Abstract
Purpose:
To determine whether a hypofractionated proton therapy regimen will control early-stage disease and maintain low rates of side effects similar to results obtained using standard-fraction proton therapy at our institution.
Materials and Methods:
A cohort of 146 patients with low-risk prostate cancer according to National Comprehensive Cancer Network guidelines (Gleason score <7, prostate-specific antigen [PSA] <10, tumor stage of T1–T2a) received 60 Gy (cobalt Gy equivalent) of proton therapy (20 fractions of 3.0 Gy per fraction) in 4 weeks, a dose biologically equivalent to standard fractionation (44–45 fractions of 1.8 Gy to a total of 79.2 to 81 Gy in 0 weeks). Patients were evaluated at least weekly during treatment, at which time documentation of treatment tolerance and acute reactions was obtained. Follow-up visits were conducted every 3 months for the first 1 years, every 6 months for the next 3 years, then annually. Follow-up visits consisted of history and physical examination, PSA measurements, and evaluation of toxicity.
Results:
The median follow-up time was 42 months (range, 3–96 months). Acute grade 2 urinary toxicity occurred in 16% (20/120) of the patients; acute grade 2 or higher gastrointestinal toxicity was seen in 1.7% (2/120). At 9 months, 1 patient had late grade 3 urinary toxicity, which resolved by 12 months; no grade 3 gastrointestinal toxicities occurred. The 3-year biochemical survival rate was 99.3% (144/145). The median time to PSA nadir was 30 months.
Conclusion:
Hypofractionated proton therapy of 60 Gy in 20 fractions was safe and effective for patients with low-risk prostate cancer.
Keywords: prostate cancer, proton therapy, hypofractionation
Introduction
Prostate cancer is one of the most commonly diagnosed cancer in men, and many of these patients have early, low-risk disease. Prostate cancer at these stages is very treatable: local control rates >90% and rates of significant late morbidity ≤5% are commonly reported for a variety of treatment modalities, including surgery, proton radiation therapy, external beam x-rays, and brachytherapy. Some studies have identified a very low risk group of patients, which suggests that observational strategies such as active surveillance may be reasonable alternatives to any form of interventional treatment [1, 2].
Even so, the increase in detected cancers has resulted in increased use of radiation and surgery for patients in these stages. The increased demand for therapeutic services, in turn, has led to increased monetary costs to society and increased numbers of people exposed to the potential risks that are associated with any form of interventional treatment. These increased costs and risks have led to searches for ways to minimize both.
Proton radiation therapy has proved to be an excellent option for low-risk prostate cancer as it delivers high control rates with very little toxicity [3–10]. Because of its physical dose distribution characteristics arising from charge and mass, a high degree of controllability inheres in the proton beam, thus providing the physician with superb ability to conform the beam where desired and spare much of the surrounding clinically normal tissue completely. If one accepts that radiation-related sequelae do not occur in unirradiated tissues, proton beam thereby enhances the physician's opportunity to minimize risks for the patient.
The downside of protons, however, is the monetary cost to the individual insurer or to the patient. The primary factor driving the cost of proton delivery in prostate cancer is the number of daily treatments (fractions) required; at our institution, this has been 40 to 45 fractions delivered over 8 to 9 weeks. Accordingly, for several years we have conducted a study to assess the safety and toxicity of hypofractionated proton therapy for low-risk prostate cancer. An ultimate goal was to determine whether the cost differential might be significantly decreased or eliminated while maintaining the results we have obtained over the years with our standard method of delivering proton radiation therapy for this disease.
Hypofractionation is the process of delivering higher doses of radiation per fraction, but using fewer daily fractions. The potential downside of hypofractionation is the risk of late toxicity of the surrounding healthy tissues. At Loma Linda University (LLU) we have successfully used hypofractionated proton therapy for several diseases, including cancers of the breast, lung, and liver. In each instance, control and survival rates have been maintained and untoward sequelae have not increased [11–13]. This experience prompted us to investigate hypofractionation for prostate cancer.
Prostate cancer lends itself well to hypofractionation because of the unique radiobiologic sensitivity of prostate cancer cells. Fowler [14] and Fowler and colleagues [15] investigated whether the α/β ratio (radiosensitivity to size of dose per fraction) is sufficiently low to justify using a few large dose fractions instead of the traditional many small doses. In critiquing 3 large statistical overviews of thousands of patients with prostate adenocarcinoma, they found that all overviews agreed that α/β values are low and do not vary significantly with risk factors. They concluded that high sensitivity to dose per fraction is an intrinsic property of prostate cancers, thus supporting the use of hypofractionation to increase the therapeutic gain for the primary tumors, provided that steps are taken to reduce the risk of late complications in rectum and bladder [14, 15]. Our experience in treating prostate cancer with protons is that the conformability of the proton beam, combined with measures to keep as low as possible the volume of bladder and rectum receiving any radiation dose, yields high rates of disease control and low rates of severe gastrointestinal and genitourinary complications. Since previous studies at LLU resulted in 10-year biochemical disease-free survival of low-risk patients of >93% and ≥ grade 3 toxicity ≤1% [7, 8, 10], and the primary factor affecting cost of proton delivery in prostate cancer is the number of individual fractions administered, a trial of hypofractionated proton therapy for early-stage prostate cancer seemed warranted.
The purpose of this study was to primarily assess the toxicity and efficacy of hypofractionated proton therapy for prostate cancer.
Materials and Methods
A phase I/II trial was initiated in 2009 (ClinicalTrials.gov Identifier: NCT00831623). Patient eligibility was limited to those with low-risk prostate cancer according to National Comprehensive Cancer Network guidelines, defined as Gleason score <7, prostate-specific antigen [PSA] <10, and tumor stage of T1-T2a.
Intervention
Dose rationale
Our standard dose for low-risk prostate cancer, based on Proton Radiation Oncology Group/American College of Radiology 95-09, is 44–45 fractions of 1.8 Gy (cobalt Gy equivalent) to a total of 79.2 to 81 Gy in 9 weeks [10]. Using an α/β ratio of 1.5 Gy for prostate cancer and 4 Gy for normal late effects to rectum, a dose of 20 fractions of 3.0 Gy (cobalt Gy equivalent) was derived, resulting in a total of 60 Gy in 4 weeks; this was calculated to yield a biologically effective dose for prostate cancer of 178 versus 180 Gy and a late effects biologically effective dose of 117 versus 105 Gy for the conventional dose and hypofractionated dose, respectively.
Immobilization and simulation
Immobilization and computed tomography scanning have been previously described [4, 5]. Briefly, all patients were immobilized in a full-body immobilization device while positioned supine. A rectal balloon filled with water was used to immobilize the rectum and minimize heterogeneity along the path of protons. Patients were encouraged to fill their bladders by drinking 8–16 ounces of water before the computed tomography scan, but no specific bladder-filling guidelines were mandated. Fiducial markers were not used for patient positioning; some patients, as participants in another trial, had fiducials placed in the prostate.
Target volumes
The clinical target volume consisted of the entire prostate alone. A margin of 5 mm in all dimensions was added to account for patient motion, variation in daily set-up positioning, and beam physics.
Treatment
Two parallel opposed proton beams were used, with both beams being used during each daily treatment. Daily treatment localization was performed with orthogonal radiographs immediately before each treatment.
Outcomes
The primary outcome for the phase I component was actuarial late grade 3 toxicity at 3 years after treatment. This was assessed via clinic visits every 3 months for the first 2 years after completing treatment, every 6 months for the next 3 years after completing treatment, and annually thereafter. The primary outcome for the phase II component was 3-year actuarial biochemical progression-free survival using the ASTRO Phoenix definition of biochemical failure. Secondary outcomes included overall survival, acute toxicity, and late toxicity over time. NCI CTCAE version 4.0 was used for adverse events.
Statistical Methods
Sample size
Sample size for the phase I primary outcome was calculated based on an expected 3-year grade 3 toxicity rate of 1% with an unacceptable toxicity rate of 5%. Statistical (α) error was set at 10% and (β) error was set at 10%, using the 1-sided test of significance. This sample size was calculated to be 105 patients.
Sample size for the phase II primary outcome was calculated based on an expected 3-year failure rate of 5% with an unacceptable failure rate of 10%. Statistical (α) error was set at 10% and (β) error was set at 10%, using the 1-sided test of significance. This sample size was calculated to be 187 patients.
Interim analyses and stopping guidelines
Stopping guidelines were set based on the acute toxicity rate. Our institutional acute toxicity rate was ∼12% grade 2 and 0.8% grade 3. The stopping rate was based on a 10% increase of grade 2 toxicity, from 15% to 25%. Interim analyses were performed every 6 months until the final analysis.
Follow-up and Analysis
Patients were seen and evaluated at least weekly during treatment; documentation of treatment tolerance and acute reactions was obtained at these times. As noted, follow-up visits were conducted every 3 months for the first 2 years after treatment, every 6 months for the next 3 years, and annually thereafter. During follow-up visits, history was taken, a physical exam was performed, PSA was measured, and toxicity was evaluated.
Acute toxicity was defined as toxicity during treatment and within the 3-month post-treatment period. Late toxicity was defined as toxicity occurring >3 months after treatment.
Results
Participant Flow
In total, 167 patients were enrolled in the study from 2009 to 2017. Nine patients withdrew after registration, leaving 158 who were treated according to protocol. This report includes 146 patients who completed treatment at least 2 years earlier. One patient was lost to follow-up, leaving 145 patients who were analyzed for late toxicity and biochemical failure (Figure 1). On further analysis, 4 patients were found to have intermediate-risk disease but were included in the analysis.
Figure 1.
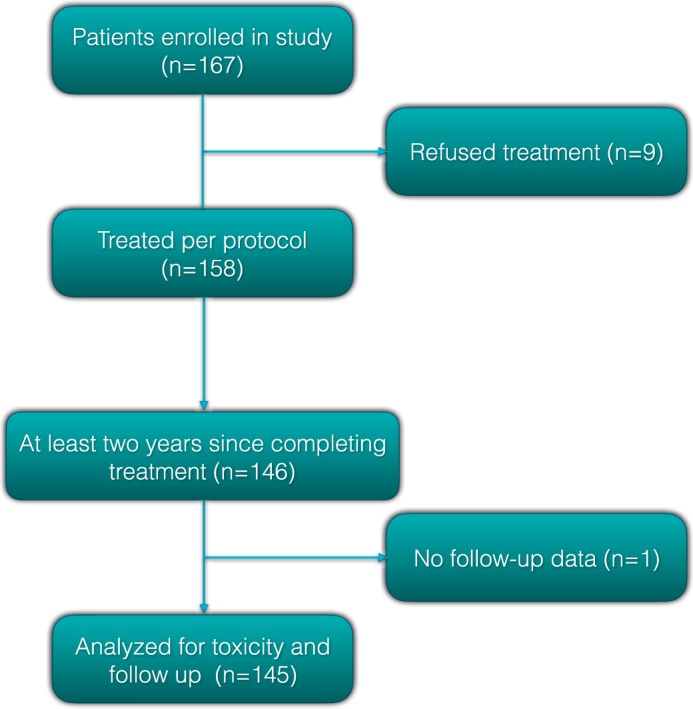
Patient flow.
Patient Characteristics
A complete listing of patient characteristics is shown in Table 1. The median follow-up time was 42 months (range, 3–96 months). The median age of patients was 65 years, and the median PSA was 4.98 ng/mL. Most patients were diagnosed based on PSA screening as evidenced by 83% having tumor stage of T1c. Seven patients (4.8%) had prior transurethral resection of the prostate, and approximately half (48.6%) had radiographic nodal staging. Of the intermediate-risk patients included, 2 had a Gleason score of 7, 1 had a PSA value >10, and 1 had stage T2b disease.
Table 1.
Patient characteristics.
|
Characteristic |
No (%) |
| Age, years | |
| 30–39 | 2 (1.4) |
| 40–49 | 4 (2.7) |
| 50–59 | 37 (25.3) |
| 60–69 | 68 (46.6) |
| 70–79 | 35 (24.0) |
| Median | 65 |
| Range | 33–80 |
| Prostate-specific antigen (ng/mL) | |
| <4 | 41 (28.1) |
| 4 to <10 | 104 (71.2) |
| Median | 4.98 |
| Range | 0.3-10.2 |
| Karnofsky Performance Status | |
| 90–100 | 143 (97.9) |
| 70–80 | 1 (0.7) |
| 50–60 | 0 (0.0) |
| <50 | 1 (0.7) |
| Not available | 1 |
| Gleason | |
| Grade group 1 (3 + 3 = 6) | 144 (98.6) |
| Grade group 2 (3 + 4 = 7) | 1 (0.7) |
| Grade group 3 (4 + 3 = 7) | 1 (0.7) |
| Prior transurethral resection of the prostate | |
| Yes | 7 (4.8) |
| No | 136 (93.2) |
| Not available | 3 (2.1) |
| Tumor stage | |
| T1a | 4 (2.7) |
| T1b | 1 (0.7) |
| T1c | 121 (82.9) |
| T2a | 19 (13.0) |
| T2b | 1 (0.7) |
| T2c | 0 (0.0) |
| N stage radiographic | |
| N0 | 71 (48.6) |
| N1 | 0 (0.0) |
| NX | 75 (49.3) |
| Percentage of positive biopsy cores | |
| <50% | 117 (80.1) |
| >50% | 15 (10.3) |
| Mean, % | 25.2 |
| Not available | 14 |
Toxicity
Acute grade 2 urinary toxicity was seen in 16% (20/120) of patients, and acute grade 2 gastrointestinal toxicity was seen in 1.7% (2/120) of patients. No ≥grade 3 acute urinary or gastrointestinal toxicities were seen.
At the time of analysis 1 patient had late grade 3 urinary toxicity (gross hematuria in a patient with a history of nephrolithiasis and nephrolithiasis found on cystoscopy) that occurred at the 9-month follow-up, which was resolved by the 12-month follow-up; there were no grade 3 gastrointestinal toxicities. The 3-year actuarial rate of late grade 3 genitourinary toxicity was 0.7%m and the rate of grade 3 gastrointestinal toxicity was 0. The 3-year actuarial grade 2 genitourinary toxicity was 9.5%; grade 2 gastrointestinal toxicity was 5.1%. The grade 2 genitourinary toxicities were predominantly void-related symptoms; there were no cases of grade 2 hematuria. Of the grade 2 gastrointestinal toxicities, 3 were cases of rectal bleeding (2.2%), 2 were rectal or pelvic pain (1 of which was the aforementioned patient with nephrolithiasis), 1 was nausea and vomiting likely related to another medical illness, and 1 consisted of irritable bowel-like symptoms.
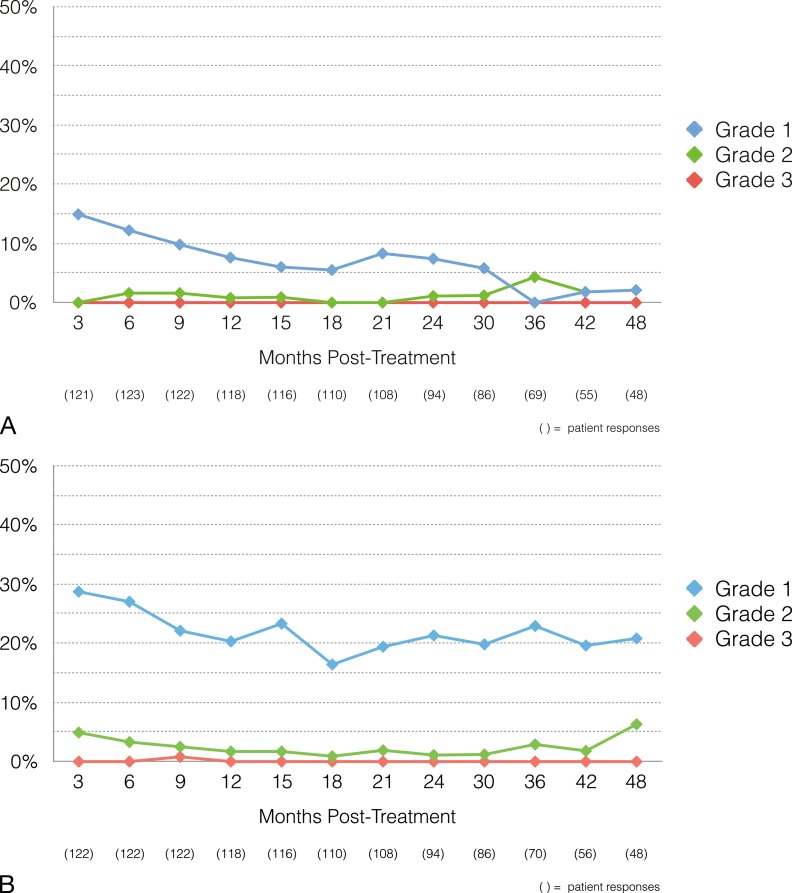
Given that many of the toxicities were limited in duration, we also compared the incidence of toxicities over time (Figure 2). The maximum incidence of grade 2 urinary toxicity at any time point was 4% (2/46), and the maximum incidence of grade 2 gastrointestinal toxicity was 4% (3/68).
Figure 2.
Toxicity rates over time: (A) gastrointestinal (B) genitourinary.
Biochemical Outcomes
At the time of analysis, 5 patients had biochemical failure. The 3-year biochemical progression-free survival rate was 99.3%, and the 5-year biochemical progression-free survival was 97.9%. The median time to PSA nadir for patients undergoing hypofractionation was 30 months. The rates of PSA nadir <1.0 and <0.5 at 24 months were 71% and 27%, respectively. The rates of PSA nadir <1.0 and <0.5 at 36 months were 83% and 41%, respectively.
Of the 5 patients with biochemical failure, 1 patient who failed at 48 months has had a labile PSA throughout his posttreatment period. He has not required any salvage therapy to date, and his PSA values have remained < 4.0 ng/mL for the past 3 years. One patient at 36 months had PSA failure, which led to additional workup. He underwent a magnetic resonance imaging–guided biopsy, which revealed a single core of biopsy-proven grade group 4 (Gleason 4 + 4 = 8) prostate cancer and subsequently underwent salvage radical prostatectomy. His prostatectomy specimen revealed right seminal vesicle invasion and 20% of the prostate volume involved with a grade group 3 (4 + 3 = 7) adenocarcinoma; the pathology report noted no significant treatment effect. The third patient had a PSA of 3.8 ng/mL at 84 months, which was an increase from 0.66 ng/mL the year prior. At 96 months, his PSA was 1.26 ng/mL, and his PSA continues to be <2.0 ng/mL without salvage treatment. One patient, whose PSA has continued to rise, has opted against salvage treatment, and the last patient had a failure but has not yet undergone additional treatment or workup.
Discussion
This study in men with prostate cancer demonstrates that a hypofractionated dose of 60 Gy in 20 fractions using protons results in a low incidence of late toxicity and does not appear to compromise disease control. This is demonstrated by the actuarial late grade 3 toxicity rate of 0.7% for genitourinary toxicity and 0% for gastrointestinal toxicity.
Several other prospective studies have looked at hypofractionated radiation treatment for prostate cancer. The Radiation Therapy Oncology Group (RTOG) 0415 trial compared 70 Gy in 28 fractions (2.5 Gy per fraction) with 73.8 Gy in 41 fractions in low-risk patients with prostate cancer. The 5-year disease-free survival rate in the hypofractionated arm of this study was 86.3% (95% confidence interval, 83.1–89), and the rates of late grade 3 or higher toxicity were 4.1% for gastrointestinal toxicity and 3.5% for genitourinary toxicity [16].
Another study comparing intensity-modulated radiation therapy doses of 3 Gy per fraction for 19 or 20 fractions and 2 Gy per fraction for 37 fractions showed that 60 Gy in 20 fractions was not inferior to 74 Gy in 37 fractions. The rates of late grade 3 or higher toxicity in the 20-fraction arm were 3% for gastrointestinal and 6% for genitourinary [17]. These toxicity rates are higher than was seen in our study; however, it should be noted that a different method of toxicity scoring was used in this study compared with the present study (RTOG toxicity criteria versus Common Terminology Criteria for Adverse Events). Additionally, the median follow-up time was longer at 62.4 months compared with 42 months in the present study. These differences, as well as differences in modalities, may contribute to the difference in toxicity.
Most recently, the University of Florida group published their data on hypofractionated treatment for prostate cancer using protons [18]. Similar to the RTOG study, that group administered a dose of 70–72.5 Gy in 2.5 Gy per fraction for 28–29 fractions depending on the risk group. They showed rates of late grade 3 or higher gastrointestinal and genitourinary toxicity of 0.5% and 1.7%, respectively. The freedom from disease progression rate for the low-risk patients was 98.3% at 5 years. They found a median time to nadir of 39 months, consistent with previously published data for standard fractionation, and found that 73.9% of patients had a PSA nadir <0.5 ng/dL.
Our study adds to this body of data. Specifically, with respect to proton hypofractionation, we were able to demonstrate low rates of toxicity with high rates of disease control similar to the rates that the University of Florida group demonstrated but in a slightly more hypofractionated regimen (20 fractions versus 28 fractions). This reduction in the number of fractions delivered may further decrease the cost of treatment without compromising outcomes or adding toxicity.
A limitation of this study was that we did not meet our desired sample size for the phase II portion of the study. This is due in part to offering active surveillance for men with low-risk prostate cancer. This study was limited to low-risk patients; however, the toxicity results are applicable to patients of other risk groups. Additionally, this was a single-arm, single-institution study. Strengths of the study include the prospective nature of data collection.
Proton radiation therapy has established itself as one of the treatments of choice for cancer of the prostate. Multiple studies over the past few decades have shown the feasibility and results of using protons in low-, intermediate-, and high-risk patients [4–8, 10]. Although most of these results are at least equivalent to the best photon therapy outcomes in terms of disease control, while significantly decreasing the dose of radiation to nontarget tissues, there has been resistance from insurers to cover proton therapy for prostate cancer, presumably because of increased treatment costs. The cost of a proton facility is often cited as the obstacle in limiting proton coverage; however, it is the cost per patient treated that is ultimately the issue with federal and private insurers.
Many factors are involved in determining overall cost per patient, including the length and complexity of daily treatments, the time required to set up and deliver daily treatments, and the overall number of treatments delivered. At our institution, the length of daily treatments with protons for prostate cancer is less than or equal to that of complex photon cases, such as those treated with intensity-modulated radiation therapy. The main issue affecting cost per patient treated with protons, then, is the overall number of fractions delivered. As the daily number of treatments is the single most expensive component in a course of protons for prostate cancer, a positive outcome in this trial could decrease the overall treatment cost by more than half. Additionally, reducing the use of proton facilities by a single patient can have a significant impact on accessibility of this scarce resource to other potential patients.
The present study adds to the body of data on hypofractionation for prostate cancer and specifically hypofractionation using protons. We were able to demonstrate low rates of toxicity and similar efficacy while delivering a fewer number of treatments. This reduction in the number of treatments delivered may further decrease the cost of treatment.
Conclusion
Hypofractionated proton therapy of 60 Gy in 20 fractions was safe and effective for patients with low-risk prostate cancer. A prospective multi-institutional randomized study is being conducted to confirm these results.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest Statement: The authors have no relevant conflicts of interest to disclose.
Acknowledgments: The authors would like to acknowledge Sandra Teichman, RN, BSN, and William Preston, EdD, for their editorial assistance.
Funding: This research was sponsored by the Ken Venturi Endowed Chair.
Ethical Approval: All patient data have been collected under internal review board approved protocol.
References
- 1.Kariburyo F, Wang Y, Cheng I-NE, Wang L, Morgenstern D, Xie L, Meadows E, Danella J, Cher ML. Observation versus treatment among men with favorable risk prostate cancer in a community-based integrated health care system: a retrospective cohort study. BMC Urol. 2018;18:55. doi: 10.1186/s12894-018-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klaff R, Rosell J, Varenhorst E, Sandblom G. The long-term disease-specific mortality of low-risk localized prostate cancer: a prospective population-based register study over 2 decades. Urology. 2016;91:77–82. doi: 10.1016/j.urology.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Kil WJ, Nichols RC, Jr, Hoppe BS, Morris CG, Marcus RB, Jr, Mendenhall W, Mendenhall NP, Li Z, Costa JA, Williams CR, Henderson RH. Hypofractionated passively scattered proton radiotherapy for low- and intermediate-risk prostate cancer is not associated with post-treatment testosterone suppression. Acta Oncol. 2013;52:492–7. doi: 10.3109/0284186X.2013.767983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendenhall NP, Hoppe BS, Nichols RC, Mendenhall WM, Morris CG, Li Z, Su Z, Williams CR, Costa J, Henderson RH. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602. doi: 10.1016/j.ijrobp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Mendenhall NP, Li Z, Hoppe BS, Marcus RB, Jr, Mendenhall WM, Nichols RC, Morris CG, Williams CR, Costa J, Henderson R. Early outcomes from three prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:213–21. doi: 10.1016/j.ijrobp.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Shipley WU, Verhey LJ, Munzenrider JE, Suit HD, Urie MM, McManus PL, Young RH, Shipley JW, Zietman AL, Biggs PJ, Heney NM, Goitein M. Advanced prostate cancer: the results of a randomized comparative trial of high dose irradiation boosting with conformal protons compared with conventional dose irradiation using photons alone. Int J Radiat Oncol Biol Phys. 1995;32:3–12. doi: 10.1016/0360-3016(95)00063-5. [DOI] [PubMed] [Google Scholar]
- 7.Slater JD, Rossi CJ, Jr, Yonemoto LT, Bush DA, Jabola BR, Levy RP, Grove RI, Preston W, Slater JM. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Slater JD, Rossi CJ, Jr, Yonemoto LT, Reyes-Molyneux NJ, Bush DA, Antoine JE, Miller DW, Teichman SL, Slater JM. Conformal proton therapy for early-stage prostate cancer. Urology. 1999;53:978–84. doi: 10.1016/s0090-4295(99)00014-x. [DOI] [PubMed] [Google Scholar]
- 9.Slater JD, Yonemoto LT, Rossi CJ, Jr, Reyes-Molyneux NJ, Bush DA, Antoine JE, Loredo LN, Schulte RW, Teichman SL, Slater JM. Conformal proton therapy for prostate carcinoma. Int J Radiat Oncol Biol Phys. 1998;42:299–304. doi: 10.1016/s0360-3016(98)00225-9. [DOI] [PubMed] [Google Scholar]
- 10.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, Bush DA, Lunt M, Spiegel DY, Skowronski R, Jabola BR, Rossi CJ. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush DA, Cheek G, Zaheer S, Wallen J, Mirshahidi H, Katerelos A, Grove R, Slater JD. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys. 2013;86:964–8. doi: 10.1016/j.ijrobp.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Bush DA, Do S, Lum S, Garberoglio C, Mirshahidi H, Patyal B, Grove R, Slater JD. Partial breast radiation therapy with proton beam: 5-year results with cosmetic outcomes. Int J Radiat Oncol Biol Phys. 2014;90:501–5. doi: 10.1016/j.ijrobp.2014.05.1308. [DOI] [PubMed] [Google Scholar]
- 13.Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, Grove R, de Vera ME. Randomized clinical trial comparing proton beam radiation therapy with rransarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. Int J Radiat Oncol Biol Phys. 2016;95:477–82. doi: 10.1016/j.ijrobp.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–76. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 15.Fowler JF, Toma-Dasu I, Dasu A. Is the α/β ratio for prostate tumours really low and does it vary with the level of risk at diagnosis? Anticancer Res. 2013;33:1009–11. [PubMed] [Google Scholar]
- 16.Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, Shah AB, D'Souza DP, Michalski JM, Dayes IS, Seaward SA, Hall WA, Nguyen PL, Pisansky TM, Faria SL, Chen Y, Koontz BF, Paulus R, Sandler HM. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34:2325–32. doi: 10.1200/JCO.2016.67.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, Graham J, Kirkbride P, Logue J, Malik Z, Money-Kyrle J, O'Sullivan JM, Panades M, Parker C, Patterson H, Scrase C, Staffurth J, Stockdale A, Tremlett J, Bidmead M, Mayles H, Naismith O, South C, Gao A, Cruickshank C, Hassan S, Pugh J, Griffin C, Hall E. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–60. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson RH, Bryant C, Hoppe BS, Nichols RC, Mendenhall WM, Flampouri S, Su Z, Li Z, Morris CG, Mendenhall NP. Five-year outcomes from a prospective trial of image-guided accelerated hypofractionated proton therapy for prostate cancer. Acta Oncol. 2017;56:963–70. doi: 10.1080/0284186X.2017.1287946. [DOI] [PubMed] [Google Scholar]