Abstract
The heavy-ion medical accelerator in Chiba (HIMAC), Japan, has been using carbon-ion radiation therapy since 1994, and the number of patients treated with this technique has reached around 10,000. The HIMAC employs single beam wobbling as a beam-delivery method. Based on the broad-beam method, respiratory-gating and layer-stacking irradiation methods were subsequently developed, which have contributed to significantly increasing irradiation accuracy. During the study and research and development to downsize carbon-ion radiation therapy facilities, a spiral beam-wobbling method was developed, which has been employed in compact carbon-ion radiation therapy facilities constructed in Japan. Toward the further development of the HIMAC treatment, the National Institute of Radiological Sciences has developed new treatment technologies, such as phase-controlled rescanning, based on a fast 3-deminsional (3D) scanning method with a pencil beam toward adaptive cancer radiation therapy. A heavy-ion rotating gantry, combined with 3D-scanning, is currently under development. These technologies developed by the National Institute of Radiological Sciences will hopefully boost the use of heavy-ion radiation therapy worldwide.
Keywords: carbon-ion radiotherapy, broad-beam irradiation, pencil-beam 3D scanning
Introduction
Heavy-ion beams are particularly suitable for deeply seated cancer radiation therapy because of their high-dose localization and high biological effect in the Bragg-peak region. The National Institute of Radiological Sciences (NIRS), therefore, constructed the heavy-ion medical accelerator in Chiba (HIMAC), Japan [1], as the world's first heavy-ion accelerator facility dedicated to medical applications. The NIRS has conducted radiation therapy with HIMAC since 1994. The NIRS selected a carbon-ion beam as the therapeutic method, based on fast-neutron radiation therapy experience. Single beam wobbling [2] was adopted as a beam-delivery method because of its robustness against beam errors and its easy dose management. A respiratory-gated irradiation process [3] and a layer-stacking method [4–6] were developed for moving tumor treatment and more-conformal dose distribution, mainly in head and neck tumors, respectively. Because of these developments, clinical studies at HIMAC have progressed significantly, resulting in more protocols. In 2003, the Japanese government approved the use of carbon-ion radiation therapy with HIMAC as a highly advanced medical technology. Because of this, the NIRS subsequently proposed a standard carbon-ion radiation therapy facility in Japan [7] to boost the applications of carbon-ion radiation therapy, with an emphasis on the development of a more-compact facility to reduce the construction costs. Concerning a beam delivery system in this work, a spiral beam-wobbling method with a broad beam was developed [8, 9], which has been used in compact carbon-ion radiation therapy facilities constructed in Japan. Since 2006, the NIRS has been engaged in a new treatment research project [10] focusing on “adaptive cancer radiation therapy” [11] for both static and moving tumors, leading to the development of a phase-controlled rescanning (PCR) method [12] based on fast 3-dimensional (3D) scanning with a pencil beam. A new treatment research facility was constructed to carry out both clinical studies and treatments with the 3D scanning technology developed. Since 2011, the clinical studies and treatments for static tumors have successfully progressed with fast 3D scanning, whereas those for moving tumors have progressed with the PCR method since March 2015. A compact, heavy-ion, rotating gantry is currently being developed with superconducting technology for shorter-course treatments with higher accuracy. This article reviews the heavy-ion radiotherapy technologies developed with HIMAC.
Development of the Broad-Beam Method
Particle radiation therapy requires a 3D biological-dose distribution within several percentage points of the uniformity on a tumor, with as low a dose as possible in the surrounding normal tissue. For this purpose, various beam-delivery methods have been proposed and used [13]. Among them, HIMAC employed a single beam-wobbling method, which is one of the broad-beam methods, resulting in easy dose management and robustness against beam fluctuations in position, size, and intensity.
Single-Beam Wobbling Irradiation Method
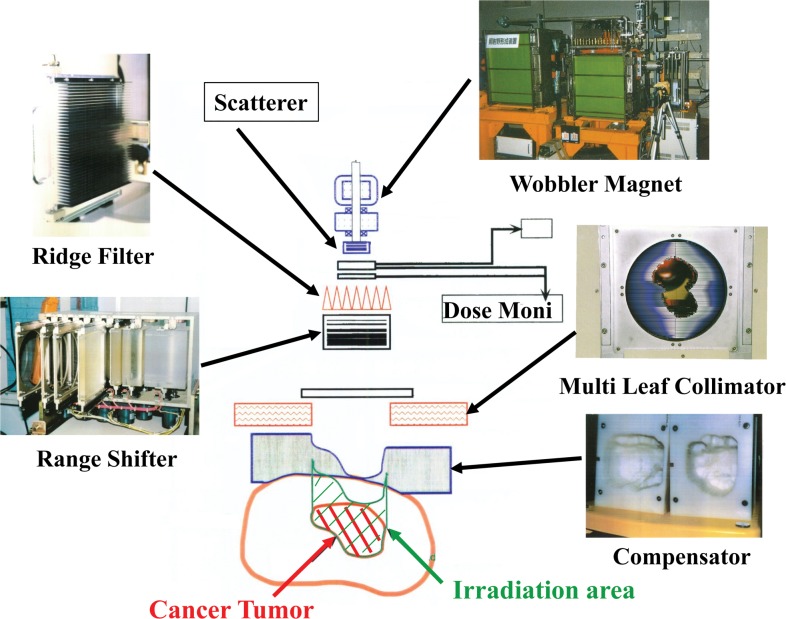
The single-beam wobbling method consists of two functions: single beam-wobbling to form a lateral-dose distribution and a ridge filter to form a distal-dose one through a spread-out Bragg peak (SOBP). A pair of beam-wobbling magnets moves a beam on a single circular orbit at a high frequency of 56.4 Hz to generate a pseudostationary broad beam in conjunction with a scatterer. A ridge filter, consisting of identical aluminum bar ridges, modulates a beam range in an irradiation field to produce an SOBP. A range-shifter system inserts variable-thickness energy absorbers to adjust the beam range. A multileaf collimator with movable metal elements or a customized patient collimator or both define the field aperture. A patient compensator, a sculptured, plastic device, compensates residual ranges to conform to the distal part of the target volume in the field. At HIMAC, the maximum size of the lateral field and the SOBP are designed to be 22 cm in diameter within ± 2.5% of uniformity and 15 cm of thickness at the isocenter. Figure 1 shows a schematic drawing of the HIMAC beam-delivery system.
Figure 1.
A schematic illustration of the heavy-ion medical accelerator in Chiba, Japan, beam-delivery system with the single beam-wobbling method.
In the case of heavy-ion beams, the SOBP is composed of various linear energy transfer (LET) components with different weighting factors at each depth. The survival rate under a mixing LET radiation field can be described by a formalism proposed in the theory of dual radiation action according to the linear-quadratic model [14], which was experimentally proven. The SOBP of mixed ions with different LETs has been designed through the procedure proposed in Chu et al [13] and the formalism Kanai et al [14].
Layer-Stacking Irradiation Method
In the beam-wobbling method, as shown in Figure 1, it is inevitable that the SOBP with a fixed thickness brings an undesirable dosage to the normal tissue in front of the target because the thickness of an actual target varies within the irradiation field. Therefore, the layer-stacking irradiation method was developed to remove the undesirable dosage. This method can conform to a variable SOBP in a target volume by dynamically controlling conventional beam-modifying devices. A thin SOBP with several millimeters in water equivalent length, which is produced by a miniridge filter, is longitudinally scanned over the target volume in a stepwise manner. The target volume is longitudinally divided into slices and the thin SOBP conforms to each of them using the multileaf collimator and the range shifter, and a variable SOBP coincides with the target volume then formed.
Respiratory-Gated Irradiation Method
Damage to normal tissues surrounding the tumor was inevitable in the treatment of a tumor moving along with the patient's respiration. Therefore, the respiratory-gated irradiation method, with the single beam-wobbling method, was developed. In this application, an infrared–light-emitting diode sensor is set on the surface of the patient's body and its movement is monitored by a position-sensitive detector, which results in obtaining the respiratory signal. The beam should be delivered according to the gate signal produced only when the target is in the design position during a flattop (FT) period that can extract the beam from the synchrotron. For this purpose, the NIRS developed the radiofrequency knockout slow extraction method [15] that can turn the beam on/off within 1 ms in response to respiration. At present, approximately 40% of all patients in HIMAC are treated with the respiratory-gated irradiation method.
Spiral Beam-Wobbling Method
Because the single beam-wobbling method inevitably loses residual range because of the relatively thick scatterer, a spiral beam-wobbling method with a broad beam was developed in HIMAC [8, 9]. The beam size in this method is much smaller than that in the single beam-wobbling method, enabling it to deliver a uniform dose distribution in a lateral direction while the beam moves along on a spiral orbit. The range loss through the scatterer is small because the smaller beam size requires a thin scatterer. Consequently, this method can obtain a longer residual range even with similar quantities of energy and irradiation field compared with the single beam-wobbling method. The spiral method requires amplitude modulation of a wobbling excitation current because the wobbling radius is proportional to the square root of time under conditions of a constant irradiation area in unit time. The amplitude modulation and angular frequencies were optimized through simulation and experimental studies and determined to be 59 and 23 Hz, respectively.
Development of 3D Scanning Method
In radiation therapy, shrinkage in the tumor size as well as a change in the tumor shape during the course of treatment has been observed. To maintain a uniform dose distribution in such cases, strict requirements were made to make treatment planning compulsory before each fractional irradiation, which is now referred to as adaptive cancer radiation therapy. It is well known that a pencil-beam 3D scanning method is very suitable for this purpose because it does not require the use of both the compensator and patient collimators, which required long manufacturing times. The pencil-beam 3D scanning method has resulted in high treatment accuracy in the case of a fixed target [16–18] but has not yet been put into practice for moving tumor treatment. Because the HIMAC facility should carry out treatments not only for static targets but also for moving ones, we developed a 3D scanning method with a new technique. In cooperation with a rotating gantry, the 3D scanning method can achieve higher treatment accuracy, even for a target close to critical organs, using intensity-modulated particle therapy [19], as compared with conventional carbon-ion radiation therapy. Therefore, we have also developed a rotating gantry with the 3D scanning method [20].
Pencil-Beam 3D Scanning for Moving-Tumor Treatment
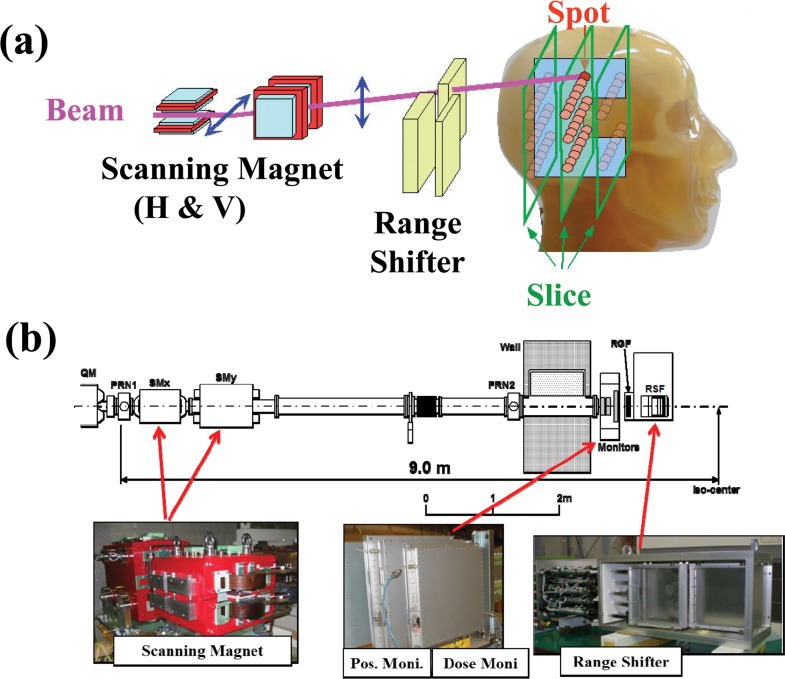
Pencil-beam 3D scanning is an irradiation method that covers the dose distribution with a small beam and narrow Bragg peak, which allows full advantage of the heavy ions. This irradiation procedure is shown schematically in Figure 2. The pencil beam is laterally scanned to form a lateral irradiation field with orthogonal scanning dipole magnets and then longitudinally scanned by either a range shifter or by energy change through the accelerator itself or both. The beam scanning path and the deposit dose in each spot have been precisely determined in treatment planning to deliver the dose distribution planned. The scanning magnets, therefore, should be controlled as a function of the dose detected by the dose-monitoring system. Fast and synchronous control of dose monitoring, magnetic scanning, and beam extraction systems with precision and resolution > 1% and 1 ms is generally required for clinically practical beam deliveries at HIMAC.
Figure 2.
(a) A schematic illustration of the pencil-beam 3D scanning method. (b) Layout of the pencil-beam 3D scanning system in heavy-ion medical accelerator in Chiba, Japan, which has been used in both the range-shifter depth scanning and hybrid depth scanning methods.
There are 2 main approaches for lateral beam scanning: the spot scanning and the raster scanning methods. Another technique, which is referred to as hybrid raster scanning [12, 18], was developed and has been in practical use. The hybrid raster scanning method is essentially the same as the spot scanning method with dose-driven scanning; however, the beam is continually delivered even during spot-position change. Conversely, beam delivery is stopped during each slice change.
Phase-Controlled Rescanning Method
The PCR method was proposed and developed for adaptive cancer radiation therapy for static and moving tumors. In this method, schematically shown in Figure 3, rescanning completes irradiation on 1 isoenergy slice during 1 respiratory-gated period. Because the average 3D movement of the target is close to zero, a uniform dose distribution can be achieved even under irradiation on the moving target.
Figure 3.
A schematic diagram of the phase-controlled rescanning method. From the top view, the respiratory signal, gate signal, beam intensity, scanning trajectory on each slice, and the operation pattern of synchrotron are shown.
The PCR method consists of 2 main technologies: (1) the intensity-modulation technique for constant irradiation time on each isoenergy slice with different cross-sections, shown in Figure 3, and (2) the fast scanning technique for completing multiple rescanning within a defined amount of time.
Intensity modulation: In the radiofrequency knockout slow-extraction method, increasing the amplitude of a transverse radiofrequency (amplitude modulation [AM]) increases the extraction rate. Based on this principle, a spill-control system was developed [21, 22] to deliver the beam with intensity modulation. The core part of this system requires the following functions: (1) calculation and output of an AM signal according to request signals from an irradiation system, (2) real-time processing with a time resolution of > 1 ms, and (3) feed-forward and feedback controls to realize the extracted intensity, as requested. This system allows us to control the beam intensity dynamically.
Fast 3D scanning: For fast pencil-beam 3D scanning, 3 key technologies were developed as follows: (1) a new treatment planning system (TPS) for hybrid raster scanning, (2) an extended flattop operation of the synchrotron, and (3) a high-speed scanning-magnet system.
New TPS [23]: The biological dose distribution of a pencil beam of carbon ions is obtained by combining the measured physical-dose distribution and the RBE obtained through measuring α and β values in the linear-quadratic model. Using the biological dose distribution, the new TPS optimizes the assignment of spot positions and their weights to provide the prescribed dose on a target and to significantly reduce the dose distribution on surrounding normal tissues. The TPS employs the fast 3D scanning method through hybrid raster scanning, which can save the beam-off period during the spot-position transition. The raster scanning method, however, is necessary to deliver an extra dose to the position between the spot positions. Increasing the extra dose disturbs the dose distribution. Because the extra dose is proportional to the beam intensity, it was not easy to increase the beam intensity. The HIMAC synchrotron, however, has high reproducibility and a uniform time structure for the extracted beam through the spill control system. Therefore, the extra dose can be predicted and incorporated into the TPS, and the beam intensity can be increased without disturbing the dose distribution, which results in fast 3D scanning. Applying a modified “travelling-salesman problem,” the path length of raster scanning could be shortened by 20% to 30%. Consequently, the new TPS can increase the scanning speed by a factor of about 5.
Extended FT operation: The pencil-beam 3D scanning method can complete a single-fractional irradiation of almost all treatment procedures when approximately 2 × 1010 carbon ions are delivered. Thus, the beam intensity was upgraded up to 2 × 1010 in HIMAC, which made a single-operation cycle of the synchrotron possible. This single-cycle operation, which can be realized by using a clock-stop technique in the FT period, can increase the treatment efficiency especially for respiratory-gated irradiation. This extended FT operation can shorten the irradiation time by a factor of 2 and make intensity modulation easy.
High-speed scanning magnet: The scanning speed is designed to be 100 mm/ms and 50 mm/ms in the horizontal and vertical directions, respectively. To increase the scanning speed, a scanning magnet with slits in both ends of the magnetic poles was designed, according to a thermal analysis, such as an eddy current loss and hysteresis loss. Because of the test, the temperature rise was measured to be around 30° maximum, which is consistent with the thermal analysis.
Depth Scan
The 3D scanning method essentially requires an isoenergy slice change for depth scanning. For the first stage of 3D scanning treatment with HIMAC, the range shifter, as the energy degrader set just in front of the patient, was used for the slice change because it cuts the commissioning time. It has been recognized, however, that the variable-energy operation by the accelerator itself has great advantages over that of the energy degrader because it (1) maintains a small spot size, (2) suppresses the secondary neutron yield, and (3) shortens the irradiation time because of the high speed slice change. By applying the extended FT operation of the HIMAC synchrotron, a multiple-energy operation was developed. In this method, the beam energy can be changed in a stepwise energy pattern at the FT of the synchrotron operation. In the second step, thus, an 11-step energy operation from 430 to 140 MeV/n was developed [24], which has been routinely used for hybrid depth scanning. In the hybrid depth scanning method, a range of > 3 cm is changed by the energy change in the synchrotron, whereas that of < 3 cm is changed by the thin energy degrader. In the third step, a 201-step energy pattern, which can change the energy from 430 to 56 MeV/n, was developed [24, 25]. One energy step corresponds to a range shift of 2 or 3 mm and takes < 100 ms for 1 slice change. The 201-step multiple-energy operation will be applied to a clinical study, which will be initiated in 2016. As shown in Figure 4, the full-energy depth scanning method gives the best treatment-planning result, compared with the hybrid depth scanning and range-shifter depth scanning methods.
Figure 4.
Comparison of (a) the range-shifter depth scanning, (b) the hybrid depth scanning, and (c) the full-energy depth scanning methods on treatment planning. (d) D-volume histograms for the 3 methods.
Rotating Gantry
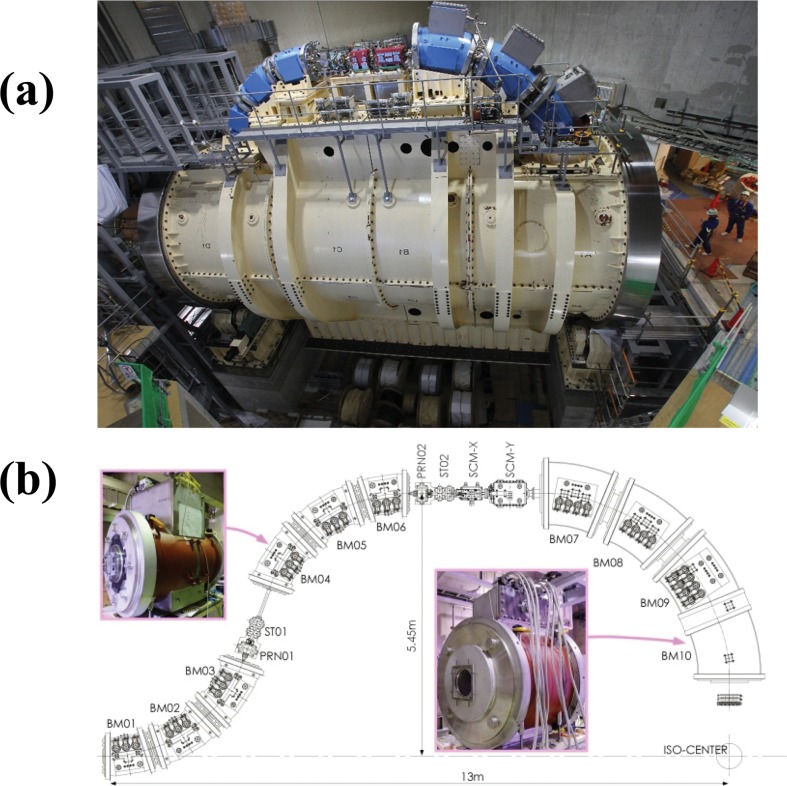
An isocentric superconducting rotating gantry has been developed [12]. This rotating gantry is designed to transport carbon ions having 430 MeV/n to an isocenter with irradiation angles of > 180° and is further capable of performing fast 3D scanning with a scan size of approximately 20 cm2 at the isocenter. Figure 5 shows a photograph and layout of the rotating gantry. The gantry consists of 10 combined-function superconducting magnets, a pair of scanning magnets, and 2 pairs of beam profile-monitor and steering magnets, allowing a compact geometry with a length of 13 m and a diameter of 11 m. Before manufacturing all magnets, a test magnet was designed and manufactured to verify the stability of a superconducting state under mechanical rotation and vibrations on a rotating gantry and the stability of the temperature under fast slewing of the magnetic field to follow the multiple-energy operation. No quench phenomena were observed in either test. Following these tests, both the dipole and quadrupole fields were measured, and the tests results were correlated with the calculated results.
Figure 5.
(a) Photograph of the superconducting rotating gantry for carbon-ion radiotherapy installed in the new treatment research facility. (b) Layout of the rotating gantry.
Conclusion
More than 600,000 persons are diagnosed with cancer every year in Japan, and it is predicted that this number will continue to rise. To meet these challenges, the NIRS has developed heavy-ion radiotherapy technologies and carried out clinical studies with carbon-ion radiotherapy, which has led to a pilot facility of a compact carbon-ion radiotherapy facility in Gunma University. Following the pilot facility, the Saga Heavy-Ion Medical Accelerator in Tosu was constructed and has been successfully operated since 2013, and the ion-beam radiation oncology center in Kanagawa is currently under construction, which will use 3D scanning technology developed by NIRS with a similarly designed accelerator as that of the pilot facility. The first patient of the ion-beam radiation oncology center was treated in December 2015. Other carbon-ion radiotherapy facilities, along with HIMAC, are expected to boost the applications of carbon-ion radiotherapy in Japan.
Acknowledgments
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The author has no conflicts of interest to disclose.
Acknowledgments: The author would like to express his thanks to the members of both the Department of Accelerator and Medical Physics and Next-generation Heavy-Ion Treatment Study Program at NIRS for their work toward the advancement of medicine. The experimental study of this work was performed as part of the research project with heavy-ion at the NIRS-HIMAC.
References
- 1.Hirao Y, Ogawa H, Yamada S, Sato Y, Yamada T, Sato K, Itano A, Kanazawa M, Noda K, Kawachi K, Endo M, Kanai T, Kohno T, Sudou M, Minohara S, Kitagawa A, Soga F, Takada E, Watanabe S, Endo K, Kumada M, Matsumoto S. Heavy ion synchrotron for medical use—HIMAC project at NIRS-Japan. Nucl Phys A. 1992;538:541–50. [Google Scholar]
- 2.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, Furusawa Y, Ando K, Suzuki M, Soga F, Kawachi K. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44:201–10. doi: 10.1016/s0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 3.Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy-ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097–103. doi: 10.1016/s0360-3016(00)00524-1. [DOI] [PubMed] [Google Scholar]
- 4.Kanai T, Kawachi K, Matsuzawa H, Inada T. Broad beam three-dimensional irradiation for proton radiotherapy. Med Phys. 1983;10:344–6. doi: 10.1118/1.595254. [DOI] [PubMed] [Google Scholar]
- 5.Kanematsu N, Endo M, Futami Y, Kanai T, Asakura H, Oka H, Yusa K. Treatment planning for the layer-stacking irradiation system for three-dimensional conformal heavy-ion radiotherapy. Med Phys. 2002;29:2823–9. doi: 10.1118/1.1521938. [DOI] [PubMed] [Google Scholar]
- 6.Kanai T, Kanematsu N, Minohara S, Komori M, Torikoshi M, Asakura H, Ikeda N, Uno T, Takei Y. Commissioning of a conformal irradiation system for heavy-ion radiotherapy using a layer-stacking method. Med Phys. 2006;33:2989–97. doi: 10.1118/1.2219771. [DOI] [PubMed] [Google Scholar]
- 7.Noda K, Furukawa T, Fujisawa T, Iwata Y, Kanai T, Kanazawa M, Kitagawa A, Komori M, Minohara S, Murakami T, Muramatsu M, Sato S, Takei Y, Tashiro M, Torikoshi M, Yamada S, Yusa K. New accelerator facility for carbon-ion cancer-therapy. J Radiat Res. 2007;48(suppl A):A43–54. doi: 10.1269/jrr.48.a43. [DOI] [PubMed] [Google Scholar]
- 8.Komori M, Furukawa T, Kanai T, Noda K. Optimization of spiral wobbler system for heavy-ion radiotherapy. Jpn J Appl Phys. 2004;43:6463–7. [Google Scholar]
- 9.Yonai S, Kanematsu N, Komori M, Kanai T, Takei Y, Takahashi O, Isobe Y, Tashiro M, Koikegami H, Tomita H. Evaluation of beam wobbling methods for heavy-ion radiotherapy. Med Phys. 2008;35:927–38. doi: 10.1118/1.2836953. [DOI] [PubMed] [Google Scholar]
- 10.Noda K, Furukawa T, Fujimoto T, Inaniwa T, Iwata Y, Kanai T, Kanazawa M, Mino-hara S, Miyoshi T, Murakami T, Sano Y, Sato S, Takada E, Takei Y, Torikai K, Torikoshi M. New treatment facility for heavy-ion cancer therapy at HIMAC. Nucl Instrum Methods Phys Res B. 2008;266:2182–5. [Google Scholar]
- 11.Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol. 1997;42:123–32. doi: 10.1088/0031-9155/42/1/008. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa T, Inaniwa T, Sato S, Tomitani T, Minohara S, Noda K, Kanai T. Design study of a raster scanning system for moving target irradiation in heavy-ion radiotherapy. Med Phys. 2007;34:1085–97. doi: 10.1118/1.2558213. [DOI] [PubMed] [Google Scholar]
- 13.Chu WT, Ludewigt BA, Renner TR. Instrumentation for treatment of cancer using proton and light-ion beams. Rev Sci Instrum. 1993;64:2055–122. [Google Scholar]
- 14.Kanai T, Furusawa Y, Fukutsu K, Itsukaichi H, Eguchi-Kasai K, Ohara H. Irradiation of mixed beam and design of spread-out Bragg peak for heavy-ion radiotherapy. Radiat Res. 1997;147:78–85. [PubMed] [Google Scholar]
- 15.Noda K, Kanazawa M, Itano A, Takada E, Torikoshi M, Araki N, Yoshizawa J, Sato K, Yamada S, Ogawa H, Itoh H, Noda A, Tomizawa M, Yoshizawa M. Slow beam extraction by a transverse RF field with AM and FM. Nucl Instrum Methods Phys Res A. 1996;374:269–77. [Google Scholar]
- 16.Kanai T, Kawachi K, Kumamoto Y, Ogawa H, Yamada T, Matsuzawa H, Inada T. Spot scanning system for proton radiotherapy. Med Phys. 1980;7:365–9. doi: 10.1118/1.594693. [DOI] [PubMed] [Google Scholar]
- 17.Pedroni E, Bacher R, Blattmann H, Bohringer T, Coray A, Lomax A, Lin S, Munkel G, Scheib S, Schneider U, et al. The 200-MeV proton therapy project at the Paul Scherrer Institute: conceptual design and practical realization. Med Phys. 1995;22:37–53. doi: 10.1118/1.597522. [DOI] [PubMed] [Google Scholar]
- 18.Haberer T, Becher W, Schardt D, Kraft G. Magnetic scanning system for heavy ion therapy. Nucl Instrum Methods Phys Res A. 1993;330:296–305. [Google Scholar]
- 19.Lomax A. Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44:185–205. doi: 10.1088/0031-9155/44/1/014. [DOI] [PubMed] [Google Scholar]
- 20.Iwata Y, Noda K, Shirai T, Murakami T, Furukawa T, Mori S, Fujita T, Itano A, Shouda K, Mizushima K, Fujimoto T, Ogitsu T, Obana T, Amemiya N, Orikasa T, Takami S, Takayama S, Watanabe I. Design of a superconducting rotating gantry for heavy-ion therapy. Phys Rev ST Accel Beams. 2012;15:044701. [Google Scholar]
- 21.Sato S, Furukawa T, Noda K. Dynamic intensity control system with RF-knockout slow-extraction in the HIMAC synchrotron. Nucl Instrum Methods Phys Res A. 2007;574:226–31. [Google Scholar]
- 22.Mizushima K, Sato S, Shirai T, Furukawa T, Katagiri K, Takeshita E, Iwata Y, Himukai T, Noda K. Development of beam current control system in RF-knockout slow extraction. Nucl Instrum Methods Phys Res B. 2011;269:2915–8. [Google Scholar]
- 23.Inaniwa T, Furukawa T, Sato S, Tomitani T, Kobayashi M, Minohara S, Noda K, Kanai T. Development of treatment planning for scanning irradiation at HIMAC. Nucl Instrum Methods Phys Res B. 2008;266:2194–8. [Google Scholar]
- 24.Iwata Y, Kadowaki T, Uchiyama H, Fujimoto T, Takada E, Shirai T, Furukawa T, Mizushima K, Takeshita E, Katagiri K, Sato S, Sano Y, Noda K. Multiple-energy operation with extended flattops at HIMAC. Nucl Instrum Methods Phys Res A. 2010;624:33–8. [Google Scholar]
- 25.Mizushima K, Katagiri K, Iwata Y, Furukawa T, Fujimoto T, Sato S, Hara Y, Shirai T, Noda K. Experimental studies of systematic multiple-energy operation at HIMAC synchrotron. Nucl Instrum Methods Phys Res B. 2014;331:243–7. [Google Scholar]