Abstract
Purpose:
To model whether in vivo range verification could reduce high-grade rectal toxicity for patients with prostate cancer treated with pencil beam scanning proton therapy by allowing novel proton beam arrangements compared to standard lateral opposed beams.
Materials and Methods:
Proton plans were generated for 8 patients with prostate cancer previously treated with photons by volumetric-modulated arc therapy (VMAT). The VMAT plans were generated by using a uniform 6-mm planning target volume (PTV) expansion. For the proton plans an additional distal margin (3.5% of beam range) was added to the uniform 6-mm PTV to account for range uncertainty, using 3 beam arrangements: (1) lateral opposed beams (LAT), (2) left and right anterior oblique beams (LAO/RAO), and (3) a single anterior-posterior beam (AP). Assuming use of in vivo range verification, plans were generated by using a reduced distal PTV and distal range uncertainty expansion (2 mm each) with AP (AP–2 mm) and LAO/RAO (LAO/RAO–2 mm) beam arrangements. Estimates of normal tissue complication probability (NTCP) for ≥grade 2 rectal bleeding were generated by using the Lyman-Kutcher-Burman model.
Results:
Each proton and photon plan was able to achieve all prespecified rectal and bladder constraints. For the VMAT, LAT, AP, and LAO/RAO plans, estimated NTCP values for ≥grade 2 rectal bleeding were 0.19, 0.21, 0.24, and 0.2, respectively. For the AP–2 mm and LAO/RAO–2 mm plans, NTCP values were reduced to 0.11 and 0.1 with respect to ≥grade 2 rectal bleeding.
Conclusion:
Presuming that in vivo range verification for pencil beam scanning proton therapy could localize the distal falloff of the Bragg peak to within 2 mm, novel beam arrangements (AP and LAO/RAO) may reduce the risk of serious rectal bleeding, compared to VMAT and LAT proton treatment techniques. These are achieved without an increase in modeled bladder complication rates.
Introduction
The main clinical advantage of proton radiation therapy (RT) over x-ray RT arises from the proton Bragg peak (BP). The finite range and sharp dose falloff at the distal end of the BP increase our ability to conform radiation dose to the tumor and minimize damage to neighboring critical organs, thus increasing the therapeutic ratio (the probability of tumor control divided by the probability of normal tissue complications) of proton RT relative to x-ray RT.
However, uncertainties exist in our ability to precisely locate the BP and its distal dose gradient in the patient [1–3]. These uncertainties arise in both (1) treatment delivery owing to patient setup errors or changes in patient anatomy; and (2) treatment planning dose calculation owing to uncertainty in the position of the distal dose falloff at the end of beam range. These uncertainties may lead to geometric misses of the tumor and delivery of excessive dose to adjacent healthy tissues and critical organs at risk (OARs), such as the rectum in the case of prostate cancer treatment. To mitigate the effects of these uncertainties, additional proximal and distal margins are used. The extent of these safety margins is dependent on the beam range (safety margin = ∼3.5% × beam range + 2 mm). For deep-seated tumors (>20 cm), such as those of the prostate, the safety margin can be >1 cm.
Distal range uncertainty has direct effects on proton treatment planning and beam arrangement. It is routine to avoid beam angles that may lead to high dose unintentionally ranging into downstream OARs. For prostate cancer, opposed lateral beams are routinely used to avoid potentially delivering increased dose to the rectum owing to distal range uncertainty. In essence, by choosing opposed lateral beams, a tradeoff is made favoring increased robustness over potentially superior normal tissue sparing. While lateral beams are more robust, they invariably deliver a higher dose to a larger amount of the anterior rectal wall. Furthermore, the use of lateral beams relies on the lateral penumbra of the beam, which is much broader (up to 1 cm or more at 20-cm depth) than the distal falloff of the BP (<5 mm), which greatly reduces sparing of the rectum for proton RT.
If distal range uncertainty could be eliminated or at least significantly minimized, then the robustness of plans using anterior beam arrangements could be improved and could potentially offer dramatic reduction in dose to OARs, especially the rectum. Thus, if the delivered range of the proton beam could be verified in vivo on a daily basis, then distal range uncertainty margins could be greatly reduced or even eliminated. This would make it possible to ensure that the beam stops at the distal edge of the target volume and therefore does not range into adjacent OARs, thus making it possible to safely use more optimal beam angles. This means that if we could verify that the beam stopped at the distal edge of the prostate (without ranging into the rectum), more optimal anterior beams could safely be used for proton RT of the prostate to not only potentially reduce rectal dose but also permit dose escalation to the prostate.
To this end, many researchers have been working to develop in vivo range verification techniques for proton RT. These techniques include the use of special rectal balloons containing an array of diode detectors that could be positioned along the internal edge of the rectal wall to provide an in vivo measurement of any dose delivered to the rectum [4], and in vivo imaging of secondary prompt γ rays emitted from the patient coupled with daily cone-beam computed tomography (CT) imaging to verify the beam range with respect to the daily location of the patients internal anatomy [5–7]. With these range verification system, it is envisioned that a small test dose (∼10-20 cGy) would be given for a treatment field, and an in vivo measurement or image would be acquired to determine if the beam range is adequate and whether or not it ranges into an adjacent critical organ (such as the rectum). If the beam delivery is acceptable, then the full treatment field would be delivered. If not, then the patient setup could be adjusted to account for daily anatomic variations, and if need be, the patient could be evaluated for possible adaptive re-planning. The purpose of this article was to evaluate differences using proton therapy for prostate cancer with anterior beams compared to lateral beams, assuming the use of daily beam range verification.
Materials and Methods
This institutional review board–approved study included clinical and imaging data from 8 patients with prostate cancer previously treated with volumetric-modulated arc therapy (VMAT). Planning of the VMAT treatments for each patient was performed with the Raystation treatment planning system (version 4.5.1, Raysearch Laboratories, Stockholm, Sweden), using an adaptive convolved dose calculation algorithm with heterogeneity corrections. For each patient, the prostate only was contoured on the planning CT data set and defined as the clinical target volume (CTV) and expanded uniformly by 6 mm to form the planning target volume (PTV). Pelvic nodal irradiation was not performed. The dose of 79.2 Gy delivered in 44 fractions was prescribed for each patient, with the requirement that a minimum of 95% of the PTV shall receive the prescribed dose. Additionally, dose-volume constraints to the adjacent OARs were applied as follows. For the bladder, the volume receiving 80 Gy or more (V80) was limited to ≤15%, with V75 ≤ 25%, V70 ≤ 35%, and V65 ≤ 50%. For the rectum, constraints of V75 ≤ 15%, V70 ≤ 25%, V65 ≤ 35%, and V60 ≤ 50% were used. The maximum femoral head dose could not exceed 50 Gy. No constraint was used for the penile bulb.
Proton Treatment Planning
The treatment planning CT data set and all contours for each patient were imported into the Eclipse Proton treatment planning system (version 11, Varian Medical Systems Inc., Palo Alto, California) to perform comparative proton treatment planning studies with the same dose prescription used for the VMAT treatments (Figure 1a). The volumes of the prostate, rectum, and bladder for each patient are listed in Table 1, as well as the PTV volumes used for standard proton treatments and treatments using range verification. Proton treatment plans using several beam arrangements were studied as shown in Figure 1. For each patient, treatment plans for pencil beam scanning proton RT with a standard arrangement of 2 parallel opposed lateral (LAT) beams (Figure 1b) were generated by using single-field uniform dose optimization. For these plans the range of the spread out BP delivered by each field was determined from the water-equivalent path length to the proximal and distal edge of the PTV plus an additional 3.5% of the water-equivalent path length to account for range uncertainty. For the treatment cases studied, this additional uncertainty margin represented an additional 7 to 9 mm added to the range for each beam, depending on the size of the patient. These plans were used to determine the achievable results for the current standard treatment procedures used clinically for pencil beam scanning proton RT of the prostate.
Figure 1.
The 6 treatment scenarios considered, including the standard (a) photon volumetric-modulated arc therapy plan and (b) proton treatment scenarios with lateral opposed beams, single anterior beam (c) without (AP) and (d) with (AP–2 mm) range verification, and 2 anterior oblique fields (e) without (LAO/RAO) and (f) with (LAO/RAO–2 mm) range verification. Yellow dots indicate location of individual point measurements used to determine average skin dose for each beam delivery scenario. Dose contours in percentage of prescription dose: 105%, orange; 100%, red; 95%, dark blue; 80%, pink; 50%, cyan; 25%, green. Abbreviations: AP, single anterior beam; AP–2 mm, single anterior beam with range verification; LAO/RAO, 2 anterior oblique fields; LAO/RAO–2 mm, 2 anterior oblique fields with range verification.
Table 1.
Volume of organs and targets for standard and range-verified treatments (cm3).
|
Patient |
Prostate |
Rectum |
Bladder |
Planned target volume |
|
|
Standard |
Range verified |
||||
| 1 | 17.2 | 31.5 | 147.3 | 58.9 | 53.8 |
| 2 | 40.5 | 135.3 | 168.6 | 181.9 | 169.5 |
| 3 | 156.9 | 129.3 | 252.9 | 276.0 | 251.6 |
| 4 | 91.0 | 89.8 | 223.9 | 226.0 | 197.8 |
| 5 | 40.2 | 118.8 | 425.1 | 96.0 | 88.6 |
| 6 | 63.8 | 95.8 | 71.3 | 145.0 | 131.6 |
| 7 | 44.0 | 42.4 | 98.4 | 94.5 | 85.0 |
| 8 | 27.7 | 48.6 | 197.5 | 78.9 | 68.0 |
| Mean | 60.2 | 87.6 | 198.1 | 144.7 | 168.2 |
| Median | 42.3 | 97.3 | 183.1 | 120.5 | 110.1 |
To determine the effect that daily range verification could have on prostate treatments with proton RT, intensity-modulated proton therapy treatment plans were generated by using multifield optimization for 2 alternative beam arrangements, namely, (1) left and right anterior oblique (LAO/RAO) beams (Figure 1e) with gantry angles of 300° and 60°, respectively, and (2) a single anterior-posterior (AP) directed beam (Figure 1c). Treatment plans were generated for the LAO/RAO and AP plans for 2 scenarios: (1) standard treatment delivery (no range verification; Figure 1c and 1e) and (2) treatment delivery in which daily image guidance and range verification (Figure 1d and 1f) are used to localize the prostate and verify the beam range with an accuracy of 2 mm. For standard treatment delivery, the same PTV margins and distal range uncertainty margins used for the LAT treatment plan were applied. For range-verified treatment delivery, only a 2-mm PTV expansion was used along the distal edge of the CTV plus 2-mm increase in the beam water-equivalent path length to account for range uncertainty, since the beam delivery will be imaged in vivo before full-dose delivery to verify that the beam range is aligned with the distal edge of the prostate. Current proposed methods for range verification (eg, rectal balloon dosimeter arrays and in vivo imaging) only provide information about the distal dose falloff at the end of the proton range and do not provide information about dose levels along the full path of the proton beam. Therefore, we only modified the distal end of the PTV margin and made no modifications to the proximal PTV margin to account for the effects of range verification. For the range-verified treatment scenario, we denote the plans for the AP and LAO/RAO beam arrangements as AP–2 mm and LAO/RAO–2 mm, respectively.
Dose-Volume Analysis
Dose-volume histograms (DVHs) were calculated for the CTV, rectum, and bladder for all treatment scenarios (VMAT, LAT, LAO/RAO, AP, LAO/RAO–2 mm, AP–2 mm) studied. For plan comparison, the V95 and the maximum dose (Dmax) values for CTV taken from the DVH for each treatment scenario were compared. Additionally, DVH parameters corresponding to published QUANTEC (Quantitative Analyses of Normal Tissue Effects in the Clinic) treatment planning guidelines for the rectum [8] and bladder [9] were also extracted for each treatment scenario for comparison purposes. Skin dose values for each treatment scenario were estimated by taking the average of 5 to 6 point dose measurements on the patient surface at the beam entrance point.
Rectal Toxicity Analysis
Rectal toxicity rates for each planning scenario were determined by using the Lyman-Kutcher-Burman (LKB) methodology [10]. The LKB model describes the normal tissue complication probability (NTCP) after uniform radiation of a fractional volume (v) of normal tissue to a dose (D), using the following equation:
 |
where
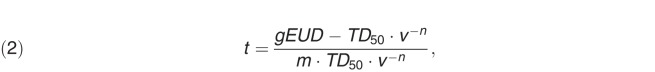 |
and TD50 is the dose at which there is a 50% probability of developing a specified rectal complication after a uniform whole-organ irradiation, m models the slope of the dose response curve for the rectum, and n is the volume effect factor, which models how the tolerance dose changes as the fractional volume of the rectum that is irradiated changes. The term gEUD is the generalized equivalent uniform dose, which accounts for the fact that the rectum does not receive a uniform dose during treatment and is calculated according to the Kutcher/Burman histogram dose reduction method [10]:
 |
where Nvoxels is the number of voxels, Di is the dose to the ith voxel, and n is volume effect factor.
Results
Treatment Plan Comparisons
For each treatment scenario studied, plans providing ≥95% coverage of the PTV with the prescription dose, along with all constraints for each listed critical structure, were achievable. The maximum dose to the femoral heads was below 50 Gy for all treatment plans. The skin dose was lowest for the VMAT plan (20% of prescription dose), with the 2 field plans (LAT, LAO/RAO, LAO/RAO–2 mm) being 37% of the prescription dose, and the single-field plans (AP, AP–2 mm) being the highest with 65% of the prescription dose.
Dose-Volume Histogram Analysis
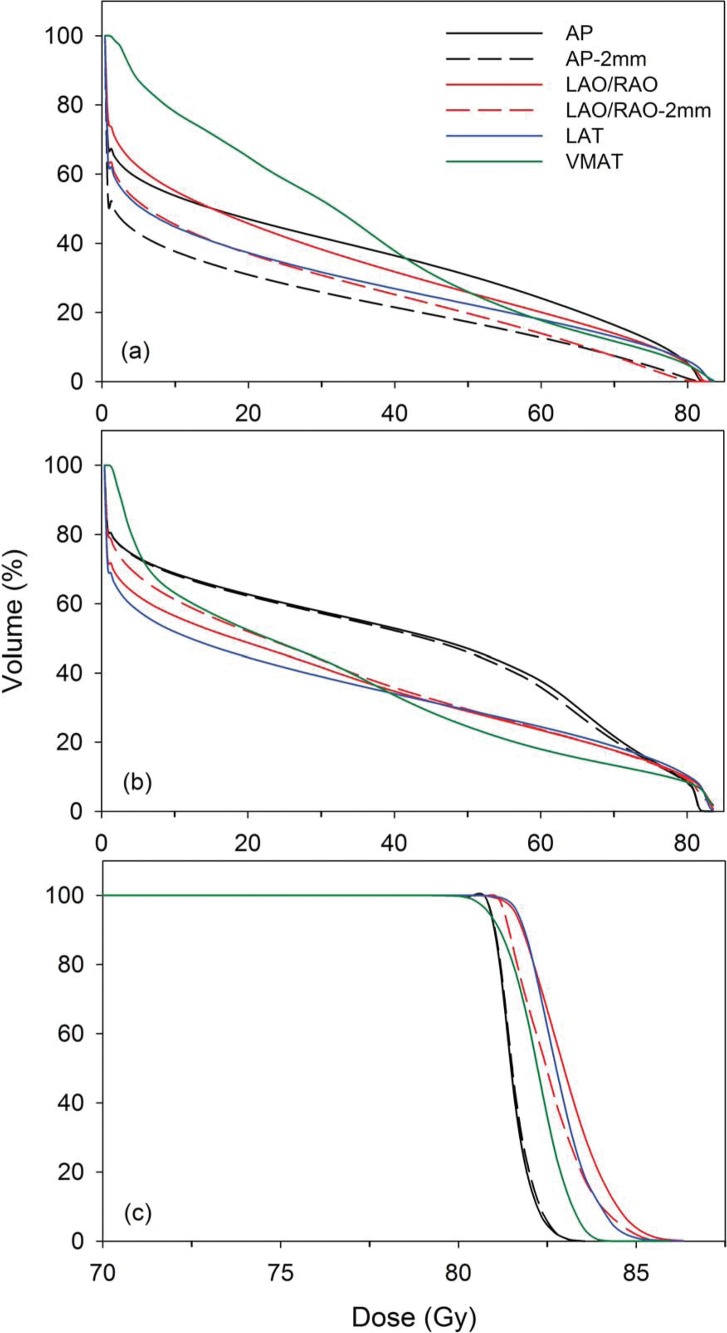
Shown in Figure 2 are the average DVHs for all 8 patients for the CTV (Figure 2c), rectum (Figure 2a) , and bladder (Figure 2b) for each treatment scenario. For all treatment scenarios, 100% of the CTV volume received the full prescription dose. For the AP and AP–2 mm treatment plans, an average Dmax of 83.2 Gy was delivered to the CTV, while the average Dmax was 84 Gy for VMAT, and between 85 Gy and 85.5 Gy for the LAT, LAO/RAO, and LAO/RAO–2 mm plans.
Figure 2.
Average dose-volume histograms for 8 patients for (a) rectum; (b) bladder; and (c) clinical target volume for each treatment scenario. Abbreviations: AP, single anterior beam; AP–2 mm, single anterior beam with range verification; LAO/RAO, 2 anterior oblique fields; LAO/RAO–2 mm, 2 anterior oblique fields with range verification; LAT, lateral opposed beams; VMAT, volume-modulated arc therapy.
Listed in Table 2 are the published QUANTEC [8] V60, V65, V70, and V75 DVH guidelines for the rectum and V65, V70, V75, and V80 guidelines for the bladder [9] along with the corresponding DVH values for each planning scenario studied. Rectum Dmax values were very similar for each planning scenario with values ranging from 80.8 Gy (102% of prescription dose) for the LAO/RAO–2 mm, up to 83.6 Gy (105.7% of prescription dose) for the VMAT and LAT plans. Additionally, bladder Dmax values ranged from 81.8 Gy for the AP and AP–2 mm plans to 84.3 Gy for the VMAT plan. For both the bladder and rectum, all plans achieved DVH values well below the QUANTEC recommendations. From Figure 2 and Table 2, it can be seen that the AP–2 mm and LAO/RAO–2 mm plans both achieved the lowest DVH values at higher doses (>60 Gy), which have been shown to be the most correlated to rectal toxicity rates [8], with the single AP beam having the highest DVH values above 60 Gy. As for the bladder, the lowest DVH values were seen with the VMAT plan, with the highest values coming from the AP and AP–2 mm plan. This was expected since the AP and AP–2 mm treatment scenarios use a single beam that passes directly through the bladder. However, even for the AP and AP–2 mm treatment scenarios, the bladder V65, V70, V75, and V80 values are still 40% to 50% below the QUANTEC guidelines.
Table 2.
Dose-volume histogram parameters for each standard and range verification treatment scenario along with QUANTEC guidelines.
|
QUANTEC |
Standard |
Range verification |
|||||
|
VMAT |
LAT |
AP |
LAO/RAO |
AP–2 mm |
LAO/RAO–2 mm |
||
| Rectum | |||||||
| Dmax (%Rxa) | - | 105.7 (1.1) | 105.6 (0.6) | 103.5 (0.3) | 103.6 (0.5) | 102.7 (1.1) | 102 (0.5) |
| V60 (%) | 35 | 17.5 (4.9) | 17.8 (5.4) | 24.1 (7.7) | 19.8 (5.4) | 12.6 (4) | 12.6 (2.8) |
| V65 (%) | 25 | 14.2 (4.2) | 15.4 (4.8) | 20 (6.4) | 16.8 (5.4) | 10 (3.4) | 9.4 (2) |
| V70 (%) | 20 | 10.9 (4.2) | 11.5 (5.6) | 16 (5.2) | 13.6 (4.2) | 7.2 (3) | 6.2 (1.3) |
| V70 (%) | 15 | 8.5 (3.1) | 10 (3.1) | 11.4 (4) | 10 (3.5) | 4.3 (2.4) | 2.8 (0.8) |
| Bladder | |||||||
| Dmax (%Rx) | - | 1.06 (1.1) | 1.05 (0.6) | 1.03 (0.4) | 1.05 (0.7) | 1.03 (0.3) | 1.05 (1.1) |
| V65 (%) | 50 | 14.5 (8.7) | 19.7 (12) | 30.1 (18.8) | 20.7 (12.4) | 27.5 (16.4) | 20.4 (12.6) |
| V70 (%) | 35 | 12.5 (7.8) | 16.9 (10.7) | 21.83 (13) | 17.2 (10.5) | 19.6 (12.1) | 17.1 (10.7) |
| V75 (%) | 25 | 10.5 (7.1) | 13.5 (9.2) | 14.5 (8.9) | 13.8 (9.1) | 13.9 (9.1) | 13.8 (9.2) |
| V80 (%) | 15 | 7.6 (6.1) | 8.8 (6.9) | 7.7 (6) | 9.1 (6.7) | 7.5 (6.1) | 9 (6.8) |
Abbreviations: QUANTEC, Quantitative Analyses of Normal Tissue Effects in the Clinic; VMAT, volumetric-modulated arc therapy; LAT, lateral opposed beams; AP, anterior-posterior; LAO/RAO, left and right anterior oblique beams.
%Rx is the percentage of the full prescription dose (79.2 Gy).
Vxx is the percentage of the structure volume receiving at least a dose of xx Gy.
Parentheses represent 1-sigma standard deviation of list value.
Rectal Toxicity Analysis
Table 3 lists the average (over all 8 patients) rectal gEUD for each treatment scenario and the resulting NTCP for ≥ grade 2 rectal toxicity, with the NTCP values also plotted in Figure 3.
Table 3.
Comparison of rectal gEUD and rectal NTCP values for >grade 2 rectal bleeding for each treatment scenario.
|
Treatment scenario |
gEUDa (Gy) |
NTCPa |
ΔNTCPc (TS – VMAT) |
PΔNTCPd (TS – VMAT) |
ΔNTCP (TS – LAT) |
PΔNTCPd (TS – LAT) |
| Standard | ||||||
| VMAT | 57.5 (1.3) | 0.19 (0.04)b | - | - | −0.05 | 0.18 |
| LAT | 58.9 (1) | 0.24 (0.04) | 0.05 | 0.18 | - | - |
| AP | 58 (1.2) | 0.21 (0.05) | 0.02 | 0.58 | −0.03 | 0.58 |
| LAO/RAO | 57.7 (1.3) | 0.2 (0.04) | 0.01 | 0.51 | −0.04 | 0.51 |
| Range verification | ||||||
| AP–2 mm | 54.3 (1.6) | 0.11 (0.03) | −0.08 | 0.04 | −0.13 | 0.01 |
| LAO/RAO–2 mm | 54 (0.8) | 0.1 (0.02) | −0.09 | 0.02 | −0.13 | 0.01a |
Abbreviations: gEUD, generalized equivalent uniform dose; NTCP, normal tissue complication probabilities; TS, treatment scenario; VMAT, volume-modulated arc therapy; LAT, lateral opposed beams; AP, anterior-posterior; LAO/RAO, left and right anterior oblique beams.
gEUD and NTCP represent average value for 8 patients.
Values in parentheses represent 1 standard deviation of the values for all 8 patients.
ΔNTCP denotes NTCP difference between listed treatment scenarios.
PΔNTCP denotes level of significance of the ΔNTCP for listed treatment scenarios.
Figure 3.
Calculated NTCP for ≥grade 2 rectal toxicity for each treatment scenario studied. Error bars represent the maximum and minimum NTCP values for each treatment scenario. Abbreviations: AP, single anterior beam; AP–2 mm, single anterior beam with range verification; LAO/RAO, 2 anterior oblique fields; LAO/RAO–2 mm, 2 anterior oblique fields with range verification; LAT, lateral opposed beams; NTCP, normal tissue complication probability; VMAT, volumetric-modulated arc therapy.
From Figure 3, we see that by simply using proton treatment scenarios with alternative beam arrangements with no range verification (AP and LAO/RAO), no statistically significant (P > .1) difference in rectal toxicity (ΔNTCP) is seen over the standard scenarios used for photon (VMAT) and proton (LAT) treatments. However, if range verification is incorporated into these treatment scenarios (AP–2 mm and LAO/RAO–2 mm), the rectal gEUD is reduced by ∼4 Gy, which correlated to a ΔNTCP = 0.1 (P < .05) over the AP and LAO/RAO techniques, as well as the standard VMAT and LAT treatments. This represented a 50% reduction in the rate of ≥ grade 2 rectal toxicities if range verification was available for proton radiation therapy.
Discussion
While the benefits of radiation dose escalation are well established for the treatment of localized prostate cancer, the associated increase in risk of rectal toxicity is equally well recognized [11, 12]. Because of the proximity of the rectum to the prostate and the need to add various setup and uncertainty margins to a proton beam plan, a portion of the anterior rectal wall will receive at least the prescription dose in most patients. Limiting the rectal volume that receives high dose will reduce the probability of significant rectal toxicity such as proctitis and bleeding. In that regard, there are well-described rectal dosimetric parameters that correlate strongly to clinical toxicity [8].
Proton therapy offers the potential for significant normal tissue dose savings, compared to photon therapy, through the steep dropoff in dose distal to the Bragg peak. While proton therapy is effective and safe for prostate cancer [13–16], there is no categorical evidence that proton therapy is actually superior to photon therapy, particularly with respect to reduced toxicity. For instance, studies by Loma Linda University [16] and Zeitman et al [12] have reported grade 2+ gastrointestinal toxicity rates after proton therapy, which are similar to rectal toxicity rates reported from intensity-modulated radiation therapy experiences [17, 18].
The potential of protons to increase the therapeutic ratio for prostate cancer has not been fully realized in large part because the typical proton treatment plan uses opposed lateral beams. However, the benefits of using anterior proton beams for prostate cancer treatment have been demonstrated by several studies. In the only published clinical experience using anterior proton beams, Cuaron et al [19] recently published favorable outcomes for 20 patients with prostate cancer after hip replacement who received proton therapy typically using a lateral beam and a low-weighted anterior oblique beam (median weight of 27.5% [range, 12.5%-50%] of the total dose). While median follow-up was short at 6.4 months, treatment was tolerated very well with only 1 patient developing late grade 2 proctitis within the anterior rectal wall. Additionally, Cao and colleagues [20] published a dosimetric comparison suggesting that optimized 3-angle plans (anterior, lateral, and posterior beams) compared to opposed lateral plans could reduce the rectal V50, V60, and V70 by 25.7%, 23.1%, and 15.9%, respectively, with no significant difference in bladder dose. Tang et al [4] reported that proton plans using a single AP beam or anterior oblique beams (assuming in vivo range verification) substantially reduced the anterior rectal wall volume receiving high dose, as compared to opposed lateral beams, while maintaining acceptable target volume coverage. We highlight that unlike most of the studies mentioned above, our proton plans were generated by using pencil beam scanning—which provides greater proximal dose conformality than passive scattering—which could be clinically significant, especially for the bladder, when using anteriorly oriented beams.
Routine use of anteriorly oriented proton beams to treat prostate cancer will not become reality unless fears about distal range uncertainty are addressed, perhaps through the use of daily pretreatment in vivo range verification in which the distal edge of the Bragg peak can be verified to lie outside of the rectum before each treatment delivery. Efforts are ongoing to develop accurate and reliable methods of in vivo proton range verification (eg, rectal diode arrays, prompt γ imaging), which would allow for improved normal tissue sparing through the elimination of, or at least reduction in, uncertainty margins. Further, such beam arrangements could be used in concert with the use of a rectal spacer, which can consistently displace the anterior rectal wall posteriorly, thereby improving our ability to reduce dose to the anterior rectal wall.
Assuming that a reliable and accurate method for in vivo range verification was available, in this study we demonstrate that significant rectal sparing can be achieved by using anterior beam arrangements compared to standard opposed lateral beams. Such rectal sparing was specifically achieved by the presumed ability to safely limit the distal uncertainty margin to 2 mm, as shown in the AP versus AP–2 mm plans and LAO/RAO versus LAO/RAO–2 mm plans. We illustrate not only that this strategy of combining anterior beams and reduced margins, using in vivo range verification, achieved a significant reduction in rectal dose, but also that this dose reduction could translate into a significant reduction in the incidence of late grade 2+ rectal toxicity of approximately 50%.
One disadvantage of anteriorly oriented proton beams is that they are less robust than opposed lateral beams. Anatomic reproducibility can be challenging for anteriorly oriented beams, especially owing to variations in bladder filling, since anterior beams would at least partly traverse through the bladder. Image-guided proton therapy, such as by using cone-beam CT, will be invaluable to assess bladder and rectal filling, especially if using anterior beams. Furthermore, an anteriorly oriented approach may not be ideal in overweight men if beams would pass through lower abdominal skin folds, which could be a challenge to set up reproducibly on a daily basis.
Conclusions
In conclusion, our data add to the growing literature supporting the combination of anterior beam arrangements and daily in vivo range verification to reduce rectal dose; moreover, they evidence that a clinically significant reduction in rectal toxicity, compared to standard proton and photon treatment techniques, is possible. A departure from the typical opposed lateral beam arrangement is critical to maximizing the potential of proton therapy by finally taking full advantage of the Bragg peak. Our data suggest that further evaluation of this strategy is warranted and could eventually provide a strong and clear rationale for the use of proton therapy over intensity-modulated radiation therapy to treat prostate cancer.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: Minesh Mehta has served as a consultant for Bristol-Meyers-Squibb, Celldex, Roche, Elekta, Novartis, Cavion, Novocure; was on the Board of Directors of Pharmacyclics (with stock options); and has research funding from Novocure, and Cellectar.
References
- 1.Bednarz B, Daartz J, Paganetti H. Dosimetric accuracy of planning and delivering small proton therapy fields. Phys Med Biol. 2010;55:7425–38. doi: 10.1088/0031-9155/55/24/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paganetti H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys Med Biol. 2012;57:R99–117. doi: 10.1088/0031-9155/57/11/R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaffner B, Pedroni E. The precision of proton range calculations in proton radiotherapy treatment planning: experimental verification of the relation between CT-HU and proton stopping power. Phys Med Biol. 1998;43:1579–92. doi: 10.1088/0031-9155/43/6/016. [DOI] [PubMed] [Google Scholar]
- 4.Tang S, Both S, Bentefour H, Paly JJ, Tochner Z, Efstathiou J, Lu HM. Improvement of prostate treatment by anterior proton fields. Int J Radiat Oncol Biol Phys. 2012;83:408–18. doi: 10.1016/j.ijrobp.2011.06.1974. [DOI] [PubMed] [Google Scholar]
- 5.Moteabbed M, Espana S, Paganetti H. Monte Carlo patient study on the comparison of prompt gamma and PET imaging for range verification in proton therapy. Phys Med Biol. 2011;56:1063–82. doi: 10.1088/0031-9155/56/4/012. [DOI] [PubMed] [Google Scholar]
- 6.Perali I, Celani A, Bombelli L, Fiorini C, Camera F, Clementel E, Henrotin S, Janssens G, Prieels D, Roellinghoff F, Smeets J, Stichelbaut F, Vander Stappen F. Prompt gamma imaging of proton pencil beams at clinical dose rate. Phys Med Biol. 2014;59:5849–71. doi: 10.1088/0031-9155/59/19/5849. [DOI] [PubMed] [Google Scholar]
- 7.Polf J, Avery S, Mackin D, Beddar S. Imaging of prompt gamma rays emitted during delivery of clinical proton beams with a Compton camera: feasibility studies for range verification. Phys Med Biol. 2015. In press. [DOI] [PubMed]
- 8.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–22. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutcher GJ, Burman C, Brewster L, Goitein M, Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–46. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 11.Kuban DA, Levy LB, Cheung MR, Lee AK, Choi S, Frank S, Pollack A. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer: who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–7. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, Bush DA, Lunt M, Spiegel DY, Skowronski R, Jabola BR, Rossi CJ. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol. 2010;28:1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colaco RJ, Hoppe BS, Flampouri S, McKibben BT, Henderson RH, Bryant C, Nichols RC, Mendenhall WM, Li Z, Su Z, Morris CG, Mendenhall NP. Rectal toxicity after proton therapy for prostate cancer: an analysis of outcomes of prospective studies conducted at the university of Florida proton therapy institute. Int J Radiat Oncol Biol Phys. 2015;91:172–81. doi: 10.1016/j.ijrobp.2014.08.353. [DOI] [PubMed] [Google Scholar]
- 14.Mendenhall NP, Hoppe BS, Nichols RC, Mendenhall WM, Morris CG, Li Z, Su Z, Williams CR, Costa J, Henderson RH. Five-year outcomes from 3 prospective trials of image-guided proton therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:596–602. doi: 10.1016/j.ijrobp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Nihei K, Ogino T, Onozawa M, Murayama S, Fuji H, Murakami M, Hishikawa Y. Multi-institutional phase II study of proton beam therapy for organ-confined prostate cancer focusing on the incidence of late rectal toxicities. Int J Radiat Oncol Biol Phys. 2011;81:390–6. doi: 10.1016/j.ijrobp.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Slater JD, Rossi CJ, Jr, Yonemoto LT, Bush DA, Jabola BR, Levy RP, Grove RI, Preston W, Slater JM. Proton therapy for prostate cancer: the initial Loma Linda University experience. Int J Radiat Oncol Biol Phys. 2004;59:348–52. doi: 10.1016/j.ijrobp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.De Meerleer GO, Fonteyne VH, Vakaet L, Villeirs GM, Denoyette L, Verbaeys A, Lummen N, De Neve WJ. Intensity-modulated radiation therapy for prostate cancer: late morbidity and results on biochemical control. Radiother Oncol. 2007;82:160–6. doi: 10.1016/j.radonc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Vora SA, Wong WW, Schild SE, Ezzell GA, Halyard MY. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–8. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Cuaron JJ, Harris AA, Chon B, Tsai H, Larson G, Hartsell WF, Hug E, Cahlon O. Anterior-oriented proton beams for prostate cancer: a multi-institutional experience. Acta Oncol. 2015. 1-7. [DOI] [PubMed]
- 20.Cao W, Lim GJ, Li Y, Zhu XR, Zhang X. Improved beam angle arrangement in intensity modulated proton therapy treatment planning for localized prostate cancer. Cancers (Basel) 2015;7:574–84. doi: 10.3390/cancers7020574. [DOI] [PMC free article] [PubMed] [Google Scholar]