions, compared with the IMPT-without-spacer plans, for all rectal dose parameters examined, and they were equivalent to IMPT-with-spacer plans in terms of the volumes of rectum receiving high doses (V70 and V80). Comparing the IMPT-with-spacer and PSPT-with-spacer plans, however, the use of the spacer did not eliminate differences in all rectal dose metrics. Notably, the rectal V70 values in our study were comparable for the IMPT-with-spacer and PSPT-with-spacer plans, which is relevant owing to prospective clinical data demonstrating that rectal V70 was most strongly associated with grade ≥2 rectal toxicity [24].
Our findings regarding the distances between the AP, MP, and BP and the anterior rectal wall are in agreement with a prior study that showed that displacement was largest at the base [19]. In seeking the optimal displacement in terms of the greatest dosimetric effect, we determined that a separation of at least 8 mm resulted in a rectal V70 below 5.1% for both the PSPT and IMPT plans. Similarly, Christodouleas et al [22] noted that displacement of at least 7 mm with a hydrogel spacer resulted in rectal V60 values of 3% to 8% for uniform scanning (ie, a scanning beam broadened with magnets and shaped with collimators) and 0% to 1% for IMPT depending on beam arrangement. Our findings are also comparable with prior data demonstrating that 9 mm of separation yielded a rectal dose of <5% for IMPT plans, with a significant decrease in the modeled probability of grade 2 toxicity [13]. Notable differences between these studies and our study are that we included imaging both before and after spacer injection, and we delineated our CTV to include the prostate and seminal vesicles to model high-risk prostate cancer.
This study has several limitations. This was a retrospective analysis of diagnostic images in a limited series of patients, which limits the statistical power for determining dosimetric correlates. Additional studies with larger patient populations are necessary to strengthen the conclusions from the current study. Three patients were not included in this analysis because the prespecified rectal dose constraints could not be met for the PSPT-without-spacer plans; for 1 of these patients, the V60 was 36.3% and the V70 was 25.4%; the V70 values for the 2 other patients were 15.9% and 20.6%. (Our dose constraints were V60 ≥ 35% and V70 ≥ 15%). We excluded these patients as the plans with failed constraints would not have been used in actual clinical scenarios and we believed it would reduce the generalizability of the results when comparing dosimetric parameters. For these patients, constraints were met with IMPT plans or with the addition of a spacer, further demonstrating the utility for treatment planning. Also, we did not use laterally oriented fields for the IMPT plans, as robustness considerations favor laterally oriented fields over anteriorly oriented beam arrangements when spacers are used [13].
We included the entire seminal vesicles in the CTV for all patients to model high-risk prostate cancer to model a worst-case scenario for estimating rectal dose. However, hydrogel spacers are not commonly used in patients with extracapsular extension owing to concerns for tumor cell seeding or concerns for creating adequate space [15]. Reducing the size of the CTV to exclude the seminal vesicles would likely have reduced rectal dose for all plans and may have changed the degree to which hydrogel spacers provided a dosimetric advantage. Although concern has been expressed about including the full seminal vesicle volume in the CTV because of increased dose to surrounding normal structures [25, 26], we found that the plans, particularly the postinjection plans, could meet the rectal dose constraints. A 1.2-cm lateral CTV to PTV expansion was used for planning. Proton planning often requires additional margins on the distal and proximal side of the CTV to account for range uncertainties (0.035 × beam range + 3 mm). When left and right lateral field arrangement is used, the range uncertainty can be incorporated into the PTV lateral expansion. If a lateral expansion of 5 to 6 mm is used, there is potential to underdose the distal edge of the CTV in the worst-case scenario for range uncertainty ±3.5% [27].
Finally, it remains to be determined whether the lower rectal doses provided by proton therapy, with or without biodegradable spacers, will translate into clinically meaningful outcomes with regard to reduced rectal toxicity [28]. Future prospective studies with larger groups of patients will be needed to answer this question. Injectable tissue spacers such as SpaceOAR offer advantages when compared to existing techniques including endorectal balloons. Endorectal balloons immobilize the prostate to reduce interfractional motion and improve dosimetry to the rectal wall, such as improving the delineation of the PTV [29]. Reducing the volume of rectum in the intermediate- and high-dose regions by using endorectal balloons has been reported to reduce late rectal toxicities [30]. However, endorectal balloons must be inserted daily for the duration of radiation therapy, which introduces challenges both for patient comfort and treatment logistics [31]. In contrast, injectable tissue spacers require a single injection into the rectoprostatic fascia before starting radiation therapy while also providing a dosimetric benefit to the rectal wall. A recent randomized trial demonstrated significant reductions in rectal dose with the spacer present, correlating with 75% reduction in grade >1 rectal toxicity and improved bowel-related quality of life with the spacer [16]. Because the spacer is biodegradable, no additional procedures are required after completing radiation to remove the device.
In conclusion, use of biodegradable hydrogels offers a complementary approach to allow further rectal dose sparing in proton therapy for prostate cancer. We observed substantial reductions in rectal dose for both PSPT and IMPT plans when a spacer was used, and we found that displacement of ≥8 mm achieved a low V70 for both proton modalities. PSPT with a hydrogel spacer led to greater rectal dose reductions than IMPT without a spacer and may further reduce rectal toxicities associated with prostate cancer radiation therapy.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Steven J. Frank, MD, is an Associate Editor of The International Journal of Particle Therapy. Dr Frank reports personal fees from Varian, grants and personal fees from C4 Imaging, grants from Eli Lilly, grants from Elekta, and grants from Hitachi, outside the submitted work.
Acknowledgments: The authors thank Augmenix for providing the spacers and de-identified CT and MRI images for analysis; Dr Patrick Campbell for his expertise regarding relevant studies; and Christine Wogan, MS, for editorial support on the manuscript. Praveen Polamraju, BS, and Alexander F. Bagley, MD, PhD, contributed equally to this work.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, Huddart RA, Jose CC, Matthews JH, Millar J, Moore AR, Morgan RC, Russel JM, Scrase CD, Stephens RJ, Syndikus I, Parmar MK. RT01 Collaborators. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8:475–87. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 2.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, Cheung MR, Lee AK, Pollack A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 4.Nathan-Kim DW, Straka C, Cho LC, Timmerman RD. Stereotactic body radiation therapy for prostate cancer: review of experience of a multicenter phase I/II dose-escalation study. Front Oncol. 2014;4:319. doi: 10.3389/fonc.2014.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas C, Fryer A, Mahajan C, Indelicato D, Horne D, Chellini A, McKenzie C, Lawlor P, Henderson R, Li Z, Lin L, Oliver K, Keole S. Dose-volume comparison of proton therapy and intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:744–51. doi: 10.1016/j.ijrobp.2007.07.2335. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall NP, Li Z, Hoppe BS, Marcus RB, Jr, Mendenhall WM, Nichols RC, Morris CG, Williams CR, Costa J, Henderson R. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–21. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Slater JD, Rossi CJ, Jr, Yonemoto DA, Bush DA, Jabola BR, Levy RP, Grove RI, Preston W, Slater JM. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Hoppe BS, Bryant C, Sandler HM. Radiation for prostate cancer: intensity modulated radiation therapy versus proton beam. J Urol. 2015;193:1089–91. doi: 10.1016/j.juro.2015.01.069. [DOI] [PubMed] [Google Scholar]
- 9.Cella L, Lomax A, Miralbell R. Potential role of intensity modulated proton beams in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49:217–23. doi: 10.1016/s0360-3016(00)01368-7. [DOI] [PubMed] [Google Scholar]
- 10.Choi S, Amin M, Palmer M, Zhu XR, Nguyen Q, Pugh TJ, Kuban DA, Lee AK. Comparison of intensity modulated proton therapy (IMPT) to passively scattered proton therapy (PSPT) in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:S154–5. [Google Scholar]
- 11.Fagundes MA, Price SG, Robison B, Case S, Blakey M, Siddiqui M, Simmons J, Stamper J, Meek AG, Schreuder N. Evolving rectal sparing in fiducial-based image guided proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2016;96:E279. [Google Scholar]
- 12.Fagundes MA, Robison B, Price SG. High-dose rectal sparing with transperineal injection of hydrogel spacer in intensity modulated proton therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2015;93:E230. [Google Scholar]
- 13.Chung H, Polf J, Badiyan S, Biagioli M, Fernandez D, Latifi K, Wilder R, Mehta M, Chuong M. Rectal dose to prostate cancer patients treated with proton therapy with or without rectal spacer. J Appl Clin Med Phys. 2017;18:32–9. doi: 10.1002/acm2.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017;7:195–202. doi: 10.1016/j.prro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Hamstra DA, Mariados N, Sylvester J, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg G, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Daignault-Newton S, Fischer-Valuck BW, Chundury A, Gay H, Bosch W, Michalski J. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–85. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Weber DC, Zilli T, Vallee JP, Rouzaud M, Miralbell R, Cozzi L. Intensity modulated proton and photon therapy for early prostate cancer with or without transperineal injection of a polyethylen glycol spacer: a treatment planning comparison study. Int J Radiat Oncol Biol Phys. 2012;84:e311–8. doi: 10.1016/j.ijrobp.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Mariados N, Sylvester J, Shah D, Karsh L, Hudes R, Beyer D, Kurtzman S, Bogart J, Hsi RA, Kos M, Ellis R, Logsdon M, Zimberg S, Forsythe K, Zhang H, Soffen E, Francke P, Mantz C, Rossi P, DeWeese T, Hamstra DA, Bosch W, Gay H, Michalski J. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–7. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Susil RC, McNutt TR, DeWeese TL, Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1251–8. doi: 10.1016/j.ijrobp.2009.07.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinkawa M, Piroth MD, Holy R, Escobar-Corral N, Caffaro M, Djukic V, Klotz J, Eble MJ. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106:220–4. doi: 10.1016/j.radonc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:1918–22. doi: 10.1016/j.ijrobp.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Pinkawa M. Current role of spacers for prostate cancer radiotherapy. World J Clin Oncol. 2015;6:189–93. doi: 10.5306/wjco.v6.i6.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christodouleas JP, Tang S, Susil RC, McNutt TR, Song DY, Bekelman J, Deville C, Vapiwala N, Deweese TL, Lu HM, Both S. The effect of anterior proton beams in the setting of a prostate-rectum spacer. Med Dosim. 2013;38:315–9. doi: 10.1016/j.meddos.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan R, Grosshans D. Proton therapy—present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Laans HP, van den Bergh A, Schilstra C, Vlasman R, Meertens H, Langendijk JA. Grading-system dependent volume effects for late radiation-induced rectal toxicity after curative radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1138–45. doi: 10.1016/j.ijrobp.2007.07.2363. [DOI] [PubMed] [Google Scholar]
- 25.Kestin L, Goldstein N, Vicini F, Yan D, Korman H, Martinez A. Treatment of prostate cancer with radiotherapy: should the entire seminal vesicles be included in the clinical target volume? Int J Radiat Oncol Biol Phys. 2002;54:686–97. doi: 10.1016/s0360-3016(02)03011-0. [DOI] [PubMed] [Google Scholar]
- 26.Gluck I, Vineberg KA. Ten Haken RK, Sandler HM. Evaluating the relationships between rectal normal tissue complication probability and the portion of seminal vesicles included in the clinical target volume in intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:334–40. doi: 10.1016/j.ijrobp.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Moyers MF, Miller DW. Range, range modulation, and field radius requirements for proton therapy of prostate cancer. Technol Cancer Res Treat. 2003;2:445–7. doi: 10.1177/153303460300200509. [DOI] [PubMed] [Google Scholar]
- 28.Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR, Chen RC. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teh BS, McGary JE, Dong L, Mai WY, Carpenter LS, Lu HH, Chiu JK, Woo SY, Grant WH, Butler EB. The use of rectal balloon during the delivery of intensity modulated radiotherapy (IMRT) for prostate cancer: more than just a prostate gland immobilization device? Cancer J. 2002;8:476–83. doi: 10.1097/00130404-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 30.van Lin EN, Kristinsson J, Philippens ME, de Jong DJ, van der Vight LP, Kaanders JH, Leer JW, Visser AG. Reduced late rectal mucosal changes after prostate three-dimensional conformal radiotherapy with endorectal balloon as observed in repeated endoscopy. Int J Radiat Oncol Biol Phys. 2007;67:799–811. doi: 10.1016/j.ijrobp.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Hedrick SG, Fagundes M, Robison B, Blakey M, Renegar J, Artz M, Schreuder N. A comparison between hydrogel spacer and endorectal balloon: an analysis of intrafraction prostate motion during proton therapy. J Appl Clin Med Phys. 2017;18:106–12. doi: 10.1002/acm2.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]