Abstract
Purpose:
Postoperative radiation therapy can improve control for adenoid cystic carcinoma (ACC) of the head and neck; however, delivering adequate dose to the tumor bed must be balanced with limiting dose to nearby critical organs. Intensity-modulated proton therapy (IMPT) may help improve the therapeutic ratio, though concerns exist regarding tissue heterogeneity and other sources of uncertainty in several head and neck subsites. We report control and toxicity outcomes for patients with ACC of the head and neck treated at a single institution with postoperative IMPT and robust planning and analysis.
Patients and Methods:
Sixteen patients with head and neck ACC treated with postoperative IMPT were identified. Intensity-modulated proton therapy was delivered by using multifield optimization. Robust planning and analysis were performed. The median dose was 60 (range, 60 to 70) Gy (RBE) (Gy [relative biological effectiveness]). Adjuvant IMPT was given with (N = 12) or without (N = 4) platinum-based chemotherapy. Tumor control outcomes were recorded from the medical record, and acute and chronic toxicities were graded weekly during treatment and upon follow-up per Common Terminology Criteria for Adverse Events, version 4.0 (CTCAE v4).
Results:
Median follow-up is 24.9 (range, 9.2 to 40.2) months. One patient developed local and distant recurrence and subsequently died. The remaining 15 patients are alive without evidence of disease. Four patients experienced acute grade 3 toxicities: dermatitis (N = 3) and oral mucositis (N = 1). One patient developed a chronic grade 4 optic nerve disorder. There were no grade 5 toxicities.
Conclusions:
Intensity-modulated proton therapy is a feasible option for patients with ACC of the head and neck in the postoperative setting. Robust treatment planning and plan analysis can be performed such that uncertainties and tissue heterogeneities do not appear to limit safe and effective IMPT delivery. Safety and efficacy appear comparable to those of other types of radiation therapy, but further follow-up of clinical outcomes is needed.
Keywords: proton radiotherapy, intensity-modulated proton therapy, adenoid cystic carcinoma, head and neck neoplasms, robust planning
Introduction
Postoperative radiation therapy (RT) has been shown to improve control rates for adenoid cystic carcinoma (ACC) of the head and neck, compared to surgery alone, with the greatest advantage conferred to patients with positive margins or more advanced stage disease [1, 2]. Proton radiation therapy (PRT) has been used for cancers in challenging head and neck locations for more than 2 decades; it is thought to be a desirable option due to the sharp dose fall-off in the proton Bragg peak, which allows for delivery of a higher radiation dose with greater normal tissue sparing distal to the tumor [3, 4]. Proton radiation therapy has more recently been implemented in head and neck ACC as adjuvant treatment with promising outcomes [5, 6].
The available technology for treatment planning, delivery, and evaluation varies by institution but in general can be divided into passive scatter and active scanning proton delivery techniques. Active scanning refers to a proton beam that moves by 2 magnets across the height, width, and depth of the target volume [7]. Optimization techniques for active scanning treatment plans include single-field optimization and multiple-field optimization (MFO) [8]. Single-field optimization involves optimizing the spot intensities for each beam individually, while MFO optimizes the intensities of all beams simultaneously to balance the doses to targets and organs at risk (OARs), based on stated objectives [9]. Active scanning with MFO is often referred to as intensity-modulated proton therapy (IMPT) because the intensity of the proton beam can be varied from spot to spot to deliver the desired dose in the target volume after adding the contributions of all treatment fields. IMPT is different from intensity-modulated radiation therapy (IMRT) in that the energy as well as the intensity can be varied.
Although IMPT is favored in certain treatment situations, it is highly sensitive to setup and range uncertainties [10]. There are clinical concerns regarding how this may affect target coverage and dose to nearby critical structures, particularly those near the distal edge of the beam [11]. First, there are uncertainties in the proton range related to the conversion of computed tomography (CT) numbers to stopping power ratios [12]. Although CT number and stopping power ratio are both related to electron density, there is not a perfect correlation between CT number and stopping power ratio in human tissue, which makes stopping power estimation susceptible to variations in tissue composition. Head and neck tumors are often situated close to areas of tissue heterogeneity such as the paranasal sinuses. Uncertainties related to stopping power rations compound with additional uncertainties relevant to treating tumors of the head and neck, such as patient weight loss and tumor shrinkage, which can contribute to interfractional daily setup variation. A “robust” treatment plan is one in which the target volume is adequately treated while the normal tissues are adequately spared even while a variety of conditions, such as patient setup and stopping power ratio, are altered. Some have raised concerns regarding the robustness of IMPT plans, particularly in the head and neck [10]. Despite recent advances in optimization techniques [13, 14], further clinical correlation with patient outcomes is needed for validation.
Therefore, we sought to evaluate our experience using MFO-IMPT with planning and analysis procedures to maximize robustness in the treatment of ACCs of the head and neck after surgical resection. We describe our treatment techniques as well as patient outcomes with regard to control and toxicity.
Methods
Patient Selection
Patient cases with histologically proven ACC who underwent postoperative MFO-IMPT at a single institution between 2011 and 2014 were reviewed. Patients all underwent surgical resection with curative intent. Patients with a prior history of head and neck radiation were excluded as were patients who received prior therapy for their ACC and were treated for disease recurrence. Approval was obtained from the institutional review board.
Simulation and Target Delineation
Computed tomography scan simulation was performed for all patients. Patients were immobilized in the supine position by using a custom-fitted head and neck mask and bite block. The IMPT plans were generated by using Eclipse proton therapy planning system (version 8.9, Varian Medical Systems, Palo Alto, California). The median prescription dose was 60 (range, 60 to 70) Gy (RBE) (Gy [relative biological effectiveness]), assuming a uniform RBE value of 1.1 for protons. Clinical target volume (CTV) 1 included the tumor bed plus a margin to encompass areas at high risk for recurrence, while CTV2 included the entire operative bed and other areas at intermediate risk, and CTV3 included an additional margin to include the anatomically relevant cranial nerve tracks. Planned target volume (PTV) 1, PTV2, and PTV3 were then created by expanding the corresponding CTVs by 3 mm. For patients who underwent margin negative resections (R0), PTV1, PTV2, and PTV3 were prescribed 60, 57, and 53 Gy (RBE), respectively, in 30 fractions using simultaneous integrated boost. For patients who had microscopically positive margins (R1), the areas of positivity were prescribed 66 Gy (RBE), and any areas of gross residual disease (R2) were prescribed 70 Gy (RBE).
Robust Treatment Planning
At our institution, we define the process of robust treatment planning to be the thoughtful selection of beam angles such that robustness can be maximized. Basic principles include avoiding directing beams at critical OARs and avoiding passing beams directly through areas of tissue heterogeneity whenever possible. Three beam angles were chosen for each plan on the basis of principles of robust treatment planning. Goal target coverage was >95% of the PTV covered by the prescription dose, while keeping doses to OARs within institutional constraints.
Robust Plan Analysis
Plans were then analyzed for robustness to determine the effect of the tested perturbations on target coverage and dose to OARs. Robust analysis is different from robust optimization in that it is performed after the treatment plan is optimized by using the Eclipse treatment planning software, but before the treatment plan is approved by the treating physician. Robust optimization is an algorithm in which the “worst-case” dose distribution is used to compute an objective function for a given iteration. Iterations include different combinations of isocenter shifting (±2 mm in the anterior-posterior, superior-inferior, and lateral directions) and scaling the stopping power ratios by ±3.5% [12]. Robust optimization cannot currently be performed by using Eclipse, and we do not yet routinely implement this process for patients with head and neck cancer. Instead, we use the process of robust analysis to assist the treating physician in recognizing the potential effects of setup and stopping power uncertainties on target coverage as well as dose to OARs.
During the robust analysis process, dose-volume histograms (DVHs) are created for the treatment plan as optimized in Eclipse as well as after applying several perturbations to the plan, including isocenter shifts ±3 mm in the anterior-posterior, superior-inferior, and lateral positions as well as stopping power ratio scaling of ±3.5%. Figures 1 and 2 show 2 examples of composite DVHs generated from the robust analysis process. To evaluate the robustness of the plan, the treating physician then evaluates the DVHs for target volumes as well as OARs under all combinations of perturbations tested including isocenter shifts and stopping power ratios. The treatment plan is ultimately deemed acceptable if 95% of the target volume is covered by 95% of the prescription dose and OARs do not exceed their tolerances even in the “worst-case” scenario. As always, sometimes clinical judgment must be used by the treating physician if compromises must be made regarding dose to OARs to improve target coverage or if compromises to target coverage must be made to spare OARs.
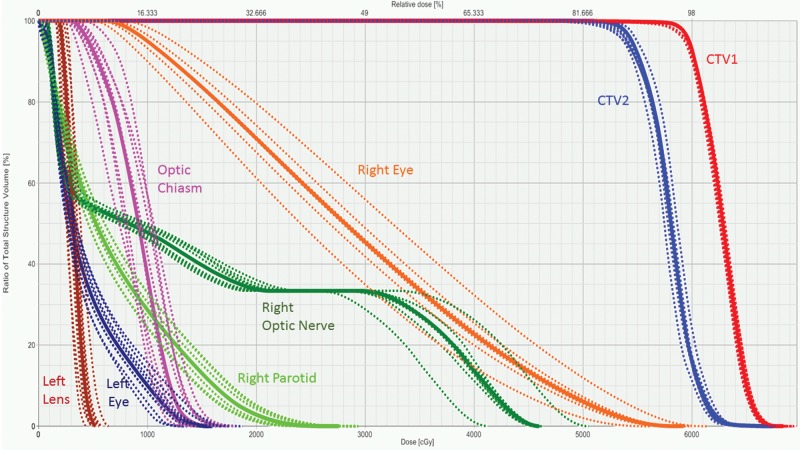
Figure 1.
Dose-volume histogram generated to display the results of robust analysis performed on the multifield optimization intensity-modulated proton therapy treatment plan generated for a patient with adenoid cystic carcinoma of the right maxillary sinus (patient 1). The solid lines indicate the primary treatment plan and the dotted lines demonstrate the different perturbations tested during robust analysis (±3-mm isocenter shift and ±3.5% stopping power ratio). There was adequate target coverage of CTV1, which included the tumor bed plus a margin to encompass areas at high risk for recurrence; and CTV2, which included the entire operative bed and other areas at intermediate risk. The right eye and optic nerves were the closest organs at risk, and the dose to these structures in all perturbations studied was deemed acceptable for treatment. Abbreviation: CTV, clinical target volume.
Chemotherapy
Concurrent chemotherapy was given at the discretion of the treating medical oncologist. When given, it consisted of cisplatin or carboplatin administered weekly during radiation therapy.
Follow-up
Patients underwent weekly examinations during treatment. Follow-up evaluations occurred approximately 1 month after treatment and then every 3 to 6 months thereafter. Patients were examined for recurrent disease status and acute or late (occurring or persisting >90 days following initiation of RT) toxicities. The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4) was used during follow-up visits for weekly grading of acute and late toxicities.
Results
Patient and Treatment Characteristics
Sixteen patients with head and neck ACC received postoperative PRT at our institution between 2011 and 2014. Primary tumor sites included the lacrimal gland or sac (N = 5), paranasal sinuses (N = 4), parotid gland (N = 4), submandibular gland (N = 2), and buccal mucosa (N = 1). No patients had positive lymph nodes or distant metastatic disease at presentation. Six patients had solid-type ACC, and 10 had cribriform and/or tubular ACC. Individual patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics and treatment information.
|
Patient No. |
Sex |
Tumor location |
Surgical resection |
PNI |
BOS involvement |
Histologic subtype |
Dose, Gy (RBE) |
Local failure |
Distant failure |
G3+ toxicity |
| 1 | F | Paranasal sinus | R0 | Unknown | No | Tubular | 60 | No | No | None |
| 2 | M | Paranasal sinus | R1 | Yes | Yes | Solid | 66 | No | No | None |
| 3 | M | Paranasal sinus | R2 | Yes | Yes | Solid | 70 | No | No | G4 late optic nerve disorder |
| 4 | F | Paranasal sinus | R1 | Yes | Yes | Solid | 60 | No | No | None |
| 5 | M | Parotid gland | R0 | Yes | No | Cribriform | 60 | No | No | None |
| 6 | M | Parotid gland | R1 | No | No | Solid | 60 | No | No | None |
| 7 | F | Parotid gland | R1 | Yes | No | Cribriform and tubular | 64 | No | No | G3 acute radiation dermatitis |
| 8 | F | Parotid gland | R0 | No | No | Cribriform | 60 | No | No | None |
| 9 | M | Submandibular gland | R1 | Yes | No | Cribriform | 66 | No | No | G3 acute oral mucositis |
| 10 | F | Submandibular gland | R1 | Yes | No | Cribriform and tubular | 60 | No | No | None |
| 11 | F | Buccal mucosa | R1 | No | No | Cribriform and tubular | 60 | No | No | None |
| 12 | F | Lacrimal gland | R0 | No | No | Cribriform | 60 | No | No | G3 acute radiation dermatitis |
| 13 | M | Lacrimal gland | R0 | Yes | No | Solid | 60 | No | No | None |
| 14 | M | Lacrimal gland | R0 | Yes | No | Cribriform and tubular | 60 | No | No | G3 acute radiation dermatitis |
| 15 | F | Lacrimal gland | R0 | Yes | No | Cribriform | 60 | No | No | None |
| 16 | F | Lacrimal sac | R0 | Yes | No | Solid | 60 | Yes | Yes | None |
Abbreviations: PNI, perineural invasion; BOS, base of skull; RBE, relative biological effectiveness; G, grade; R0, margin negative resection; R1, microscopic positive margin resection; R2, incomplete resection with gross residual disease.
Eight patients had an R0 resection; 7, R1; and 1, R2. All patients were treated with MFO-IMPT and received a median dose of 60 (range, 60 to 70) Gy (RBE). Ten patients received concurrent cisplatin, 2 received carboplatin, and 4 did not receive chemotherapy. Figures 3 and 4 display the pretreatment imaging and representative sections from the IMPT plan for patients 1 and 13 in Table 1, respectively. The robust analysis results for patients 1 and 13 are also presented in Figures 1 and 2, respectively.
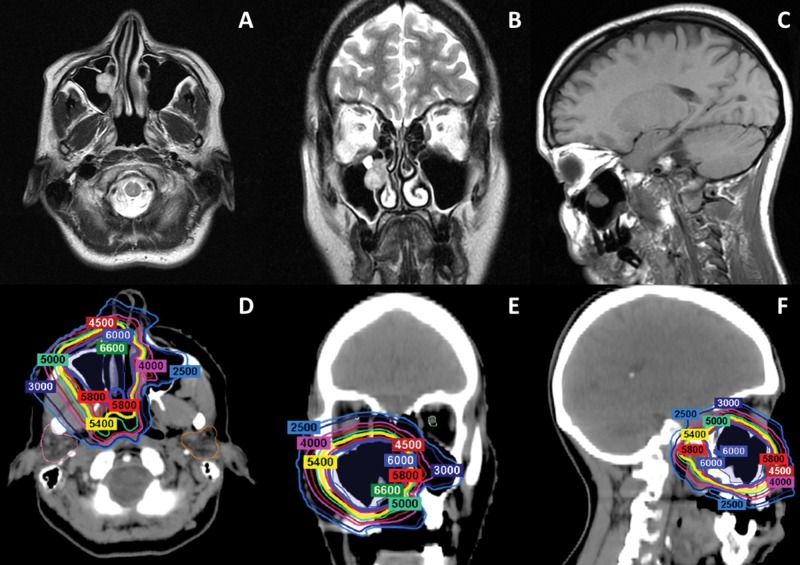
Figure 3.
(A–C) Axial, coronal, and sagittal images from the preoperative MRI performed on a patient with a 2.5-cm adenoid cystic carcinoma with predominantly tubular pattern in the right maxillary sinus (patient 1). The patient underwent endoscopic sinus surgery, and negative margins were achieved. She was treated with multifield optimization intensity- modulated proton therapy to a total dose of 60 Gy (RBE) in 30 fractions. (D–F) Axial, coronal, and sagittal images from the treatment plan. Abbreviations: MRI, magnetic resonance imaging; RBE, relative biological effectiveness.
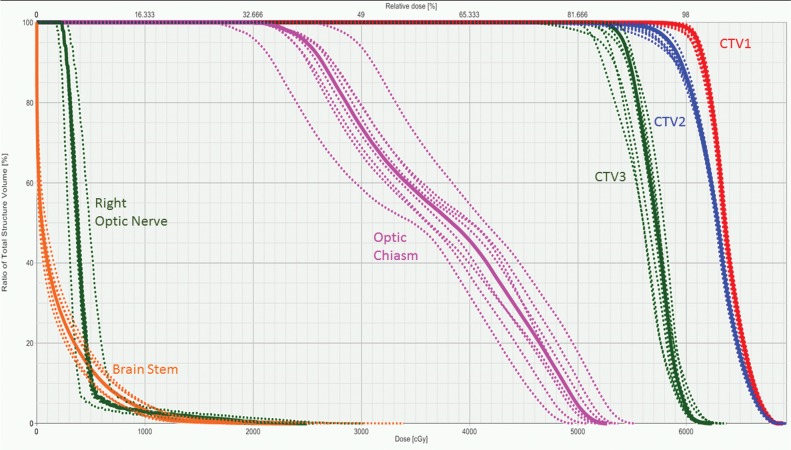
Figure 2.
Dose-volume histogram generated to display the results of robust analysis performed on the multifield optimization intensity-modulated proton therapy treatment plan generated for a patient with adenoid cystic carcinoma of the left lacrimal gland (patient 13). The solid lines indicate the primary treatment plan and the dotted lines demonstrate the different perturbations tested during robust analysis (±3-mm isocenter shift and ±3.5% stopping power ratio). There was adequate target coverage of CTV1, which included the tumor bed plus a margin to encompass areas at high risk for recurrence; CTV2, which included the entire operative bed and other areas at intermediate risk; and CTV3, which included an additional margin to include the nerve tracks to the skull base, as this patient had perinerual invasion found on surgical pathology. The optic chiasm was the closest critical organ at risk, and the potential dose to the optic chiasm in all perturbations was acceptable for treatment. Extra care was taken during planning to minimize dose to the right eye and optic nerve, as the patient underwent a left orbital exenteration. Abbreviation: CTV, clinical target volume.
Control and Toxicity Outcomes
Median follow-up is 24.9 (range, 9.2 to 40.2) months. Fifteen of 16 patients (94%) are currently without evidence of disease. One patient with lacrimal sac ACC had disease recurrence 9 months after completion of PRT with concurrent cisplatin. She developed extensive in-field local recurrence within the tumor bed, ipsilateral retropharyngeal and supraclavicular nodal involvement, and spread to the facial bones, and she died 18 months following treatment.
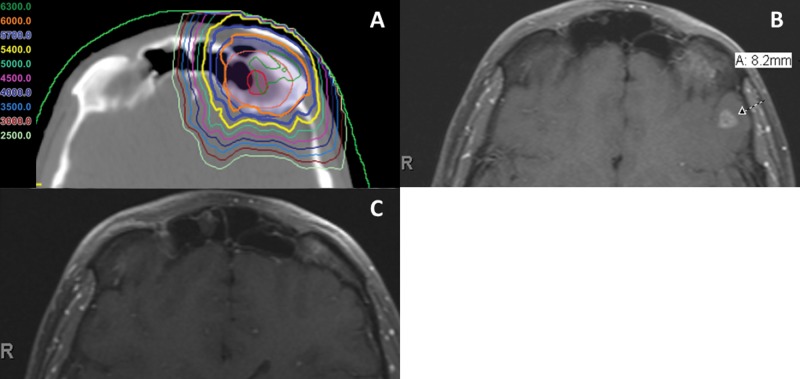
Four patients experienced acute grade 3 toxicities: dermatitis (N = 3) and oral mucositis (N = 1). One patient developed a chronic grade 4 optic nerve disorder (blindness or vision ≤ 20/200 in the affected eye), but the patient was counseled and consented for this expected side effect of delivering a curative dose for an incompletely resected paranasal sinus ACC. The optic nerve received a maximum point dose of 73.7 Gy (RBE) and a mean dose of 68.0 Gy (RBE). Patient 13 (Figure 4) treated for ACC of the lacrimal gland developed asymptomatic frontal lobe necrosis 18 months after completion of IMPT. The area of necrosis was just posterior to the operative bed and received 54 to 60 Gy (RBE). This patient remained asymptomatic, and on follow-up magnetic resonance imaging at 24 months, the area of frontal lobe necrosis had nearly completely resolved (Figure 5).
Figure 4.
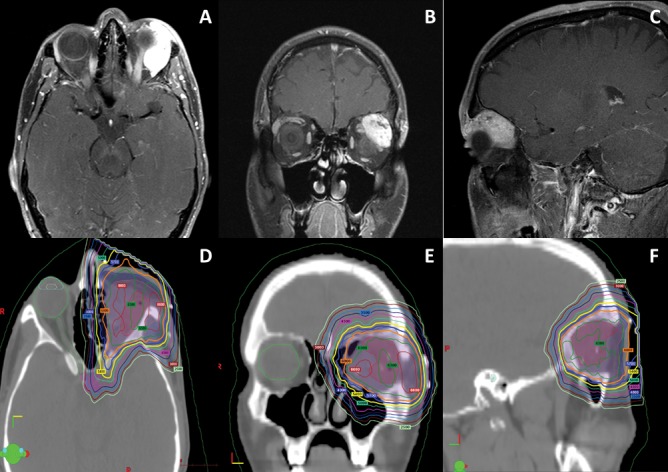
(A–C) Axial, coronal, and sagittal images from the preoperative MRI performed on a patient with a 3.0-cm adenoid cystic carcinoma of the left lacrimal gland (patient 13). The patient underwent a left orbital exenteration. Operative pathology revealed solid histology, perineural invasion, and negative margins. The patient was treated with multifield optimization intensity-modulated proton therapy to a total dose of 60 Gy (RBE) in 30 fractions. (C–F) Axial, coronal, and sagittal images from the treatment plan. Abbreviation: RBE, relative biological effectiveness.
Figure 5.
Axial slices from the radiation treatment plan (A) and follow-up MRI scans at 18 (B) and 24 (C) months from a patient with a 3.0-cm solid-type adenoid cystic carcinoma of the left lacrimal gland with perineural invasion (patient 13). The patient was treated with multifield optimization IMPT to a total dose of 60 Gy (RBE) in 30 fractions. The area that developed an 8.2-mm area of frontal lobe necrosis (arrow) 18 months after completion of IMPT (B) occurred in an area that received between 54 to 60 Gy (RBE). The patient's frontal lobe necrosis resolved without the need for intervention on repeated imaging at 24 months (C). Abbreviations: IMPT, intensity-modulated proton therapy; MRI, magnetic resonance imaging; RBE, relative biological effectiveness.
Discussion
Our results demonstrate that patients with ACC of the head and neck treated with postoperative MFO-IMPT at a large, tertiary referral center have excellent local control outcomes at 2 years as well as high freedom from significant toxicity. Despite the challenging location of these tumors, often in areas of tissue heterogeneity and in close proximity to critical structures, robust treatment planning and plan analysis methods can result in high levels of confidence, such that setup and range uncertainties do not affect our ability to effectively deliver a curative dose to the target volume while keeping doses to OARs within tolerance.
Adenoid cystic carcinoma of the head and neck is a challenging clinical entity best treated with multimodality treatment [15]. Surgical resection followed by postoperative photon-based RT results in 5- and 15-year local control rates of 95% and 79%, respectively [16], although the degree of improvement resulting from postoperative RT appears to vary by factors such as margin status [1, 17] and stage [2]. The ability to deliver a postoperative dose sufficient for eradication of microscopic or gross residual disease, however, is often limited by concerns of exceeding normal tissue tolerance in nearby critical structures [18]. Even with highly conformal techniques such as IMRT, there is excess dose deposited in the path of the beam, which can lead to clinically meaningful toxicity in patients being treated for head and neck cancers [19]. Furthermore, a systematic review and meta-analysis of paranasal and nasal cavity malignancies observed that patients treated with charged particle therapy had significantly improved local regional control, disease-free survival, and overall survival as compared to patients treated with photon therapy [20]. Specifically, a local-regional control and disease-free survival benefit was seen with proton therapy. Although the meta-analysis was a review of noncomparative observational studies, the data suggest that charged particle therapy may lead to improved outcomes for patients with tumors in critical locations. Proton therapy has been investigated in several head and neck subsites and found to be a promising modality for the unique anatomic challenges of this complex region [21].
The use of proton therapy in the postoperative treatment of head and neck ACC has been previously described by other groups with excellent clinical results. Linton et al [6] achieved a 2-year local control rate of 95% for patients with newly diagnosed head and neck ACC after surgical resection or biopsy only, and Pommier et al [5] reported a 5-year local control rate of 93% after postoperative PRT for skull base ACC. Our control rates are comparable to those of these studies, but several differences in patient population and radiation technique must be considered. In the cohort of Linton et al [6], 7 patients were treated for recurrent disease and 24 patients had either positive margins or gross residual disease. The median dose delivered was 72 Gy (RBE). Chronic toxicity rates were similar to those of our cohort, but Linton et al [6] did describe one grade 5 toxicity (cerebrospinal fluid leak–induced meningitis) in their cohort. The cohort of Pommier et al [5] was even less favorable than that of Linton et al [6]; all patients had base of skull invasion, and 20 or 23 had gross tumor present at the time of radiation. The median dose delivered was even higher at 75.9 Gy (RBE), and radiation was delivered by using a combination of photon and proton radiation with varying fractionation schemes. The proton component was delivered with passive scatter technique. Pommier et al [5] reported higher rates of toxicity including 1 patient requiring hospitalization for gastrostomy tube placement due to mucositis, 7 patients with grade 3 neurologic toxicities such as seizures and short-term memory problems, and 1 patient with grade 5 temporal lobe necrosis during a 5-year period after the completion of radiation.
Patients in these previously published studies potentially experienced more severe toxicities than our cohort due to higher doses of proton therapy, accelerated hyperfractionated fractionation, and/or the combination of proton therapy and photon RT. Studies using photon or neutron therapy in the head and neck have also reported more severe complications including tracheal stenosis, temporal lobe necrosis, vision loss, and fistula formation [22–24]. One additional potential contributing factor to the increased toxicity reported in previously published studies compared to our cohort is the difference in proton therapy technique (passive scatter versus active scanning IMPT).
Many physicians are faced with the decision of whether or not to intentionally accept target underdosage to avoid higher critical organ dose and the corresponding complication risk when treating ACC, particularly when the skull base must be treated. The increased conformity achievable with IMPT in the head and neck, compared with passive scatter proton therapy, may allow for delivery of the full intended dose and has been shown to significantly reduce dose to nearby OARs in previous dosimetric analyses [25, 26]. This may be one way IMPT could lead to improved local control and cure rates, although longer follow-up will be needed to answer this question.
Clinical use of MFO-IMPT for tumors of the head and neck was first described by members of our group in a heterogeneous cohort of 15 patients with squamous cell carcinoma (N = 10) or ACC (N = 5) of the head and neck. Early clinical results were similarly encouraging to patients in our cohort [27]. Excitement over the advantages offered by IMPT is tempered by concerns regarding potential challenges of IMPT such as sensitivity to organ motion, setup variability, tissue heterogeneity, and range uncertainty [11]. Members of our group have published extensively on improving IMPT plan robustness by moving from PTV-based optimization to the robust optimization described above [9, 12, 14, 28]. It is important to note that the 16 patients in this current cohort underwent planning with Eclipse without the use of such robust optimization techniques. Although we feel comfortable that our robust planning and analysis allow us to compare IMPT plans and choose the most robust one, correlation with further clinical outcomes is needed to better determine what is “robust enough.”
Our rates of chronic grade 3 and 4 toxicity were low, and we saw no unanticipated toxicities from IMPT in our cohort. One patient did develop asymptomatic frontal lobe necrosis in an area just adjacent to the 60–Gy (RBE) isodose line (Figure 5) 18 months after completing radiation (patient 13 in Table 1). There are not extensive data available for frontal lobe necrosis specifically, but temporal lobe necrosis has long been well documented when treating nasopharyngeal cancer or other tumors at the base of skull [29–31]. Dosimetric studies in the setting of IMRT have suggested a dose constraint of D 0.5 cm3 (maximum dose to 0.5 cm3) = 69 Gy for the temporal lobe [32], although other studies suggest that V45 (volume receiving 45 Gy or greater) should be kept to <15.1 cm3 as well [31]. For the patient in our series who developed frontal lobe necrosis, the maximum dose to the brain was only 62.3 Gy (RBE), and there was not a high volume of moderate dose to brain tissue, so both of these constraints were comfortably met. However, in the setting of IMPT, some have concern that range uncertainties regarding the higher RBE at the end of the Bragg peak, as well as uncertainties regarding the neurological RBE, may play a role [33]. There is also a question of whether the safest possible field arrangement was used, as typically we avoid directing beams through areas of tissue heterogeneity such as the frontal sinuses and bone-brain interface. Few dosimetric studies have been published examining temporal lobe necrosis after proton therapy [34, 35]. In a cohort of skull base chordoma, chondrosarcoma, and ACC, McDonald et al [35] reported that the risk of temporal lobe necrosis increased sharply when the V60 > 5.5 cm3 or V70 > 1.7 cm3. At our own institution, 2 of 10 patients treated with IMPT for nasopharyngeal carcinoma developed temporal lobe necrosis, largely in the areas receiving 66 to 70 Gy (RBE) [36]. The case of frontal lobe necrosis in our current series did not have a V60 > 5.5 cm3, and the maximum point dose to the frontal lobe was 62.9 Gy (RBE).
Conclusion
We present the first outcomes of postoperative MFO-IMPT in the treatment of head and neck ACC. Local control appears excellent, as 94% of patients are without evidence of disease at a median of 2 years follow-up. However, longer follow-up is required, as recurrences for ACC are common in the 2 to 5 year posttreatment time interval. The treatment was well tolerated among patients, with no unexpected grade 3 or greater chronic toxicities. Intensity-modulated proton therapy demonstrated comparable efficacy and safety when compared to other radiation modalities including other proton therapy delivery techniques. Processes of robust treatment planning and analysis currently serve to identify and account for setup, tissue heterogeneity, and range uncertainties. Robust optimization methods may serve to improve the quality of MFO-IMPT plans for head and neck cancers in the future. Further clinical study will be necessary to validate the long-term effects of these procedures to produce robust IMPT plans in the head and neck.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no conflicts of interest to disclose.
Acknowledgments: The authors would like to acknowledge Marka Anderson for her assistance with data collection and verification. This manuscript was presented as an abstract at the 54th Annual Conference of the Particle Therapy Cooperative Group in San Diego, California.
References
- 1.Silverman DA, Carlson TP, Khuntia D, Bergstrom RT, Saxton J, Esclamado RM. Role for postoperative radiation therapy in adenoid cystic carcinoma of the head and neck. Laryngoscope. 2004;114:1194–9. doi: 10.1097/00005537-200407000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623–8. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]
- 3.Slater JM, Slater JD, Archambeau JO. Proton therapy for cranial base tumors. J Craniofac Surg. 1995;6:24–6. doi: 10.1097/00001665-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Niemierko A, Urie M, Goitein M. Optimization of 3D radiation therapy with both physical and biological end points and constraints. Int J Radiat Oncol Biol Phys. 1992;23:99–108. doi: 10.1016/0360-3016(92)90548-v. [DOI] [PubMed] [Google Scholar]
- 5.Pommier P, Liebsch NJ, Deschler DG, Lin DT, McIntyre JF, Barker FG, II, Adams JA, Lopes VV, Varvares M, Loeffler JS, Chan AW. Proton beam radiation therapy for skull base adenoid cystic carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132:1242–9. doi: 10.1001/archotol.132.11.1242. [DOI] [PubMed] [Google Scholar]
- 6.Linton OR, Moore MG, Brigance JS, Summerlin DJ, McDonald MW. Proton therapy for head and neck adenoid cystic carcinoma: initial clinical outcomes. Head Neck. 2015;37:117–24. doi: 10.1002/hed.23573. [DOI] [PubMed] [Google Scholar]
- 7.Smith A, Gillin M, Bues M, Zhu XR, Suzuki K, Mohan R, Woo S, Lee A, Komaki R, Cox J, Hiramoto K, Akiyama H, Ishida T, Sasaki T, Matsuda K. The M. D. Anderson proton therapy system. Med Phys. 2009;36:4068–83. doi: 10.1118/1.3187229. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XR, Poenisch F, Li H, Zhang X, Sahoo N, Wu RY, Li X, Lee AK, Chang EL, Choi S, Pugh T, Frank SJ, Gillin MT, Mahajan A, Grosshans DR. A single-field integrated boost treatment planning technique for spot scanning proton therapy. Radiat Oncol. 2014;9:202. doi: 10.1186/1748-717X-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–91. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomax AJ. Intensity modulated proton therapy and its sensitivity to treatment uncertainties 1: the potential effects of calculational uncertainties. Phys Med Biol. 2008;53:1027–42. doi: 10.1088/0031-9155/53/4/014. [DOI] [PubMed] [Google Scholar]
- 11.Kraan AC, van de Water S, Teguh DN, Al-Mamgani A, Madden T, Kooy HM, Heijmen BJ, Hoogeman MS. Dose uncertainties in IMPT for oropharyngeal cancer in the presence of anatomical, range, and setup errors. Int J Radiat Oncol Biol Phys. 2013;87:888–96. doi: 10.1016/j.ijrobp.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Frank SJ, Li X, Li Y, Park PC, Dong L, Ronald Zhu X, Mohan R. Effectiveness of robust optimization in intensity-modulated proton therapy planning for head and neck cancers. Med Phys. 2013;40:051711. doi: 10.1118/1.4801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2008;53:1689–700. doi: 10.1088/0031-9155/53/6/013. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Frank SJ, Li X, Li Y, Zhu RX, Mohan R. PTV-based IMPT optimization incorporating planning risk volumes vs robust optimization. Med Phys. 2013;40:021709. doi: 10.1118/1.4774363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen C, Xu T, Huang C, Hu C, He S. Treatment outcomes and prognostic features in adenoid cystic carcinoma originated from the head and neck. Oral Oncol. 2012;48:445–9. doi: 10.1016/j.oraloncology.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–26. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 17.Lin YC, Chen KC, Lin CH, Kuo KT, Ko JY, Hong RL. Clinicopathological features of salivary and non-salivary adenoid cystic carcinomas. Int J Oral Maxillofac Surg. 2012;41:354–60. doi: 10.1016/j.ijom.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mamgani A, van Rooij P, Sewnaik A, Tans L, Hardillo JA. Adenoid cystic carcinoma of parotid gland treated with surgery and radiotherapy: long-term outcomes, QoL assessment and review of the literature. Oral Oncol. 2012;48:278–83. doi: 10.1016/j.oraloncology.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, Morrison WH, Ang KK, Garden AS. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–55. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, Alahdab F, Altayar O, Nabhan M, Schild SE, Foote RL. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1027–38. doi: 10.1016/S1470-2045(14)70268-2. [DOI] [PubMed] [Google Scholar]
- 21.Holliday EB, Frank SJ. Proton radiation therapy for head and neck cancer: a review of the clinical experience to date. Int J Radiat Oncol Biol Phys. 2014;89:292–302. doi: 10.1016/j.ijrobp.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 22.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 23.Laramore GE, Krall JM, Griffin TW, Duncan W, Richter MP, Saroja KR, Maor MH, Davis LW. Neutron versus photon irradiation for unresectable salivary gland tumors: final report of an RTOG-MRC randomized clinical trial: Radiation Therapy Oncology Group: Medical Research Council. Int J Radiat Oncol Biol Phys. 1993;27:235–40. doi: 10.1016/0360-3016(93)90233-l. [DOI] [PubMed] [Google Scholar]
- 24.Douglas JG, Koh WJ, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg. 2003;129:944–8. doi: 10.1001/archotol.129.9.944. [DOI] [PubMed] [Google Scholar]
- 25.Cozzi L, Fogliata A, Lomax A, Bolsi A. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61:287–97. doi: 10.1016/s0167-8140(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 26.Mock U, Georg D, Bogner J, Auberger T, Potter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147–54. doi: 10.1016/s0360-3016(03)01452-4. [DOI] [PubMed] [Google Scholar]
- 27.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, Munsell MF, Palmer MB, Sahoo N, Zhang X, Liu W, Zhu XR. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89:846–53. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Mohan R, Park P, Liu Z, Li H, Li X, Li Y, Wu R, Sahoo N, Dong L, Zhu XR, Grosshans DR. Dosimetric benefits of robust treatment planning for intensity modulated proton therapy for base-of-skull cancers. Pract Radiat Oncol. 2014;4:384–91. doi: 10.1016/j.prro.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takiar V, Ma D, Garden AS, Li J, Rosenthal DI, Beadle BM, Frank SJ, Fuller CD, Gunn GB, Morrison WH, Hutcheson K, El-Naggar AK, Gold KA, Kupferman ME, Phan J. Disease control and toxicity outcomes for T4 carcinoma of the nasopharynx treated with intensity-modulated radiotherapy. Head Neck. 2015] doi: 10.1002/hed.24128. [published online ahead of print May 20. [DOI] [PubMed]
- 30.Zheng Y, Han F, Xiao W, Xiang Y, Lu L, Deng X, Cui N, Zhao C. Analysis of late toxicity in nasopharyngeal carcinoma patients treated with intensity modulated radiation therapy. Radiat Oncol. 2015;10:17. doi: 10.1186/s13014-014-0326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Ou X, Xu T, Wang X, Shen C, Ding J, Hu C. Effect of dosimetric factors on occurrence and volume of temporal lobe necrosis following intensity modulated radiation therapy for nasopharyngeal carcinoma: a case-control study. Int J Radiat Oncol Biol Phys. 2014;90:261–9. doi: 10.1016/j.ijrobp.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Zhou GQ, Qi ZY, Zhang L, Huang SM, Liu LZ, Li L, Lin AH, Ma J. Radiation-induced temporal lobe injury after intensity modulated radiotherapy in nasopharyngeal carcinoma patients: a dose-volume-outcome analysis. BMC Cancer. 2013;13:397. doi: 10.1186/1471-2407-13-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirkovic D, Peeler C, Grosshans D, Titt U, Taleei R, Mohan R. SU-E-T-549: modeling relative biological effectiveness of protons for radiation induced brain necrosis. Med Phys. 2015;42:3461. [Google Scholar]
- 34.Pehlivan B, Ares C, Lomax AJ, Stadelmann O, Goitein G, Timmermann B, Schneider RA, Hug EB. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys. 2012;83:1432–40. doi: 10.1016/j.ijrobp.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 35.McDonald MW, Linton OR, Calley CS. Dose-volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int J Radiat Oncol Biol Phys. 2015;91:261–7. doi: 10.1016/j.ijrobp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Holliday EB, Garden AS, Rosenthal DI, Fuller CD, Morrison WH, Gunn GB, Phan J, Beadle BM, Zhu XR, Zhang X, Hanna E, Glisson BS, Hutcheson KA, El-Naggar AK, Hong J-H, Hung T-M, Uzel EK, Lewis G, Frank SJ. Proton therapy reduces treatment-related toxicities for patients with nasopharyngeal cancer: a case-match control study of intensity-modulated proton therapy and intensity-modulated photon therapy. Int J Particle Ther. 2015;2:19–28. [Google Scholar]