Abstract
Background:
Serious chronic medical conditions occur in childhood cancer survivors. We investigated incidence of and risk factors for end-organ damage resulting in wait-listing for or receiving solid organ transplantation (SOT) and five-year survival following SOT.
Methods:
The Childhood Cancer Survivor Study (CCSS) is a cohort of five-year survivors of childhood cancers, diagnosed < 21 years of age. We linked data from US-treated participants diagnosed January 1, 1970 - December 31, 1980, without SOT prior to cohort entry, to the Organ Procurement and Transplantation Network (OPTN) – a database of all US organ transplants. Primary objectives were: SOT/wait-listing cumulative incidence, risk factor hazard ratios (HR), and 5-year Kaplan-Meier survival following SOT.
Findings:
Of 13,318 eligible survivors, one-hundred had 103 SOT (50 kidney, 37 heart, 9 liver, 7 lung) and 67 were wait-listed without transplant (21 kidney, 25 heart, 15 liver, 6 lung). Cumulative incidence (95% CI) of SOT/wait-listing was: kidney 0·54% (0·40–0·67), heart 0·49% (0·36–0·62), liver 0·19% (0·10–0·27), and lung 0·10% (0·04–0·16) by 35 years from cancer diagnosis. Risk factors (HR, 95% CI) for kidney transplantation were unilateral nephrectomy (4·2, 2·3–7·7), ifosfamide (24·9, 7·4–83·5), total body irradiation (6·9, 2·3–21·1), and mean kidney radiation >15 Gy; for heart transplantation, anthracycline and mean heart radiation >20 Gy (dose-dependent, both p<0·0001); for liver transplantation, dactinomycin (3·8, 1·3–11·3) and methotrexate (3·3, 1·0–10·2); for lung transplantation, carmustine (12·3, 3·1–48·9) and mean lung radiation >10 Gy (15·6, 2·6–92·7). Five-year overall survival (95% CI) after SOT were: kidney 93·5% (81·0–97·9), heart 80·6% (63·6–90·3), liver 27·8% (4·4–59·1), and lung 34·3% (4·8–68·6).
Interpretation:
SOT is uncommon among aging childhood cancer survivors. Organ-specific exposures were associated with increased SOT incidence. Survival outcomes demonstrate that SOT should be considered for five-year childhood cancer survivors with life-limiting end-organ failure.
Funding:
National Institute of Health, American Lebanese Syrian Associated Charities, Health Resources & Services Administration.
Introduction
With treatment, >80% of children diagnosed with malignancy will become long-term survivors.1 However, risks of long-term morbidity and premature mortality from renal, cardiac, hepatic, pulmonary and other medical conditions are well-established as this population ages.2–7 We previously reported that by 20 years following cancer diagnosis, the cumulative incidence of severe, disabling, or life-threatening conditions or death due to chronic conditions was 27·5% (95% CI, 26·4–28·6) among survivors diagnosed from 1990–1999.8 When clinically ascertained for serious, disabling, or life-threatening conditions at 45 years of age, cumulative prevalence was 80·5% (95% CI, 73·0–86·6).9
Crude prevalence of adverse health outcomes has been described in an organ-specific fashion including 65·2% (95% CI 60·4–69·8) abnormal pulmonary function, 56·4% (95% CI, 53·5–59·2) cardiac conditions, 13·0% (95% CI, 10·8–15·3) liver dysfunction, and 5·0% (95% CI 4·0–6·3) kidney dysfunction.9 However, these reports do not adequately highlight end-organ failure sufficient to require therapeutic intervention. Solid organ transplantation (SOT) is an option for end-organ failure that results from childhood cancer therapy.2,10–12 Only case reports of a limited number of cancer survivors undergoing kidney, heart, liver, or lung transplantation are available.10,12–16
The Childhood Cancer Survivor Study (CCSS; http://ccss.stjude.org) is a retrospective cohort of five-year survivors of childhood cancer, diagnosed 1970–1986, <21 years of age at diagnosis at one of 26 institutions. The Organ Procurement and Transplantation Network (OPTN; http://optn.transplant.hrsa.gov) administered by the United Network for Organ Sharing (UNOS) in contract with the Health Resources and Services Administration (HRSA), actively tracks occurrence and outcomes of >30,000 SOTs each year in the US. The OPTN website notes that >400 abstracts and manuscripts relating to, or based on, OPTN data have been published. These two large databases, each a unique resource with a wealth of information, have never previously been linked. The OPTN has been linked to other large databases for studies in organ transplantation,17 but the only similar linkage between two large databases comparable to the current study occurred with the National Wilms Tumor Study and the United States Renal Data System to look at end-stage renal disease in survivors of Wilms tumor, inclusive of kidney transplantation.2
Incidence of, risk factors for, and long-term outcomes following SOT in childhood cancer survivors are unknown. This study establishes the first data linkage between CCSS and OPTN in order to identify and describe a large population of childhood cancer survivors who subsequently underwent kidney, heart, liver or lung transplantation. Knowledge of survival following SOT will provide evidence to health care providers and survivors regarding the consideration of SOT in this population.
Methods
Study design and participants.
The CCSS includes five-year survivors diagnosed at age less than 21 years, between January 1, 1970 and December 31,1986, with leukemia, lymphoma, malignant CNS tumors, neuroblastoma, Wilms tumors, and bone and soft tissue sarcomas. This cohort has been previously well described.18 Survivors with other tumors, including hepatoblastoma, retinoblastoma and germ cell tumors were not eligible for CCSS. The eligible participants are childhood cancer survivors (n= 13,318) in the CCSS cohort who were not from Canada (n=1046) and who did not have a solid organ transplant prior to entry into CCSS (n=8).
Procedures.
All institutions obtained IRB approval for CCSS. After written informed consent, participants completed questionnaires. CCSS systematically abstracted treatment data at the local sites, including chemotherapy, surgery, and radiation therapy, from medical records of survivors who authorized release using local, trained study personnel.18 No additional abstraction was performed for this current study. All questionnaires are available at https://ccss.stjude.org/tools-and-documents/questionnaires.html.
The OPTN launched on October 25, 1999, and contains data regarding every organ donation and transplant event occurring in the US since October 1, 1987 (https://optn.transplant.hrsa.gov/data/about-data/). Determination of SOT came through OPTN +/− CCSS data after October 1, 1987 (n= 100; 54 having both CCSS and OPTN reporting and 46 with only OPTN information, of whom 33 occurred after last contact with CCSS. For SOT prior to OPTN inception, SOT was determined by self-report on a questionnaire (n=3, with some information in OPTN for one of those) or medical record abstraction (n= 0). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the OPTN, and is overseen by the US Department of Health and Human Services.
Through a data-sharing agreement with OPTN, SOT and wait-list outcomes were obtained for all eligible CCSS participants through 12/31/2013. After OPTN database coverage extended nationwide, this analysis utilized only SOT cases that were verified via linkage to a record in the OPTN database, based on matching name, sex, date of birth and, if available, social security number. All matches were reviewed for additional agreement between available clinical data. A few participants reported SOT (2 kidney, 1 heart, 1 liver) after 1988 but could not be linked to an OPTN record and were excluded from the analyses. More detail on the methodology for linkage between CCSS and OPTN is provided in the supplemental methods (web appendix, pages 1–2).
Outcomes.
The objectives of this study were to determine (1) the incidence of wait-listing for or receiving a kidney, heart, liver or lung transplant in five-year pediatric cancer survivors, (2) the treatment risk factors for organ damage severe enough to warrant eligibility for SOT, and (3) the 5-year survival of participants receiving SOT. Two primary endpoints were evaluated: 1) first registration of a transplant candidate on the wait-list for an organ, and 2) first transplant received.
Kidney, heart, liver and lung transplantations were analyzed separately. Total anthracycline dose was calculated with equivalent doxorubicin to daunorubicin doses and idarubicin doses multiplied by 3. For each participant who received radiotherapy, doses were reconstructed on age-specific phantoms using previously described methods: for the kidney, heart, and lungs, mean organ doses were estimated, with blocking taken into account using standardized blocking by field. For liver doses, maximum tumor dose to the abdomen was used rather than mean organ dose as details on liver blocking were incomplete.6,19
Statistical analysis.
Demographic and cancer treatment factors relevant to each organ transplant type were summarized with descriptive statistics. Cumulative incidence summarized the proportion of survivors who received SOT and had organ damage severe enough to be considered for SOT by being wait-listed over time after study entry, treating death as a competing risk and censoring at 12/31/2013. Survivors undergoing transplant surgery not preceded by a wait-list registration event, or for which there were no wait-list registration data available, had their candidate registration date set to the transplant surgery date, making the cumulative incidence of wait-list a composite endpoint. Among survivors who received a transplant, Kaplan-Meier curves estimated overall survival following first SOT using mortality data obtained from the National Death Index through 12/31/2013.
Cox proportional hazards models estimated Hazard Ratios (HRs) and 95% CI for associations between cancer treatments, demographic factors, and risk of becoming a SOT candidate or recipient. Sensitivity analyses were performed to evaluate how study results might vary if the study included unconfirmed self-reports. If an included risk factor was missing, the subject was not included in that analysis. Factors with p<0·10 in univariate analyses were included in multivariable models. Those not significantly associated with SOT were removed from the final model, unless doing so caused ≥10% change in the HR estimate for other factor(s) in the model. The proportional hazards assumption was verified for each factor included in final models. All p-values presented are two-sided. P-values <0·05 were considered statistically significant and no adjustment was made for multiple comparisons. Statistics were performed using SAS Version 9.4, copyright ©2016 SAS Institute Inc., Cary, NC, USA, and Stata 15.1, StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.
Role of funding sources
Funding sources for this study had no role in design, collection, analysis, and interpretation of data, writing of the report, or decision to submit for publication. The corresponding author had full access to all of data and the final responsibility to submit for publication.
Results
Characteristics of the included CCSS participants (n=13,318) enrolled from January 1, 1970 and December 31, 1986, are shown in Table 1. (Four eligible participants were excluded who self-reported SOT without data in OPTN.) Participants were more likely to be male, white non-Hispanic, with median age at diagnosis of 6 years (interquartile range [IQR], 3–13) and median age at follow-up of 39 years (IQR 33–46). Table 1 summarizes details of therapeutic exposures. There were 103 solid organ transplants for 100 survivors identified after linkage (Table 2). Three survivors with a kidney transplant previously underwent a different SOT. An additional 67 were wait-listed without receiving a transplanted organ, with some survivors being listed for more than one organ. The most common SOT was kidney, followed by heart, liver, and lung. Table 3 provides age at original cancer diagnosis, age at SOT, cancer diagnosis preceding SOT, and treatment exposures based on SOT organ group. The supplemental table (web appendix, pages 3–4) shows results of univariate analyses for those variables that were included in the multivariable analyses reported in Table 4. For each organ transplant risk factor analysis outlined below, sensitivity analyses were performed and showed the same results (data not shown).
Table 1:
Demographic and treatment characteristics of 13,318 five-year survivors of childhood cancer at risk for solid organ wait-list and transplantation
| Characteristic | N | %† |
|---|---|---|
| Total | 13,318 | |
| Sex | ||
| Female | 6,177 | 46·4% |
| Male | 7,141 | 53·6% |
| Race | ||
| Non-Hispanic, White | 11,449 | 86·6% |
| Non-Hispanic, Black | 679 | 5·1% |
| Hispanic | 743 | 5·6% |
| Other or mixed | 352 | 2·7% |
| Unknown | 95 | |
| Age at primary cancer diagnosis (years) | ||
| 0–4 | 5,295 | 39·8% |
| 5–9 | 2,922 | 21·9% |
| 10–14 | 2,687 | 20·2% |
| 15–20 | 2,414 | 18·1% |
| Age at last follow-up (years) | ||
| <20 | 612 | 4·6% |
| 20–29 | 989 | 7·4% |
| 30–39 | 5,147 | 38·7% |
| 40–49 | 4,805 | 36·1% |
| >=50 | 1,758 | 13·2% |
| Unknown* | 7 | |
| Primary cancer diagnosis | ||
| Leukemia | 4,502 | 33·8% |
| CNS tumor | 1,639 | 12·3% |
| Hodgkin lymphoma | 1,846 | 13·9% |
| Non-Hodgkin lymphoma | 1,022 | 7·7% |
| Kidney (Wilms) tumor | 1,162 | 8·7% |
| Neuroblastoma | 866 | 6·5% |
| Soft tissue sarcoma | 1,167 | 8·8% |
| Bone tumor | 1,114 | 8·4% |
| Chemotherapy exposure: | ||
| Dactinomycin | 2,300 | 19·8% |
| Anthracycline | 4,574 | 39·6% |
| Carmustine (BCNU) | 460 | 4·0% |
| Bleomycin | 658 | 5·7% |
| Busulfan | 22 | 0·2% |
| Lomustine (CCNU) | 395 | 3·4% |
| Cisplatin | 604 | 5·2% |
| Cyclophosphamide | 5,132 | 44·4% |
| Ifosfamide | 62 | 0·5% |
| Melphalan | 116 | 1·0% |
| Methotrexate (IV/IM) | 2,501 | 21·6% |
| 6-mercaptopurine | 3,657 | 31·5% |
| 6-thioguanine | 1,073 | 9·2% |
| Radiation dose: | ||
| Kidney (mean dose) | ||
| None | 3,849 | 34·1% |
| >0 to 10 Gy | 6,832 | 60·4% |
| > 10 to 20 Gy | 546 | 4·8% |
| > 20 Gy | 76 | 0·7% |
| Heart (mean dose) | ||
| None | 3,853 | 34·0% |
| > 0 to 10 Gy | 4,847 | 42·8% |
| > 10 to 20 Gy | 939 | 8·3% |
| > 20 to 30 Gy | 627 | 5·5% |
| > 30 Gy | 1,054 | 9·3% |
| Abdomen (max tumor dose) | ||
| None | 3,848 | 33·8% |
| >0 to 20 Gy | 5,531 | 48·6% |
| > 20 Gy | 1,993 | 17·5% |
| Lung (mean dose) | ||
| None | 3,850 | 34·0% |
| >0 to 10 Gy | 5,761 | 50·9% |
| > 10 Gy | 1,703 | 15·1% |
| Total Body Irradiation | ||
| No | 11,196 | 98·4% |
| Yes | 185 | 1·6% |
IV – intravenous, IM – intramuscular.
End of follow-up is 12/31/2013 or the death date for participants who died before the end of 2013. Seven participants are omitted due to missing death date.
Denominators for all percentage calculations are the number of participants for whom information was available. Approximately 2000 (15%) survivors did not have available treatment information, primarily because they or their proxy did not sign a medical release and less frequently because of missing information needed to calculate radiotherapy dosimetry to a specific organ.
Table 2:
Survivors who were recipients (n=103) or placed of a wait-list (n=67) for a solid organ transplantation greater than five years from diagnosis of childhood cancer*
| Organ type | Organ transplantations |
Wait-list registrations, never received an organ |
Total number of transplantations and wait-list registrations |
|---|---|---|---|
| Kidney | 50 | 21 | 71 |
| Heart | 37 | 25 | 62 |
| Liver | 9 | 15 | 24 |
| Lung | 7 | 6 | 13 |
Three survivors with a kidney transplant previously underwent a different solid organ transplant. For those wait-listed without receiving a transplanted organ, some survivors were listed for more than one organ.
Table 3:
Cancer treatment exposures for participants in the CCSS survivor cohort who received or registered on the wait-list for an organ transplantation (n=170)
| Survivors needing kidney transplantation (n=71) | |||
|---|---|---|---|
| Demographic Characteristics | |||
| Age at Cancer Diagnosis (median, IQR) in years | 2 (<1–9) | ||
| Age at Transplantation (median, IQR) in years | 25 (20–35) | ||
| Years from Cancer Diagnosis to Transplantation (median, IQR) | 21 (17–29) | ||
| Years after Wait-List or Transplantation for participants alive at end of follow-up (median, IQR) | 9 (5–14) | ||
| Years after Wait-List or Transplantation for participants dead at end of follow-up (median, IQR) | 7 (1–10) | ||
| Diagnosis | N | Percent | |
| Kidney tumor | 33 | 46·5% | |
| Acute lymphoblastic leukemia | 9 | 12·7% | |
| Acute myeloid and other leukemia | 4 | 5·6% | |
| Non-Hodgkin lymphoma | 8 | 11·3% | |
| Neuroblastoma | 7 | 9·9% | |
| Bone cancer | 7 | 9·9% | |
| Soft tissue sarcoma | 2 | 2·8% | |
| Central nervous system tumor | 1 | 1·4% | |
| Cancer treatment | N | Percent | |
| Cisplatin | No | 59 | 93·7% |
| Yes | 4 | 6·3% | |
| Ifosfamide | No | 59 | 93·7% |
| Yes | 4 | 6·3% | |
| Methotrexate (IV/IM) | No | 55 | 87·3% |
| Yes | 8 | 12·7% | |
| Nephrectomy | No | 39 | 61·9% |
| (all unilateral) | Yes | 24 | 38·1% |
| Mean Kidney Radiation Dose*† (Gy) | None | 26 | 41·3% |
| > 0 to 10 | 18 | 28·6% | |
| > 10 to 15 | 8 | 12·7% | |
| > 15 to 20 | 9 | 14·3% | |
| > 20 | 2 | 3·2% | |
| TBI | No | 59 | 93·7% |
| Yes | 4 | 6·3% | |
| treatment exposure data availability: complete (n=61), partial (n=4), none (n=6) | |||
| Survivors needing heart transplantation (n=62) | |||
| Demographic Characteristics | |||
| Age at Cancer Diagnosis (median, IQR) in years | 6 (3–11) | ||
| Age at Transplantation (median, IQR) in years | 28 (21–32) | ||
| Years from Cancer Diagnosis to Transplantation (median, IQR) | 17 (13–26) | ||
| Years after Wait-List or Transplantation for participants alive at end of follow-up (median, IQR) | 11 (6–20) | ||
| Years after Wait-List or Transplantation for participants dead at end of follow-up (median, IQR) | 1 (0–5) | ||
| Diagnosis | N | Percent | |
| Bone cancer | 15 | 24·2% | |
| Acute lymphoblastic leukemia | 8 | 12·9% | |
| Acute myeloid and other leukemia | 6 | 9·7% | |
| Hodgkin lymphoma | 12 | 19·4% | |
| Non-Hodgkin lymphoma | 10 | 16·1% | |
| Kidney tumor | 7 | 11·3% | |
| Soft tissue sarcoma | 3 | 4·8% | |
| Neuroblastoma | 1 | 1·6% | |
| Cancer treatment | N | Percent | |
| Anthracyclines | None | 9 | 17·0% |
| (mg/m2) | >0 to 150 | 3 | 5·7% |
| >150 to 300 | 4 | 7·5% | |
| >300 to 450 | 16 | 30·2% | |
| >450 | 21 | 39·6% | |
| Cisplatin | No | 53 | 94·6% |
| Yes | 3 | 5·4% | |
| Cyclophosphamide | None | 27 | 50·0% |
| (mg/m2) | >0 to 10000 | 10 | 18·5% |
| >10000 to 20000 | 12 | 22·2% | |
| >20000 | 5 | 9·3% | |
| Mean Heart Radiation Dose* (Gy) | None | 10 | 18·9% |
| >0 to 10 | 25 | 47·2% | |
| > 10 to 20 | 3 | 5·7% | |
| > 20 to 30 | 4 | 7·5% | |
| > 30 | 11 | 20·8% | |
| TBI | No | 52 | 98·1% |
| Yes | 1 | 1·9% | |
| treatment data availability: complete (n=52), partial (n=5), none (n=5) | |||
| Survivors needing liver transplantation (n=24) | |||
| Demographic Characteristics | |||
| Age at Cancer Diagnosis (median, IQR) in years | 6 (4–9) | ||
| Age at Transplantation (median, IQR) in years | 37 (25–38) | ||
| Years from Cancer Diagnosis to Transplantation (median, IQR) | 27 (20–29) | ||
| Years after Wait-List or Transplantation for participants alive at end of follow-up (median, IQR) | 6 (1–20) | ||
| Years after Wait-List or Transplantation for participants dead at end of follow-up (median, IQR) | 1 (0–3) | ||
| Diagnosis | N | Percent | |
| Acute lymphoblastic leukemia | 8 | 33·3% | |
| Acute myeloid and other leukemia | 3 | 12·5% | |
| Bone cancer | 4 | 16·7% | |
| Soft tissue sarcoma | 4 | 16·7% | |
| Kidney tumor | 3 | 12·5% | |
| Non-Hodgkin lymphoma | 1 | 4·2% | |
| Neuroblastoma | 1 | 4·2% | |
| Cancer treatment | N | Percent | |
| Dactinomycin | No | 12 | 57·1% |
| Yes | 9 | 42·9% | |
| Busulfan | No | 21 | 100·0% |
| Yes | 0 | 0·0% | |
| Cyclophosphamide | None | 5 | 25·0% |
| (mg/m2) | >0 to 10000 | 12 | 60·0% |
| >10000 to 20000 | 2 | 10·0% | |
| >20000 | 1 | 5·0% | |
| Melphalan | No | 21 | 100·0% |
| Yes | 0 | 0·0% | |
| Methotrexate (IV/IM) | No | 11 | 52·4% |
| Yes | 10 | 47·6% | |
| Max Abdomen Tumor Radiation Dose* (Gy) | None | 8 | 42·1% |
| >0 to 20 | 6 | 31·6% | |
| > 20 | 5 | 26·3% | |
| TBI | No | 18 | 94·7% |
| Yes | 1 | 5·3% | |
| treatment data availability: complete (n=19), partial (n=2), none (n=3) | |||
| Survivors needing lung transplantation (n=13) | |||
| Demographic Characteristics | |||
| Age at Cancer Diagnosis (median, IQR) in years | 12 (<1–16) | ||
| Age at Transplantation (median, IQR) in years | 30 (27–37) | ||
| Years from Cancer Diagnosis to Transplantation (median, IQR) | 21 (14–29) | ||
| Years after Wait-List or Transplantation for participants alive at end of follow-up (median, IQR) | 5 (1–15) | ||
| Years after Wait-List or Transplantation for participants dead at end of follow-up (median, IQR) | 3 (1–4) | ||
| Diagnosis | N | Percent | |
| Acute lymphoblastic leukemia | 3 | 23·1% | |
| Acute myeloid and other leukemia | 2 | 15·4% | |
| Hodgkin lymphoma | 3 | 23·1% | |
| Kidney tumor | 2 | 15·4% | |
| Non-Hodgkin lymphoma | 1 | 7·7% | |
| Neuroblastoma | 1 | 7·7% | |
| Central nervous system tumor | 1 | 7·7% | |
| Cancer treatment | N | Percent | |
| Carmustine | No | 8 | 72·7% |
| Yes | 3 | 27·3% | |
| Bleomycin | No | 10 | 90·9% |
| Yes | 1 | 9·1% | |
| Busulfan | No | 11 | 100·0% |
| Yes | 0 | 0·0% | |
| Lomustine | No | 11 | 100·0% |
| Yes | 0 | 0·0% | |
| Cisplatin | No | 10 | 90·9% |
| Yes | 1 | 9·1% | |
| Cyclophosphamide | None | 4 | 44·4% |
| (mg/m2) | >0 to 10000 | 4 | 44·4% |
| >10000 to 20000 | 1 | 11·1% | |
| >20000 | 0 | 0·0% | |
| Methotrexate (IV/IM) | No | 7 | 63·6% |
| Yes | 4 | 36·4% | |
| Mean Lung Radiation Dose*† (Gy) | None | 2 | 18·2% |
| >0 to 10 | 4 | 36·4% | |
| >10 | 5 | 45·5% | |
| TBI | No | 9 | 81·8% |
| Yes | 2 | 18·2% | |
| treatment data availability: complete (n=11), partial (n=0), none (n=2) | |||
IQR – interquartile range, IV – intravenous, IM – intramuscular, TBI – total body irradiation.
Radiation received as TBI is included in the organ-specific dose calculations.
If the right and left kidneys (lungs) received different amounts of radiation, the survivor was classified based on the kidney (lung) with the lesser degree of radiation exposure.
Table 4:
Risk factors associated with organ-specific wait-list registration or transplantation in survivors of childhood cancer
| *Kidney wait-list registration or transplantation | ||||
|---|---|---|---|---|
| Treatment | Exposure | Hazard Ratio |
95% Confidence Interval |
p-value |
| Ifosfamide | No | 1·0 | referent | |
| Yes | 24.9 | (7·4, 83·5) | <0·0001 | |
| IV or IM Methotrexate | No | 1·0 | referent | |
| Yes | 0·6 | (0·3, 1·5) | 0·30 | |
| Mean Kidney Radiation Dose | None | 1·0 | referent | |
| (Gy) | >0 to 10† | 0·4 | (0·2, 0·7) | 0·0040 |
| > 10 to 15† | 1.6 | (0·6, 4·0) | 0.35 | |
| > 15 to 20† | 3.6 | (1·5, 8·5) | 0·0041 | |
| > 20† | 4·6 | (1·1, 19·6) | 0·040 | |
| TBI | 6.9 | (2·3, 21.1) | 0·0007 | |
| Nephrectomy | No | 1·0 | referent | |
| (all unilateral) | Yes | 4·2 | (2·3, 7·7) | <0·0001 |
| Age at Primary Cancer | 0–4 | 1·5 | (0·6, 3·7) | 0·38 |
| Diagnosis (years) | 5–9 | 1·2 | (0·4, 3·3) | 0·72 |
| 10–14 | 0·8 | (0·2, 2·6) | 0·71 | |
| 15–20 | 1·0 | referent | ||
| *Heart wait-list registration or transplantation | ||||
| Treatment factor | Level of exposure | Hazard Ratio |
95% Confidence Interval |
p-value |
| Cyclophosphamide (mg/m2) | None | referent | ||
| >0 to 10000 | 0·3 | (0·1, 0·6) | 0·0018 | |
| >10000 to 20000 | 0·8 | (0·4, 1·7) | 0·57 | |
| >20000 | 1·3 | (0·5, 3·7) | 0·61 | |
| Anthracyclines (mg/m2) | None | referent | ||
| >0 to 150 | 8·4 | (2·2, 32·6) | 0·002 | |
| >150 to 300 | 5·0 | (1·3, 19·5) | 0·021 | |
| >300 to 450 | 26·5 | (9·9, 71.0) | <0·0001 | |
| >450 | 94·2 | (35·3, 251·2) | <0·0001 | |
| Mean Heart Radiation Dose | None | referent | ||
| (Gy) | >0 to 10 | 2·2 | (1·0, 4·8) | 0·050 |
| > 10 to 20 | 1·9 | (0·5, 7·3) | 0·33 | |
| > 20 to 30 | 6·1 | (1·8, 20·6) | 0·0035 | |
| > 30 | 19·7 | (7·1, 54·2) | <0·0001 | |
| *Liver wait-list registration or transplantation | ||||
| Treatment | Exposure | Hazard Ratio |
95% Confidence Interval |
p-value |
| Dactinomycin | No | 1·0 | referent | |
| Yes | 3·8 | (1·3, 11·3) | 0·016 | |
| Cyclophosphamide | No | 1·0 | referent | |
| Yes | 2·4 | (0·8, 7·5) | 0·13 | |
| IV or IM Methotrexate | No | 1·0 | referent | |
| Yes | 3·3 | (1·0, 10·2) | 0·041 | |
| Antimetabolites (6-MP or 6-TG) | No | 1·0 | referent | |
| Yes | 1·6 | (0·5, 5·1) | 0·46 | |
| Max Abdomen Tumor | None | 1·0 | referent | |
| Radiation Dose (Gy) | >0 to 20† | 0·4 | (0·1, 1·5) | 0·18 |
| > 20† | 2·2 | (0·6, 7·8) | 0·21 | |
| TBI | 2·2 | (0·3, 18·6) | 0·48 | |
| Sex | Male | 1·0 | referent | |
| Female | 0·4 | (0·2, 1·2) | 0·11 | |
| *Outcome event: first lung wait-list registration or transplantation | ||||
| Treatment factor | Level of exposure | Hazard Ratio |
95% Confidence Interval |
p-value |
| Carmustine | No | 1·0 | referent | |
| Yes | 12·3 | (3·1, 48·9) | 0·0004 | |
| IV or IM Methotrexate | No | 1·0 | referent | |
| Yes | 2·7 | (0·8, 9·9) | 0·12 | |
| Mean Lung Radiation Dose | None | 1·0 | referent | |
| (Gy) | >0 to 10 | 1·5 | (0·3, 8·3) | 0·63 |
| > 10 | 15·6 | (2·6, 92·7) | 0·0025 | |
| Age at primary cancer diagnosis | 0–4 yrs old | 9·7 | (1·0, 93·9) | 0·050 |
| 5–9 yrs old | 1·9 | (0·1, 31·3) | 0·65 | |
| 10–14 yrs old | 4·2 | (0·4, 40·5) | 0·22 | |
| 15–20 yrs old | 1·0 | referent | ||
| Sex | Male | 1·0 | referent | |
| Female | 3·3 | (0·9, 12·4) | 0·083 | |
IV – intravenous, IM – intramuscular, TBI – total body irradiation.
Only cancer treatment exposures associated with statistically significant increases in risk for SOT are displayed. Other factors examined include: sex, age at primary cancer diagnosis, cisplatin, cyclophosphamide and busulfan (multiple models); antimetabolites, melphalan and abdominal radiation (liver model); bleomycin and lomustine (lung model).
non-TBI
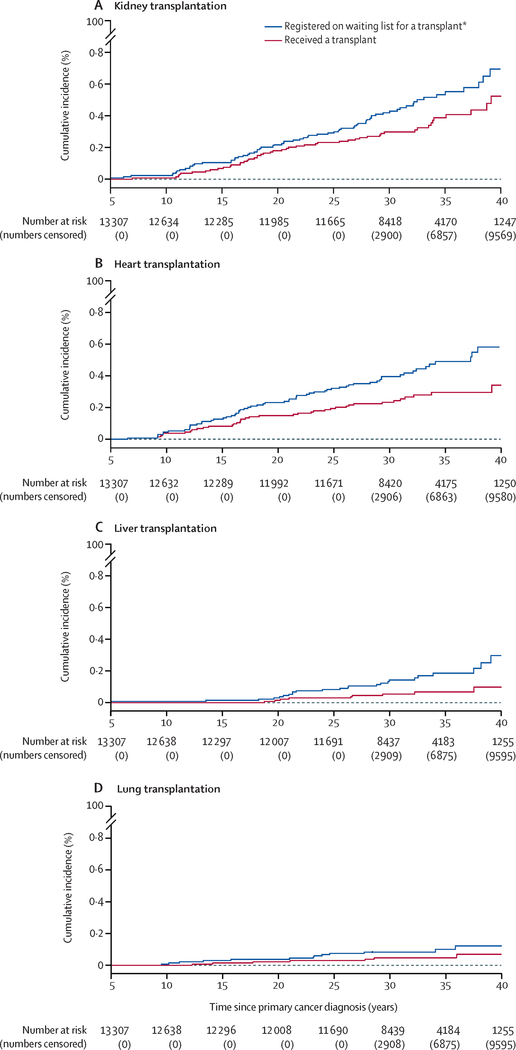
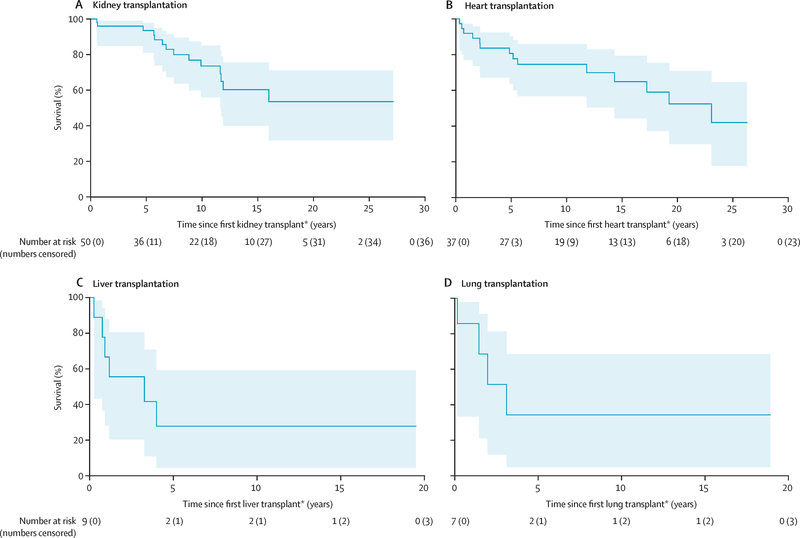
Seventy-one survivors had end-stage kidney disease warranting kidney transplantation. Of those, 21 were placed on a wait-list without receiving a kidney, while 50 survivors received a kidney transplant (Table 2). At 35 years after cancer diagnosis, cumulative incidence of kidney transplantation was 0·39% (95% CI 0·27–0·51) and cumulative incidence of being wait-listed and/or receiving a kidney was 0·54% (95% CI 0·40–0·67; Figure 1a). Exposure to ifosfamide and total body irradiation (TBI) were associated with the highest risk of being wait-listed and and/or receiving a kidney transplant. Additional significant associations included unilateral nephrectomy and non-TBI kidney radiation doses >15 Gy. The 5 and 10-year survival estimates from time of kidney transplantation were 93·5% (95% CI 81·0–97·9%) and 73·6% (95% CI 56·0–85·0; Figure 2a). Of those wait-listed, but not receiving a kidney, 10 (47.6%) out of 21 died before the end of follow-up with a median time from wait-list to death of 5.9 (IQR 0.3 to >21.8) years.
Figure 1:
Cumulative incidence of transplantation and transplant wait-listing for (a) kidney, (b) heart, (c) liver, and (d) lung.
Figure 2:
Overall survival following (a) kidney, (b) heart, (c) liver, and (d) lung transplantation (numbers above lines show numbers censored, numbers below lines show numbers at risk, shaded area is the 95% CI).
A total of 62 survivors had end-stage heart failure warranting heart transplantation. Of those, 25 were wait-listed without receiving a heart, while 37 survivors received a heart transplant (Table 2). At 35 years after cancer diagnosis, cumulative incidence of heart transplantation was 0·30% (95% CI 0·20–0·40) and cumulative incidence of being wait-listed and/or receiving a heart was 0·49% (95% CI 0·36–0·62%; Figure 1b). The HR increased in a dose dependent manner with anthracycline exposure (p<0·0001, Table 4) and heart radiation dose (p<0·0001). The 5-year survival estimate from time of heart transplantation was 80·6% (95% CI 63·6–90·3, Figure 2b). Of those wait-listed, but not receiving a heart, 20 (80%) out of 25 died before the end of follow-up with a median time from wait-list to death of 0.7 (IQR 0.1–4. 5) years.
A total of 24 survivors had end-stage liver disease warranting liver transplantation. Of those, 15 were wait-listed without receiving a liver, while nine survivors had a liver transplant (Table 2). At 35 years after cancer diagnosis, cumulative incidence of liver transplantation was 0·07% (95% CI 0·02–0·12) and cumulative incidence of being wait-listed and/or receiving a liver was 0·19% (95% CI 0·10–0·27%; Figure 1c). Significant associations were identified for dactinomycin and methotrexate. The 5-year survival estimate from time of liver transplantation was 27·8% (95% CI 4·4–59·1%), with the full survival curve shown in Figure 2c. Of those wait-listed, but not receiving a liver, 11 (73%) out of 15 died before the end of follow-up with a median time from wait-list to death of 0.9 (IQR 0.2–4.0) years.
A total of 13 survivors had pulmonary damage significant enough to warrant lung transplantation. Of those, six were wait-listed without receiving a lung, while seven survivors received a lung transplant (Table 2). At 35 years after cancer diagnosis, cumulative incidence of lung transplantation was 0·05% (95% CI 0·01–0·08) and cumulative incidence of being wait-listed and/or receiving a lung was 0·10% (95% CI 0·04–0·16%; Figure 1d). Exposure to carmustine and lung radiation over 10 Gy were significantly associated with lung transplantation. The 5-year survival estimate from time of lung transplantation was 34·3% (95% CI 4·8–68·6%; Figure 2d). Of those wait-listed, but not receiving a lung, 5 (83.3%) out of 6 died before the end of follow-up with a median time from wait-list to death of 0.7 (IQR 0.3–3.1) years.
Discussion
To our knowledge, this is the first linkage of a population of survivors of common childhood cancers with the OPTN providing outcomes from the largest series of cancer survivors with SOT to date. Incidence of kidney, heart, liver, or lung transplantation is low among five-year childhood cancer survivors at a median age of 39 years. There are clear organ-specific radiation and chemotherapy exposures that increase risk of requiring SOT following cure of childhood cancer. Direct comparison of five-year survival after SOT to those recipients that were not childhood cancer survivors was not performed due to unavailability of a relevant data set with covariates. However, publicly available OPTN data as of April 26, 2019 (https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/) shows that for kidney and heart transplants, the five-year survival and 95% CI for 18–34-year-olds transplanted from 2008–2015 for any diagnosis was comparable to those among childhood cancer survivors (kidney 95.5% [95.0–95.9%] and heart 74.9% [71.8–77.7%]). For liver and lung transplants, the five-year survival was higher in the general population (liver 79.5% [77.2–81.6%] and lung 54.9% [51.2–58.5%]). In this cohort, less than ten childhood cancer survivors received a liver or lung transplant, which limits ability to draw meaningful conclusions relative to the general population. Survival following kidney, heart, liver, or lung transplantation demonstrates that SOT should be considered for five-year survivors with life-limiting end organ failure, particularly for those with renal and cardiac failure.
Established general guidelines exist regarding the evaluation of SOT candidates.20–22 However, guidelines regarding cancer involve excluding patients with untreated or untreatable malignancy and often mention oncology clearance. Ambiguity can result in different practice among treating institutions, owing in part to lack of evidence available regarding these populations. SOT may be a part of frontline therapy, such as with hepatoblastoma, where outcomes look promising (US Scientific Registry of Transplant Recipients and University of Pittsburgh registry)23 or may be performed when organ failure is a consequence of acute toxicity. The CCSS cannot assess all SOT following cancer diagnosis since cohort entry is five years after diagnosis. These findings inform oncologists and organ transplant physicians about survivors who are at least five years out from original diagnosis regarding risk factors and outcomes.
While kidney transplantation is reported in survivors of childhood cancer,10–12 cumulative incidence and survival after kidney transplantation is understudied. Risk factors for renal toxicity, in general, are well-established and include TBI,24 nephrectomy,12 radiotherapy, platinum agents, and ifosfamide.24,25 This study further details risk of ifosfamide, modest radiation exposure of the kidneys and unilateral nephrectomy leading to end-organ failure severe enough to require SOT. With radiotherapy, we identified increased risk at exposures >15 Gy. There was no risk associated with platinum agents, though with increasing age and follow-up, that risk may emerge. Notably, survival continues to steadily drop even after the five-year mark, which is similar to observations in overall analyses of kidney transplantation with respect to graft and overall survival.26,27
There are more reports on heart transplantation in the literature for childhood cancer survivors than any other SOT, often associated with good long-term survival.10,13–15 While not as common as kidney transplantation in our cohort, it was the second most common organ transplanted. Here we establish excellent 5-year survival, comparable to the only other adequately sized previous study, which showed 74% survival at 5-years and 67% at 10 years after.13 Known risk factors for heart failure were also identified as risk factors for heart transplantation including anthracyclines and cardiac radiotherapy, each in a dose dependent manner. It would be ideal to have a better understanding of these risks as independent or potentiating within each dose level, however, there were not enough events in separate strata to examine this relationship. No other exposures were identified to be associated with SOT in this study.
While liver transplantation can play a significant role in treatment of pediatric cancer, particularly advanced stage hepatoblastoma (not captured in CCSS),23 its use to treat late hepatotoxicity in survivors of childhood cancer is less frequently reported,10 consistent with the much smaller cumulative incidence found in this study. Even though this is the largest series of liver transplants to date, the small number of survivors with a liver transplant make it difficult to interpret survival estimates and risk factor analyses. Importantly, we observed that long-term survival is possible after liver transplantation in survivors of childhood cancer. The most significant risk factors for hepatotoxicity include radiation therapy, HSCT, and infectious hepatitis.4,28 While methotrexate and dactinomycin are known to cause acute hepatotoxicity, this study demonstrates the risk these two agents carry for long-term hepatotoxicity, not recognized to date. Abdominal radiation was not identified as a risk factor, possibly due to low incidence of SOT. Further assessment should be performed as this population ages.
Lung transplantation was the least common procedure. Previously reported in case reports or case series,10,13 very little is known regarding this therapeutic intervention in childhood cancer survivors. While the current analysis is the largest reported, low incidence limits interpretation of survival and risk factor analyses. Despite this, we demonstrate long-term survival is possible after lung transplantation in cancer survivors. Risk factors for the most serious lung toxicities include carmustine and lung radiation.6 Now, these two exposures are additionally associated with lung damage severe enough to require lung transplantation.
A concern for any survivor considering SOT is the required use of prolonged immunosuppression, and whether that could lead to relapse of primary cancer or development of secondary malignancies. Original reports regarding these issues might have limited survivors’ access to SOT.29 While secondary malignancies can be a significant issue in childhood cancer survivors as well as survivors of SOT, the majority of these secondary malignancies are skin cancers,30 for which good screening is available. The current study was not able to assess risks of relapse or secondary malignancies as many survivors had SOT after the last recorded CCSS contact. There are very limited data available regarding this, and additional research is needed.
Study of this rare outcome in the second, more recently treated cohort of pediatric cancer survivors, would be valuable in determining if changes in treatment exposure would reduce the risk for SOT.
Limitations of this study include that relatively small numbers of survivors receive SOT, especially for liver and lung transplantation. CCSS is often limited by self-reported outcomes, but linkage to OPTN with direct assessment of SOT overcomes that limitation. This study is also limited to the US population, and it is unknown if results can be generalized or will be different in other populations. The exclusion of children diagnosed with hepatoblastoma, retinoblastoma and germ cell tumors from the CCSS cohort may impact the total number of five-year survivors who received SOT. The use of SOT in cancer therapy or for acute complications of treatment is open for further study outside the context of CCSS. The strengths of this study include the ability to link two large databases, each with a wealth of information, in order to define this large cohort and create the first assessments, to our knowledge, of cumulative incidence, survival, and risk factor analyses for this unique survivor population.
This study provides evidence for health care providers regarding risk of life-threatening solid organ failure and transplantation occurring five years or longer after diagnosis of common pediatric cancers. It provides evidence for efficacy of SOT in recipients who have a history of pediatric cancer, informing practice in organ transplant subspecialties, and data for third party payers, who may be resistant to payment for these rare life-saving procedures after a cancer diagnosis, based on old and limited number of case reports.
Supplementary Material
Research in Context:
Evidence before this study:
The authors reviewed published literature prior to analysis using PubMed from 1966 up until Jan 1, 2019 with search terms “pediatric cancer survivors” and “liver transplantation”, “kidney transplantation”, “lung transplantation”, and/or “heart transplantation”. We subsequently examined the bibliographies of references gathered. Most of the literature found consisted of case reports and small case series that were descriptive in nature, without detail regarding risk factors or group summary statistics.
Added value of this study:
This study is the first to link the Childhood Cancer Survivor Study population, the largest north American cohort of pediatric cancer survivors, with the Organ Procurement and Transplantation Network, representing all solid organ transplants performed in the United States. To our knowledge, this is the largest analysis to date to define the incidence of, risk factors for, and survival after end-organ failure and transplantation in survivors of childhood cancer, enabling providers and patients to better understand risks after cancer therapy as well as survival after this potentially life-saving intervention.
Implications of all the available evidence:
This study provides evidence for health care providers regarding risk of life-threatening solid organ failure and transplantation after diagnosis and treatment of common pediatric cancers. It provides evidence for efficacy of organ transplantation in recipients with a history of pediatric cancer, informing practice in the organ transplant subspecialties. Additionally, it provides data for third party payers who may be resistant to pay for these rare, life-saving procedures after a pediatric cancer diagnosis based on old and very limited case reports published in the literature.
Funding and Acknowledgements
This work was supported by U24 CA-55727 (G.T. Armstrong, MD, MSCE, Principal Investigator), National Institute of Health, Bethesda, MD, Cancer Center Support (CORE) Grant (CA-21765) to St. Jude Children’s Research Hospital, and the American Lebanese Syrian Associated Charities (ALSAC), Memphis, TN. This work was also supported in part by Health Resources and Services Administration contract 234–2005-37011C. The data reported here have been in part supplied by UNOS as the contractor for the OPTN. The content, interpretation, and reporting of these data are the responsibility of the authors alone, in no way should be seen as an official policy of or interpretation by the OPTN or the US Government, and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. No authors are employees of the NIH. G T Armstrong, L L Robison and K C Oeffinger are Principal Investigators of NIH grants. The CCSS is a publicly available data resource. Investigators may apply for specific analyses through a proposal process available on the website https://ccss.stjude.org. The data set specific to these analyses is not publicly available.
Footnotes
Declaration of Interests
Subsequent to this work, Dr. Dietz became employed by bluebird bio, Inc. which provided no support and had no oversight over or input into the completion of this study.
The authors have no other conflicts of interest to declare.
References
- 1.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014; 14(1): 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breslow NE, Collins AJ, Ritchey ML, Grigoriev YA, Peterson SM, Green DM. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol 2005; 174(5): 1972–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005; 23(12): 2629–36. [DOI] [PubMed] [Google Scholar]
- 4.Castellino S, Muir A, Shah A, et al. Hepato-biliary late effects in survivors of childhood and adolescent cancer: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2010; 54(5): 663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339: b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz AC, Chen Y, Yasui Y, et al. Risk and impact of pulmonary complications in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2016; 122(23): 3687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med 2016; 374(9): 833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2018; 19(12): 1590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013; 309(22): 2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitinjaneh A, Burns LJ, Majhail NS. Solid organ transplantation in survivors of hematopoietic cell transplantation: a single institution case series and literature review. Clin Transplant 2010; 24(4): E94–102. [DOI] [PubMed] [Google Scholar]
- 11.Aronson DC, Slaar A, Heinen RC, de Kraker J, Heij HA. Long-term outcome of bilateral Wilms tumors (BWT). Pediatr Blood Cancer 2011; 56(7): 1110–3. [DOI] [PubMed] [Google Scholar]
- 12.Grigoriev Y, Lange J, Peterson SM, et al. Treatments and outcomes for end-stage renal disease following Wilms tumor. Pediatr Nephrol 2012; 27(8): 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitt G, Anazodo A, Burch M, Bunch K. Cardiac or cardiopulmonary transplantation in childhood cancer survivors: an increasing need? Eur J Cancer 2009; 45(17): 3027–34. [DOI] [PubMed] [Google Scholar]
- 14.Arico M, Pedroni E, Nespoli L, Vigano M, Porta F, Burgio GR. Long term survival after heart transplantation for doxorubicin induced cardiomyopathy. Arch Dis Child 1991; 66(8): 985–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor DO, Farhoud HH, Kfoury G, et al. Cardiac transplantation in survivors of lymphoma: a multi-institutional survey. Transplantation 2000; 69(10): 2112–5. [DOI] [PubMed] [Google Scholar]
- 16.Koerner MM, Tenderich G, Minami K, et al. Results of heart transplantation in patients with preexisting malignancies. Am J Cardiol 1997; 79(7): 988–91. [DOI] [PubMed] [Google Scholar]
- 17.Massie AB, Kucirka LM, Kuricka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014; 14(8): 1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27(14): 2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006; 166(1 Pt 2): 141–57. [DOI] [PubMed] [Google Scholar]
- 20.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006; 25(7): 745–55. [DOI] [PubMed] [Google Scholar]
- 21.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant 2016; 35(1): 1–23. [DOI] [PubMed] [Google Scholar]
- 22.Kasiske BL, Cangro CB, Hariharan S, et al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant 2001; 1 Suppl 2: 3–95. [PubMed] [Google Scholar]
- 23.Vinayak R, Cruz RJ, Ranganathan S, et al. Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981–2015). Liver Transpl 2017; 23(12): 1577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, et al. Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev 2013; (10): CD008944. [DOI] [PubMed] [Google Scholar]
- 25.Dekkers IA, Blijdorp K, Cransberg K, et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol 2013; 8(6): 922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald SP, Craig JC, Association AaNZPN. Long-term survival of children with end-stage renal disease. N Engl J Med 2004; 350(26): 2654–62. [DOI] [PubMed] [Google Scholar]
- 27.Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med 2014; 371(6): 549–58. [DOI] [PubMed] [Google Scholar]
- 28.Mulder RL, Kremer LC, Koot BG, et al. Surveillance of hepatic late adverse effects in a large cohort of long-term survivors of childhood cancer: prevalence and risk factors. Eur J Cancer 2013; 49(1): 185–93. [DOI] [PubMed] [Google Scholar]
- 29.Penn I. Renal transplantation for Wilms tumor: report of 20 cases. J Urol 1979; 122(6): 793–4. [DOI] [PubMed] [Google Scholar]
- 30.Ploos van Amstel S, Vogelzang JL, Starink MV, Jager KJ, Groothoff JW. Long-Term Risk of Cancer in Survivors of Pediatric ESRD. Clin J Am Soc Nephrol 2015; 10(12): 2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.