Abstract
Purpose:
The incidence of anal cancer in patients with kidney transplants has increased. The definitive treatment for anal cancer is chemotherapy and intensity-modulated radiation therapy. In kidney transplant recipients, sparing the pelvic kidney in the process of delivering radiation to the anus can be challenging. Intensity-modulated proton therapy (IMPT) has been proposed as an alternative to intensity-modulated radiation therapy for the treatment of anal cancer in this population, given its increased ability to spare organs-at-risk.
Case Series:
We present 4 cases of patients with transplanted pelvic kidneys who subsequently developed anal cancer and were treated with IMPT from 2017 to 2019.
Conclusion:
Use of IMPT appears to be an acceptable option for the treatment of anal cancer in patients with a pelvic kidney.
Keywords: intensity-modulated proton therapy, anal cancer, anal neoplasia, kidney transplant, pelvic kidney
Introduction
In 2018, there were 36 527 organ transplants performed in the United States, an annual record for the sixth straight year [1]. Of those, 21 167 (58%, .58 = 21,167/36,527) were kidney transplants [2]. Not only are more transplantations being performed, but as immunosuppressive therapy has improved, recipients of kidney transplants are living longer [3–6].
Chronic immunosuppression confers an increased risk of malignancy, and there is a well-established relationship between organ transplants and de novo cancer development [3, 5–17]. It has been estimated that, for all patients with solid organ transplants, the risk of developing cancer is increased 2-fold to 4-fold above that of the general population [3]. Recipients of kidney transplants are no different, with multiple publications discussing the greater frequency of cancer in this population.* Patients with kidney transplants are at higher risk of developing multiple human papillomavirus–mediated and non–human papillomavirus–mediated squamous cell carcinomas. The presence of a transplanted pelvic kidney can be a therapeutic challenge in patients who require pelvic radiation as management. In that scenario, the dose required to the region at risk must be balanced against the potential risk of kidney injury, which may result in dialysis or necessitate a second transplant.
In this case series, we review 4 patients with squamous cell carcinoma of the anal canal and kidney transplant who were treated with intensity-modulated proton therapy (IMPT) as part of definitive chemoradiation.
Case Series
All patients with anal cancer treated from 2017 to 2019 by the Department of Radiation Oncology at the University of Cincinnati (Cincinnati, Ohio) were retrospectively reviewed. We identified 4 patients treated with IMPT in the setting of a transplanted pelvic kidney. All patients were treated with definitive intent with standard 5-flourouracil (5-FU)/mitomycin–based chemoradiation. The radiation dose and fractionation were delivered through a simultaneous integrated boost technique in 28 to 30 fractions, per the Radiation Therapy Oncology Group (RTOG) 0529 schema [18]. The IMPT technique has been previously described [19]. The approach used a 3-field multifield-optimized split-target technique. A posterior field was used to cover the primary tumor, the mesorectum, internal iliac lymph nodes, and posterior external iliac lymph targets. Right and left anterior oblique fields were used to cover the right and left inguinal lymph nodes and the anterior, external iliac lymph node station. The summary demographic and treatment characteristics are outlined in Tables 1 and 2.
Table 1.
Summary of demographics, treatment characteristics, and pelvic kidney dosimetry.
|
Patient |
Age, y |
Stage |
Chemotherapy |
Radiation dose, Gy |
Radiation fraction |
Kidney mean, Gy |
Kidney V6, % |
Kidney V20, % |
| 1 | 63 | IIIB T3N2M0 | 5-FU/mitomycin | 54 | 30 | 3.6 | 18.26 | 5.79 |
| 2 | 62 | II T2N0M0 | 5-FU/mitomycin | 54 | 30 | .95 | 4.16 | 0.02 |
| 3 | 60 | IIB T3N0M0 | 5-FU/mitomycin | 54 | 30 | .58 | 2.47 | 0 |
| 4 | 60 | II T3N0M0 | None | 50.4 | 28 | .19 | 0.22 | 0 |
Abbreviation: 5-FU, 5-flourouracil.
Table 2.
Summary of clinical status and kidney function before and after treatment.
|
Patient no. |
PET-CT, moa |
PET-CT: clinical status |
Pretreatment creatinine, mg/dLb |
Posttreatment creatinine, mg/dLb |
Posttreatment creatinine, moa |
| 1 | 3 | No residual disease | 0.67 | 0.56 | 17 |
| 2 | 4 | Overall decreased uptake in anorectal region; some residual uptake | 2.16 | 2.27 | 0 |
| 3 | N/A | 3-mo follow-up pending | 1.69 | 1.18 | 0 |
| 4 | 27 | No residual disease | 3–4.5c | 3–4.5d | 28 |
Abbreviations: PET-CT, positron emission tomography–computed tomography; N/A, not available.
Months after treatment completion.
To convert to micromoles per liter, multiply by 88.4.
Baseline listed. Pretreatment creatinine range (6 months): 2.13 to 4.28 mg/dL.b
Baseline listed. Posttreatment creatinine range 2.76 to 5.3 mg/dL.b Most recent creatinine level, 2.76 mg/dL.b
Case 1
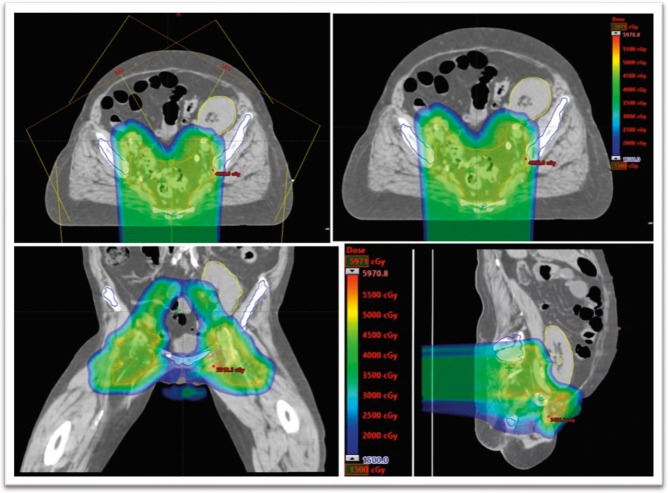
A 63-year-old, white woman with a history of end-stage renal disease (ESRD), secondary to hypertensive nephropathy, with a pelvic kidney transplant 20 years before diagnosis, presented with stage IIIB T3N2M0 squamous cell carcinoma of the anal canal. A positron emission tomography–computed tomography (PET-CT) scan showed a hypermetabolic primary tumor at the anus and the anorectal junction, measuring 5.3 cm with 1 positive left inguinal lymph node, without evidence of metastasis. The patient was treated with 5-FU/mitomycin–based chemoradiation with IMPT (Figure 1). The primary tumor was treated with 54 Gy in 30 fractions, and the elective nodal regions received 45 Gy in 30 fractions. The mean transplanted kidney dose was 3.6 Gy, with a maximum dose of 40.7 Gy. The kidney V6 was 18.26%, and the V20 was 5.79%. Pretreatment and posttreatment creatinine levels were 0.67 and 0.56 mg/dL (to convert to micromoles per liter, multiply by 88.4), respectively. A PET-CT in January 2018 showed no residual disease.
Figure 1.
Intensity-modulated proton therapy plan for patient 1.
Case 2
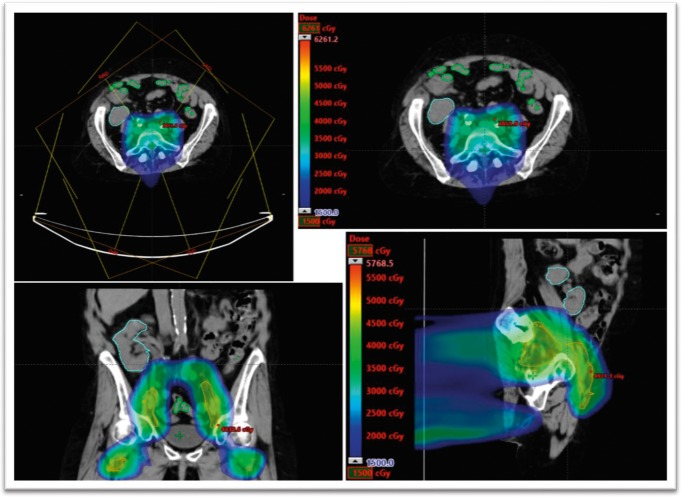
A 62-year-old woman with a history of ESRD, secondary to lupus nephritis, with pelvic kidney transplant 27 years before diagnosis presented with stage II T2N0M0 squamous cell carcinoma of the anal canal. Of note, the patient had a previous bout of squamous cell carcinoma of the anus in situ that was excised in 2006. The magnetic resonance and PET imaging revealed a 3.5-cm, hypermetabolic mass extending beyond the anal verge, with involvement of the external sphincter. She was previously seen at another institution and underwent radiation treatment planning; however, her kidney dose was not acceptable, and she was referred for proton therapy. The patient was treated with 5-FU/mitomycin–based chemoradiation using IMPT (Figure 2). The patient was treated in 30 fractions, with the primary tumor receiving 54 Gy, and the elective nodal regions receiving 45 Gy. The mean transplanted kidney dose was 0.95 Gy, with a maximum dose of 22 Gy. The kidney V6 was 4.16%, and the V20 was 0.02%. Pretreatment and posttreatment creatinine levels were 2.16 and 2.27 mg/dL (to convert to micromoles per liter, multiply by 88.4), respectively. A PET scan in January 2019 showed decreased uptake in the anorectal primary site, consistent with an excellent treatment response.
Figure 2.
Intensity-modulated proton therapy plan for patient 2.
Case 3
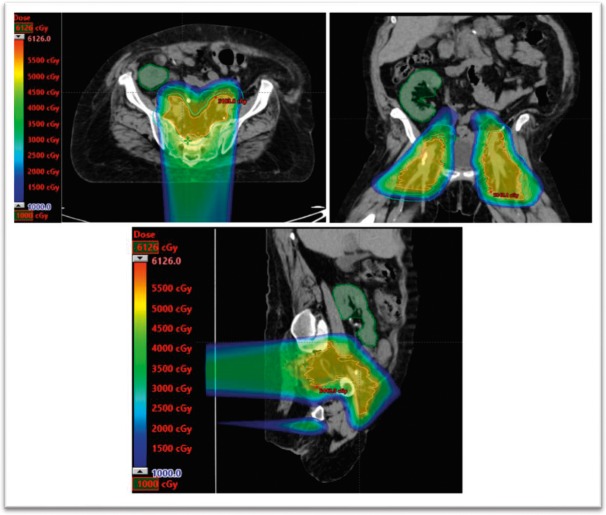
A 60-year-old, white woman with a kidney transplant 20 years before diagnosis presented with stage IIB T3N0M0 squamous cell carcinoma of the anal canal. The patient was treated with 5-FU/mitomycin–based chemoradiation using IMPT (Figure 3). The primary tumor was treated to 54 Gy in 30 fractions. The mean transplanted kidney dose was 0.58 Gy, with a maximum dose of 19.5 Gy. The kidney V6 was 2.47%, and the V20 was 0%. Pretreatment and posttreatment creatinine levels were 1.69 and 1.18 mg/dL (to convert to micromoles per liter, multiply by 88.4), respectively. Despite having a break in treatment because of neutropenic fever, the patient was able to complete all therapy. The 3-month follow-up is pending.
Figure 3.
Intensity-modulated proton therapy plan for patient 3.
Case 4
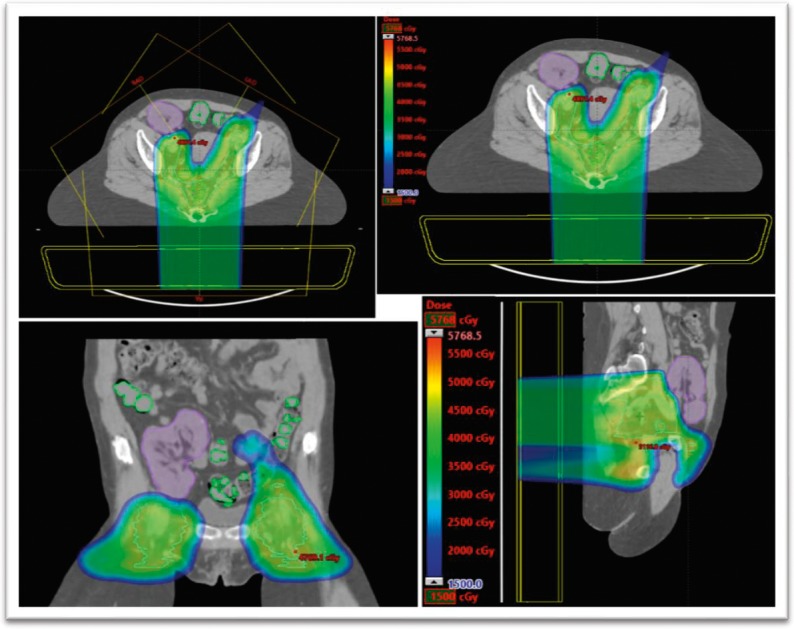
A 60-year-old, white woman with a history of ESRD, secondary to vesicoureteral reflux and glomerulonephritis, with 2 kidney transplants (initial transplant failed, secondary to rejection) 33 and 23 years before diagnosis, presented with stage II T3N0M0 squamous cell carcinoma of the anal canal. After a multidisciplinary discussion, the patient was treated with IMPT alone because of her multiple comorbidities (Figure 4). The primary tumor received 50.4 Gy in 28 fractions. The mean transplanted kidney dose was 0.19 Gy, with a maximum dose of 10 Gy. The kidney V6 was 0.22%, and the V20 was 0%. The patient's creatinine level fluctuated, secondary to her multiple metabolic and cardiac comorbidities. Her posttreatment creatinine baseline (3–4.5 mg/dL [to convert to micromoles per liter, multiply by 88.4]) remained unchanged from her pretreatment level. The PET scans in January 2018 and January 2019 revealed no evidence of local recurrent or distant metastatic disease, consistent with a highly favorable outcome.
Figure 4.
Intensity-modulated proton therapy plan for patient 4.
Discussion
In this case series, we describe the use of pencil-beam IMPT in the treatment of anal carcinoma in 4 patients with transplanted pelvic kidneys who presented to our institution. A literature review did not reveal any studies documenting the use of proton therapy to treat anal cancer in patients with transplanted pelvic kidneys. To our knowledge, this is the first description of this therapeutic approach.
In the United States, there were 8580 new diagnoses of anal cancer in 2018 [20]. An increased incidence of anal cancer in patients with kidney transplants has been reported in multiple studies.† The risk is often reported as a standard incidence ratio, which is the incidence in transplant recipients compared with age-matched controls [17]. In the literature, reported standard incidence ratios for anal cancer in patients with kidney transplants for a variety of populations range from 2 to 10 [5, 10, 11, 14, 16], with one 2007 meta-analysis reporting a standard incidence ratio of 4.85 [14].
The modern standard of care for anal carcinoma generally follows RTOG 0529 guidelines and consists of 5-FU/mitomycin C–based chemoradiation [18, 23–28]. Given the lower abdominal location of most transplanted kidneys, pelvic radiation presents greater challenges in this patient population. In many cases, the kidneys are the dose-limiting organs for radiotherapy to gastrointestinal cancers, gynecologic cancers, lymphomas and sarcomas of the upper abdomen, and during total body irradiation (TBI). Per Quantitative Analysis of Normal Tissue Effects in the Clinic guidelines, the suggested dose-volume constraints for non-TBI, bilateral kidney irradiation for an estimated nephropathy risk of < 5% are a mean kidney dose of < 18 Gy, a V20 of < 32%, and a V6 of < 30% [29]. Despite that, studies examining renal toxicity in the context of TBI have demonstrated increased rates of nephropathy in patients receiving as little as 8 to 12 Gy to the kidneys, although it should be noted that these results are often confounded by concurrent chemotherapy regimens. Given the relative radiosensitivity of native kidneys, the tissue-sparing effects of proton therapy are of utmost importance in the treatment of patients with pelvic kidneys and pelvic malignancies.
Proton therapy is being investigated as an alternative to intensity-modulated radiation therapy because of its potential to minimize radiation exposure to the transplanted kidney and other organs at risk. Previous dosimetric studies have demonstrated improved organ-at-risk sparing with IMPT, compared with intensity-modulated radiation therapy, that do not sacrifice coverage of the target volumes [19]. Of note, there are 3 on-going clinical trials investigating the use of proton therapy in the treatment of anal cancer in the population at large (NCT03018418, NCT01858025, and NCT03690921). In our study, radiation dosage to the pelvic kidney was well below proposed dose-volume constraints, with a maximum mean kidney dose of 3.6 Gy, a maximum V20 of 5.79%, and a maximum V6 of 18.26%. Clinical target volume coverage was 100% for patients 1 to 3, and 99.5% for patient 4.
Although short-term kidney function was preserved, radiation-induced kidney injury is subclinical and frequently presents during the subacute (3–18 month) and chronic (> 18 month) periods [29]. Because of recent treatment completion and the receipt of follow-up care in another state, subacute or chronic posttreatment creatinine levels were only obtained for 2 patients. Although immediate results are encouraging, on-going evaluation will be needed to assess long-term kidney function.
Based on these 4 patient cases, IMPT appears to be an acceptable option for patients with squamous cell carcinoma of the anal canal and a transplanted pelvic kidney. In each case, minimal radiation exposure of the renal transplant was achieved with relative preservation of kidney function and no sacrifice of target volumes.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Ethical approval: All patient data were collected under internal review board–approved protocol.
Footnotes
References
- 1.United Network for Organ Sharing. Organ transplants in United States set sixth consecutive record in 2018. 2019 https://unos.org/news/organ-transplants-in-united-states-set-sixth-consecutive-record-in-2018 Updated January 8, Accessed May 18, 2019.
- 2.United Network for Organ Sharing. Transplant trends. 2019 https://unos.org/data/transplant-trends Updated May 9, Accessed May 18, 2019.
- 3.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. J Am Med Assoc. 2011;306:1891–901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 5.Ogilvie JW, Park IU, Downs LS, Anderson KE, Hansberger J, Madoff RD. Anal dysplasia in kidney transplant recipients. J Am Coll Surg. 2008;207:914–21. doi: 10.1016/j.jamcollsurg.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Meeuwis KA, Melchers WJ, Bouten H, van de Kerkhof PC, Hinten F, Quint WG, Massuger LF, Hoitsma AJ, van Rossum MM, de Hullu JA. Anogenital malignancies in women after renal transplantation over 40 years in a single center. Transplantation. 2012;93:914–22. doi: 10.1097/TP.0b013e318249b13d. [DOI] [PubMed] [Google Scholar]
- 7.Hoshida Y, Tsukuma H, Yasunaga Y, Xu N, Fujita MQ, Satoh T, Ichikawa Y, Kurihara K, Imanishi M, Matsuno T, Aozasa K. Cancer risk after renal transplantation in Japan. Int J Cancer. 1997;71:517–20. doi: 10.1002/(sici)1097-0215(19970516)71:4<517::aid-ijc3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Kyllonen L, Salmela K, Pukkala E. Cancer incidence in a kidney-transplanted population. Transpl Int. 2000;13(suppl 1):S394–8. doi: 10.1007/s001470050369. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay F, Fernandes M, Habbab F, De Edwardes MDB, Loertscher R, Meterissian S. Malignancy after renal transplantation: incidence and role of type of immunosuppression. Ann Surg Oncol. 2002;9:785–8. doi: 10.1007/BF02574501. [DOI] [PubMed] [Google Scholar]
- 10.Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–31. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 12.Taioli E, Piselli P, Arbustini E, Boschiero L, Burra P, Busnach G, Caldara R, Citterio F, De Juli E, Dissegna D, Gotti E, Marchini F, Maresca MC, Marsano L, Montagnino G, Montanaro D, Sandrini S, Pedotti P, Scalamogna M, Serraino D. Incidence of second primary cancer in transplanted patients. Transplantation. 2006;81:982–5. doi: 10.1097/01.tp.0000203321.42121.14. [DOI] [PubMed] [Google Scholar]
- 13.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–8. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 15.Vajdic CM, Van Leeuwen MT. What types of cancers are associated with immune suppression in HIV? lessons from solid organ transplant recipients. Curr Opin HIV AIDS. 2009;4:35–41. doi: 10.1097/coh.0b013e328319bcd1. [DOI] [PubMed] [Google Scholar]
- 16.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x. [DOI] [PubMed] [Google Scholar]
- 17.Asch WS, Bia MJ. Oncologic issues and kidney transplantation: a review of frequency, mortality, and screening. Adv Chronic Kidney Dis. 2014;21:106–13. doi: 10.1053/j.ackd.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H, Willett CG. RTOG. 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier TM, Mascia AP, Wolf EM, Kharofa JM. Dosimetric comparison of intensity-modulated proton therapy and volumetric-modulated arc therapy in anal cancer patients and the ability to spare bone marrow. Int J Part Ther. 2017;4:11–7. doi: 10.14338/IJPT-17-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 21.Patel HS, Silver AR, Northover JM. Anal cancer in renal transplant patients. Int J Colorectal Dis. 2007;22:1–5. doi: 10.1007/s00384-005-0023-3. [DOI] [PubMed] [Google Scholar]
- 22.Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med. 2013 doi: 10.1101/cshperspect.a015677. [DOI] [PMC free article] [PubMed]
- 23.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report: 1974. Dis Colon Rectum. 1993;36:709–11. doi: 10.1007/BF02238600. [DOI] [PubMed] [Google Scholar]
- 24.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, Quivey J, Rotman M, Kerman H, Coia L, Murray K. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 25.UK Co-ordinating Committee on Cancer Research; UKCCCR Anal Cancer Trial Working Party. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348:1049–54. [PubMed] [Google Scholar]
- 26.Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M, Pierart M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 27.Gunderson LL, Winter KA, Ajani JA, Pedersen JE, Moughan J, Benson AB, III, Thomas CR, Jr, Mayer RJ, Haddock MG, Rich TA, Willett CG. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–51. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, Maughan T, McDonald A, Essapen S, Leslie M, Falk S, Wilson C, Gollins S, Begum R, Ledermann J, Kadalayil L, Sebag-Montefiore D. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–24. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 29.Dawson LA, Kavanagh BD, Paulino AC, Das SH, Miften M, Li XA, Pan C, Ten Haken RK, Schultheiss TE. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010;6:108–15. doi: 10.1016/j.ijrobp.2009.02.089. [DOI] [PubMed] [Google Scholar]