Abstract
Oprozomib is an oral proteasome inhibitor with activity in multiple myeloma (MM). Our phase 1b/2 study examined the safety and efficacy of oprozomib with dexamethasone in patients with relapsed and refractory MM. Oprozomib was administered with a 5/14 or 2/7 schedule with dexamethasone. Phase 1b primary objectives were to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of oprozomib; phase 2 primary objectives were to determine overall response rate (ORR) and safety/tolerability of the RP2D. Between July 2, 2013, and August 29, 2016, data were available on 65 enrolled patients (5/14 schedule, n = 19; 2/7 schedule, n = 46). In phase 1b, MTD was 180mg (5/14 schedule) and not reached (2/7 schedule); RP2D was 300mg (2/7 schedule). In phases 1b and 2, ORR across dosing cohorts (210–330mg) for the 2/7 schedule was 58.7% overall and 46.4% for bortezomib-refractory patients (n = 28). All patients reported ≥1 treatment-emergent adverse event (AE); the most common AEs were gastrointestinal. Grade ≥3 AEs occurred in 78.9% and 82.6% of patients on the 5/14 and 2/7 schedules, respectively. The oprozomib and dexamethasone combination has encouraging activity and could be an important MM therapy if gastrointestinal tolerability is improved.
Keywords: Oprozomib, Oral proteasome inhibitor, Dexamethasone, Multiple myeloma, Maximum tolerated dose
1. Introduction
Proteasome inhibitors are an important component of treatment strategies for multiple myeloma (MM) due to their effectiveness and manageable safety profiles [1,2]. Preclinical and clinical studies support the therapeutic advantage of combining a proteasome inhibitor with dexamethasone [3–9]. Combination regimens of bortezomib or carfilzomib are approved treatment options for patients with relapsed or refractory MM (RRMM) [10]. Among patients with MM who had received prior treatments, carfilzomib has demonstrated greater clinical benefit compared with bortezomib [11]. However, both carfilzomib-and bortezomib-containing regimens have less activity in patients previously exposed to bortezomib compared with those naive to bortezomib, suggesting that proteasome inhibitor retreatment with these regimens may be limited by varying levels of cross resistance [5,12]. Furthermore, peripheral neuropathy is a concern for some patients receiving bortezomib [3,13], while the intravenous route of administration of carfilzomib may be undesirable to some patients. Given these limitations, there is a continued need for active proteasome inhibitors with improved ease of administration.
Oral administration of proteasome inhibitors can improve the accessibility of MM treatment. Currently, ixazomib is the only orally administered proteasome inhibitor approved in the United States for the treatment of MM [14]. Oprozomib, a structural analog of carfilzomib, is an orally administered epoxyketone proteasome inhibitor that irreversibly binds to its target [15]. Oprozomib led to an antitumor response similar to intravenously administered carfilzomib in mouse syngeneic and human tumor xenograft models [15]. Importantly, oprozomib has also shown single-agent antitumor activity in patients with advanced MM in a phase 1b/2 study, with overall response rates (ORRs) of up to 34%, depending on the dosing schedule [16]. The most common adverse events (AEs) with single-agent oprozomib were gastrointestinal (GI) in nature [16]; however, the addition of a steroid (e.g., dexamethasone) to a proteasome inhibitor has been shown to reduce GI toxicity [17].
Given the demonstrated single-agent activity of oprozomib and the potential for enhanced efficacy and reduced GI toxicity with the addition of a corticosteroid, the current study evaluated the safety and efficacy of oprozomib in combination with low-dose dexamethasone in patients with RRMM. This study consisted of a phase 1b portion to determine the maximum tolerated dose (MTD) and recommended phase 2 dosing of oprozomib given in combination with dexamethasone, and a phase 2 portion to determine the efficacy and safety of the recommended phase 2 dose.
2. Materials and methods
2.1. Study design and participants
In this open-label, phase 1b/2, multicenter study (NCT01832727), patients with RRMM were recruited from 18 centers in the United States and France. Patients aged 18 years or older with Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 who had received 1–5 prior lines of therapy were eligible. Prior therapy must have consisted of ≥ 1 regimen that included lenalidomide and/or bortezomib, and relapsed patients must have achieved at least minimal response on ≥ 1 line of therapy. Refractory patients were allowed, but patients were not required to be refractory to their last therapy. Primary refractory patients were allowed in the phase 1b portion of the study only.
For the phase 1b portion, the primary objectives were to determine the MTD and recommended phase 2 dosing of oprozomib in combination with dexamethasone, and to evaluate the safety and tolerability of the regimen. The MTD was defined as the highest dose at which < 33% of patients experience dose-limiting toxicities (DLTs) after one treatment cycle. Secondary endpoints for phase 1b included pharmacokinetics (PK) of oprozomib. The primary objectives for the phase 2 portion was to determine ORR (partial response [PR] or better, defined by Uniform Response Criteria) [18], as well as to evaluate safety and tolerability of the regimen. Secondary endpoints for phase 2 included clinical benefit rate (CBR; minimal response or better), duration of response, progression-free survival (PFS), time to progression, and time to response.
2.2. Procedures
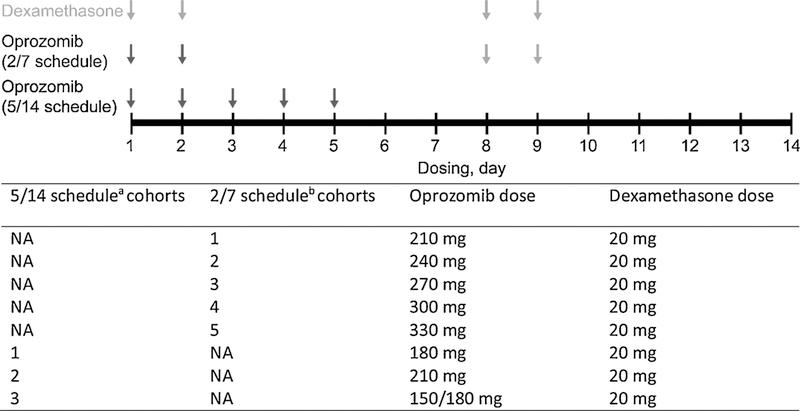
Oprozomib was administered once daily with one of two extended release (ER) tablet formulations, identified as oprozomib tablet or oprozomib ER tablet, on days 1–5 (5/14 schedule) or on days 1, 2, 8, and 9 (2/7 schedule) of a 14-day cycle, with both continuous and step-up (initial lower dose followed by a step-up to the target dose) dosing of oprozomib for both schedules (Fig. 1). For both schedules, the oprozomib dose was escalated using a standard 3 + 3 dose-escalation scheme. The phase 1b portion was initiated using the oprozomib tablet at 210 mg for both schedules. All subsequent cohorts were escalated or de-escalated by 30 mg oprozomib until the MTD was reached. Step-up dosing for the 5/14 schedule was introduced with the switch from oprozomib tablets to oprozomib ER tablets, with the first cohort of subjects receiving the first cycle at 150 mg and subsequent cycles at 180 mg (150/180 mg cohort). For the 2/7 schedule, the oprozomib ER tablet was used in the cohorts that had not been given the original oprozomib tablet, unless the MTD for continuous dosing for oprozomib tablets had been previously determined prior to the introduction of the oprozomib ER tablets. The dose was then escalated in 30 mg increments to determine the MTD.
Fig. 1.
Oprozomib with low-dose dexamethasone dosing schedule (14-day cycle). NA, not available. a Cohort 2 in the 5/14 schedule represents patients who were given the starting oprozomib dose of 210 mg for the 5/14 schedule. The dose was de-escalated by 30 mg for cohort 1, and cohort 3 represents patients who were given the step-up oprozomib dosing of 150/180 mg. b Cohort 1 in the 2/7 schedule represents patients who were given the starting oprozomib dose of 210 mg for the 2/7 schedule; each subsequent cohort on the 2/7 schedule was given higher oprozomib doses in 30 mg increments.
Dexamethasone (20 mg) was administered orally on days 1, 2, 8, and 9 of a 14-day cycle. Patients were treated until disease progression, unacceptable toxicity, or treatment discontinuation. AEs were graded and classified according to the Common Terminology Criteria for Adverse Events, version 4.03 [19] and were coded using the Medical Dictionary for Regulatory Activities. Patients were evaluated for response following the International Myeloma Working Group-International Uniform Response Criteria and European Group for Blood and Marrow Transplant criteria [18,20,21]. For PK analyses, blood samples were collected at predose and at time points up to 24 h after cycle 1, day 1 dosing. PK analyses included terminal half-life (t1/2), time to maximum observed concentration (Tmax), maximum observed concentration (Cmax), and area under the plasma concentration-time curve to the last measurable concentration (AUClast).
The study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent, and the study was approved by the independent ethics committee or relevant institutional review board at each participating site.
2.3. Concomitant medications for GI conditions
Patients were premedicated when necessary with 5-HT3 inhibitors (e.g., ondansetron, granisetron) prior to administration of oprozomib, with additional doses as needed to prevent and treat nausea. Lansoprazole or some other orally administered proton-pump inhibitor was required for the treatment duration to prevent peptic disease or other acid-related GI toxicities, unless the patient was hypersensitive or intolerant to proton-pump inhibitors. Medications allowed for GI conditions included antiemetics, antidiarrheals, and/or laxatives.
2.4. Statistical analysis
The safety population included all subjects who received at least one dose of study treatment and was the primary population for all safety and efficacy analyses. The ORR and its two-sided 95% exact binomial confidence interval (CI) for each of the recommended dose groups were determined. The Kaplan-Meier method was used to analyze time-to-event endpoints, including PFS, duration of response, and time to progression. Safety and tolerability assessments included extent of exposure to study treatment, AEs, and DLTs.
2.5. Data sharing
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
2.6. Role of the funding source
Amgen Inc. was the study sponsor and had a role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
3. Results
3.1. Patients and enrollment
Between July 2, 2013, and August 29, 2016 (data cutoff for this study), 65 patients were enrolled and had data available for analysis (5/14 schedule, n = 19; 2/7 schedule, n = 46) (Table 1). In the phase 1b portion, 19 patients received oprozomib on the 5/14 schedule: nine at 180 mg/day, seven at 210 mg/day, and three at 150/180 mg/day. For the 5/14 schedule, step-up dosing was introduced with oprozomib ER tablets, in which the first cohort received the first cycle at 150 mg and subsequent cycles at 180 mg (150/180 mg). In the phase 1b portion, 28 patients received oprozomib on the 2/7 schedule: four at 210 mg/day, four at 240 mg/day, six at 270 mg/day, eight at 300 mg/day, and six at 330mg/day. An additional 18 patients received oprozomib 300 mg on the 2/7 schedule in phase 2. For the 2/7 schedule, oprozomib ER tablets were escalated in 30 mg increments to determine the MTD.
Table 1.
Patient disposition.
| 5/14 schedule 150–210 mg/day OPZ |
2/7 schedule 210–330 mg/day OPZ |
|
|---|---|---|
| Number of patients enrolled | 19 | 46 |
| Continuing treatment | 0 | 8 (17.4) |
| Discontinued treatment | 19 (100) | 38 (82.6) |
| Adverse event | 11 (57.9) | 12 (26.1) |
| Disease progression | 5 (26.3) | 19 (41.3) |
| Lost to follow-up | 0 | 1 (2.2) |
| PI decision | 1 (5.3) | 2 (4.3) |
| Patient request | 2 (10.5) | 4 (8.7) |
OPZ, oprozomib; PI, principal investigator.
Patient demographic characteristics are shown in Table 2. Median patient age was 64.0 years for patients on the 5/14 schedule and 65.0 years for patients on the 2/7 schedule. The median oprozomib treatment duration was 5.7 (range: 0.7–43.0) weeks for patients on the 5/14 schedule and 29.8 (range: 1.1–137.3) weeks for patients on the 2/7 schedule. Patients had received a median of two prior regimens (range: 1–5) in each of the schedules. The mean time from initial diagnosis to first treatment dose was 4.6 years on the 5/14 schedule and 5.4 years on the 2/7 schedule. On the 5/14 schedule, 100% of patients had received prior bortezomib, and 52.6% were refractory to bortezomib. On the 2/7 schedule, 89.1% of patients had received prior bortezomib, and 60.9% were refractory to bortezomib. Sixty percent of the patients (39 of 65 patients) received bortezomib in their last line of the regimen. Prior to taking oprozomib, 69.2% of these patients progressed while on bortezomib treatment or after it. No patients had received prior carfilzomib on either schedule.
Table 2.
Patient demographics and disease characteristics (phase 1 and 2).
| Characteristic | 5/14 schedule 150–210 mg/day OPZ (n = 19) |
2/7 schedule 210–330 mg/day OPZ (n = 46) |
|---|---|---|
| Age, years | ||
| Median (range) | 64.0 (50–92) | 65.0 (45–87) |
| Age group, n (%) | ||
| < 65 years | 11 (57.9) | 22 (47.8) |
| 65 to < 75 years | 5 (26.3) | 15 (32.6) |
| ≥ 75 years | 3 (15.8) | 9 (19.6) |
| Sex, n (%) | ||
| Male | 11 (57.9) | 27 (58.7) |
| Race, n (%) | ||
| White | 17 (89.5) | 30 (65.2) |
| Black | 1 (5.3) | 7 (15.2) |
| Asian | 1 (5.3) | 0 |
| Other | 0 | 2 (4.3) |
| Not reported | 0 | 7 (15.2) |
| ECOG performance status, n (%) | ||
| 0 | 11 (57.9) | 28 (60.9) |
| 1 | 7 (36.8) | 18 (39.1) |
| 2 | 1 (5.3) | 0 |
| ISS stage at initial diagnosis, n (%) | ||
| 1 | 4 (21.1) | 11 (23.9) |
| 2 | 8 (42.1) | 18 (39.1) |
| 3 | 6 (31.6) | 9 (19.6) |
| Unknown | 1 (5.3) | 8 (17.4) |
| Time from initial diagnosis to first dose, years | ||
| Mean | 4.6 | 5.4 |
| Prior therapies | ||
| Number of prior therapies | ||
| Median | 2 (1–5) | 2 (1–5) |
| Exposure to prior therapies, n (%) | ||
| Bortezomib | 19 (100) | 41 (89.1) |
| Carfilzomib | 0 | 0 |
| Lenalidomide | 19 (100) | 42 (91.3) |
| Prior transplant, n (%) | 12 (63.2) | 27 (58.7) |
| No. of patients grouped by total no. of prior therapies, n (%) | ||
| 1 | 2 (10.5) | 9 (19.6) |
| 2 | 12 (63.2) | 15 (32.6) |
| ≥ 3 | 5 (26.3) | 22 (47.8) |
| Patients with refractory MM, n (%) | ||
| Refractory to bortezomib | 10 (52.6) | 28 (60.9) |
| Refractory to bortezomib or immunomodulatory agents in any prior regimena | 14 (73.6) | 36 (78.3) |
| Refractory to last regimen, n (%) | 14 (73.6) | 31 (67.4) |
| Refractory to bortezomib in last prior regimen | 7 (36.8) | 18 (39.1) |
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System; OPZ, oprozomib.
Includes lenalidomide, thalidomide, pomalidomide, and immunomodulatory agents.
3.2. PK
Oprozomib was absorbed rapidly, with a median Tmax of 1–2 h following oprozomib tablet and oprozomib ER tablet administration, and was eliminated rapidly, with a mean t1/2 of 0.59–1.6 h after oprozomib tablet administration and 0.49–0.86 h after oprozomib ER tablet administration. After the initial administration of oprozomib tablets, there was a dose-related increase in exposure (as assessed by AUClast and Cmax) of oprozomib over the dose range of 180–300 mg. In contrast, observed exposure (AUClast and Cmax) of oprozomib did not increase over the dose range of 150–330 mg for the oprozomib ER tablets. However, high intersubject variability was observed by percent of coefficient of variation (CV%) of the geometric mean up to 197% for oprozomib tablets and up to 71% for oprozomib ER tablets across the cohorts for AUClast was observed during cycle 1. These results should be interpreted with caution considering the observed intersubject variability and small number of subjects evaluated (as low as three patients per dose level).
3.3. Dose escalation
During the phase 1b portion examining the 5/14 schedule, seven patients were enrolled at the starting oprozomib dose of 210 mg. Three patients (43%) reported a total of five DLTs (alanine aminotransferase increased in one patient; aspartate aminotransferase increased in one patient; and hypertension, subarachnoid hemorrhage, and thrombocytopenia in one patient, without thrombotic thrombocytopenic purpura/thrombotic microangiopathy) in this cohort, prompting a decrease to a starting dose of 180 mg. Of the nine patients who received 180 mg of oprozomib on the 5/14 schedule, none experienced DLTs. The MTD was 180 mg for the 5/14 schedule without the use of step-up dosing. To reduce GI toxicity, the formulation was switched from oprozomib tablets to oprozomib ER tablets, and the protocol was amended to include step-up dosing (i.e., 150 mg for cycle 1 with escalation to 180 mg at cycle 2 and beyond, if tolerated). One patient of three reported a DLT in the 150/180 mg group (mental status changes). No further dose escalations were attempted in the 5/14 schedule as the DLTs in this schedule were deemed unacceptably toxic.
On the 2/7 schedule, which did not include step-up dosing, enrollment progressed until one patient reported a DLT of thrombocytopenia in the 270 mg cohort (n = 6). No further DLT was noted in the expansion of the 270 mg cohort. The 300 mg cohort (n = 8) was successfully completed without DLTs. In the 330 mg cohort (n = 6), two patients reported a total of three DLTs (nausea and vomiting, upper respiratory tract infection, and vomiting). Based on safety and efficacy, the 2/7 schedule was selected as the recommended phase 2 schedule for oprozomib with dexamethasone. The MTD was not reached on the 2/7 schedule because no more than 33% of patients in a dose group reported a DLT. A recommended phase 2 dosing of 300 mg oprozomib for the 2/7 schedule was selected following a review of safety for both schedules across all cohorts.
3.4. Safety
All patients on the 5/14 schedule (n = 19; included 180 mg, 210 mg, and de-escalated 150/180 mg cohorts) and 2/7 schedule (n = 46; included cohorts escalated in 30 mg increments from 210 mg to 330 mg) reported at least one treatment-emergent AE. The most common AEs experienced by patients enrolled in the study were GI related, including nausea (5/14 schedule, 94.7%; 2/7 schedule, 80.4%), diarrhea (5/14 schedule, 84.2%; 2/7 schedule, 84.8%), and vomiting (5/14 schedule, 73.7%; 2/7 schedule, 52.2%) (Table 3). Any-grade cardiac disorders occurred in 21.1% of patients in the 5/14 schedule and 13.0% of patients in the 2/7 schedule; for any-grade dyspnea also, these percentages were 21.1% and 13.0%, respectively. Grade ≥ 2 peripheral neuropathy occurred in 0% of patients in the 5/14 schedule and 2.2% of patients in the 2/7 schedule. After 18 patients had enrolled in the phase 2 portion of the study, further enrollment was halted due to GI toxicity and limitations of the ER formulations observed with the current formulation of oprozomib, as well as high intrapatient PK variability observed in an ongoing phase 1 oprozomib study in patients with advanced malignancies (ClinicalTrials.gov, NCT02244112). Grade ≥ 3 AEs were reported by 15 patients (78.9%) on the 5/14 schedule and 38 patients (82.6%) on the 2/7 schedule. Serious AEs occurred in eight patients (42.1%) on the 5/14 schedule and 15 patients (32.6%) on the 2/7 schedule. On the 5/14 and 2/7 schedules, 11 patients (57.9%) and 12 patients (26.1%), respectively, had an AE that led to treatment discontinuation. Diarrhea was the most common AE that led to treatment discontinuation on both the 5/14 (10.5%) and 2/7 (8.7%) schedules. One patient (5.3%) on the 5/14 schedule and one patient (2.2%) on the 2/7 schedule had a fatal AE of sepsis.
Table 3.
Most frequent treatment-emergent adverse events occurring in ≥ 25% of patients (by schedule) or grade ≥ 3 adverse events occurring in ≥ 2 patients (by schedule) (phase 1 and 2).
| Treatment-emergent adverse event |
5/14 schedule 150–210 mg/day OPZ (n = 19) |
2/7 schedule 210–330 mg/day OPZ (n = 46) |
||
|---|---|---|---|---|
| Any grade n (%) |
Grade ≥ 3 n (%) |
Any grade n (%) |
Grade ≥ 3 n (%) |
|
| Nausea | 18 (94.7) | 6 (31.6) | 37 (80.4) | 4 (8.7) |
| Diarrhea | 16 (84.2) | 6 (31.6) | 39 (84.8) | 11 (23.9) |
| Vomiting | 14 (73.7) | 2 (10.5) | 24 (52.2) | 5 (10.9) |
| Fatigue | 12 (63.2) | 3 (15.8) | 28 (60.9) | 0 |
| Decreased appetite | 8 (42.1) | 1 (5.3) | 12 (26.1) | 0 |
| Platelet count decreased or thrombocytopenia | 8 (42.1) | 4 (21.1) | 14 (30.4) | 6 (13.0) |
| Anemia | 6 (31.6) | 3 (15.8) | 18 (39.1) | 8 (17.4) |
| Hypertension | 6 (31.6) | 2 (10.5) | 8 (17.4) | 5 (10.9) |
| Hypokalemia | 6 (31.6) | 0 | 5 (10.9) | 0 |
| Constipation | 5 (26.3) | 0 | 22 (47.8) | 4 (8.7) |
| Headache | 2 (10.5) | 0 | 13 (28.3) | 0 |
| Dysgeusia | 3 (15.8) | 0 | 12 (26.1) | 0 |
| Pneumonia | 4 (21.1) | 4 (21.1) | 4 (8.7) | 4 (8.7) |
| White blood cell count decreased | 2 (10.5) | 2 (10.5) | 3 (6.5) | 0 |
| Hypophosphatemia | 2 (10.5) | 2 (10.5) | 4 (8.7) | 3 (6.5) |
| Asthenia | 2 (10.5) | 1 (5.3) | 10 (21.7) | 3 (6.5) |
| Urinary tract infection | 2 (10.5) | 0 | 3 (6.5) | 2 (4.3) |
| Alanine aminotransferase increased | 2 (10.5) | 1 (5.3) | 5 (10.9) | 2 (4.3) |
| Aspartate aminotransferase increased | 2 (10.5) | 1 (5.3) | 4 (8.7) | 2 (4.3) |
| Neutrophil count decreased | 1 (5.3) | 1 (5.3) | 3 (6.5) | 2 (4.3) |
OPZ, oprozomib.
3.5. Efficacy
For the 5/14 schedule in phase 1b, the ORR was 15.8%. At a median follow up of 6.0 (95% CI: 1.1-not estimable [NE]) months, the median PFS and median time to progression were both 7.4 (1.8–10.2) months. For the 2/7 schedule in phase 1b + 2, the ORR was 58.7% for the total group across dosing cohorts, and 65.4% for patients who received 300 mg of oprozomib (Table 4). The median PFS for the 2/7 schedule in phase 1b + 2 was 9.1 months for the total group and 10.8 months for patients who received 300 mg of oprozomib. Median time to progression values were the same as median PFS values. The median duration of response was 10.9 months and 10.1 months for these groups, respectively.
Table 4.
Treatment response for patients in 2/7 schedule.
| Phase 1b |
Phase 2 300 mg |
Phase 1b + phase 2 300 mg |
Total 210–330 mg |
Total bortezomib- refractory patients 210–330 mg |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 210 mg |
Cohort 2 240 mg |
Cohort 3 270 mg |
Cohort 4 300 mg |
Cohort 5 330 mg |
|||||
| n | 4 | 4 | 6 | 8 | 6 | 18 | 26 | 46 | 28 |
| BOR, n (%) | |||||||||
| sCR | 0 | 0 | 1 (16.7) | 0 | 1 (16.7) | 0 | 0 | 2 (4.3) | 0 |
| CR | 0 | 0 | 0 | 0 | 0 | 1 (5.6) | 1 (3.8) | 1 (2.2) | 1 (3.6) |
| nCR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VGPR | 0 | 0 | 0 | 1 (12.5) | 3 (50.0) | 2 (11.1) | 3 (11.5) | 6 (13.0) | 0 |
| PR | 2 (50.0) | 1 (25.0) | 2 (33.3) | 4 (50.0) | 0 | 9 (50.0) | 13 (50.0) | 18 (39.1) | 12 (42.9) |
| MR | 1 (25.0) | 0 | 0 | 0 | 0 | 1 (5.6) | 1 (3.8) | 2 (4.3) | 1 (3.6) |
| SD | 0 | 3 (75.0) | 2 (33.3) | 1 (12.5) | 2 (33.3) | 2 (11.1) | 3 (11.5) | 10 (21.7) | 10 (35.7) |
| PD | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (5.6) | 1 (3.8) | 2 (4.3) | 2 (7.1) |
| Not evaluable | 1 (25.0) | 0 | 0 | 2 (25.0) | 0 | 2 (11.1) | 4 (15.4) | 5 (10.9) | 2 (7.1) |
| ORR, % (95% CI) | 50.0 (6.8–93.2) | 25.0 (0.6–80.6) | 50.0 (11.8–88.2) | 62.5 (24.5–91.5) | 66.7 (22.3–95.7) | 66.7 (41.0–86.7) | 65.4 (44.3–82.8) | 58.7 (43.2–73.0) | 46.4 (27.5–66.1) |
| DOR, months | |||||||||
| Median (95% CI) | NE (2.8-NE) | 11.5 (NE-NE) | 7.2 (6.0-NE) | 9.7 (6.0-NE) | NE (4.2-NE) | NE (6.8-NE) | 10.1 (7.3-NE) | 10.9 (7.3-NE) | 7.9 (6.0-NE) |
| CBR, % (95% CI) | 75.0 (19.4–99.4) | 25.0 (0.6–80.6) | 50.0 (11.8–88.2) | 62.5 (24.5–91.5) | 66.7 (22.3–95.7) | 72.2 (46.5–90.3) | 69.2 (48.2–85.7) | 63.0 (47.5–76.8) | 50.0 (30.6–69.4) |
BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DOR, duration of response; MR, minimal response; nCR, near CR; NE, not estimable; OPZ, oprozomib; ORR, overall response rate; PD, progressive disease; PR, partial response; sCR, stringent CR; SD, stable disease; VGPR, very good PR.
Of the 26 patients who received 300 mg oprozomib in the phase 1b + phase 2 portions (2/7 schedule), 17 patients achieved a best overall response of PR or better, yielding an ORR of 65.4% (95% CI: 44.3–82.8). The CBR on this schedule was 69.2% (95% CI: 48.2–85.7). The median duration of response was 10.1 (7.3-NE) months. At a median follow up of 13.4 (95% CI: 9.7–14.1) months, the median PFS and time to progression were both 10.8 (6.7–12.7) months for the 26 patients who received 300 mg oprozomib (phase 1b + phase 2, 2/7 schedule). The ORR for all patients (N = 46) from both phase 1b and phase 2 on the 2/7 schedule was 58.7% (95% CI: 43.2–73.0).
Among bortezomib-refractory patients who received oprozomib 210–330 mg in phase 1b + 2 (2/7 schedule; n = 28), 13 patients achieved a best overall response of PR or better (Table 4), resulting in an ORR of 46.4% (95% CI: 27.5–66.1). Complete response was achieved by one patient (3.6%), and the median duration of response was 7.9 months (95% CI: 6.0-NE). The CBR on this schedule was 50.0% (95% CI: 30.6–69.4). At a median follow up of 13.4 (95% CI: 7.9–13.9) months, the median PFS and median time to progression were both 6.7 (95% CI: 3.7–9.1) months.
4. Discussion
In this study investigating oprozomib with dexamethasone in patients with RRMM, the MTD was 180 mg for the 5/14 schedule without step-up dosing and not reached for the 2/7 schedule. DLTs prevented successful dose escalation on the 5/14 schedule, whereas patients on the 2/7 schedule underwent successful dose escalation and received higher doses of oprozomib. Based on the available safety and efficacy data, the 300 mg oprozomib dose in the 2/7 schedule was chosen as the recommended phase 2 dosing with dexamethasone.
GI AEs were the most common any-grade AEs observed, and included nausea, vomiting, and diarrhea. These AEs occurred despite aggressive GI management, diarrhea being the most common cause of AE-related treatment discontinuation on both the 5/14 and 2/7 schedules. A substantial percentage of patients also experienced grade ≥ 3 GI AEs, further highlighting the concerning nature of these toxicities. GI AEs were also among the most common AEs observed in other oprozomib studies [16,22]. In a phase 1b/2 study of single-agent oprozomib in patients with hematologic malignancies, two patients died on study due to treatment-related GI hemorrhage [16]. Overall, the safety profile of oprozomib on the 2/7 schedule was similar to that in previous reports of single-agent oprozomib and oprozomib in combination with pomalidomide and dexamethasone [16,22]. All-grade GI-related AEs on the 2/7 schedule occurred less frequently with oprozomib and dexamethasone than reported for similar doses of single-agent oprozomib [16], perhaps in part due to the antiemetogenic properties of dexamethasone.
Oprozomib in combination with dexamethasone showed promising antimyeloma activity in patients with RRMM, especially in patients who were on the 2/7 schedule. Response assessment was limited in the 5/14 schedule due to toxicity. The ORR was 65.4% for patients on the 2/7 schedule (phase 1 and 2) who received the recommended phase 2 dose of 300 mg oprozomib. With the caveat that cross-trial comparisons should be interpreted cautiously, treatment with oprozomib and dexamethasone resulted in higher ORR and CBR, as well as longer median PFS, for the 2/7 schedule in this study than what was seen with similar doses of single-agent oprozomib [16]. In another study investigating oprozomib, pomalidomide, and dexamethasone in patients with RRMM, the ORR and CBR were both 85.7%, indicating encouraging antimyeloma activity if pomalidomide was added to the oprozomib-dexamethasone backbone [22]. In our study, promising activity was also seen in bortezomib-refractory patients, a difficult-to-treat population, with an ORR of 46.4%.
Oprozomib was absorbed and eliminated rapidly when administered as tablets or ER tablets, and exhibited high intersubject PK variability in cycle 1. These findings are in line with a previous study that reported relatively high interpatient variability with single-agent oprozomib in patients with advanced solid tumors [23].
This study was limited by its relatively small sample size, sub-optimal safety profile, short follow-up time with patients due to the termination of the trial, and DLTs that occurred during the study, particularly in the 5/14 schedule. The GI AEs reported here and in other oprozomib studies, together with the limitations of the ER formulations and high intrapatient PK variability in an ongoing oprozomib study (ClinicalTrials.gov, NCT02244112), led to a halt in further enrollment in the phase 2 portion of this study. Although step-up dosing was implemented and appeared to be safe, the benefit in reducing GI toxicities was difficult to ascertain given the relatively small sample size; future studies will not use with the current oprozomib formulation.
This study demonstrated continued promising efficacy with oprozomib in combination with dexamethasone for patients with RRMM. Improved GI tolerability and more predictable and consistent PK properties will be critical for this agent to be used routinely in patients with MM. To optimize the safety and PK profiles of oprozomib, new oprozomib formulations with two different dissolution profiles are currently being evaluated in a phase 1b, dose-exploration study of patients with RRMM treated with oprozomib, pomalidomide, and dexamethasone (ClinicalTrials.gov, NCT02939183).
Acknowledgments
Medical writing and editorial assistance were provided by Sachi Yim and Andrew Gomes of BlueMomentum, an Ashfield company, part of UDG Healthcare PLC, and by Yin Lin of Amgen Inc., and funded by Amgen Inc.
Funding
This study and the manuscript development were supported by Amgen Inc., Thousand Oaks, CA, USA.
Abbreviations:
- AE
adverse event
- AUClast
area under the plasma concentration-time curve to the last measurable concentration
- CBR
clinical benefit rate
- CI
confidence interval
- Cmax
maximum observed concentration
- CV%
percent of coefficient of variation
- DLT
dose-limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- ER
extended release
- GI
gastrointestinal
- MM
multiple myeloma
- MTD
maximum tolerated dose
- NE
not estimable
- ORR
overall response rate
- PFS
progression-free survival
- PK
pharmacokinetics
- PR
partial response
- RP2D
recommended phase 2 dose
- RRMM
relapsed or refractory MM
- t1/2
terminal half-life
- Tmax
time to maximum concentration
References
- [1].Merin NM, Kelly KR, Clinical use of proteasome inhibitors in the treatment of multiple myeloma, Pharmaceuticals (Basel) 8 (2014) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sonneveld P, Goldschmidt H, Rosinol L, et al. , Bortezomib-based versus non-bortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials, J. Clin. Oncol 31 (2013) 3279–3287. [DOI] [PubMed] [Google Scholar]
- [3].Richardson PG, Barlogie B, Berenson J, et al. , A phase 2 study of bortezomib in relapsed, refractory myeloma, N. Engl. J. Med 348 (2003) 2609–2617. [DOI] [PubMed] [Google Scholar]
- [4].Mikhael JR, Belch AR, Prince HM, et al. , High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program, Br. J. Haematol 144 (2009) 169–175. [DOI] [PubMed] [Google Scholar]
- [5].Kuhn DJ, Chen Q, Voorhees PM, et al. , Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin proteasome pathway, against preclinical models of multiple myeloma, Blood 110 (2007) 3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hideshima T, Richardson P, Chauhan D, et al. , The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells, Cancer Res. 61 (2001) 3071–3076. [PubMed] [Google Scholar]
- [7].Berenson JR, Cartmell A, Bessudo A, et al. , CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma, Blood 127 (2016) 3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dimopoulos MA, Moreau P, Palumbo A, et al. , Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study, Lancet Oncol. 17 (2016) 27–38. [DOI] [PubMed] [Google Scholar]
- [9].Siegel D, Martin T, Nooka A, et al. , Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies, Haematologica 98 (2013) 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].KYPROLIS® (carfilzomib) prescribing information, Onyx Pharmaceuticals Inc., an Amgen Inc. subsidiary.
- [11].Moreau P, Joshua D, Chng WJ, et al. , Impact of prior treatment on patients with relapsed multiple myeloma treated with carfilzomib and dexamethasone vs bortezomib and dexamethasone in the phase 3 ENDEAVOR study, Leukemia 31 (2017) 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Knopf KB, Duh MS, Lafeuille MH, et al. , Meta-analysis of the efficacy and safety of bortezomib re-treatment in patients with multiple myeloma, Clin. Lymphoma Myeloma Leuk 14 (2014) 380–388. [DOI] [PubMed] [Google Scholar]
- [13].Richardson PG, Sonneveld P, Schuster MW, et al. , Bortezomib or high-dose dexamethasone for relapsed multiple myeloma, N. Engl. J. Med 352 (2005) 2487–2498. [DOI] [PubMed] [Google Scholar]
- [14].NINLARO® (ixazomib) prescribing information, Takeda; 2015.
- [15].Zhou HJ, Aujay MA, Bennett MK, et al. , Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047), J. Med. Chem 52 (2009) 3028–3038. [DOI] [PubMed] [Google Scholar]
- [16].Ghobrial IM, Savona MR, Vij R, et al. , Final results from a multicenter, open-label, dose-escalation phase 1b/2 study of single-agent oprozomib in patients with hematologic malignancies, Blood 128 (2016) 2110. [Google Scholar]
- [17].Jagannath S, Richardson RG, Barlogie B, et al. , Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone, Haematologica 91 (2006) 929–934. [PubMed] [Google Scholar]
- [18].Durie BGM, Harousseau JL, Miguel JS, et al. , International uniform response criteria for multiple myeloma, Leukemia 20 (2006) 1467–1473. [DOI] [PubMed] [Google Scholar]
- [19].Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03, National Cancer Institute, Bethesda, 2010. NIH Publication No. 09–5410. [Google Scholar]
- [20].Kyle RA, Rajkumar SV, Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma, Leukemia 23 (2009) 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blade J, Samson D, Reece D, et al. , Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant, Br. J. Haematol 102 (1998) 1115–1123. [DOI] [PubMed] [Google Scholar]
- [22].Shah J, Niesvizky R, Stadtmauer E, et al. , Oprozomib, pomalidomide, and dexamethasone (OPomd) in patients (pts) with relapsed and/or refractory multiple myeloma (RRMM): initial results of a phase 1b study (NCT01999335), Presented at the 21st Annual European Society of Hematology Congress, (2016), p. 133258. [Google Scholar]
- [23].Infante JR, Mendelson DS, Burris HA 3rd et al. , A first-in-human dose-escalation study of the oral proteasome inhibitor oprozomib in patients with advanced solid tumors, Invest. New Drugs 34 (2016) 216–224. [DOI] [PubMed] [Google Scholar]