Abstract
To address the extent to which functional connectivity measures an absolute brain state, we observed the effect of prior performance of a language task on resting‐state networks in regions associated with language. Six subjects were imaged during rest before and after a block‐design language task. Connectivity maps were generated for each of four language regions (identified from analysis of the language activation portion of the study) in each subject for both rest periods. Conjunction analysis demonstrated distinct networks of voxels for each seed region, indicating separate functional subnetworks associated with the different regions. In a comparison of rest before and after the activation task widespread and significant changes were observed in all individuals, suggesting that the measured resting state network reflects a dynamic image of the current brain state. At the group level, an extended network was observed that was largely persistent over time. Even at the group level an increase in connectivity was observed between left and right middle frontal gyri, and between posterior cingulate cortex and medial frontal cortex in the rest after the language task. These results suggest that functional connectivity may be a powerful measure of cognitive state, sensitive to differences between controls and patients together with the particular cognitive processing occurring during the rest state. Hum. Brain Mapping 24:59–68, 2005. © 2004 Wiley‐Liss, Inc.
Keywords: functional MRI, brain networks, functional connectivity, BOLD, rest
INTRODUCTION
A trend in functional magnetic resonance imaging (fMRI) research has been to develop ever more sophisticated behavioural designs to isolate regions specific to particular function. One problem with this approach is that it only reveals regions specifically active in the task state. Cognitive elements that are actively involved in both states are absent from our functional maps, despite possibly being crucial to task performance.
More recently, functional connectivity (FC) has been suggested as an alternative approach for exploring the interrelation between functionally related regions of the brain [Biswal et al., 1995; Friston et al., 1995]. This approach exploits the fact that there exists a synchrony between low frequency (<0.08 Hz) fluctuations in fMRI signal intensity in spatially separated brain regions when the brain is free from any externally imposed task [Biswal et al., 1995; Lowe et al., 1998; Xiong et al., 1999]. This synchrony or connectivity has been seen within a variety of cortical regions, including motor cortex [Biswal et al., 1995; Xiong et al., 1999], visual cortex [Lowe et al., 1998], but also in subcortical regions [Stein et al., 2000] and the language system [Cordes et al., 2000]. Detecting these resting state networks (RSN) may identify interregional relationships not seen in conventional activation study designs.
Several recent studies have applied FC techniques to various patient groups [Lawrie et al., 2002; Li et al., 2000; Lowe et al., 2002; Quigley et al., 2001; Rowe et al., 2002]. FC maps may be of clinical utility because they detect the amount of correlation in signal intensity between regions within a network but are insensitive to the ability of a subject to carry out a specific cognitive task, because they are obtained at rest. FC maps may even allow the study of a function (e.g., language) in patients unable to carry out tasks related to that function (e.g., in patients suffering aphasia) to probe where the functional deficit may be located. The fact that FC maps identify co‐varying brain regions without the need to directly compare and contrast two cognitive states may make them a more robust measure of intactness of cognitive networks. FC maps may also help to identify cognitive strategies (such as brain reorganisation) that may have overcome the challenges of a brain lesion affecting one node in a network. Despite this clear potential, there are unresolved issues in how to interpret findings in the presence of pathology. Observed differences in functional connectivity between patients and controls may represent permanent cognitive changes associated with brain pathology or may simply be due to behavioural changes associated with a patient's experience in the scanner, in terms of task performance and subjective feeling, which may influence the resting state in a measurable way. To be sure that changes observed reflect permanent network changes rather than behavioural differences in the resting state, we need to know that the FC map of a particular RSN is consistent and stable in control subjects.
In studies of FC during continual task performance compared to those undertaken at rest, differences due to cognitive state have been suggested, but this has not been tested statistically [Greicius et al., 2003; Lowe et al., 2000]. We cannot be certain that FC maps represent fixed networks associated with particular cognitive function, and we do not know if different cognitive states engage such a network in a different way. If, as indicated by the work of Lowe et al. [2000], performance of a task increases the level of FC due to cognitive processing, will this change persist into a subsequent rest period?
We specifically address the issue of variability in FC maps, examining the effect of cognitive activity immediately before a resting state on the FC pattern subsequently observed, both in individuals and in a group analysis. Specifically, we ask two questions: (1) whether for any individual subject, their FC map is altered after performance of a cognitive task; and (2) if this variability invalidates connectivity analysis at a group level.
SUBJECTS AND METHODS
Subjects
Six healthy volunteers (three women, three men; all right‐handed) participated in the study, after giving written informed consent. Handedness was assessed using the Edinburgh handedness inventory [Oldfield, 1971]. Subjects had no cerebral abnormalities on screening MRI and no history of past or present neurologic or psychiatric disorder. The study was approved by the Austin Health Human Research Ethics Committee, and conformed to the guidelines of the National Health and Medical Research Council and the Declaration of Helsinki.
Image Acquisition
The fMRI studies were carried out with a 3T GE Signa LX whole body scanner (General Electric, Milwaukee, WI), using a standard birdcage quadrature head coil. Stimuli were back‐projected through the shielded window in the Faraday cage onto a screen at the foot of the scanning table. Structural imaging included T1‐weighted, 2‐D, spin‐echo images, acquired with the same geometric orientation and slice thickness as the subsequent functional images. Functional images were acquired as a series of gradient‐recalled echo planar imaging (GR‐EPI) volumes (TR/TE = 3,600/40 ms, flip angle = 60 degrees, 25 oblique slices 4 mm thick + 1‐mm gap, 24‐cm field of view (FOV), 128 × 128 matrix), with one imaging volume being acquired every 3.6 s. The first four acquisitions were discarded to allow the tissue magnetisation to reach equilibrium.
Task
Each subject was scanned continuously for 16:12 min. This session involved 5:24 min (90 volumes) of resting state scanning (subject instructed to lie still and not to carry out any specific cognitive task, with eyes open), followed by a 5:24‐min block‐design language task, then a further 5:24 min of resting state data. During the language task, 9 blocks of 10 acquisitions (10 × 3.6 = 36 s) were acquired. A visual fixation (baseline) block was carried out five times interleaved with a block of orthographic lexical retrieval (OLR) carried out four times. In each OLR block, the subject was shown a new letter every 18 s (two per block). Subjects were instructed to silently generate as many novel words as possible beginning with each displayed letter. They were instructed not to repeat words, even in different forms, and not to use proper nouns. Task performance of the OLR paradigm was assessed for each subject in a separate out‐of‐scanner behavioural study. This was carried out to assess the ability of each subject to carry out the task. Due to the covert nature of the paradigm, in‐scanner performance measures were not obtained. During the rest periods, subjects were instructed not to engage in any cognitive activity, specifically not to engage in language production, as in the OLR task.
After the scanning session, subjects completed a short questionnaire to ascertain their cognitive state during the two rest periods. No subjects were conscious of falling asleep during scanning, and all felt they complied with the instruction not to engage in language production.
Image Analysis
Standard block‐design fMRI analysis of the OLR task was carried out using MATLAB (MathWorks Inc., Natick, MA) and SPM99 (Wellcome Department of Cognitive Neurology; online at http://www.fil.ion.ucl.ac.uk/spm). The time series for each subject was first preprocessed. This involved motion correction, where the rigid body transformation set of six parameters (three translations and three rotations) that minimised the squared difference between each image and the first were obtained, and then used to reslice the images. The volumes were then nonlinearly transformed into a space approximating the SPM standard space (Montreal Neurological Institute [MNI]). This was done in two stages due to the signal drop out in 3T EPI volumes. The main problem for registration of 3T EPI volumes is the significant signal loss in the inferior frontal and temporal lobes, which leads to a misregistration. To overcome this, a 3T standard space template was created using the EPI images of a group of 30 subjects. Each individual subject had an EPI acquired together with a T1 image with the same orientation and slice thickness. By comparing these two volumes, it was possible to identify regions of signal loss in the EPI. A mask was generated to indicate these regions for each of the 30 subjects. This mask was excluded from consideration in the registration process, leading to a series of 30 individual EPIs in standard space. These 30 images were averaged and then smoothed to 8‐mm full‐width half‐maximum (FWHM) to generate a local 3T template. Each subject being considered in the connectivity study was registered to this 3T template. Finally, the data were spatially smoothed by convolution with an 8‐mm isotropic Gaussian kernel to improve the signal‐to‐noise ratio, as well as to allow the use of Gaussian random field theory to generate corrected P values.
Statistical analysis of the activation study (task vs. rest) of each individual and the group were carried out using SPM99, which uses the general linear model and Gaussian random field theory to determine voxels significantly correlated with the task (convolved with a canonical hemodynamic response function). Motion correction parameters were also included in the design matrix for each subject as covariates of no interest. Individual results were thresholded at P < 0.05, corrected for multiple comparisons. Group data were assessed using conjunction analysis. A cognitive conjunction tests whether all of the subjects' “activations” are jointly significant, which identifies the probability of activation occurring jointly in all subjects within a group, and is thus less sensitive to outliers than a standard fixed effects analysis [Price and Friston, 1997]. Group voxel thresholds were chosen at the P < 0.05 level (corrected). By acquiring T1 images with the same orientation and slice thickness as the EPI images, we were able to identify relevant anatomic landmarks, which aid in interpretation, given the approximate nature of the registration to standard space.
To select regions to use as seed regions in the connectivity analysis, we chose the four voxels of highest z score in the positive and negative group analysis, which lay in the left middle frontal gyrus (MFG), left inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC). To generate regions of interest (ROIs), for each subject, we included significantly active voxels for that individual within a 5‐mm radius of the maximum voxels above (thresholded at P < 0.05, corrected). This choice of ROI is somewhat similar to the approach of Greicius et al. [2003].
Connectivity analysis was carried out using SPM99 and iBrain [Abbott and Jackson, 2001]. Before carrying out the statistical analysis, each dataset was low‐pass filtered in iBrain using a finite impulse response filter to remove the effect of high‐frequency noise (f < 0.08 Hz). Seed time‐courses for each region and subject were then generated by averaging the signal within the ROI at each time point. These time‐courses were then regressed against all brain voxel time‐courses using SPM99 to obtain four connectivity maps for each subject. The regression of each seed time‐course was carried out separately to detect all voxels significantly correlated with the seed. This allowed detection of the full network, and any overlap between the four networks could subsequently be considered. The individual FC maps were thresholded at P < 0.05 (corrected) for the main effect, and P < 0.0001 (uncorrected) for the contrast of before language (REST2) versus after language (REST1). In the group analysis, again carried out using a conjunction, all contrasts were thresholded at P < 0.001 (uncorrected) at the voxel level. Only clusters containing at least one voxel significant at P < 0.05 at the voxel level, corrected for multiple comparisons, are reported in the text.
A measure of the percentage overlap between each of the possible pairs (A and B) of FC maps was calculated as:
| (1) |
where n A∩B is the number of voxels in the intersection A∩B of the two maps, and n A∪B is the number of voxels in the union A∪B.
RESULTS
Task‐Related Activation
Task‐related changes in signal intensity are shown in Figure 1, where significantly activated or deactivated voxels are projected onto the surface of a single subject's 3‐D‐rendered brain in MNI space (Fig. 1A), as well as overlaid on the mean EPI of the six subjects (Fig. 1B). The OLR activation above rest (Fig. 1A) shows extensive left MFG activation, as well as bilateral IFG activation, and activation in the region of the ACC. In the contrast of rest above OLR, strong “deactivation” is seen in the PCC. All of these activations and deactivations are typical of the response to this task, and are consistent with published data [Cabeza and Nyberg, 2000].
Figure 1.
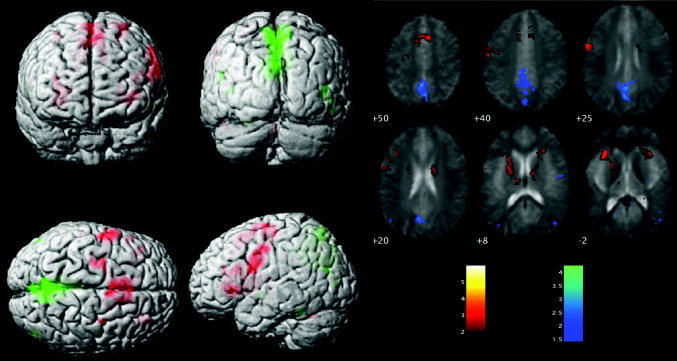
Brain activity during the OLR task. Main effect of OLR task above (activation) and below (deactivation) the baseline fixation condition. A: Results projected onto the surface of a single rendered brain (activation, red; deactivation, green). B: Results are overlaid onto the average EPI of the six subjects, with activation (task > rest) shown in “hot” colours and deactivation in “cold” colours.
Individual Subject FC Maps
FC maps of each subject were generated separately to assess individual variability. Two of the subjects are presented in Figures 2 and 3.
Figure 2.
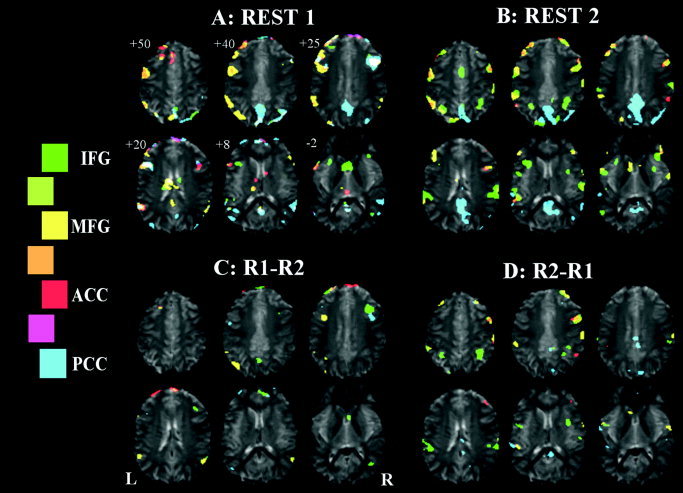
Seeded functional connectivity maps of Subject A, showing regions correlated with ACC, PCC, left IFG, and left MFG ROI average signal time‐courses shown in red, blue, green, and yellow, respectively. A: Initial rest period. B: Rest period immediately after an OLR vs. baseline paradigm. C: Comparison of the two rest periods, REST1 > REST2. D: REST2 > REST1. Legend shows the colour scale, with intermediate colours (between two regions) representing the overlap of those two maps.
Figure 3.
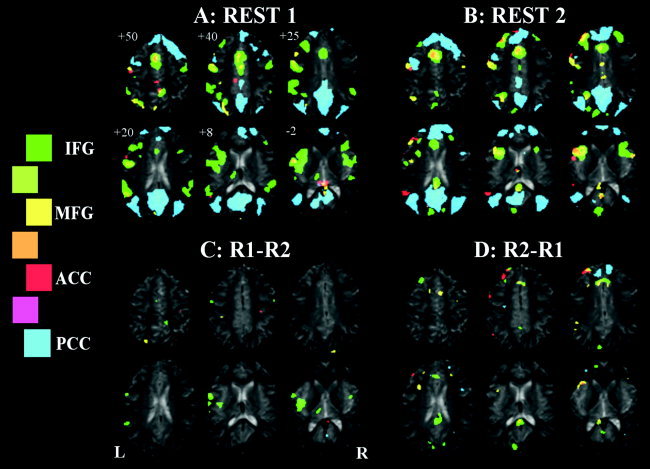
Seeded functional connectivity maps of Subject B, showing regions correlated with ACC, PCC, left IFG, and left MFG ROI average signal time‐courses shown in red, blue, green, and yellow, respectively. A: Initial rest period. B: Rest period immediately after an OLR vs. baseline paradigm. C: Comparison of the two rest periods, REST1 > REST2. D: REST2 > REST1.
The first subject (Fig. 2) shows a large degree of overlap in all four FC maps in the initial resting period, as indicated by the white colour in Figure 2A. This connectivity pattern includes the PCC network including the MFG region bilaterally. This pattern changes after performance of the language task (Fig. 2B), where increased involvement of the ACC, left MFG, and left IFG networks with the right MFG region, and PCC connectivity reverts to a more posterior pattern. These results are verified in Figure 2C and 2D, where we see significant right MFG decrease in connectivity to the left IFG and PCC seed regions and increase in ACC and left MFG maps after the language task. Indeed, it seems that these changes may reflect a change in the anatomic location of involvement, from a more inferior position before the language task to a more superior position afterward. This subject also shows only a limited region of ACC involvement in any of the FC maps.
The results of the second subject are shown in Figure 3. This subject shows large regions of connectivity, especially in the PCC and left IFG FC maps. The left IFG FC map in particular shows diffuse and widespread connectivity in the inferior frontal region, involving the insula, before the language task. This FC map becomes more focal after the language task, and shows greater overlap with the left MFG map. This subject also clearly and consistently displays functional segregation within the left MFG (upper left slice in Fig. 3A,B) where segregated regions of involvement of the ACC, PCC, and left MFG FC maps are seen. These regions are remarkably constant between the two independent resting state periods. For this subject, few between‐session changes reach statistical significance (Fig. 3C,D). Most obvious are the decreased connectivity of the left IFG seed with the left insula, increased connectivity with the PCC region, and increased ventral ACC involvement in the PCC connectivity map. There also seems to be an increase in involvement of the left IFG within the left MFG map after the language task.
In a visual comparison of results from these two subjects (e.g., comparing Fig. 2A and 3A), the second subject had little involvement in right MFG (no FC maps showing right MFG activation), but had extensive involvement of the PCC FC map in the ventral aspect of the ACC region, both of which are at odds with the results of the first subject. Although this suggests the extent of the functional networks varies considerably between individuals, this difference could also be due to variation in the residual level of error variance between the subjects.
Group FC Maps
The conjunction across the six subjects of the four FC maps are presented in Figure 4, where the PCC FC map is shown in blue, left IFG in green, left MFG in yellow, and ACC in red. The connectivity maps (e.g., the map in Fig. 4A showing connectivity during the resting period before the language task) show a large degree of overlap with the task activation map (Fig. 1), together with several additional regions. The MFG, ACC, and IFG maps each involve ACC, left MFG, and bilateral IFG regions, together with left angular gyrus. The left MFG map further involves right MFG and bilateral occipital/parietal lobe adjacent to PCC, neither of which is seen in the task activation map. The PCC FC map shows strong correlation with a large region of PCC/retrosplenial cortex, bilateral parietal cortex, as well as ventral ACC regions and some lateral frontal cortical areas. On visual inspection, the map in Figure 4B, obtained from the resting state after OLR performance, shows similar FC maps for the four seed ROIs.
Figure 4.
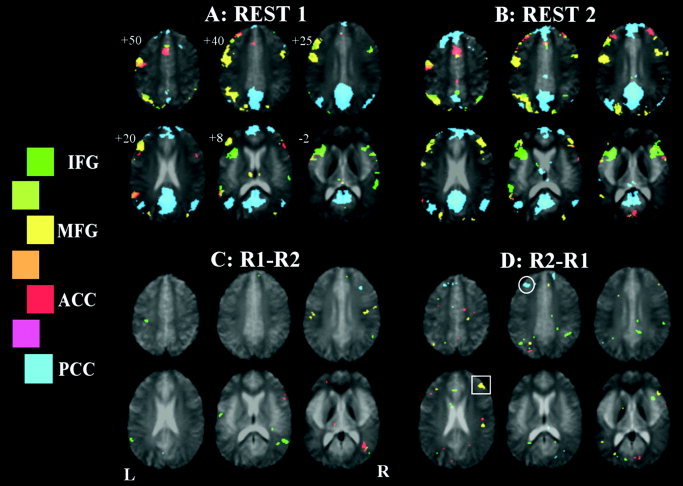
Seeded functional connectivity over the six subjects, showing regions correlated with ACC, PCC, left IFG, and left MFG ROI average signal time‐courses shown in red, blue, green, and yellow, respectively. A: Initial rest period. B: Rest period immediately after an OLR vs. baseline paradigm. C: Comparison of the two rest periods, REST1 > REST2. D: REST2 > REST1. Significant increases in connectivity are seen for the PCC map (cluster in medial frontal cortex circled), and the left MFG map (cluster in the right MFG, indicated by a square)
Subregions
In Figure 4, there are distinct subregions within each functional area (as defined in a task vs. rest comparison) that show higher correlation with other seed ROI time‐courses than with the functional area's own mean time‐course. For instance, in Figure 4A, there are regions within the left IFG that are correlated significantly with the left MFG (yellow) and ACC (red) time‐courses, but not with the left IFG time‐course (green). Similar observations are made in the ACC and left MFG.
Changes in group FC maps after language task
In Figure 4C and 4D, group results are presented for the contrasts REST1–REST2 and REST2–REST1, respectively, reflecting the decrease and increase in connectivity after the language task. It is seen that for the PCC FC map, there is an increase in medial frontal connectivity (circle) after language performance. For the MFG map, there is an increase in connectivity with the contralateral homologous region after language task performance (square).
Overlap of maps
Another way to assess connectivity patterns is to look at the overlap between FC maps associated with different ROI regions. This overlap is quantified in Figure 5 (and using equation [1]), where the average (across‐subject) fraction of voxels that overlap between pairs of FC maps is plotted. It is seen that the PCC network is relatively distinct, showing significantly less overlap than that seen between other maps (P < 0.0001, Student's t‐test). Between each of the other three maps, there was 5–14% overlap. The changes after language task performance can be seen also in the changes in the overlap between functional networks (Fig. 5B). None of the differences seen were significant, although there was a trend toward an increase in the overlap between IFG and MFG networks after language task performance (P = 0.052, Student's t‐test).
Figure 5.
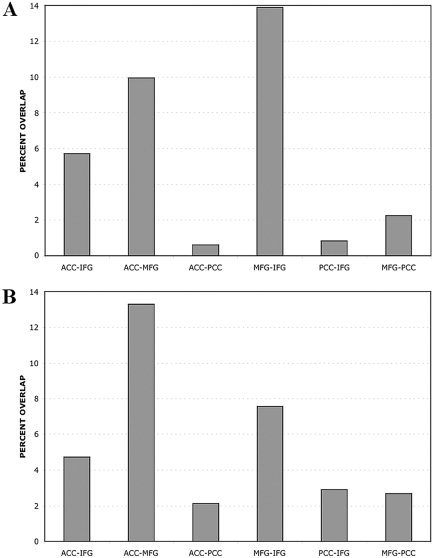
Average percentage overlap between each of the FC maps in Figure 4, and how these overlaps vary between the two resting periods. From left to right: bars represent overlap between ACC and left IFG, ACC and left MFG, ACC and PCC, IFG and MFG, IFG and PCC, and MFG and PCC. A: Overlap before the language task. B: Overlap after the task period.
DISCUSSION
We observed that functional connectivity maps show significant variation across subjects, and at an individual level can show marked changes after performance of the OLR task. However, when studied at the group level, the FC maps are extended and largely consistent when compared between two sessions separated by a language task.
Inter‐ and Intra‐Subject Variability
All subjects studied showed significant changes in functional connectivity after carrying out a language task, suggesting that FC maps are not exclusively measuring an absolute brain state. Rather, the degree of connectivity between brain regions can vary, even though measured during a continuous resting state. This may be due in part to physiologic factors such as cardiac or respiratory noise or bulk head motion, and cognitive factors such as attention or motivation changes influencing the observed connectivity. The low sampling rate associated with acquiring whole brain coverage (TR = 3 s) creates a potential for physiologic noise, primarily cardiac variance, to be aliased to low frequency and to survive the filtering process. These signals, if present in the data, would be more global in nature with a focus in the ventricles and brainstem. This is not seen in our data, leading us conclude that the effects of this artefact are negligible.
One possible explanation we considered for the greater variability of individual maps was that a group map has greater statistical power. In both individuals and the group, we carried out a statistical test of the difference between sessions. A lack of power can only account for a lack of an observed effect. We actually observed statistically significant differences in our individuals and fewer significantly different voxels in the group (e.g., compare Fig. 2C,D with Fig. 4C,D). This cannot be explained by a lack of power in the individual analyses.
The resting state is known to involve uncontrolled cognitive processing, and is associated especially with episodic and autobiographic memory recall [Andreasen et al., 1995; Binder et al., 1999; Mazoyer et al., 2001; Shulman et al., 1997]. In fact, the “network” of regions found to be more active in rest than in a variety of cognitive tasks in all these studies seems almost identical to the PCC network we found in Figure 4, involving ventral ACC and bilaterally in the region of the angular gyrus. It is interesting to consider what the level of association between the PCC and the other networks (MFG, IFG) within this system may mean. We have detected a level of interaction for instance between the PCC and left MFG. This implies that the rest network of Mazoyer et al. is in direct connection with elements that are more active during complicated tasks. Further speculation on this is beyond the scope of the present study, but future work focusing on connectivity analysis during continuous rest and task periods may shed light on how these brain networks interact as a function of cognitive state.
The important point we have demonstrated is that when studying an individual, connectivity maps change over time, and are possibly dependent on the prior cognitive processing of the subject. This finding is especially important if we aim to use resting state FC maps to monitor network‐level changes in cognitive function as a result of pathology, such has been attempted by several groups [Lawrie et al., 2002; Lowe et al., 2002; Quigley et al., 2001; Rowe et al., 2002].
Resting Networks Associated With Language Regions
Despite the variability in FC maps across sessions in a single individual and between subjects, the group results were very robust. The FC networks obtained before and after language processing were both widespread and varied, including, but not limited to, regions associated with performance of the OLR task. This network was largely consistent across the two sessions, with only some small focal differences detected. This consistency suggests that group analysis of functional connectivity is a stable measure, despite FC being a dynamic measure of brain state.
FC maps associated with the four ROIs (left IFG, left MFG, ACC, and PCC) were largely distinct in their distribution, suggesting that FC analysis may be sensitive to detecting functional subnetworks within a larger network. We assume that these networks are involved in language‐related processing, because we have seeded with regions identified in a language production task. Previous studies have described FC maps as qualitatively representing the same regions seen in block‐design activation studies of the functions, such as sensorimotor cortex and supplementary motor area involvement in the motor system [Biswal et al., 1995; Lowe et al., 1998]. Our findings suggest a richer system, including regions not identified in an activation paradigm, as well as a division into different subnetworks. Study of the functional roles of these subnetworks may offer further insight into brain function at a system level. It is our hope that this information obtained using FC be included amongst inputs when constructing neurocognitive models for complex cognitive processes such as language and memory [Mesulam, 1990].
Subregional Functional Specificity
It was interesting to observe anatomically segregated distributions of functional connectivity within areas interpreted as single functional regions in a traditional fMRI activation study of language. For example, within the functional unit of the left MFG as identified in the activation study, there exist three different populations of voxels. Firstly, there are voxels that solely correlate (significantly) with the left MFG ROI time‐course. There are also overlapping voxels, which are correlated with both left MFG and left IFG time‐courses, and cross‐talk voxels, which are correlated with left IFG or ACC, but not with the left MFG ROI average time‐course. One can speculate about what this means in terms of brain function. There may be subregions within MFG that are involved in a feedback system, monitoring or regulating other related processing, e.g., in the ACC and IFG. This is consistent with our knowledge from activation studies that these regions carry out different functions but cooperate in performance of the OLR task. This study does not attempt to determine all possible connections within the language system or to explore the limits of functional segregation of language. Having identified additional detail in the map of cognitive function, our next goal is to understand its generalisability across subjects, in both position and extent.
In conclusion, this study has used brain regions involved in language production as seeds in a functional connectivity analysis, and explored the resting state networks associated with these regions. These networks were found to vary in individuals in a manner that might depend on the prior cognitive state, suggesting that FC maps represent measurements of a dynamically varying brain state. Despite this, it was found that group analysis of FC maps was robust and stable across sessions. In addition, it was found that there are at least four subnetworks involving language regions that are largely distinct in their spatial distributions. These connectivity maps identified the signal correlation of additional brain regions to those seen in the language activation study. Finally, we identified subregions within each language region that showed significant correlation with other ROIs, but not with the local time‐course, suggesting a possible functional link in coordinating the level of interaction between these different language networks.
These results suggest that connectivity analysis may have the sensitivity to identify nodes involved in interaction between brain regions during cognitive processing. The challenge remains to explore how best to identify disease‐associated changes in connectivity and to understand the cognitive mechanisms that result in the presence of functionally connected networks.
REFERENCES
- Abbott D, Jackson G (2001): iBrain–software for analysis and visualisation of functional MR images. Neuroimage 13: 59. [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD (1995): Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry 152: 1576–1585. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11: 80–95. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition. II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol 21: 1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R (1995): Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 2: 166–172. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC (2002): Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry 51: 1008–1011. [DOI] [PubMed] [Google Scholar]
- Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, Salmeron BJ, Stein EA (2000): Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med 43: 45–51. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD (2000): Correlations in low‐frequency BOLD fluctuations reflect cortico‐cortical connections. Neuroimage 12: 582–587. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP (2002): Multiple sclerosis: low‐frequency temporal blood oxygen level‐dependent fluctuations indicate reduced functional connectivity initial results. Radiology 224: 184–192. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1990): Large‐scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 28: 597–613. [DOI] [PubMed] [Google Scholar]
- Oldfield R (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997): Cognitive conjunction: a new approach to brain activation experiments. Neuroimage 5: 261–270. [DOI] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Wendt G, Turski P, Moritz C, Haughton V, Meyerand ME (2001): Effect of focal and nonfocal cerebral lesions on functional connectivity studied with MR imaging. AJNR Am J Neuroradiol 22: 294–300. [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R (2002): Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain 125: 276–289. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Peterson SE (1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Stein T, Moritz C, Quigley M, Cordes D, Haughton V, Meyerand E (2000): Functional connectivity in the thalamus and hippocampus studied with functional MR imaging. AJNR Am J Neuroradiol 21: 1397–1401. [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT (1999): Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp 8: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]


