Abstract
Inflammasomes are cytoplasmic multi-protein complexes that coordinate inflammatory responses, including those that take place during pregnancy. Inflammasomes and their downstream mediators caspase-1 and IL-1β are expressed by gestational tissues (e.g. the placenta and chorioamniotic membranes) during normal pregnancy. Yet, only the activation of the NLRP3 inflammasome in the chorioamniotic membranes has been partially implicated in the sterile inflammatory process of term parturition. In vivo and ex vivo studies have consistently shown that the activation of the NLRP3 inflammasome is a mechanism whereby preterm labor and birth occur in the context of microbial- or alarmin-induced inflammation. In the placenta, the activation of the NLRP3 inflammasome is involved in the pathogenesis of preeclampsia and other pregnancy syndromes associated with placental inflammation. This evidence suggests that inhibition of the NLRP3 inflammasome or its downstream mediators may foster the development of novel anti-inflammatory therapies for the prevention or treatment of pregnancy complications.
AN OVERVIEW OF THE INFLAMMASOMES
Inflammasomes are cytosolic multiprotein complexes that typically consist of a sensor molecule (e.g. a pattern recognition receptor), the adaptor protein (apoptosis-associated speck-like protein containing a caspase recruitment domain; ASC), and the pro-inflammatory caspase-1 (1). Inflammasome sensor molecules are responsible for recognizing pathogen-associated molecular patterns (PAMPs) or endogenous danger signals/alarmins/damage-associated molecule patterns (DAMPs) (2–11). Upon recognition, oligomerization of the inflammasome complex and activation of caspase-1 occur (2–7), which initiates downstream responses including the processing and release of interleukin (IL)-1β and IL-18 (12–18) as well as pyroptosis, a lytic form of cell death (19–22). Inflammasomes were thought to be exclusive to innate immune signaling (1, 23); however, recent reports showed that these platforms also promote adaptive immune responses (24–26). Several members of the nucleotide-binding oligomerization domain leucine-rich repeat-containing protein (NLR) family function as the sensor molecules of the inflammasome (1, 27, 28); therefore, it was initially thought that NLR signaling was inherent to inflammasome activation (2). Consequently, multiple NLR-dependent inflammasomes were described, namely nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein (NLRP)-1 (1), NLRP3 (28), and NLR family caspase-activation-and-recruitment domain (CARD)-domain-containing protein-4 (NLRC4) (27, 29). Yet, NLR-independent inflammasomes that are driven by alternative sensor molecules such as absent in melanoma-2 (AIM2) (30–33) and pyrin (34) have also been described.
To date, five distinct inflammasomes have been well characterized, each identified by its specific sensor molecule: NLRP1, NLRP3, NLRC4, AIM2, and pyrin (2–7). Other inflammasomes that require further characterization of their specific ligands, mechanisms of action, and roles in disease include NLRP6 (35), NLRP7 (36), NLRP12 (37), retinoic acid-inducible gene-I (RIG-I) (38, 39), and interferon-γ (IFNγ)-inducible protein-16 (IFI16) (40, 41). Next, we will provide a brief overview of the NLRP1, NLRC4, AIM2, pyrin, and NLRP3 inflammasomes.
The NLRP1 inflammasome was the first to be described (1) and exists as a single protein in humans, whereas mice express multiple NLRP1 paralogues (42). Initial reports showed that NLRP1 responds to the lethal toxin of Bacillus anthracis (42), and subsequent studies indicated that this inflammasome also responds to Toxoplasma gondii (43), Listeria monocytogenes, and Shigella flexneri (44). The NLRP1 inflammasome can also be activated by the microbial product muramyl-dipeptide, a component of peptidoglycan (45). Interestingly, mutations in NLRP1 have been associated with severe inflammatory skin disorders (46), which may be due to the high expression of this molecule in keratinocytes (46). Therefore, the NLRP1 inflammasome is implicated in host defense against pathogens and skin homeostasis.
NLRC4 was first characterized as an apoptotic-protease activating factor-1 (APAF1)-related protein (27), and was shown to induce inflammasome activation in response to Salmonella typhimurium infection in mice (29). Subsequent reports indicated that the murine NLRC4 inflammasome was activated in response to flagellin (47) as well as multiple components of the bacterial type 3 secretion system (T3SS) (48). The NLRC4 inflammasome is unique in that it relies on multiple NLR family apoptosis inhibitory proteins (NAIPs) (49) to detect specific bacterial proteins (e.g. T3SS rod protein in mice (48, 50) and T3SS needle subunit in humans (48). NAIPs can then interact with NLRC4 to trigger the assembly of this inflammasome (48, 50). Humans express only one NAIP with at least two reported isoforms (51), which recognize Chromobacterium violaceum and Salmonella flagellin (48, 51). The assembly of the NLRC4 inflammasome may also require the phosphorylation of NLRC4 (52), highlighting the complexity of the mechanisms by which this inflammasome is activated.
The AIM2 inflammasome is unique in that it is activated by cytosolic DNA of microbial or host origin independently of NLRP3 and TLR signaling (30–32, 53). In the absence of cytosolic DNA, AIM2 exists in an auto-inhibitory state with its HIN200 domain tightly bound to the pyrin domain (PYD) (54, 55). The binding of cytosolic DNA to HIN200 releases the protected PYD, allowing for self-oligomerization and interaction with ASC in order to initiate inflammasome assembly (54, 55). The AIM2 inflammasome orchestrates host defense against DNA viruses such as cytomegalovirus and vaccinia virus, as well as infections with intracellular bacterial pathogens (30–32, 53, 56, 57). In addition, the AIM2 inflammasome is implicated in the pathogenesis of psoriasis (58) and prostate cancer (59). Hence, the AIM2 inflammasome participates in host defense and tumor progression (5).
The most recently discovered of the well characterized inflammasomes is the Pyrin inflammasome (34, 60). This inflammasome indirectly responds to Burkholderia cenocepacia and Clostridium difficile (34, 60) by sensing the bacterial modification and inactivation of Rho GTPases (60). Such modifications include glycosylation, adenylation, and ADP-ribosylation, all of which result in activation of the Pyrin inflammasome; yet, direct interactions between Rho and Pyrin have not been detected (60). Interestingly, recent reports indicate that the activation of the Pyrin inflammasome can occur in response to microtubule disruption and other cytoskeletal modifications resulting from microbial infection, rather than in response to the pathogen itself (61, 62). More recently, it was shown that specific bile acid analogs can directly activate the Pyrin inflammasome, suggesting a new mechanism whereby the production of bile acid metabolites by gut microbiota could affect host innate immune responses (63). Therefore, the Pyrin inflammasome can participate in host defense responses and gut homeostasis.
The most widely studied of the inflammasomes is the NLRP3 inflammasome (23, 28, 64–66). This inflammasome has two key characteristics: first, it can be activated by a wide range of unrelated molecules, including PAMPs (64, 67) and both endogenous and exogenous DAMPs or alarmins (23, 66, 68), as has been previously reviewed (11). Second, the NLRP3 inflammasome is highly expressed in innate immune cells such as macrophages, neutrophils, and dendritic cells (23, 69, 70), as well as in multiple tissues (23, 68, 71–73). Notably, classical or canonical activation of the NLRP3 inflammasome requires two distinct steps: priming and assembly (74, 75). The priming step is initiated by inflammatory stimuli via surface PRRs such as TLRs, which induce NF-κB activation resulting in the increase of NLRP3 and pro-IL-1β (65, 76). The second step includes multiple signaling events occurring upon recognition of the PAMP or DAMP which, in turn, promotes the assembly of the inflammasome complex, the cleavage of caspase-1, and subsequent processing and release of IL-1β and IL-18 (11). The activation of the NLRP3 inflammasome has been associated with multiple cellular events including potassium efflux (77, 78), lysosomal rupture (79), mitochondrial dysfunction (80), calcium influx (81, 82), and decreased cellular cAMP levels (82), many of which seemed to depend on the activating stimulus. A later study suggested that potassium efflux is a common cellular event associated with NLRP3 inflammasome activation by showing that multiple microbial and endogenous signals induce a drop in cytosolic potassium that is sufficient to activate this inflammasome (83). Yet, even potassium efflux-independent pathways of NLRP3 inflammasome activation have been described (84). Further studies are required to elucidate all of the cellular pathways associated with the canonical activation of this inflammasome.
In addition to the canonical activation pathway of the NLRP3 inflammasome, this inflammasome can also be indirectly triggered by caspase-11 in mice (85) (or the homologues caspase-4 and caspase-5 in humans (85, 86)), which has been termed the non-canonical activation pathway (87). The non-canonical pathway was first described in murine macrophages infected with Escherichia coli, Citrobacter rodentium, and Vibrio cholera (87). This report showed that caspase-11 was required for the non-canonical activation of the NLRP3 inflammasome, which subsequently leads to the cleavage of caspase-1 and release of IL-1β and IL-18 (87). Notably, in the non-canonical pathway, caspase-11 directly recognizes and binds to intracellular lipopolysaccharide (LPS) (88, 89), resulting in its oligomerization and activation by auto-proteolytic cleavage (90). Active caspase-11 can then directly induce the cleavage of gasdermin D (GSDMD) to cause pyroptosis (e.g. release of caspase-1-processed IL-1β and IL-18) (87, 91).
In summary, inflammasomes mediate central processes during host defense against pathogens and immunoregulation, whose processes are essential for homeostasis (92). Hence, aberrations in inflammasome activation can be implicated in the pathogenesis of disease (92). In this review, we focus on describing the role of inflammasomes during normal pregnancy and its complications, including preterm labor and birth, the leading cause of perinatal morbidity and mortality worldwide (93, 94), and pregnancy disorders associated with placental inflammation.
INFLAMMASOMES DURING NORMAL PREGNANCY
Inflammation is a key process in reproductive success since it is required for implantation (95), pregnancy maintenance (96), and parturition (97–99). Therefore, it is tempting to propose that inflammasomes are involved in each of the above processes and, consequently, their components are expressed in the gestational tissues.
Inflammasome components in the gestational tissues
Inflammasome components have been detected during pregnancy in both maternal and fetal compartments. Initial reports showed that NLRP3 (100–102), NLRC4 (103), and NLRP1 (102) are expressed by peripheral leukocytes of pregnant women. In the placenta (organ serving as the lungs, liver, and kidney for the fetus (104)), a tissue-wide survey revealed that multiple sensor molecules including NLRP1, NLRP3, and NLRC4 were expressed (105). In the first trimester, in vitro studies have shown that placental cells (e.g. trophoblasts) expressed NLRP1, NLRP3, and NLRC4 (106, 107), as well as NLRP2 (108). At term (≥37 weeks of gestation), placental tissues also expressed these sensor molecules (106, 109–114). Mirroring the expression of the NLRs, the adaptor protein ASC (or PYCARD) is also expressed in the placenta throughout pregnancy (105–107, 113, 114). The chorioamniotic membranes (also known as the extraplacental membranes: fetal tissues forming the amniotic cavity (115)) expressed sensor molecules of the inflammasome, namely NLRP1 (116), NLRP3 (109, 116), NLRC4 (116), and AIM2 (116), as well as ASC (117). Immune cells infiltrating the chorioamniotic membranes (e.g. choriodecidual leukocytes) also expressed ASC (117). Moreover, NLRP3 is expressed by myometrial tissues from women at term pregnancy (118). Together with the fact that inflammatory caspases (caspase-1 and caspase-4) have been detected in the human placenta (110–113, 119, 120), chorioamniotic membranes (110, 116, 117, 121, 122), and myometrium (121), this evidence indicates that gestational tissues possess the machinery to initiate inflammasome-mediated immune responses during pregnancy. Figure 1 includes a schematic representation of the inflammasomes reported in the chorioamniotic membranes during normal pregnancy.
Figure 1. Inflammasomes in the chorioamniotic membranes during normal parturition.
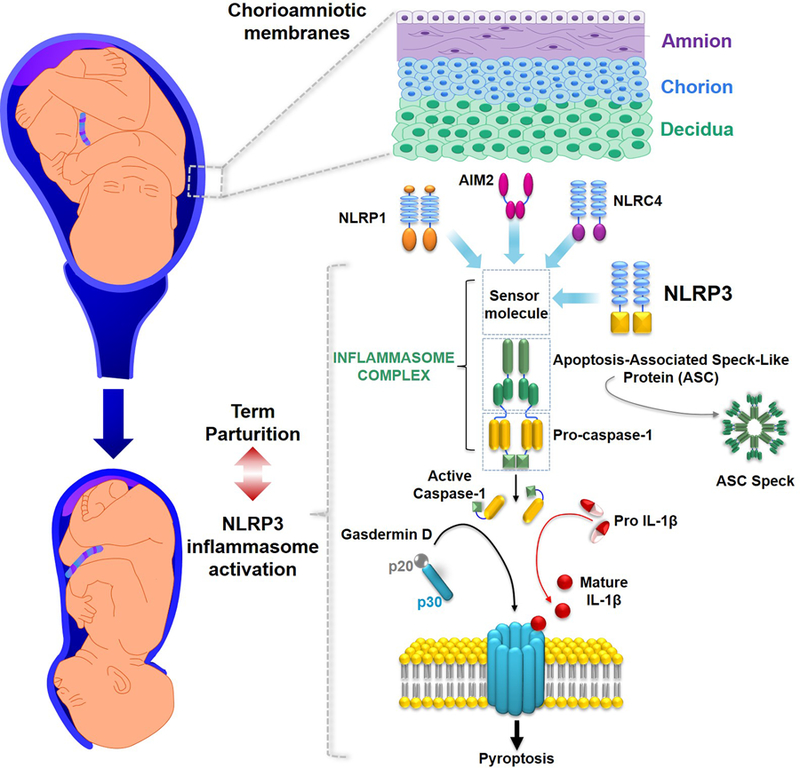
Representative image of the chorioamniotic membranes (amnion, chorion, and decidua) surrounding the amniotic cavity containing the fetus and amniotic fluid. The NLRP1, AIM2, NLRC4 and NLRP3 sensor molecules have been detected in the chorioamniotic membranes during normal pregnancy. The activation of the NLRP3 inflammasome leading to pyroptosis has been implicated in the physiological mechanisms of term parturition.
Inflammasomes in term parturition
Parturition represents a form of physiological inflammation (98, 123), which is considered sterile in nature given that the majority of women who undergo labor do not have culturable microorganisms in the amniotic cavity (124). This concept is supported by numerous studies showing an increased bioavailability of cytokines (125–134) and chemokines (135–139) in the amniotic fluid, maternal circulation (140, 141), and gestational tissues such as the placenta (142–144), chorioamniotic membranes (123, 144–152), myometrium (146, 148, 150, 151, 153), and cervix (146, 148, 151, 154, 155) during labor. This sterile inflammatory process occurs in conjunction with an influx of innate and adaptive immune cells into the choriodecidua (cell layer attached to the chorioamniotic membranes) (146, 156–167), myometrium (168–173), and cervix (148, 168, 174–182). Recent reports have established that inflammasomes also participate in the pro-inflammatory milieu of parturition (116, 117, 183). Next, we discuss the evidence supporting such a concept.
The first link between the inflammasome and parturition was reported in 2008 by Gotsch et al. (183) who measured caspase-1 in amniotic fluid (biological fluid with physiological and immune properties that surrounds the fetus throughout gestation (184–186)). These authors reported that the inflammasome-dependent caspase-1 was detected in amniotic fluid of women at term pregnancy, but not in the second trimester (183). In addition, caspase-1 concentrations in amniotic fluid were further increased in women with spontaneous labor at term (183). These findings are in line with reports showing that the main downstream product of the inflammasome, IL-1β, is elevated in women during the physiological process of labor at term (126, 127, 144). Yet, amniotic fluid concentrations of IL-18 do not increase during term parturition (187). In addition, amniotic fluid concentrations of the adaptor protein ASC and the effector protein of pyroptosis GSDMD are also increased in women with spontaneous labor at term (188, 189). The findings described above led us to hypothesize that the chorioamniotic membranes, tissues that surround the amniotic cavity, display an increased expression of the sensor molecules, the adaptor protein, and inflammatory caspases in the process of parturition at term. Consistent with this hypothesis, we and others found that the chorioamniotic membranes expressed NLRP1, NLRP3, AIM2, and NLCR4 (116) as well as the inflammatory caspase-1 (116, 121) and caspase-4 (116). Yet, only the priming and activation of the NLRP3 inflammasome, as evidenced by the upregulation of the sensor molecule and increased amounts of the active forms of caspase-1 and mature IL-1β, was observed in the chorioamniotic membranes of women with labor at term (116). The assembly of the NLRP3 inflammasome was later confirmed by localization of ASC/caspase-1 complexes and ASC specks (a readout of inflammasome activation (190)) in the chorioamniotic membranes and choriodecidual leukocytes of women with labor at term (117, 188). Subsequent studies also suggested that the NLRP3 inflammasome is involved in the inflammatory process of labor in the myometrium (118). The final piece of evidence showing a partial role for the NLRP3 inflammasome in the physiological process of labor was generated when pregnant dams were treated with an inhibitor of NLRP3 inflammasome assembly, MCC950 (191), and arrest of labor (i.e. dystocia) was observed in a subset of animals (192). Collectively, the abovementioned studies indicate that the activation of the NLRP3 inflammasome in the amniotic cavity and surrounding tissues occurs as part of the sterile inflammatory milieu that accompanies physiological labor at term (Figure 1).
Not all term pregnancies occur in the absence of pathology. A subset of women with labor at term are diagnosed with acute histologic chorioamnionitis (193). This placental lesion is associated with intra-amniotic infection (i.e. microorganisms in the amniotic fluid and inflammation) or sterile intra-amniotic inflammation (i.e. inflammation without detectable microorganisms in amniotic fluid) (194). Acute histologic chorioamnionitis is characterized by the invasion of neutrophils and macrophages into the chorioamniotic membranes (195), and is associated with elevated concentrations of pro-inflammatory cytokines such as IL-1β in amniotic fluid (196, 197). Therefore, we hypothesized that inflammasomes may be involved in the process of parturition associated with acute placental inflammation. In line with this hypothesis, NLRP3 and NLRC4 as well as the active/mature forms of caspase-1, IL-1β, and IL-18 were increased in the chorioamniotic membranes of women with labor at term and acute chorioamnionitis compared to those without this placental lesion (198). Enhanced inflammasome assembly in the chorioamniotic membranes of women with acute chorioamnionitis was later confirmed by detection of ASC/caspase-1 complexes (117). Furthermore, amniotic fluid concentrations of the adaptor protein ASC are increased in women with acute histologic chorioamnionitis at term (199). These descriptive findings are consistent with in vitro studies showing that the incubation of the chorioamniotic membranes with microbial products (e.g. LPS) induces the processing of the active forms of caspase-1 and the release of IL-1β, which is blocked by caspase-1 inhibitors (109, 121, 198, 200). These studies suggest that the NLRP3 and NLRC4 inflammasomes may be involved in the pathological inflammatory process of labor at term associated with microbial invasion. Yet, further in vivo studies are needed to investigate whether these inflammasomes are indeed implicated in the acute inflammation of the placental tissues at term pregnancy.
INFLAMMASOMES IN PRETERM LABOR AND BIRTH
Spontaneous preterm labor is a syndrome of multiple etiologies (201), which commonly leads to preterm birth, the leading cause of perinatal morbidity and mortality worldwide (93, 202, 203). The best studied cause for preterm labor is intra-amniotic inflammation (204–213), which can occur as a consequence of microbial invasion of the amniotic cavity (i.e. intra-amniotic infection) or as a result of elevated concentrations of danger signals or alarmins in amniotic fluid (i.e. sterile intra-amniotic inflammation) (214, 215). Both clinical conditions are characterized by increased cytokine concentrations (125, 127, 128, 135–137, 187, 216–222) and elevated numbers of immune cells (186, 223–230) in amniotic fluid. One of the central players in this intra-amniotic inflammatory response is IL-1β (127, 221), given that this cytokine orchestrates the production of labor mediators such as prostaglandins (231–237). Indeed, the administration of IL-1β induces preterm birth in mice (129, 238, 239) and non-human primates (207, 240–244). The abovementioned studies led us to investigate whether inflammasomes, the primary machinery of IL-1β processing, were implicated in the intra-amniotic inflammatory response that accompanies preterm labor and birth. Next, we discuss the evidence indicating a role for the inflammasome in intra-amniotic infection- and sterile intra-amniotic inflammation-associated preterm labor and birth.
Intra-amniotic infection-associated preterm labor and birth
The first evidence suggesting a role for the inflammasome in the mechanisms that lead to preterm labor and birth in the context of intra-amniotic infection was generated by Gotsch et al. (183). These authors reported that amniotic fluid concentrations of caspase-1 were increased in women with preterm labor and intra-amniotic infection compared to those without this clinical condition (183). Such findings were in line with prior studies showing that amniotic fluid concentrations of IL-1β (125, 127, 221, 245, 246) and IL-18 (187, 247) were also elevated in women with preterm labor and intra-amniotic infection. This clinical evidence led us to investigate whether inflammasomes were involved in the pathophysiology of preterm labor/birth in the context of inflammation induced by microbes. First, we showed that women with preterm labor and birth and acute chorioamnionitis (a readout of intra-amniotic infection (195, 248, 249)) displayed priming of the NLRP3 inflammasome as evidenced by the upregulation of NLRP3, caspase-1, caspase-4, IL-1β, and IL-18 in the chorioamniotic membranes (250). Next, the activation of the NLRP3 inflammasome was confirmed by increased concentrations of active caspase-1 and caspase-4 and mature forms of IL-1β and IL-18, as well as enhanced formation of ASC/caspase-1 complexes in the chorioamniotic membranes of women with preterm labor and acute chorioamnionitis (250). The increased concentrations of active caspase-4 suggest that non-canonical inflammasome activation may occur in the context of preterm labor resulting from intra-amniotic infection due to Gram-negative bacteria. Recently, we also found that amniotic fluid concentrations of the adaptor protein ASC (251) and the effector molecule of pyroptosis GSDMD (252) were increased in women with preterm labor and intra-amniotic infection compared to those without this clinical condition. Both ASC and GSDMD are also overexpressed by the chorioamniotic membranes of women with preterm labor and intra-amniotic infection. Together, these data provide descriptive evidence supporting a role for the NLRP3 inflammasome in the pathophysiology of intra-amniotic infection-associated preterm labor and birth.
Causal links between the activation of the NLRP3 inflammasome and preterm labor and birth in the context of infection include the following: 1) the intra-uterine administration of peptidoglycan and poly I:C increased the expression of NLRP3 and caspase-1, as well as increased amounts of active caspase-1, in the uterine tissues (253); 2) the deficiency of Nlrp3 protects against group B streptococcus-induced preterm birth (254); 3) the combined injection of MHV-68 and LPS induces preterm birth (255, 256) by causing exaggerated inflammation in the fetal membranes, which was suggested to occur in part through the activation of the NLRP3 inflammasome (200); and 4) the ultrasound-guided intra-amniotic administration of LPS induced priming and activation of the NLRP3 inflammasome in the fetal membranes prior to preterm birth, which was ameliorated by blocking the assembly of the NLRP3 inflammasome using MCC950 (257). Preliminary data from our group suggest that the NLRP3 inflammasome is implicated in host defense mechanisms against genital mycoplasmas (Motomura et al., unpublished data). It is worth mentioning that inhibition of the inflammasome in the context of intra-amniotic infection does not fully prevent adverse pregnancy and neonatal outcomes (257), indicating that the blockade of multiple pathways (including other inflammasomes) may be necessary to restore the normal timing of parturition. Further studies are required to investigate whether clinically-isolated bacterial cultivars associated with preterm labor and birth induce the activation of the NLRP3 inflammasome in vivo, and whether conventional treatments are effective for prevention of adverse pregnancy outcomes.
Sterile intra-amniotic inflammation-induced preterm labor and birth
A link between the NLRP3 inflammasome and the mechanisms leading to sterile intra-amniotic inflammation-associated preterm labor and birth was first hypothesized upon the observation that placentas from women with intra-amniotic inflammation without detectable microorganisms are diagnosed with acute chorioamnionitis (214, 215) and display characteristics of NLRP3 inflammasome activation (250). This hypothesis was confirmed by recent reports showing that women with preterm labor and sterile intra-amniotic inflammation have increased concentrations of ASC (251) and GSDMD (252) in amniotic fluid and the chorioamniotic membranes. These clinical observations led us to investigate the mechanisms whereby danger signals or alarmins, molecules that initiate sterile inflammation (258–260), trigger inflammatory processes in the amniotic cavity and chorioamniotic membranes. First, we showed that the ultrasound-guided intra-amniotic administration of the classical alarmin HMGB1, a molecule that is present in amniotic fluid of women with preterm labor (261), induces preterm birth in mice (262). Next, using an ex vivo model of intra-amniotic inflammation, we reported that HMGB1 causes the priming and activation of the NLRP3 inflammasome in the chorioamniotic membranes (263). Recently, we also provided in vivo evidence that the alarmin S100B can induce sterile intra-amniotic inflammation by activating the NLRP3 inflammasome in the fetal membranes prior to inducing preterm birth (192). Importantly, by inhibiting the assembly of this inflammasome using MCC950, S100B-induced preterm birth can be prevented in most cases (192). Furthermore, we have generated data showing that the ultrasound-guided intra-amniotic injection of the alarmin IL-1α induces preterm labor and birth via the NLRP3 inflammasome (Motomura et al., unpublished data). These findings have clinical implications given that we have proposed to use inhibitors of the NLRP3 inflammasome as a therapeutic strategy for sterile intra-amniotic inflammation, a condition that currently lacks treatment (192). Additional studies are required to investigate whether other alarmins [e.g. heat shock protein 70 (HSP70) (264)] present in amniotic fluid of women with preterm labor and sterile intra-amniotic inflammation can activate the NLRP3 inflammasome in the fetal membranes, inducing preterm labor and birth.
Figure 2 includes a representation of the proposed role for the canonical and non-canonical NLRP3 inflammasome pathways in the pathophysiology of preterm labor and birth in the context of intra-amniotic infection or sterile intra-amniotic inflammation.
Figure 2. The NLRP3 inflammasome in preterm labor and birth.
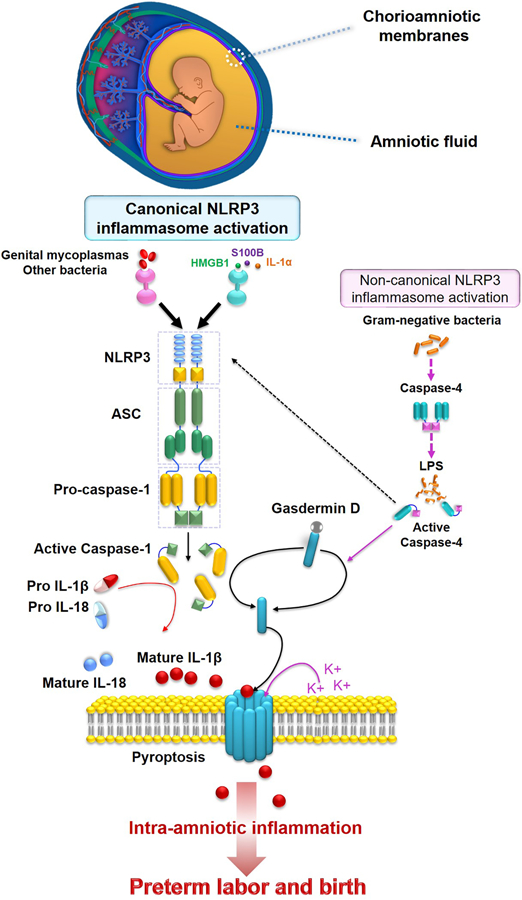
Bacteria (e.g. genital mycoplasmas) or alarmins (e.g. HMGB1, S100B, or IL-1α) can activate the canonical NLRP3 inflammasome pathway in the chorioamniotic membranes, which results in the release of active caspase-1 and mature forms of IL-1β and IL-18 into the amniotic fluid. Gram-negative bacteria may also activate the non-canonical NLRP3 inflammasome pathway. Detection of extracellular ASC and gasdermin D in the chorioamnioitic membranes and amniotic fluid have also been reported as readouts of inflammasome activation and pyroptosis, respectively.
INFLAMMASOMES IN PREGNANCY COMPLICATIONS ASSOCIATED WITH PLACENTAL INFLAMMATION
Given that inflammasome components are expressed by placental cells, as reviewed above, early studies have suggested that inflammasomes are implicated in the inflammatory responses associated with placental disease. Mulla et al. and Xie et al. were the first to demonstrate that NLRP3 inflammasome activation in trophoblasts (106) and peripheral blood (100) was implicated in the pathogenesis of preeclampsia. Indeed, it has been shown that peripheral monocytes from women with preeclampsia display enhanced expression of NLRP1 and NLRP3 (102, 265, 266), and polymorphisms in their coding genes are associated with the development of this syndrome (267, 268). In addition, women with preeclampsia had elevated levels of total cholesterol and uric acid, cellular metabolites that act as alarmins when released extracellularly (269, 270), which can potentially activate the NLRP3 inflammasome in the syncytiotrophoblast layer of the placenta (112). Descriptive studies have also shown that placentas from women with severe preeclampsia display higher expression of NLRP3, caspase-1, and IL-1β compared to normotensive pregnant women (120, 271). Further, in vivo studies (119, 272–275) have provided a link between alarmin-induced activation of placental NLRP3 inflammasomes and the resulting placental inflammation-associated pregnancy complications. In line with this evidence, a recent study using murine models and human tissues showed that endothelial-derived extracellular vesicles induce NLRP3 inflammasome activation, triggering a preeclampsia-like syndrome that can be attenuated by inhibition of this pathway (276). Taken together, these findings suggest that NLRP3 inflammasome activation is implicated in the placental inflammatory processes associated with the pathophysiology of preeclampsia (Figure 3).
Figure 3. Inflammasomes in placental inflammation.
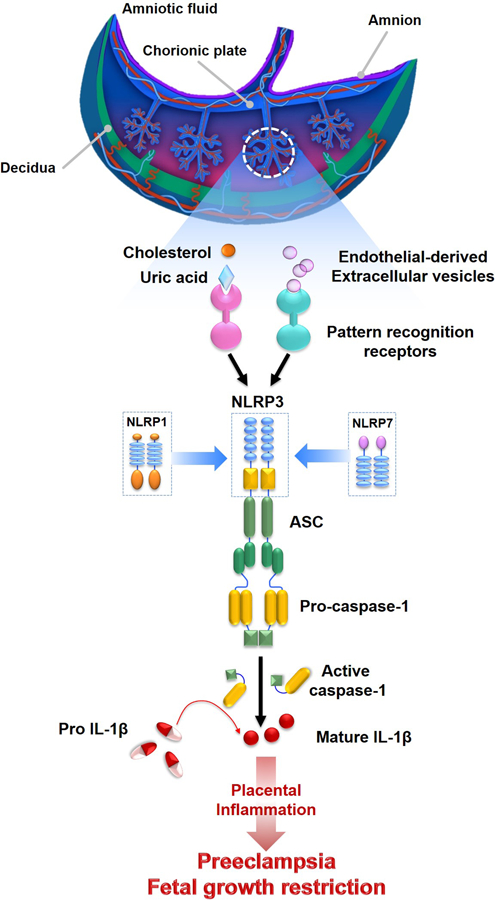
Endothelial-derived extracellular vesicles and/or alarmins (e.g. cholesterol or uric acid) can activate the NLRP3, NLRP1, and NLRP7 inflammasomes in the placenta, leading to the processing and release of active caspase-1 and mature IL-1β. The resulting inflammation may lead to placental diseases such as preeclampsia and fetal growth restriction.
Moreover, in vitro and in vivo studies have shown that inflammatory stimuli (e.g. LPS or uric acid) induce the activation of the NLRP3 inflammasome in the placenta (107, 277), which may also contribute to the mechanisms of disease of other pregnancy complications associated with placental inflammation such as anti-phospholipid syndrome (277–279), gestational diabetes (280), and fetal growth restriction (119). Recent studies showed that the NLRP7 inflammasome is a key regulator of placental development and hypoxia, the impairment of which can lead to fetal growth restriction (281). This finding suggests that the NLRP7 inflammasome, which has been previously shown to be activated by microbial products (36), may also be triggered by non-microbial signals resulting from hypoxic conditions in the placenta (281) (Figure 3). Yet, further studies are required to investigate whether the inhibition of inflammasomes can be considered as a strategy to prevent placental inflammation-associated disorders.
CONCLUSION
Growing evidence has consistently shown that inflammasomes are implicated in the physiological and pathological inflammatory processes of pregnancy. Several inflammasomes have been detected in the gestational tissues; yet, only the NLRP3 inflammasome in the chorioamniotic membranes has been implicated in the mechanisms that lead to the sterile inflammatory process of term parturition. The premature activation of the NLRP3 inflammasome in the chorioamniotic membranes is now established to be an important mechanism whereby microbes or danger signals induce preterm labor and birth. The activation of the NLRP3 inflammasome in the placenta has also been involved in the pathogenesis of preeclampsia and other placental disorders. This evidence could foster the development of novel anti-inflammatory therapies based on the inhibition of the NLRP3 inflammasome for the prevention or treatment of pregnancy complications.
ACKNOWLEDGMENTS
We are grateful to Marcia Arenas-Hernandez, M.Sc. for critical discussion of some sections included in this review.
This review was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. N. G-L is also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
3Non-standard abbreviations:
- AIM2
absent in melanoma-2
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- DAMPs
damage-associated molecule patterns
- GSDMD
Gasdermin D
- LPS
Lipopolysaccharide
- MCC950
sodium salt is a potent selective inhibitor of NLRP3
- NAIPs
NLR family apoptosis inhibitory proteins
- NLR
nucleotide-binding oligomerization domain leucine-rich repeat-containing protein
- NLRC4
NLR family caspase-activation-and-recruitment domain (CARD)-domain-containing protein-4
- NLRP
nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing protein
- PAMPs
pathogen-associated molecular patterns
- PRRs
pattern recognition receptors
- T3SS
Type 3 secretion system
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Martinon F, Burns K, and Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426. [DOI] [PubMed] [Google Scholar]
- 2.Schroder K, and Tschopp J. 2010. The inflammasomes. Cell 140: 821–832. [DOI] [PubMed] [Google Scholar]
- 3.Latz E, Xiao TS, and Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol 13: 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Zoete MR, Palm NW, Zhu S, and Flavell RA. 2014. Inflammasomes. Cold Spring Harb Perspect Biol 6: a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broz P, and Dixit VM. 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420. [DOI] [PubMed] [Google Scholar]
- 6.Sharma D, and Kanneganti TD. 2016. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. The Journal of cell biology 213: 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathur A, Hayward JA, and Man SM. 2018. Molecular mechanisms of inflammasome signaling. Journal of leukocyte biology 103: 233–257. [DOI] [PubMed] [Google Scholar]
- 8.Hegde B, Bodduluri SR, Satpathy SR, Alghsham RS, Jala VR, Uriarte SM, Chung DH, Lawrenz MB, and Haribabu B. 2018. Inflammasome-Independent Leukotriene B4 Production Drives Crystalline Silica-Induced Sterile Inflammation. J Immunol 200: 3556–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Sedlacek AL, Pawaria S, Xu H, Scott MJ, and Binder RJ. 2018. Cutting Edge: The Heat Shock Protein gp96 Activates Inflammasome-Signaling Platforms in APCs. J Immunol 201: 2209–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa Franco MM, Marim F, Guimaraes ES, Assis NRG, Cerqueira DM, Alves-Silva J, Harms J, Splitter G, Smith J, Kanneganti TD, de Queiroz N, Gutman D, Barber GN, and Oliveira SC. 2018. Brucella abortus Triggers a cGAS-Independent STING Pathway To Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J Immunol 200: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson KV, Deng M, and Ting JP. 2019. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. [DOI] [PMC free article] [PubMed]
- 12.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, and et al. 1992. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356: 768–774. [DOI] [PubMed] [Google Scholar]
- 13.Black RA, Kronheim SR, Merriam JE, March CJ, and Hopp TP. 1989. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J Biol Chem 264: 5323–5326. [PubMed] [Google Scholar]
- 14.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, Chartrain NA, and Schmidt JA. 1989. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A 86: 5227–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, and et al. 1992. Molecular cloning of the interleukin-1 beta converting enzyme. Science 256: 97–100. [DOI] [PubMed] [Google Scholar]
- 16.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, and Su MS. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science 275: 206–209. [DOI] [PubMed] [Google Scholar]
- 17.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, and Allen H. 1997. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 386: 619–623. [DOI] [PubMed] [Google Scholar]
- 18.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, and Zychlinsky A. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12: 581–590. [DOI] [PubMed] [Google Scholar]
- 19.Cookson BT, and Brennan MA. 2001. Pro-inflammatory programmed cell death. Trends Microbiol 9: 113–114. [DOI] [PubMed] [Google Scholar]
- 20.Bergsbaken T, Fink SL, and Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nature reviews. Microbiology 7: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao EA, Rajan JV, and Aderem A. 2011. Caspase-1-induced pyroptotic cell death. Immunological reviews 243: 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Q, Zheng M, Balakrishnan A, Karki R, and Kanneganti TD. 2018. Gasdermin D Promotes AIM2 Inflammasome Activation and Is Required for Host Protection against Francisella novicida. J Immunol 201: 3662–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinon F, Petrilli V, Mayor A, Tardivel A, and Tschopp J. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440: 237–241. [DOI] [PubMed] [Google Scholar]
- 24.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, and Greene WC. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbore G, West EE, Spolski R, Robertson AAB, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O’Neill LA, Coll RC, Sher A, Leonard WJ, Kohl J, Monk P, Cooper MA, Arno M, Afzali B, Lachmann HJ, Cope AP, Mayer-Barber KD, and Kemper C. 2016. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science 352: aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seydoux E, Liang H, Dubois Cauwelaert N, Archer M, Rintala ND, Kramer R, Carter D, Fox CB, and Orr MT. 2018. Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses. J Immunol 201: 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poyet JL, Srinivasula SM, Tnani M, Razmara M, Fernandes-Alnemri T, and Alnemri ES. 2001. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem 276: 28309–28313. [DOI] [PubMed] [Google Scholar]
- 28.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, and Tschopp J. 2004. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325. [DOI] [PubMed] [Google Scholar]
- 29.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, and Dixit VM. 2004. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430: 213–218. [DOI] [PubMed] [Google Scholar]
- 30.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, and Stacey KJ. 2009. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 31.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, and Fitzgerald KA. 2009. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, and Alnemri ES. 2009. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458: 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, and Superti-Furga G. 2009. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol 10: 266–272. [DOI] [PubMed] [Google Scholar]
- 34.Gavrilin MA, Abdelaziz DH, Mostafa M, Abdulrahman BA, Grandhi J, Akhter A, Abu Khweek A, Aubert DF, Valvano MA, Wewers MD, and Amer AO. 2012. Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol 188: 3469–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grenier JM, Wang L, Manji GA, Huang WJ, Al-Garawi A, Kelly R, Carlson A, Merriam S, Lora JM, Briskin M, DiStefano PS, and Bertin J. 2002. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS letters 530: 73–78. [DOI] [PubMed] [Google Scholar]
- 36.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, and Stehlik C. 2012. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36: 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS, and Bertin J. 2002. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem 277: 29874–29880. [DOI] [PubMed] [Google Scholar]
- 38.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, Hannesschlager N, Schlee M, Rothenfusser S, Barchet W, Kato H, Akira S, Inoue S, Endres S, Peschel C, Hartmann G, Hornung V, and Ruland J. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol 11: 63–69. [DOI] [PubMed] [Google Scholar]
- 39.Pothlichet J, Meunier I, Davis BK, Ting JP, Skamene E, von Messling V, and Vidal SM. 2013. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog 9: e1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerur N, Veettil MV, Sharma-Walia N, Bottero V, Sadagopan S, Otageri P, and Chandran B. 2011. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, and Greene WC. 2014. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science 343: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyden ED, and Dietrich WF. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38: 240–244. [DOI] [PubMed] [Google Scholar]
- 43.Ewald SE, Chavarria-Smith J, and Boothroyd JC. 2014. NLRP1 is an inflammasome sensor for Toxoplasma gondii. Infect Immun 82: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neiman-Zenevich J, Stuart S, Abdel-Nour M, Girardin SE, and Mogridge J. 2017. Listeria monocytogenes and Shigella flexneri Activate the NLRP1B Inflammasome. Infect Immun 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, Volkmann N, Hanein D, Rouiller I, and Reed JC. 2007. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell 25: 713–724. [DOI] [PubMed] [Google Scholar]
- 46.Zhong FL, Mamai O, Sborgi L, Boussofara L, Hopkins R, Robinson K, Szeverenyi I, Takeichi T, Balaji R, Lau A, Tye H, Roy K, Bonnard C, Ahl PJ, Jones LA, Baker PJ, Lacina L, Otsuka A, Fournie PR, Malecaze F, Lane EB, Akiyama M, Kabashima K, Connolly JE, Masters SL, Soler VJ, Omar SS, McGrath JA, Nedelcu R, Gribaa M, Denguezli M, Saad A, Hiller S, and Reversade B. 2016. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 167: 187–202 e117. [DOI] [PubMed] [Google Scholar]
- 47.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, and Nunez G. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7: 576–582. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, Liu L, and Shao F. 2011. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477: 596–600. [DOI] [PubMed] [Google Scholar]
- 49.Endrizzi MG, Hadinoto V, Growney JD, Miller W, and Dietrich WF. 2000. Genomic sequence analysis of the mouse Naip gene array. Genome Res 10: 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kofoed EM, and Vance RE. 2011. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477: 592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kortmann J, Brubaker SW, and Monack DM. 2015. Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J Immunol 195: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu Y, Misaghi S, Izrael-Tomasevic A, Newton K, Gilmour LL, Lamkanfi M, Louie S, Kayagaki N, Liu J, Komuves L, Cupp JE, Arnott D, Monack D, and Dixit VM. 2012. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490: 539–542. [DOI] [PubMed] [Google Scholar]
- 53.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, and Tschopp J. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452: 103–107. [DOI] [PubMed] [Google Scholar]
- 54.Jin T, Perry A, Jiang J, Smith P, Curry JA, Unterholzner L, Jiang Z, Horvath G, Rathinam VA, Johnstone RW, Hornung V, Latz E, Bowie AG, Fitzgerald KA, and Xiao TS. 2012. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin T, Perry A, Smith P, Jiang J, and Xiao TS. 2013. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem 288: 13225–13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, and Alnemri ES. 2010. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, and Fitzgerald KA. 2010. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, Simanski M, Glaser R, Harder J, Hornung V, Gallo RL, Ruzicka T, Besch R, and Schauber J. 2011. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med 3: 82ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, Panchanathan R, and Choubey D. 2013. AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 60.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, and Shao F. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513: 237–241. [DOI] [PubMed] [Google Scholar]
- 61.Gao W, Yang J, Liu W, Wang Y, and Shao F. 2016. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A 113: E4857–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park YH, Wood G, Kastner DL, and Chae JJ. 2016. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alimov I, Menon S, Cochran N, Maher R, Wang Q, Alford J, Concannon JB, Yang Z, Harrington E, Llamas L, Lindeman A, Hoffman G, Schuhmann T, Russ C, Reece-Hoyes J, Canham SM, and Cai X. 2019. Bile acid analogues are activators of pyrin inflammasome. J Biol Chem 294: 3359–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, and Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440: 228–232. [DOI] [PubMed] [Google Scholar]
- 65.Franchi L, Eigenbrod T, and Nunez G. 2009. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol 183: 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, and Latz E. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franchi L, Eigenbrod T, Munoz-Planillo R, Ozkurede U, Kim YG, Arindam C, Gale M Jr., Silverman RH, Colonna M, Akira S, and Nunez G. 2014. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol 193: 4214–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freeman L, Guo H, David CN, Brickey WJ, Jha S, and Ting JP. 2017. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med 214: 1351–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, and Tschopp J. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol 181: 3755–3759. [DOI] [PubMed] [Google Scholar]
- 70.Goldberg EL, Asher JL, Molony RD, Shaw AC, Zeiss CJ, Wang C, Morozova-Roche LA, Herzog RI, Iwasaki A, and Dixit VD. 2017. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep 18: 2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, and Tschopp J. 2007. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55: 443–452. [DOI] [PubMed] [Google Scholar]
- 72.Chow MT, Tschopp J, Moller A, and Smyth MJ. 2012. NLRP3 promotes inflammation-induced skin cancer but is dispensable for asbestos-induced mesothelioma. Immunol Cell Biol 90: 983–986. [DOI] [PubMed] [Google Scholar]
- 73.Man SM 2018. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat Rev Gastroenterol Hepatol 15: 721–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutterwala FS, Haasken S, and Cassel SL. 2014. Mechanism of NLRP3 inflammasome activation. Annals of the New York Academy of Sciences 1319: 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elliott EI, Miller AN, Banoth B, Iyer SS, Stotland A, Weiss JP, Gottlieb RA, Sutterwala FS, and Cassel SL. 2018. Cutting Edge: Mitochondrial Assembly of the NLRP3 Inflammasome Complex Is Initiated at Priming. J Immunol 200: 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, and Latz E. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, and Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell death and differentiation 14: 1583–1589. [DOI] [PubMed] [Google Scholar]
- 78.Gov L, Schneider CA, Lima TS, Pandori W, and Lodoen MB. 2017. NLRP3 and Potassium Efflux Drive Rapid IL-1beta Release from Primary Human Monocytes during Toxoplasma gondii Infection. J Immunol 199: 2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, and Latz E. 2008. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou R, Yazdi AS, Menu P, and Tschopp J. 2011. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225. [DOI] [PubMed] [Google Scholar]
- 81.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, and Chae JJ. 2012. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, and Nunez G. 2013. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Cikovic T, Hartjes L, Smollich J, Robertson AAB, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, and Gross O. 2016. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 45: 761–773. [DOI] [PubMed] [Google Scholar]
- 85.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, and Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514: 187–192. [DOI] [PubMed] [Google Scholar]
- 86.Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, and Mortellaro A. 2015. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat Commun 6: 8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, and Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121. [DOI] [PubMed] [Google Scholar]
- 88.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, and Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341: 1246–1249. [DOI] [PubMed] [Google Scholar]
- 89.Hagar JA, Powell DA, Aachoui Y, Ernst RK, and Miao EA. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341: 1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee BL, Stowe IB, Gupta A, Kornfeld OS, Roose-Girma M, Anderson K, Warming S, Zhang J, Lee WP, and Kayagaki N. 2018. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med 215: 2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, and Shao F. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. [DOI] [PubMed] [Google Scholar]
- 92.Strowig T, Henao-Mejia J, Elinav E, and Flavell R. 2012. Inflammasomes in health and disease. Nature 481: 278–286. [DOI] [PubMed] [Google Scholar]
- 93.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, and Black RE. 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet (London, England) 385: 430–440. [DOI] [PubMed] [Google Scholar]
- 94.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, Thorp JM, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, and Tolosa JE. 2016. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. American journal of obstetrics and gynecology 215: 103.e101–103.e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, and Wagner GP. 2017. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A 114: E6566–E6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly RW 1994. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocr Rev 15: 684–706. [DOI] [PubMed] [Google Scholar]
- 97.Lindstrom TM, and Bennett PR. 2005. The role of nuclear factor kappa B in human labour. Reproduction 130: 569–581. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, and Nien JK. 2006. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine 11: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vora S, Abbas A, Kim CJ, Summerfield TL, Kusanovic JP, Iams JD, Romero R, Kniss DA, and Ackerman W. E. t.. 2010. Nuclear factor-kappa B localization and function within intrauterine tissues from term and preterm labor and cultured fetal membranes. Reprod Biol Endocrinol 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie F, Hu Y, Turvey SE, Magee LA, Brunham RM, Choi KC, Krajden M, Leung PC, Money DM, Patrick DM, Thomas E, and von Dadelszen P. 2010. Toll-like receptors 2 and 4 and the cryopyrin inflammasome in normal pregnancy and pre-eclampsia. BJOG 117: 99–108. [DOI] [PubMed] [Google Scholar]
- 101.Maneta E, Warren AY, Hay DP, and Khan RN. 2015. Caspase-1-mediated cytokine release from gestational tissues, placental, and cord blood. Front Physiol 6: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matias ML, Romao M, Weel IC, Ribeiro VR, Nunes PR, Borges VT, Araujo JP Jr., Peracoli JC, de Oliveira L, and Peracoli MT. 2015. Endogenous and Uric Acid-Induced Activation of NLRP3 Inflammasome in Pregnant Women with Preeclampsia. PLoS One 10: e0129095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pineles B, Romero R, Montenegro D, Tarca A, Than N, Hassan S, Gotsch F, Draghici S, Espinoza J, and Kim C. 2007. “The Inflammasome” in Human Parturition. Reproductive Sciences 14. [Google Scholar]
- 104.Burton GJ, and Jauniaux E. 2015. What is the placenta? American journal of obstetrics and gynecology 213: S6 e1, S6–8. [DOI] [PubMed] [Google Scholar]
- 105.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, and Yang XF. 2009. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol 22: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, and Abrahams VM. 2011. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. American journal of reproductive immunology (New York, N.Y. : 1989) 65: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pontillo A, Girardelli M, Agostinis C, Masat E, Bulla R, and Crovella S. 2013. Bacterial LPS differently modulates inflammasome gene expression and IL-1beta secretion in trophoblast cells, decidual stromal cells, and decidual endothelial cells. Reprod Sci 20: 563–566. [DOI] [PubMed] [Google Scholar]
- 108.Tilburgs T, Meissner TB, Ferreira LMR, Mulder A, Musunuru K, Ye J, and Strominger JL. 2017. NLRP2 is a suppressor of NF-kB signaling and HLA-C expression in human trophoblastsdagger,double dagger. Biology of reproduction 96: 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bryant AH, Bevan RJ, Spencer-Harty S, Scott LM, Jones RH, and Thornton CA. 2017. Expression and function of NOD-like receptors by human term gestation-associated tissues. Placenta 58: 25–32. [DOI] [PubMed] [Google Scholar]
- 110.Zhu J, He M, Ma C, Peng F, Su Y, and Huang L. 2018. Expression and Clinical Significance of NOD-Like Receptor Protein 3 (NLRP3) and Caspase-1 in Fetal Membrane and Placental Tissues of Patients with Premature Rupture of Membrane. Med Sci Monit 24: 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tamura K, Ishikawa G, Yoshie M, Ohneda W, Nakai A, Takeshita T, and Tachikawa E. 2017. Glibenclamide inhibits NLRP3 inflammasome-mediated IL-1beta secretion in human trophoblasts. J Pharmacol Sci 135: 89–95. [DOI] [PubMed] [Google Scholar]
- 112.Stodle GS, Silva GB, Tangeras LH, Gierman LM, Nervik I, Dahlberg UE, Sun C, Aune MH, Thomsen LCV, Bjorge L, and Iversen AC. 2018. Placental inflammation in pre-eclampsia by Nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin Exp Immunol 193: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Correa-Silva S, Alencar AP, Moreli JB, Borbely AU, de SLL, Scavone C, Damasceno DC, Rudge MVC, Bevilacqua E, and Calderon IMP. 2018. Hyperglycemia induces inflammatory mediators in the human chorionic villous. Cytokine 111: 41–48. [DOI] [PubMed] [Google Scholar]
- 114.Kaneko Y, Sano M, Seno K, Oogaki Y, Takahashi H, Ohkuchi A, Yokozawa M, Yamauchi K, Iwata H, Kuwayama T, and Shirasuna K. 2019. Olive Leaf Extract (OleaVita) Suppresses Inflammatory Cytokine Production and NLRP3 Inflammasomes in Human Placenta. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bourne G 1962. The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgrad Med J 38: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, Than NG, Chiang PJ, Dong Z, Xu Z, Tarca AL, Abrahams VM, Hassan SS, Yeo L, and Gomez-Lopez N. 2018. A Role for the Inflammasome in Spontaneous Labor at Term. American journal of reproductive immunology (New York, N.Y. : 1989) 79: e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, Miller D, Abrahams VM, and Hassan SS. 2017. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. American journal of reproductive immunology (New York, N.Y. : 1989) 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim R, and Lappas M. 2018. NOD-like receptor pyrin domain-containing-3 (NLRP3) regulates inflammation-induced pro-labor mediators in human myometrial cells. American journal of reproductive immunology (New York, N.Y. : 1989) 79: e12825. [DOI] [PubMed] [Google Scholar]
- 119.Brien ME, Duval C, Palacios J, Boufaied I, Hudon-Thibeault AA, Nadeau-Vallee M, Vaillancourt C, Sibley CP, Abrahams VM, Jones RL, and Girard S. 2017. Uric Acid Crystals Induce Placental Inflammation and Alter Trophoblast Function via an IL-1-Dependent Pathway: Implications for Fetal Growth Restriction. J Immunol 198: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weel IC, Romao-Veiga M, Matias ML, Fioratti EG, Peracoli JC, Borges VT, Araujo JP Jr., and Peracoli MT. 2017. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. Journal of reproductive immunology 123: 40–47. [DOI] [PubMed] [Google Scholar]
- 121.Lappas M 2014. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. American journal of reproductive immunology (New York, N.Y. : 1989) 71: 189–201. [DOI] [PubMed] [Google Scholar]
- 122.Brickle A, Tran HT, Lim R, Liong S, and Lappas M. 2015. Autophagy, which is decreased in labouring fetal membranes, regulates IL-1beta production via the inflammasome. Placenta 36: 1393–1404. [DOI] [PubMed] [Google Scholar]
- 123.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, and Romero R. 2006. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American journal of obstetrics and gynecology 195: 394.e391–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, Insunza A, Montiel F, Behnke E, and Cassell GH. 1993. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. The Journal of reproductive medicine 38: 543–548. [PubMed] [Google Scholar]
- 125.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, and Durum SK. 1989. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. American journal of obstetrics and gynecology 160: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 126.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, Athanassiadis AP, and Mitchell MD. 1990. Amniotic fluid interleukin-1 in spontaneous labor at term. The Journal of reproductive medicine 35: 235–238. [PubMed] [Google Scholar]
- 127.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, and Dinarello CA. 1992. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. American journal of reproductive immunology (New York, N.Y. : 1989) 27: 117–123. [DOI] [PubMed] [Google Scholar]
- 128.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, and Williams J. 1992. Tumor necrosis factor in preterm and term labor. American journal of obstetrics and gynecology 166: 1576–1587. [DOI] [PubMed] [Google Scholar]
- 129.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, and Mitchell MD. 1992. The natural interleukin-1 receptor antagonist in term and preterm parturition. American journal of obstetrics and gynecology 167: 863–872. [DOI] [PubMed] [Google Scholar]
- 130.Saito S, Kasahara T, Kato Y, Ishihara Y, and Ichijo M. 1993. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 5: 81–88. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, and Cotton DB. 1994. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. American journal of obstetrics and gynecology 171: 912–921. [DOI] [PubMed] [Google Scholar]
- 132.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, and Cassell GH. 1995. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. American journal of obstetrics and gynecology 173: 606–612. [DOI] [PubMed] [Google Scholar]
- 133.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, and Romero R. 1999. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. American journal of obstetrics and gynecology 181: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 134.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, and Mitchell MD. 2003. Cytokines, prostaglandins and parturition--a review. Placenta 24 Suppl A: S33–46. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Ceska M, Avila C, Mazor M, Behnke E, and Lindley I. 1991. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. American journal of obstetrics and gynecology 165: 813–820. [DOI] [PubMed] [Google Scholar]
- 136.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, and Cotton DB. 1994. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. American journal of reproductive immunology (New York, N.Y. : 1989) 32: 108–113. [DOI] [PubMed] [Google Scholar]
- 137.Dudley DJ, Hunter C, Mitchell MD, and Varner MW. 1996. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstetrics and gynecology 87: 94–98. [DOI] [PubMed] [Google Scholar]
- 138.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, and Yoon BH. 1999. A role for the novel cytokine RANTES in pregnancy and parturition. American journal of obstetrics and gynecology 181: 989–994. [DOI] [PubMed] [Google Scholar]
- 139.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, Gonzalez R, and Adashi EY. 2003. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 14: 51–56. [DOI] [PubMed] [Google Scholar]
- 140.Unal ER, Cierny JT, Roedner C, Newman R, and Goetzl L. 2011. Maternal inflammation in spontaneous term labor. American journal of obstetrics and gynecology 204: 223.e221–225. [DOI] [PubMed] [Google Scholar]
- 141.Cierny JT, Unal ER, Flood P, Rhee KY, Praktish A, Olson TH, and Goetzl L. 2014. Maternal inflammatory markers and term labor performance. American journal of obstetrics and gynecology 210: 447.e441–446. [DOI] [PubMed] [Google Scholar]
- 142.Taniguchi T, Matsuzaki N, Kameda T, Shimoya K, Jo T, Saji F, and Tanizawa O. 1991. The enhanced production of placental interleukin-1 during labor and intrauterine infection. American journal of obstetrics and gynecology 165: 131–137. [DOI] [PubMed] [Google Scholar]
- 143.Ammala M, Nyman T, Salmi A, and Rutanen EM. 1997. The interleukin-1 system in gestational tissues at term: effect of labour. Placenta 18: 717–723. [DOI] [PubMed] [Google Scholar]
- 144.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, and Mitchell MD. 1999. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. American journal of obstetrics and gynecology 181: 1530–1536. [DOI] [PubMed] [Google Scholar]
- 145.Fidel PL Jr., Romero R, Ramirez M, Cutright J, Edwin SS, LaMarche S, Cotton DB, and Mitchell MD. 1994. Interleukin-1 receptor antagonist (IL-1ra) production by human amnion, chorion, and decidua. American journal of reproductive immunology (New York, N.Y. : 1989) 32: 1–7. [DOI] [PubMed] [Google Scholar]
- 146.Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, and Norman JE. 2002. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biology of reproduction 66: 445–449. [DOI] [PubMed] [Google Scholar]
- 147.Lonergan M, Aponso D, Marvin KW, Helliwell RJ, Sato TA, Mitchell MD, Chaiwaropongsa T, Romero R, and Keelan JA. 2003. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab 88: 3835–3844. [DOI] [PubMed] [Google Scholar]
- 148.Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, and Norman JE. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular human reproduction 9: 41–45. [DOI] [PubMed] [Google Scholar]
- 149.Nhan-Chang CL, Romero R, Tarca AL, Mittal P, Kusanovic JP, Erez O, Mazaki-Tovi S, Chaiworapongsa T, Hotra J, Than NG, Kim JS, Hassan SS, and Kim CJ. 2010. Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. American journal of obstetrics and gynecology 202: 462.e461–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, and Adashi EY. 2005. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labor. Placenta 26: 661–671. [DOI] [PubMed] [Google Scholar]
- 151.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, and Nelson S. 2009. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. American journal of obstetrics and gynecology 200: 104.e101–111. [DOI] [PubMed] [Google Scholar]
- 152.Stephen GL, Lui S, Hamilton SA, Tower CL, Harris LK, Stevens A, and Jones RL. 2015. Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. American journal of reproductive immunology (New York, N.Y. : 1989) 73: 36–55. [DOI] [PubMed] [Google Scholar]
- 153.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, and Kim CJ. 2010. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. Journal of perinatal medicine 38: 617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, Diamond MP, Sorokin Y, and Malone J Jr. 2006. The transcriptome of the uterine cervix before and after spontaneous term parturition. American journal of obstetrics and gynecology 195: 778–786. [DOI] [PubMed] [Google Scholar]
- 155.Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Vaisbuch E, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Yeo L, Draghici S, Kim JS, Uldbjerg N, and Kim CJ. 2009. The transcriptome of cervical ripening in human pregnancy before the onset of labor at term: identification of novel molecular functions involved in this process. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 22: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, and Claas FH. 2004. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. Journal of reproductive immunology 62: 125–137. [DOI] [PubMed] [Google Scholar]
- 157.Osman I, Young A, Jordan F, Greer IA, and Norman JE. 2006. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. Journal of the Society for Gynecologic Investigation 13: 97–103. [DOI] [PubMed] [Google Scholar]
- 158.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, and Vadillo-Ortega F. 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. Journal of reproductive immunology 80: 122–131. [DOI] [PubMed] [Google Scholar]
- 159.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, and Vadillo-Ortega F. 2011. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. American journal of obstetrics and gynecology 205: 235.e215–224. [DOI] [PubMed] [Google Scholar]
- 160.Gomez-Lopez N, Vadillo-Perez L, Nessim S, Olson DM, and Vadillo-Ortega F. 2011. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. American journal of obstetrics and gynecology 204: 364.e369–316. [DOI] [PubMed] [Google Scholar]
- 161.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, and Vadillo-Ortega F. 2013. Evidence for a role for the adaptive immune response in human term parturition. American journal of reproductive immunology (New York, N.Y. : 1989) 69: 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, and Arenas-Hernandez M. 2014. Immune cells in term and preterm labor. Cellular & molecular immunology 11: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, Drewlo S, Tarca AL, Hassan SS, and Gomez-Lopez N. 2016. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol 196: 1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, and Gomez-Lopez N. 2016. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol 196: 2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu CD, Tarca AL, Sanchez-Torres C, and Gomez-Lopez N. 2019. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J Immunol 202: 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leng Y, Romero R, Xu Y, Galaz J, Slutsky R, Arenas-Hernandez M, Garcia-Flores V, Motomura K, Hassan SS, Reboldi A, and Gomez-Lopez N. 2019. Are B cells altered in the decidua of women with preterm or term labor? American journal of reproductive immunology (New York, N.Y. : 1989) 81: e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, and Gomez-Lopez N. 2019. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. Journal of immunology research 2019: 3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mackler AM, Iezza G, Akin MR, McMillan P, and Yellon SM. 1999. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biology of reproduction 61: 879–883. [DOI] [PubMed] [Google Scholar]
- 169.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, and Norman JE. 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Human reproduction (Oxford, England) 14: 229–236. [PubMed] [Google Scholar]
- 170.Shynlova O, Tsui P, Dorogin A, and Lye SJ. 2008. Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 181: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 171.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, and Jones RL. 2012. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biology of reproduction 86: 39. [DOI] [PubMed] [Google Scholar]
- 172.Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Nguyen T, and Lye SJ. 2013. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. Journal of cellular and molecular medicine 17: 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, and Gomez-Lopez N. 2016. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cellular & molecular immunology 13: 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liggins G 1981. Cervical ripening as an inflammatory reaction In The cervix in pregnancy and labor: clinical and biochemical investigations. Ellwood E, and Anderson A, eds. Churchill Livingstone, Edinburgh: 1–9. [Google Scholar]
- 175.Sakamoto Y, Moran P, Bulmer JN, Searle RF, and Robson SC. 2005. Macrophages and not granulocytes are involved in cervical ripening. Journal of reproductive immunology 66: 161–173. [DOI] [PubMed] [Google Scholar]
- 176.Timmons BC, and Mahendroo MS. 2006. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biology of reproduction 74: 236–245. [DOI] [PubMed] [Google Scholar]
- 177.Yellon SM, Ebner CA, and Sugimoto Y. 2008. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biology of reproduction 78: 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Timmons BC, Fairhurst AM, and Mahendroo MS. 2009. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol 182: 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, and Yellon SM. 2011. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biology of reproduction 84: 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yellon SM, Oshiro BT, Chhaya TY, Lechuga TJ, Dias RM, Burns AE, Force L, and Apostolakis EM. 2011. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biology of reproduction 85: 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Myers DA 2012. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biology of reproduction 87: 107. [DOI] [PubMed] [Google Scholar]
- 182.Payne KJ, Clyde LA, Weldon AJ, Milford TA, and Yellon SM. 2012. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biology of reproduction 87: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, and Hassan SS. 2008. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 21: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Davis LE, McLaren LC, Stewart JA, James CG, Levine MD, and Skipper BJ. 1983. Immunological and microbiological studies of midtrimester amniotic fluid. Gynecologic and obstetric investigation 16: 261–268. [DOI] [PubMed] [Google Scholar]
- 185.Schmidt W 1992. The amniotic fluid compartment: the fetal habitat. Advances in anatomy, embryology, and cell biology 127: 1–100. [DOI] [PubMed] [Google Scholar]
- 186.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, and Hsu CD. 2018. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. American journal of reproductive immunology (New York, N.Y. : 1989) 79: e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, and Yoon BH. 2000. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. American journal of obstetrics and gynecology 183: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 188.Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, Pacora P, Erez O, Yeo L, Hassan SS, and Hsu CD. 2019. In vivo evidence of inflammasome activation during spontaneous labor at term. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 32: 1978–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gomez-Lopez N, Romero R, Panaitescu B, Miller D, Zou C, Gudicha DW, Tarca AL, Para R, Pacora P, Hassan SS, and Hsu CD. 2019. Gasdermin D: in vivo evidence of pyroptosis in spontaneous labor at term. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet: 1–11. [DOI] [PMC free article] [PubMed]
- 190.Stutz A, Horvath GL, Monks BG, and Latz E. 2013. ASC speck formation as a readout for inflammasome activation. Methods Mol Biol 1040: 91–101. [DOI] [PubMed] [Google Scholar]
- 191.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, and O’Neill LA. 2015. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, Hsu CD, and Panaitescu B. 2019. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biology of reproduction 100: 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Seong HS, Lee SE, Kang JH, Romero R, and Yoon BH. 2008. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. American journal of obstetrics and gynecology 199: 375 e371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, and Kim YM. 2015. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine 43: 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, and Kim YM. 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology 213: S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Halgunset J, Johnsen H, Kjollesdal AM, Qvigstad E, Espevik T, and Austgulen R. 1994. Cytokine levels in amniotic fluid and inflammatory changes in the placenta from normal deliveries at term. European journal of obstetrics, gynecology, and reproductive biology 56: 153–160. [DOI] [PubMed] [Google Scholar]
- 197.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, and Kim IO. 1997. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. American journal of obstetrics and gynecology 177: 19–26. [DOI] [PubMed] [Google Scholar]
- 198.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L, and Hassan SS. 2017. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci 24: 934–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, Pacora P, Tarca AL, Motomura K, Erez O, Jung E, Hassan SS, and Hsu CD. 2019. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. Journal of perinatal medicine 47: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cross SN, Potter JA, Aldo P, Kwon JY, Pitruzzello M, Tong M, Guller S, Rothlin CV, Mor G, and Abrahams VM. 2017. Viral Infection Sensitizes Human Fetal Membranes to Bacterial Lipopolysaccharide by MERTK Inhibition and Inflammasome Activation. J Immunol 199: 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Romero R, Dey SK, and Fisher SJ. 2014. Preterm labor: one syndrome, many causes. Science 345: 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, and Lawn JE. 2012. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England) 379: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 203.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, and Gulmezoglu AM. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7: e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gravett MG, Hummel D, Eschenbach DA, and Holmes KK. 1986. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstetrics and gynecology 67: 229–237. [DOI] [PubMed] [Google Scholar]
- 205.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, and Hobbins JC. 1988. Infection in the pathogenesis of preterm labor. Seminars in perinatology 12: 262–279. [PubMed] [Google Scholar]
- 206.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, and Hobbins JC. 1989. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. American journal of obstetrics and gynecology 161: 817–824. [DOI] [PubMed] [Google Scholar]
- 207.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, and Novy MJ. 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. American journal of obstetrics and gynecology 171: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 208.Gomez R, Romero R, Edwin SS, and David C. 1997. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infectious disease clinics of North America 11: 135–176. [DOI] [PubMed] [Google Scholar]
- 209.Kallapur SG, Willet KE, Jobe AH, Ikegami M, and Bachurski CJ. 2001. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 280: L527–536. [DOI] [PubMed] [Google Scholar]
- 210.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, and Waites KB. 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 16: 56–70. [DOI] [PubMed] [Google Scholar]
- 211.Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, and Rajagopal L. 2013. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med 210: 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, and Heyborne K. 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 210: 125.e121–125.e115. [DOI] [PubMed] [Google Scholar]
- 213.Cobo T, Kacerovsky M, and Jacobsson B. 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. American journal of obstetrics and gynecology 211: 708. [DOI] [PubMed] [Google Scholar]
- 214.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, and Yeo L. 2014. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. American journal of reproductive immunology (New York, N.Y. : 1989) 71: 330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, and Yeo L. 2014. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of reproductive immunology (New York, N.Y. : 1989) 72: 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, and Cerami A. 1989. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. American journal of obstetrics and gynecology 161: 336–341. [DOI] [PubMed] [Google Scholar]
- 217.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, and Appelbaum PC. 1993. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. American journal of obstetrics and gynecology 169: 1299–1303. [DOI] [PubMed] [Google Scholar]
- 218.Keelan JA, Wang K, Chaiworapongsa T, Romero R, Mitchell MD, Sato TA, Brown DA, Fairlie WD, and Breit SN. 2003. Macrophage inhibitory cytokine 1 in fetal membranes and amniotic fluid from pregnancies with and without preterm labour and premature rupture of membranes. Molecular human reproduction 9: 535–540. [DOI] [PubMed] [Google Scholar]
- 219.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, and Antsaklis A. 2010. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. European journal of obstetrics, gynecology, and reproductive biology 148: 147–151. [DOI] [PubMed] [Google Scholar]
- 220.Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, and Jacobsson B. 2013. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One 8: e60399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, and Margolis L. 2015. Evidence of perturbations of the cytokine network in preterm labor. American journal of obstetrics and gynecology 213: 836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Romero R, Chaemsaithong P, Chaiyasit N, Docheva N, Dong Z, Kim CJ, Kim YM, Kim JS, Qureshi F, Jacques SM, Yoon BH, Chaiworapongsa T, Yeo L, Hassan SS, Erez O, and Korzeniewski SJ. 2017. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. American journal of reproductive immunology (New York, N.Y. : 1989) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, and Hobbins JC. 1991. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. American journal of obstetrics and gynecology 165: 821–830. [DOI] [PubMed] [Google Scholar]
- 224.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, and et al. 1993. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology 169: 805–816. [DOI] [PubMed] [Google Scholar]
- 225.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB, and et al. 1993. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. American journal of obstetrics and gynecology 169: 839–851. [DOI] [PubMed] [Google Scholar]
- 226.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, and Cotton DB. 1994. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. American journal of reproductive immunology (New York, N.Y. : 1989) 32: 200–210. [DOI] [PubMed] [Google Scholar]
- 227.Yoon BH, Yang SH, Jun JK, Park KH, Kim CJ, and Romero R. 1996. Maternal blood C-reactive protein, white blood cell count, and temperature in preterm labor: a comparison with amniotic fluid white blood cell count. Obstetrics and gynecology 87: 231–237. [DOI] [PubMed] [Google Scholar]
- 228.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, and Panaitescu B. 2017. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. American journal of reproductive immunology (New York, N.Y. : 1989) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, and Maymon E. 2017. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? American journal of obstetrics and gynecology 217: 693.e691–693.e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, and Hassan SS. 2017. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci 24: 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, and Mitchell MD. 1989. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 37: 13–22. [DOI] [PubMed] [Google Scholar]
- 232.Hertelendy F, Romero R, Molnar M, Todd H, and Baldassare JJ. 1993. Cytokine-initiated signal transduction in human myometrial cells. American journal of reproductive immunology (New York, N.Y. : 1989) 30: 49–57. [DOI] [PubMed] [Google Scholar]
- 233.Belt AR, Baldassare JJ, Molnar M, Romero R, and Hertelendy F. 1999. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. American journal of obstetrics and gynecology 181: 359–366. [DOI] [PubMed] [Google Scholar]
- 234.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, and Strauss JF 3rd. 1999. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. The American journal of pathology 154: 1755–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hertelendy F, Rastogi P, Molnar M, and Romero R. 2001. Interleukin-1beta-induced prostaglandin E2 production in human myometrial cells: role of a pertussis toxin-sensitive component. American journal of reproductive immunology (New York, N.Y. : 1989) 45: 142–147. [DOI] [PubMed] [Google Scholar]
- 236.Heng YJ, Liong S, Permezel M, Rice GE, Di Quinzio MK, and Georgiou HM. 2014. The interplay of the interleukin 1 system in pregnancy and labor. Reprod Sci 21: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ibrahim SA, Ackerman W. E. t., Summerfield TL, Lockwood CJ, Schatz F, and Kniss DA. 2016. Inflammatory gene networks in term human decidual cells define a potential signature for cytokine-mediated parturition. American journal of obstetrics and gynecology 214: 284 e281–284 e247. [DOI] [PubMed] [Google Scholar]
- 238.Romero R, Mazor M, and Tartakovsky B. 1991. Systemic administration of interleukin-1 induces preterm parturition in mice. American journal of obstetrics and gynecology 165: 969–971. [DOI] [PubMed] [Google Scholar]
- 239.Romero R, and Tartakovsky B. 1992. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. American journal of obstetrics and gynecology 167: 1041–1045. [DOI] [PubMed] [Google Scholar]
- 240.Witkin SS, Gravett MG, Haluska GJ, and Novy MJ. 1994. Induction of interleukin-1 receptor antagonist in rhesus monkeys after intraamniotic infection with group B streptococci or interleukin-1 infusion. American journal of obstetrics and gynecology 171: 1668–1672. [DOI] [PubMed] [Google Scholar]
- 241.Baggia S, Gravett MG, Witkin SS, Haluska GJ, and Novy MJ. 1996. Interleukin-1 beta intra-amniotic infusion induces tumor necrosis factor-alpha, prostaglandin production, and preterm contractions in pregnant rhesus monkeys. Journal of the Society for Gynecologic Investigation 3: 121–126. [DOI] [PubMed] [Google Scholar]
- 242.Vadillo-Ortega F, Sadowsky DW, Haluska GJ, Hernandez-Guerrero C, Guevara-Silva R, Gravett MG, and Novy MJ. 2002. Identification of matrix metalloproteinase-9 in amniotic fluid and amniochorion in spontaneous labor and after experimental intrauterine infection or interleukin-1 beta infusion in pregnant rhesus monkeys. American journal of obstetrics and gynecology 186: 128–138. [DOI] [PubMed] [Google Scholar]
- 243.Sadowsky DW, Adams KM, Gravett MG, Witkin SS, and Novy MJ. 2006. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. American journal of obstetrics and gynecology 195: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 244.Presicce P, Senthamaraikannan P, Alvarez M, Rueda CM, Cappelletti M, Miller LA, Jobe AH, Chougnet CA, and Kallapur SG. 2015. Neutrophil recruitment and activation in decidua with intra-amniotic IL-1beta in the preterm rhesus macaque. Biology of reproduction 92: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, and Austgulen R. 1998. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. Journal of perinatal medicine 26: 17–26. [DOI] [PubMed] [Google Scholar]
- 246.Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, and da Silva MG. 2011. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. American journal of reproductive immunology (New York, N.Y. : 1989) 65: 549–556. [DOI] [PubMed] [Google Scholar]
- 247.Jacobsson B, Holst RM, Mattsby-Baltzer I, Nikolaitchouk N, Wennerholm UB, and Hagberg H. 2003. Interleukin-18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation and preterm delivery. BJOG 110: 598–603. [PubMed] [Google Scholar]
- 248.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, and Eschenbach DA. 1988. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. The New England journal of medicine 319: 972–978. [DOI] [PubMed] [Google Scholar]
- 249.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W, and Bracken MB. 1992. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. American journal of obstetrics and gynecology 166: 1382–1388. [DOI] [PubMed] [Google Scholar]
- 250.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, Than NG, Chaiworapongsa T, Panaitescu B, Dong Z, Tarca AL, Abrahams VM, Yeo L, and Hassan SS. 2017. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci 24: 1382–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, Faro J, Pacora P, Hassan SS, and Hsu CD. 2018. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. American journal of reproductive immunology (New York, N.Y. : 1989) 80: e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, Gudicha DW, Hassan SS, Pacora P, Jung E, and Hsu CD. 2019. Gasdermin D: Evidence of Pyroptosis in Spontaneous Preterm Labor with Sterile Intra-amniotic Inflammation or Intra-amniotic Infection. American journal of reproductive immunology (New York, N.Y. : 1989). [DOI] [PMC free article] [PubMed]
- 253.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, and Beaman KD. 2013. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 191: 5702–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Whidbey C, Vornhagen J, Gendrin C, Boldenow E, Samson JM, Doering K, Ngo L, Ezekwe EA Jr., Gundlach JH, Elovitz MA, Liggitt D, Duncan JA, Adams Waldorf KM, and Rajagopal L. 2015. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO molecular medicine 7: 488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, and Mor G. 2010. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 185: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, and Means RE. 2011. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. American journal of reproductive immunology (New York, N.Y. : 1989) 65: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, Xu Y, Miller D, Hassan SS, and Gomez-Lopez N. 2019. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biology of reproduction 100: 1290–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Oppenheim JJ, and Yang D. 2005. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17: 359–365. [DOI] [PubMed] [Google Scholar]
- 259.Rubartelli A, and Lotze MT. 2007. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends in immunology 28: 429–436. [DOI] [PubMed] [Google Scholar]
- 260.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, and Billiar T. 2007. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological reviews 220: 60–81. [DOI] [PubMed] [Google Scholar]
- 261.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, and Hassan SS. 2011. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 24: 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, and Hassan SS. 2016. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. American journal of reproductive immunology (New York, N.Y. : 1989) 75: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, Dong Z, Hassan SS, and Gomez-Lopez N. 2016. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biology of reproduction 95: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Than NG, Mittal P, Kim YM, Camacho N, Edwin S, Gomez R, Hassan SS, and Romero R. 2008. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 21: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Romao-Veiga M, Matias ML, Ribeiro VR, Nunes PR, VT, M. B., Peracoli JC, and Peracoli MTS. 2018. Induction of systemic inflammation by hyaluronan and hsp70 in women with pre-eclampsia. Cytokine 105: 23–31. [DOI] [PubMed] [Google Scholar]
- 266.Matias ML, Gomes VJ, Romao-Veiga M, Ribeiro VR, Nunes PR, Romagnoli GG, Peracoli JC, and Peracoli MTS. 2019. Silibinin Downregulates the NF-kappaB Pathway and NLRP1/NLRP3 Inflammasomes in Monocytes from Pregnant Women with Preeclampsia. Molecules 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Pontillo A, Reis EC, Bricher PN, Vianna P, Diniz S, Fernandes KS, Chies JA, and Sandrim V. 2015. NLRP1 L155H Polymorphism is a Risk Factor for Preeclampsia Development. American journal of reproductive immunology (New York, N.Y. : 1989) 73: 577–581. [DOI] [PubMed] [Google Scholar]
- 268.Xu L, Li S, Liu Z, Jiang S, Wang J, Guo M, Zhao X, Song W, and Liu S. 2019. The NLRP3 rs10754558 polymorphism is a risk factor for preeclampsia in a Chinese Han population. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet 32: 1792–1799. [DOI] [PubMed] [Google Scholar]
- 269.Robbins GR, Wen H, and Ting JP. 2014. Inflammasomes and metabolic disorders: old genes in modern diseases. Mol Cell 54: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Patel S 2018. Danger-Associated Molecular Patterns (DAMPs): the Derivatives and Triggers of Inflammation. Curr Allergy Asthma Rep 18: 63. [DOI] [PubMed] [Google Scholar]
- 271.Liu Z, Zhao X, Shan H, Gao H, and Wang P. 2019. microRNA-520c-3p suppresses NLRP3 inflammasome activation and inflammatory cascade in preeclampsia by downregulating NLRP3. Inflamm Res 68: 643–654. [DOI] [PubMed] [Google Scholar]
- 272.Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, Kawashima A, Ohkuchi A, Taniguchi S, and Takahashi M. 2015. NLRP3 Deficiency Improves Angiotensin II-Induced Hypertension But Not Fetal Growth Restriction During Pregnancy. Endocrinology 156: 4281–4292. [DOI] [PubMed] [Google Scholar]
- 273.Shirasuna K, Usui F, Karasawa T, Kimura H, Kawashima A, Mizukami H, Ohkuchi A, Nishimura S, Sagara J, Noda T, Ozawa K, Taniguchi S, and Takahashi M. 2015. Nanosilica-induced placental inflammation and pregnancy complications: Different roles of the inflammasome components NLRP3 and ASC. Nanotoxicology 9: 554–567. [DOI] [PubMed] [Google Scholar]
- 274.Shirasuna K, Takano H, Seno K, Ohtsu A, Karasawa T, Takahashi M, Ohkuchi A, Suzuki H, Matsubara S, Iwata H, and Kuwayama T. 2016. Palmitic acid induces interleukin-1beta secretion via NLRP3 inflammasomes and inflammatory responses through ROS production in human placental cells. Journal of reproductive immunology 116: 104–112. [DOI] [PubMed] [Google Scholar]
- 275.Seno K, Sase S, Ozeki A, Takahashi H, Ohkuchi A, Suzuki H, Matsubara S, Iwata H, Kuwayama T, and Shirasuna K. 2017. Advanced glycation end products regulate interleukin-1beta production in human placenta. The Journal of reproduction and development 63: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kohli S, Ranjan S, Hoffmann J, Kashif M, Daniel EA, Al-Dabet MM, Bock F, Nazir S, Huebner H, Mertens PR, Fischer KD, Zenclussen AC, Offermanns S, Aharon A, Brenner B, Shahzad K, Ruebner M, and Isermann B. 2016. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128: 2153–2164. [DOI] [PubMed] [Google Scholar]
- 277.Mulla MJ, Salmon JE, Chamley LW, Brosens JJ, Boeras CM, Kavathas PB, and Abrahams VM. 2013. A role for uric acid and the Nalp3 inflammasome in antiphospholipid antibody-induced IL-1beta production by human first trimester trophoblast. PLoS One 8: e65237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Leon-Martinez D, Mulla MJ, Han CS, Chamley LW, and Abrahams VM. 2018. Modulation of trophoblast function by concurrent hyperglycemia and antiphospholipid antibodies is in part TLR4-dependent. American journal of reproductive immunology (New York, N.Y. : 1989) 80: e13045. [DOI] [PubMed] [Google Scholar]
- 279.Mulla MJ, Weel IC, Potter JA, Gysler SM, Salmon JE, Peracoli MTS, Rothlin CV, Chamley LW, and Abrahams VM. 2018. Antiphospholipid Antibodies Inhibit Trophoblast Toll-Like Receptor and Inflammasome Negative Regulators. Arthritis & rheumatology 70: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Han CS, Herrin MA, Pitruzzello MC, Mulla MJ, Werner EF, Pettker CM, Flannery CA, and Abrahams VM. 2015. Glucose and metformin modulate human first trimester trophoblast function: a model and potential therapy for diabetes-associated uteroplacental insufficiency. American journal of reproductive immunology (New York, N.Y. : 1989) 73: 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abi Nahed R, Reynaud D, Borg AJ, Traboulsi W, Wetzel A, Sapin V, Brouillet S, Dieudonne MN, Dakouane-Giudicelli M, Benharouga M, Murthi P, and Alfaidy N. 2019. NLRP7 is increased in human idiopathic fetal growth restriction and plays a critical role in trophoblast differentiation. Journal of molecular medicine 97: 355–367. [DOI] [PubMed] [Google Scholar]


