Summary/Abstract
Small molecules that address fundamental defects underlying cystic fibrosis (so called “modulators,” such as the approved drugs ivacaftor, lumacaftor, and tezacaftor) have advanced dramatically during the past few years and are transforming care and prognosis among individuals with this disease. The new treatment strategies are predicated on established scientific insight concerning pathogenesis, and applying “personalized” or “precision” interventions for specific abnormalities of the cystic fibrosis transmembrane conductance regulator (CFTR). Even with the advent of highly effective triple drug combinations – which are anticipated to markedly benefit most patients with CF worldwide – challenges to precision therapy remain. These include refractory CFTR variants (premature truncation codons, splice defects, large indels, severe missense mutations, and others) not addressed by available modulators, and access to leading-edge therapeutic compounds for patients with ultrarare forms of cystic fibrosis. The CF experience is emblematic of other conditions for which personalized interventions are actively being sought.
Keywords: personalized medicine, combination drug therapy, investigational drugs
A transformative era for cystic fibrosis therapy
The emergence of modulator treatment (primarily “potentiators” and “correctors”) has dramatically changed the prognostic landscape for patients with CF1. CFTR, the responsible gene, encodes an epithelial anion channel, and ‘potentiators’ of channel function such as ivacaftor have led to dramatic clinical improvement for individuals carrying mutations such as G551D*2, as well as several other variants (described below). Clinically approved ‘corrector’ molecules (i.e., lumacaftor and tezacaftor) designed to overcome maturational processing abnormalities were initially directed towards the common F508del mutation3,4. Experimental next-generation correctors such as VX-445 (elexacaftor) and VX-659 work in concert with ivacaftor and tezacaftor, and appear to act by mechanism(s) independent of the approved agents5,6. Triple combination therapies (TCTs) (e.g., ivacaftor + tezacaftor + elexacaftor) have recently shown pronounced benefit in Phase 2 and 3 clinical testing among patients with at least one copy of F508del, leading to a new drug application currently under FDA review5–11. Individuals encoding at least one F508del variant represent a sizable majority of patients with CF7, and TCT benefit can be achieved even when the second (non-F508del) allele lacks measurable activity.
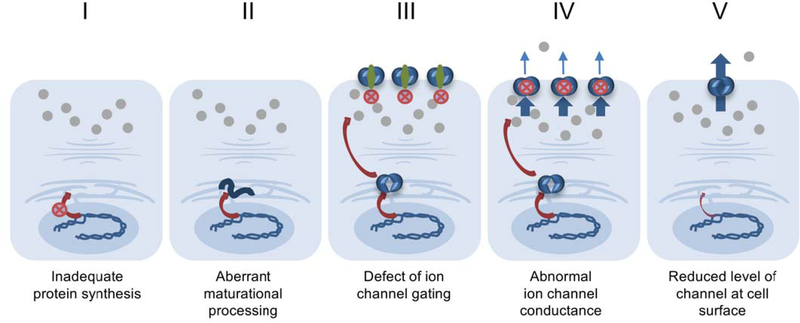
The original notion that specific drugs would be tailored (personalized) to address discrete CFTR defects has evolved substantially over the past decade. CFTR abnormalities have traditionally been grouped or binned into 5–6 subcategories defined by underlying molecular dysfunction (Figure 1). It has become increasingly clear, however, that most CFTR mutations confer numerous protein defects12,13. Moreover, active modulator drugs typically exhibit beneficial effect across a broad range of CFTR variants and disease subcategories13. Ivacaftor, for example, was originally developed for G551D CFTR (currently in use by patients with this variant who are six months of age and older), but has subsequently shown pronounced CFTR stimulatory effect and gained registration (FDA approval) as a single agent for 38 CFTR mutations (primarily class III (gating) abnormalities). Among these, ivacaftor is approved as a single agent for other (sometimes unanticipated) categories of CFTR mutation; e.g., variants such as E831X (class I), A455E (traditionally grouped in class IV), and 2789+5 G→A (class V). Moreover, the drug has become part of leading-edge combination treatments (together with lumacaftor or tezacaftor) among patients homozygous for the common F508del abnormality (class II). Twenty-six other CFTR variants have also been approved for the combination of ivacaftor with tezacaftor.
Figure 1. Traditional classification of CFTR mutations.
Note that a sixth group (plasma membrane instability) is sometimes included as a separate category1. Alternative evaluation of CFTR variants based on modulator response offers an additional means of binning CFTR molecular defects, with designations that constitute greater than six categories30,31. Modulator treatments have been shown to confer beneficial effects across many CFTR mutational subgroups, based on increased expression of well-folded CFTR (mediated by correctors) and enhanced residual ion channel activity (potentiators) in a manner that is sometimes less dependent on underlying molecular abnormality or mutation subclass.
Activity of ivacaftor against E831X is attributable to occurrence of this nonsense variant as part of a splice donor/acceptor site, with a portion of the translation product leading to full-length CFTR lacking only the aspartate at position 83114. The resulting E831del encodes a protein responsive to gating activation by ivacaftor. In similar fashion, mRNA splicing defects such as 2789 + 5G→A or 3849 + 10 Kb C→T are translated in alternatively spliced forms, some of which maintain partial CFTR activity and can be potentiated by ivacaftor.
Molecular complexity of CFTR variants
In broad terms, the concept of personalized CF treatment presupposes mutations that exhibit discrete abnormalities – many of which are remediable by a specific small molecule. Detailed studies of numerous CFTR variants, however, belie that notion. The prevalent F508del CFTR, for example, exhibits not only a class II (aberrant protein maturation) phenotype, but also defective ion channel gating (class III), and cell surface instability12. Initial characterization of P67L – a very rare mutation found predominantly among individuals of Scottish descent – described a problem with the CFTR ion conducting pore (class IV), whereas more complete studies have shown profound abnormalities involving protein instability (class II) and defective channel gating (class III)15,16. Relatively common mutations such as N1303K exhibit severely inadequate biogenesis that appears mechanistically distinct from other class II variants. Unlike F508del, for example, N1303K leads to expression of high-level immature (endoplasmic reticulum restricted) CFTR which is not rescued by tezacaftor or lumacaftor17. The clinically important finding that a single CFTR mutation typically confers numerous and complex abnormalities underscores a need to view CF precision therapeutics from an updated perspective.
High cost of modulator treatment and impact on drug access among individuals with rare variants
For the majority of FDA approved agents in general pharmaceutical use, physicians in the US are allowed flexibility to prescribe these drugs whether a treatment is “on” or “off” label (i.e., whether or not the FDA drug approval label specifically designates use of the compound for a particular clinical indication). In other words, if an approved antibiotic, anti-inflammatory, or anticancer compound is believed to offer significant benefit and safety outside the formal FDA label indications, caregivers are often provided freedom to administer the treatment. A problem arises, however, in the case of patients with “off-label” CFTR variants who might benefit from a therapy such as ivacaftor. In the era of personalized medicine, regulatory approval for compounds of this type has traditionally been directed towards specific CFTR mutations. CF clinicians may of course prescribe the drug to any patient who might benefit – even if the patient’s genotype is not formally “on label”. However, ivacaftor costs over $300,000 per year in the United States, and third-party payers have been understandably concerned about providing blanket reimbursement for these CF treatments without formal FDA or other regulatory endorsement. In practice, therefore, although many patients might benefit “off label”, drug access is usually not available to individuals who lack an approved genotype. Similarly, when clinical efficacy for a specific CF indication is argued to be less robust, the cost of CFTR modulation has sometimes impeded (or obviated) patient access (e.g., third party payment for lumacaftor/ivacaftor in the United Kingdom)18. Comparable issues have been appreciated in other disease contexts where discovery (and marketing) of new compounds for rare or ultrarare conditions involves therapies that have been approved for specific, unusual, and/or personalized treatments19.
Addressing the challenges of patient access to modulator therapy
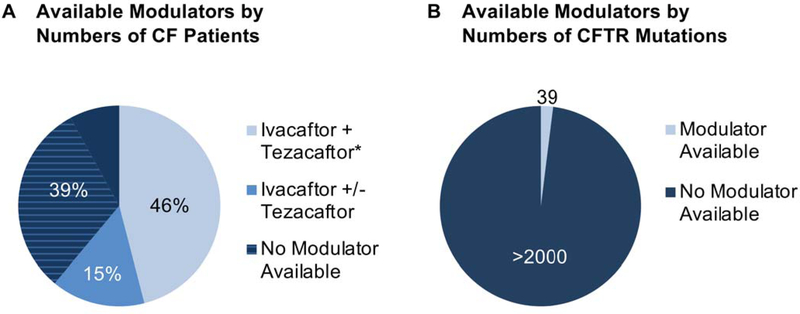
Because the regulatory review process often employs Phase 3 double-blind, placebo-controlled studies, low prevalence of many CF mutations makes clinical testing of this sort problematic – particularly when entry criteria are based strictly on genotype. It has been estimated that over 1,000 CFTR variants are represented by less than five patients each worldwide (Figure 2)20. Since a personalized approach that includes placebo-controlled testing of ultrarare genotypes could therefore be viewed as impractical, important alternatives have emerged. For example, N = 1 trials21 allow individual patients to serve as their own controls, with comparisons made for each study subject before, during, and after drug treatment. Establishing strong efficacy data in very small patient cohorts using the N = 1 format has been challenging due to features such as fluctuating respiratory infection, pulmonary inflammation, disease trajectory, rebound exacerbation following omission of drug (or beneficial effects that persist once treatment has been stopped), chronic (non-reversible) lung scarring, and/or complexity of statistical analysis. Moreover, even if an individual with CF exhibits significant benefit (e.g., FEV1 improvement) during an N = 1 study, precedent for expanding drug label on a patient-by-patient (rather than overall genotype-dependent) basis is lacking. As an alternative, recent guidance from FDA and other regulatory agencies has described use of scientifically meaningful groupings of study subjects by criteria not exclusively based on genotype22. Using this framework, evaluation of certain rare CFTR variants has incorporated clinical evidence of significant residual function (e.g., pancreatic sufficiency, less pronounced sweat chloride abnormalities) or in vitro studies of modulator effectiveness23,24, as discussed below.
Figure 2.
(A) Subset of individuals with an approved modulator from among ~30,000 patients in the U.S. Approximately 15% of individuals with CF are approved for treatment using ivacaftor alone, with a subset of these (below) also approved for ivacaftor/tezacaftor. The (*) describes F508del/F508del genotype, and denotes approval for ivacaftor together with either lumacaftor or tezacaftor (46% of patients with CF). For another ~ 39% of patients, no modulator drugs are yet available. The majority of these individuals carry a copy of F508del and are anticipated to benefit from triple combination therapy (TCTs, hatched detail of diagram, see text). (B) Subset of CF variants with an approved modulator. Among > 2000 disease-associated mutations30, thirty-nine currently are “on label.” Ivacaftor as a single agent is available for the following mutations (~ 15% of patients): E56K, P67L, R74W, D110E, D110H, R117C, E193K, L206W, R347H, R352Q, A455E, D579G, E831X, S945L, S977F, F1052V, K1060T, A1067T, R1070W, F1074L, D1152H, D1270N, 711+3A->G, 2789+5G->A, 3272–26A->G, 3849+10kb C->T, G551D, R117H, G178R, S549N, S549R, G551S, G1069R, R1070Q, G1244E, S1251N, S1255P, and G1349D. Combination treatment with ivacaftor and tezacaftor is also approved when any of the first 26 ivacaftor-responsive variants from the list above is present at either CFTR locus, or for individuals homozygous with F508del. Additional studies will be required to estimate numbers of patients with CF who encode variants not formally approved for modulator treatment, but likely to show clinical benefit following drug administration.
“Theratype” is a term coined by T. Torphy and applied to CF drug discovery and personalized intervention. Theratype simply asks whether improved lung function, fewer pulmonary exacerbations, or additional beneficial effects are observed following potentiation, correction, or other CF treatment. The concept helps address behavior of modulators insofar as rare mutations are concerned. In particular, theratype denotes whether or not a specific CFTR variant – irrespective of underlying mechanistic subcategory – exhibits improvement following drug administration. Theratype represents a concession to molecular complexity noted for many (or most) CFTR variants, and has been increasingly employed by the research community.
In vitro model systems for identifying patients likely to benefit from modulator treatment
In vitro data can be considered in specific cases when developing label expansion for rare CFTR variants. For example, CFTR modulator response in primary airway epithelial cells correlates with likelihood of improvement in FEV1 for certain genotypes tested to date25. Fischer rat thyroid cells expressing recombinant CFTR have been employed in similar fashion to provide evidence of residual ion channel function24. In Europe, epithelial organoids obtained from intestinal mucosal biopsy are being advanced to test rare variants, and constitute another valuable approach for personalized analysis26,27. Well-validated in vitro assays for predicting clinical improvement in CF may help facilitate approval of rare CFTR genotypes that respond favorably to modulator compounds. Other parameters such as intermediate sweat chloride levels, pancreatic sufficiency, and in vivo measures of ion transport or mucociliary clearance are also being studied as a means to identify patients most likely to benefit following modulator-based treatment23,25,28.
CF precision medicine and prospects for the immediate future
Remarkable progress over the past few years has set the stage for highly active triple drug combination therapies designed to offer pronounced benefit among patients with one copy of F508del, irrespective of the other allelic defect. In Phase 2 and 3 clinical testing, activity of TCTs has been robust5,6,8–11. Regulatory approval of the first TCT is hoped for within the coming year – in which case a sizable majority of patients should have access to an effective modulator formulation. Even if these expectations are met, it is essential to acknowledge (as with any therapy) that certain individuals may not exhibit pronounced benefit, or could experience side effects that limit their ability to tolerate a new, life-long treatment. Patients such as these, together with the estimated 6–8% of those encoding refractory CFTR mutations that are not clinically addressed by TCTs (e.g., recalcitrant splice or premature truncation defects, large indels, severe folding abnormalities)7,20 constitute a group that merits intensified drug discovery efforts. Novel agents for this purpose are aggressively being sought by pharmaceutical, academic, and other research laboratories.
Conclusions
Modulator compounds address fundamental protein defects responsible for cystic fibrosis, and have been advanced using innovative personalized or precision-type strategies (i.e., alignment of molecular abnormality with a specific small molecule intervention). Based on the large number of disease-associated variants, complexity of underlying mechanism, and barriers to drug access among those with rare mutations, an adapted approach to CF precision medicine has been increasingly applied. Grouping or binning unusual CFTR genotypes according to residual function holds considerable promise in this regard. Cell based (ex vivo) systems and other innovative refinements have emerged, and are already enhancing personalized approaches to the disease.
Notwithstanding the unparalleled success of CFTR modulator-based therapeutics, significant challenges remain. Even if TCTs achieve their potential, many patients with refractory CFTR variants will still lack an approved modulator. In this context, work by numerous laboratories to develop new and highly active modulator compounds, including drugs directed towards CFTR variants that remain “off label”, represents an area of considerable emphasis29. In parallel, the field continues to pursue treatment strategies less restricted by the underlying CFTR abnormality (for example, initiatives directed towards CFTR gene transfer, stem cell technology, or DNA editing). Many such approaches, in contrast to more personalized therapies, have the potential to ultimately benefit all patients with cystic fibrosis, irrespective of underlying genotype. In addition, studies that address CF tissue sequelae (chronic scarring, lung fibrosis, inflammation, or infection) comprise areas of substantial need for those without access to modulators – as well as individuals with advanced disease not fully remediable by TCTs or other CF drug formulations1,29,30. In these ways, a multifaceted approach to treatment will continue to provide new hope and optimism for patients with CF and their families in the future.
Acknowledgments
The authors thank Jan Tindall for help preparing the manuscript. We also thank Jan Tindall and Bess Renjilian for review of the paper and valuable comments.
Funding for this project was provided by NIH R01HL139876 and R01HL136414 and CFF SORSCH13XX0.
Footnotes
Nomenclature here reflects replacement of an aspartic acid (D) for glycine (G) normally found at position 551 of the 1480 amino acid CFTR.
References
- 1.Sorscher EJ. Cystic Fibrosis In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison’s principles of internal medicine. 20th edition New York: McGraw-Hill Education; 2018. Chapter 285,2 volumes (xli, 3528, I-214 pages). [Google Scholar]
- 2.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010; 363:1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A, 2011; 108:18843–18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373:1783–1784. [DOI] [PubMed] [Google Scholar]
- 5.Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, Plant BJ, Prais D, Ramsey BW, et al. VX-659-Tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018; 379:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, et al. VX-445-Tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018; 379:1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CFTR2. CFTR2 database. Available from: https://www.cftr2.org/.
- 8.Business Wire. Vertex selects triple combination regimen of VX-445, tezacaftor, and ivacaftor to submit for global regulatory approvals in cystic fibrosis. May 30, 2019. https://www.businesswire.com/news/home/20190530005252/en/
- 9.Business Wire. FDA accepts new drug application for VX-445 (elexacaftor), tezacaftor, and ivacaftor combination treatment. August 20, 2019. https://www.businesswire.com/news/home/20190820005233/en/
- 10.ClinicalTrials.gov. A study evaluating the long-term safety and efficacy of VX-445 combination therapy. 2019. ClinicalTrials.gov Identifier: NCT03525574. https://clinicaltrials.gov/ct2/show/record/NCT03525574?term=vx-17-445-102&cond=cystic+fibrosis&rank=2
- 11.ClinicalTrials.gov. A phase 3 study of VX-445 combination therapy in subjects with cystic fibrosis heterozygous for the F508del mutation and a minimal function mutation (F/MF) 2019 ClinicalTrials.gov Identifier: NCT03525444. https://clinicaltrials.gov/ct2/show/NCT03525444?term=NCT03525444&cond=Cystic+Fibrosis&rank=1
- 12.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016; 27:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA, Joynt AT, Lu Z, Cai Z, Raraigh KS, et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight 2018; 3:pii:121159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinzpeter A, Aissat A, Sondo E, Costa C, Arous N, Gameiro C, Martin N, Tarze A, Weiss L, de Becdelièvre A, et al. Alternative splicing at a NAGNAG acceptor site as a novel phenotype modifier. PLoS Genet 2010; 6:pii:e1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabusap CM, Wang W, McNicholas CM, Chung WJ, Fu L, Wen H, Mazur M, Kirk Kl, Collawn JF, Hong JS, et al. Analysis of cystic fibrosis-associated P67L CFTR illustrates barriers to personalized therapeutics for orphan diseases. JCI Insight 2016; 1:pii:e86581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren HY, Grove DE, De La Rosa O, Houck SA, Sopha P, Van Goor F, Hoffman BJ, Cyr DM. VX-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell 2013; 24:3016–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekkers JF, Gogorza Gondra RA, Kruisselbrink E, Vonk AM, Janssenss HM, de Winter-de Groot KM, van der Ent CK, Beekman JM. Optimal correction of distinct CFTR folding mutants in rectal cystic fibrosis organoids. Eur Respir J 2016; 48:451–458. [DOI] [PubMed] [Google Scholar]
- 18.BBC, News ‘Deadlock must end’ over cystic fibrosis drug Orkambi. 2019. [Google Scholar]
- 19.Gammie T, Lu CY, Babar ZU. Access to orphan drugs: a comprehensive review of legislations, regulations, and policies in 35 countries. PLoS One 2015; 10:e0140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle M CF Foundation 2017 NACFC Plenary: Matching medicines with mutations. 2017; available from: https://www.youtube.com/watch?v=tvWST858Awo.
- 21.McGarry ME, Illek B, Ly NP, Zlock L, Olshansky S, Moreno C, Finkbeiner WE, Nielson DW. In vivo and in vitro ivacaftor response in cystic fibrosis patients with residual CFTR function: N-of-1 studies. Pediatr Pulmonol 2017; 52:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuck RN, Woodcock J, Zineh I, Stein P, Jarow J, Temple R, Permutt T, LaVange L, Beaver JA, Charlab R, et al. Considerations for developing targeted therapies in low-frequency molecular subsets of a disease. Clin Pharmacol Ther 2018; 104:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, Nair N, Simard C, Han L, Ingenito EP, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017; 377:2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durmowicz AG, Lim R, Rogers H, Rosebraugh CJ, Chowdhury BA. The U.S. Food and Drug Administration’s experience with ivacaftor in cystic fibrosis. Establishing efficacy using in vitro data in lieu of a clinical trial. Ann Am Thorac Soc 2018; 15:1–2. [DOI] [PubMed] [Google Scholar]
- 25.Clancy JP, Cotton CU, Donaldson SH, Solomon GM, VanDevanter DR, Boyle MP, Gentzsch M, Nick JA, Illek B, Wallenburg JC, et al. CFTR modulator theratyping: current status, gaps, and future directions. J Cyst Fibros 2019; 18:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, Arets HGM, Marck-van der Wilt REP, Dijkema JS, Vanderschuren MM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep 2019; 26:1701–1708. [DOI] [PubMed] [Google Scholar]
- 27.van Mourik P, Beekman JM, van der Ent CK. Intestinal organoids to model cystic fibrosis. Eur Respir J 2019; pii:1802379. [DOI] [PubMed] [Google Scholar]
- 28.McCague AF, Raraigh KS, Pellicore MJ, Davis-Marcisak EF, Evans TA, Han ST, Lu Z, Joynt AT, Sharma N, Castellani C, et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med 2019; 199:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cystic Fibrosis Foundation. Drug Development Pipeline. 2019; https://www.cff.org/Trials/pipeline.
- 30.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Resp Med 2016; 4:662–674. [DOI] [PubMed] [Google Scholar]
- 31.Stanke F, Tümmler B. Classification of CFTR mutation classes 2016; 4:e36. [DOI] [PubMed] [Google Scholar]