Abstract
The relationship between cardiovascular regulation and brain activation was investigated during attempted foot lifting in paraplegic subjects and during rhythmic handgrip exercise at one‐third of maximum voluntary contraction force. Brain areas of interest were the primary sensory‐motor area and the insula, a hypothesized center for a central nervous feed‐forward mechanism involved in cardiovascular control (“central command”). This mechanism is complementary to the usual known feedback pathways such as skeletal muscle afferent signals. Regional cerebral blood flow (rCBF) was measured in eight normal and three paraplegic subjects using positron emission tomography (PET) and oxygen‐15‐labeled water. Statistical parametric maps were calculated from the images comparing rest and handgrip. Paraplegics were also scanned during attempted foot lifting, a condition without sensory feedback. During activation tasks, heart rate and mean arterial pressure increased. PET activation responses (P < 0.05, corrected for multiple comparisons) were found in the contralateral primary sensory‐motor area, the supplementary motor area, ipsilateral cerebellum, and bilaterally in the insula. A conjunction analysis showing responses common to handgrip and attempted foot lifting revealed activation in the right central insula (P < 0.05, corrected) in concordance with the concept of a central command feed‐forward hypothesis. Hum Brain Mapp 25:259–265, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: heart rate, positron emission tomography, regional cerebral blood flow, spinal cord injury, insular activation
INTRODUCTION
Heart rate and blood pressure increase simultaneously at the onset of exercise. These rapid cardiovascular responses are caused by central nervous feed‐forward mechanism involved in cardiovascular control (“central command”), first described as “cortical irradiation” [Krogh and Lindhard,1913]. There is ample evidence for this mechanism during exercise and during attempted or imagined exercise [Decety et al.,1993; Kjær and Secher,1992; Leonard et al.,1985; Nowak et al.,1999; Victor et al.,1995; Williamson et al.,1995]. The insular cortex in the brain may be a key component of this mechanism. Anatomically, the insula is characterized by its location in the lateral sulcus (covered by the operculum) and by projections to various parts of the cerebral cortex, the basal ganglia, limbic areas, and other structures [Augustine,1996]. The primate insula is divided in three cytoarchitecturally distinct fields: the rostroventral agranular field, the posterior granular field, and the transitional dysgranular field denoted herein as the central part of the insula. Each field has a distinct set of afferent and efferent projections. Of note here is that afferent projections to the central part of the insula descend from the somatosensory cortex and the superior temporal sulcus. The former may provide pathways for a feed‐forward signal to the insula, which in turn influences regulation of cardiovascular function.
From animals it is known that the insular cortex contains centers for cardiovascular control, e.g., in rats, electrical stimulation of the posterior inferior cortex increases arterial pressure and heart rate [Cechetto and Saper,1990]. Studies in humans have identified the insular cortex as a site for cortical regulation of cardiac autonomic (parasympathetic) activity [Williamson et al.,1997] and this activation response may be lateralized. Electrical stimulation of the insula during neurosurgery is reported to elicit increases in blood pressure (right posterior insula) and to cause bradycardia (left posterior insula) [Oppenheimer et al.,1992].
To examine the role of the insula in cardiovascular feed‐forward regulation during exercise, we studied regional cerebral blood flow (rCBF) as an indicator for neuronal activation employing positron emission tomography (PET) and oxygen‐15‐labeled water (H2 15O). The main focus of interest was on the insula, where we expected increased flow during tasks causing an increase in heart rate and blood pressure. We investigated normal subjects at rest and during rhythmic handgrip and paraplegic subjects with a complete neural lesion of the spinal cord (no sensation or voluntary motor function in the lower extremities) at rest, during rhythmic handgrip and attempted foot lifting. During this task, motor circuits (thus feed‐forward mechanisms) are recruited without resulting in any movement and consequently not activating feedback mechanisms. The rationale was to attempt to separate between activation caused by feed‐forward signals versus feedback signals (like muscle activity and muscle afferent feedback). Foot lifting was chosen as it was a simple movement involving a large muscle mass and thus likely to cause a measurable signal in the brain despite the fact that this task was only attempted, i.e., not actually carried out. We hypothesized that activation would give rise to increased rCBF in the insula and be linked to cardiovascular responses.
SUBJECTS AND METHODS
Three female and five male healthy volunteers (median age, 28 years; age range 21–33 years) and three male paraplegic volunteers with traumatic spinal cord injury participated after informed consent was given (Table I). The paraplegics had all complete spinal cord injuries with no sensation or voluntary motor function in the lower extremities. All subjects were right‐handed as evaluated by the Edinburgh inventory [Oldfield,1971] and underwent a PET investigation followed by an magnetic resonance imaging (MRI) investigation on a different day. The study was approved by the Ethical Committee of Copenhagen and Frederiksberg (journal no. 01‐208/97) and complied with the Declaration of Helsinki.
Table I.
Data of the three paraplegic volunteers with traumatic spinal cord injury
| Subject no. | Neurologic level of lesion | Years post‐injury | Age (yr) |
|---|---|---|---|
| 1 | Thoracic 6 | 5.5 | 52 |
| 2 | Thoracic 4 | 7.5 | 23 |
| 3 | Thoracic 7 | 26 | 51 |
Rhythmic Handgrip
The subjects carried out rhythmic handgrip with their nondominant left hand around a plastic ball applying about one‐third of their maximum voluntary contraction force (MVC) followed by complete relaxation. Because the paraplegics had remarkably stronger arm muscles, they were instructed to apply only around 10% MVC. Handgrip was paced by a metronome set to 2 Hz. The ball was connected to a pressure transducer via a rigid tube and the pressure was recorded online.
Attempted Foot Lifting
In addition to rhythmic handgrip, the paraplegic subjects also carried out attempted foot lifting. They were instructed to try to lift their feet, paced by the metronome, while supine on the bed of the scanner.
PET Scanning
The scans were carried out with an Advance PET scanner (GE, Milwaukee, WI). Each subject was exposed to a 10‐min transmission scan and six to eight intravenous bolus injections of 300 MBq H2 15O [Holm et al.,1996]. Emission scans were acquired in 3D mode with retracted collimating septa. The order of the scan conditions was randomized.
During the PET investigation, subjects were supine with pillows under the legs and were carefully instructed and trained in the procedures, especially in performing rhythmic handgrip while keeping other muscles as relaxed as possible. The head was immobilized with polystyrene foam, the eyes were covered, the room lights were dimmed, and ambient noise kept to a minimum. A venous catheter for administration of H2 15O was placed in the left brachial vein and an arterial catheter for blood sampling and blood pressure recordings was inserted in the right brachial artery.
The scan protocol is illustrated in Figure 1. Handgrip force was recorded. The tracer was administered intravenously with an automated water injection system over 5–10 s followed by 10 ml of isotonic saline for flushing. Data acquisition was triggered by the arrival of the tracer to the brain and lasted for 90 s (two frames of 45 s each). There was an interval of 10 min between repeated injections to allow for isotope decay.
Figure 1.
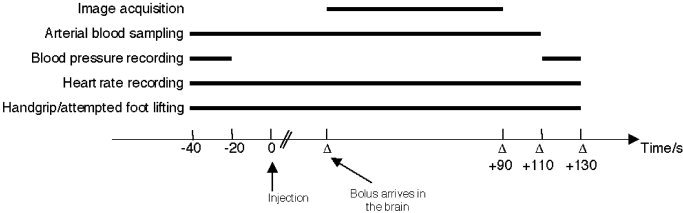
Schematic presentation of a PET scan during rhythmic handgrip/attempted foot lifting (Δ, delay between injection and arrival of the tracer in the brain, usually ∼50 s). At rest, a corresponding protocol was carried out.
MR Scanning
The scans were carried out with a clinical 1.5‐Tesla MR scanner (Magnetom Vision; Siemens, Erlangen, Germany). The subjects' heads were fixed as described above. High‐resolution anatomic scans were acquired with a T1‐weighted 3‐D MPRAGE sequence (echo time [TE] = 4.4 ms, inversion time [TI] = 300 ms, repetition time [TR] = 11.4 ms, flip angle of 12 degrees, and voxel size 1 mm3).
PET Image Processing
The PET data were reconstructed to 35 128 × 128 pixel matrices applying 8 mm Hanning filtering. The voxel size was 2.0 × 2.0 × 4.25 mm3. For calculation of absolute rCBF, an autoradiographic one‐tissue‐compartment model with correction for delay and dispersion was implemented using the first 45‐s frame [Meyer,1989]. The resulting flow images were corrected for changes in arterial pCO2 tension (3%/mm Hg) and used as an indication of regional neural activity [Fox and Raichle,1986; Mazziotta et al.,1985]. For semiquantitative rCBF analysis in the paraplegic subjects, the two frames were summed and the resulting time integrated counts (relative rCBF) normalized to a global flow of 50 ml/(100 g min) [Fox and Mintun,1989]. Statistical image analysis was carried out with the Statistical Parametric Mapping software package (SPM99; Wellcome Department, London, UK) [Frackowiak et al.,1997]. The processing steps before statistical analysis included realignment, coregistration to each subject's anatomic T1‐weighted MR scan, transformation to a standard stereotactic space and smoothing with an isotropic 3D Gaussian filter (full‐width half‐maximum [FWHM] 8 × 8 × 8 mm3).
Statistical Analysis
An ANCOVA method was used to account for global effects. The gray matter threshold was set to 80% and the evaluated contrast was handgrip minus rest or attempted foot lifting minus rest. Foci of activated areas were assessed on a voxel‐by‐voxel level using the t‐statistic comparing the expected and observed number of pixels above the chosen threshold [Friston et al.,1991]. The location of significant activation during stimulus was defined as the Talairach coordinates [Talairach and Tournoux,1988] of the peak within a confluent area that exceeded the chosen threshold of P < 0.05 and its spatial extent defined as the number of voxels above the threshold.
The resulting statistical parametric maps were also used for masking the spatially normalized, pCO2‐corrected PET images to calculate absolute rCBF in the activated areas and to calculate the correlation between heart rate and rCBF in the insula. Average global cerebral blood flow (CBF) was calculated by employing a template to define the extent of the brain. Unless otherwise stated, values are given as mean ± standard error of the mean (SEM).
RESULTS
Healthy Subjects
Handgrip was carried out at 34 ± 2% of MVC. During rhythmic handgrip, heart rate and mean arterial pressure increased by 12 ± 1 beats/min (bpm) and 11 ± 1 mm Hg, respectively. Forty PET datasets were included in the quantitative flow calculation, as some datasets had to be excluded due to inadequate fits or corrupted input curves. CBF, corrected for pCO2 tension, was 41.4 ± 1.3 ml/(min 100 g). There was no significant difference in global CBF between rhythmic handgrip and rest. Activation during rhythmic handgrip was found in the contralateral primary sensory‐motor area, ipsilateral cerebellum, supplementary motor area (SMA), bilateral insula, and ipsilateral precentral gyrus (Table II; P < 0.05 corrected for multiple comparisons). The activation in the insula was strongest on the right side, with two individual peaks in the central part of the insula. The activation on the left side was somewhat more dorsal.
Table II.
Activated areas during left rhythmic handgrip in healthy subjects
| Anatomic location of activated area | Spatial extent* | Z‐score | rCBF increase (%)** | Talairach coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| R Pre/postcentral gyrus, S1/M1 | 2,068 | >8 | 34 | 42 | −28 | 64 |
| Bottom central sulcus, S1/M1 | — | >8 | — | 38 | −26 | 50 |
| Pre/post‐central gyrus, S1/M1 | — | 7.02 | — | 54 | −18 | 48 |
| L Cerebellum | 281 | 7.07 | 28 | −20 | −52 | −20 |
| L Cerebellum | — | 5.79 | — | −8 | −54 | −14 |
| R Inferior parietal lobule, area 40 | 210 | 6.08 | — | 56 | −32 | 26 |
| Postcentral gyrus, area 40 | — | 6.08 | — | 54 | −22 | 20 |
| R Insula (central) | 54 | 5.56 | 20 | 36 | −8 | 0 |
| R Insula (central) | — | 5.02 | — | 26 | −6 | 6 |
| L Precentral gyrus, area 6 | 5 | 5.37 | — | −64 | −4 | 28 |
| Medial aspect, frontal lobe, SMA | 13 | 5.23 | 19 | 2 | −5 | 54 |
| R Precentral gyrus | 47 | 5.21 | — | 42 | −2 | 10 |
| R Precentral gyrus | — | 4.99 | — | 48 | −8 | 8 |
| L Insula (dorsal) | 8 | 5.13 | 20 | −48 | −8 | 10 |
Table is sorted in order of descending peak Z‐score in each confluent area. Voxel numbers are not shown for subpeaks (indented). Areas with less than five pixels are not listed. P < 0.05, corrected.
Spatial extent is measured in voxels of 2 × 2 × 2 mm3.
The increase in rCBF (activation minus rest) was calculated as described in the legend to Figure 2.
rCBF, regional cerebral blood flow; S1/M1, primary sensorimotor area; SMA, supplementary motor area.
During rhythmic handgrip, the average flow in the activated region in the right insula increased by 20%, from 48 ± 2 to 57 ± 2 ml/(min 100g), and in the left insula by 10%, from 52 ± 2 to 57 ± 3 ml/(min 100g).
A correlation analysis pooling all image data of all healthy subjects revealed correlation coefficients between right/left insular flow and heart rate of 0.72 and 0.71, respectively, (Fig. 2; P < 0.05, corrected for nonindependent observations). There was no evidence for a correlation between insular flow and mean arterial pressure.
Figure 2.
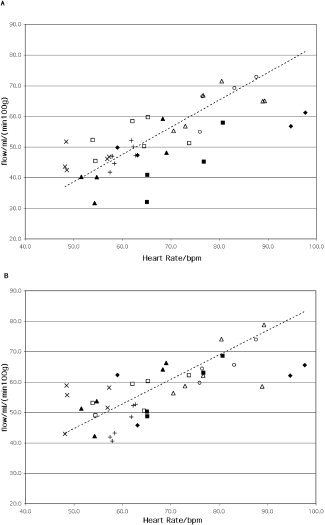
Individual rCBF in the right (A) and left (B) central insular cortex plotted against heart rate. rCBF was calculated as the average of all activated pixels in the respective region in the spatially normalized PET images before smoothing. Data are from healthy subjects at rest and during rhythmic handgrip. Heart rate is averaged over the scan interval. The correlation coefficients are 0.72 and 0.71 for right and left insular cortex, respectively (P < 0.05). Each subject is plotted with an individual marker symbol. The linear fit is plotted as a dashed line.
Paraplegic Subjects
Rhythmic handgrip was carried out at 10% of MVC. Heart rate and mean arterial pressure increased by 7 ± 8 bpm and 5 ± 2 mm Hg, respectively. Attempted foot lifting was associated with an increase of 5 ± 4 bpm and 9 ± 2 mm Hg, respectively. In one paraplegic subject it was not possible to place an arterial catheter and therefore the statistical analysis of the paraplegic subjects was carried out on semiquantitative PET images separately from the other subjects, i.e., the PET images were assumed to reflect rCBF without quantitative flow calculation.
The contrasts of handgrip minus rest and attempted foot lifting minus rest were evaluated and fed into a conjunction analysis. This type of analysis reveals jointly activated areas during the two exercise conditions by statistical evaluation of the joint probability of the individual contrasts. The right central insula and the left cerebellum were activated (P < 0.05, corrected for multiple comparisons; Table III).
Table III.
Results of the conjunction analysis between left rhythmic handgrip and attempted foot lifting in paraplegic subjects
| Anatomic location of activated area | Spatial extent* | Z‐score | Talairach coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left cerebellum | 28 | 5.56 | −14 | −48 | −18 |
| Right insula | 8 | 5.91 | 30 | −14 | 12 |
Table is sorted in order of ascending peak Z‐score in each confluent area. Areas with less than five pixels are not listed. P < 0.05, corrected.
Spatial extent is measured in voxels of 2 × 2 × 2 mm3.
DISCUSSION
This study investigated the relation between cardiovascular regulation and brain activation during rhythmic handgrip exercise with specific interest in the central part of the insular cortex.
The insula is a hypothesized site of central command and we observed robust activation in the central part of the insula during exercise along with increases in heart rate and blood pressure. On a more general level, a correlation between absolute insular blood flow and heart rate across all subjects and conditions was observed. These data alone, however, do not clarify whether the insula is operative in feed‐forward or feedback processing.
The results obtained in paraplegic subjects revealed the insula as belonging to a set of regions jointly activated during actual performed handgrip and attempted foot‐lifting exercise. Both conditions elicited increases in cardiovascular variables. There was no sensory feedback from the lower extremities during attempted exercise; thus, we conclude that this feedback is not a prerequisite for activation in the insular cortex. The observed activation of the insular cortex may be part of the feed‐forward control of the cardiovascular system. We cannot exclude the possibility, however, that activation in the insula is a response to changes in cardiovascular variables, such as feedback from baroreceptors or other systemic changes (e.g., respiration).
In early PET studies investigating hand movements, insular activation was not found consistently [Colebatch et al.,1991; Grafton et al.,1993; Nowak et al.,1999; Sadato et al.,1996a]. Activation in the insula was found only at very slow rates of movement (≤0.5 Hz), which may be related to attention [Sadato et al.,1996b] and at high frequencies of movement in a physically demanding finger‐tapping task [Blinkenberg et al.,1996]. Passive or active elbow movement [Weiller et al.,1996], median nerve stimulation [Ibáñez et al.,1995], or motor preparation [Deiber et al.,1996] do not demonstrate insular activation. One study reports activation of the insula during shoulder movements and finger opposition, whereas making a fist did not show insular activation [Colebatch et al.,1991]. These results share some characteristics despite seeming somewhat contradictory at first sight: The insula seems to be activated during difficult tasks requiring a certain degree of attention and during motor tasks provided they cause a cardiovascular response.
There are reported human single photon emission computed tomography (SPECT) studies during exercise in which insular activation was observed. The left insular cortex was pointed out as a site for cortical regulation of cardiac autonomic (parasympathetic) activity during moderate cycling in humans [Williamson et al.,1997]. Different relations for different conditions (post‐exercise muscular ischemia, low‐intensity cycling, and moderate‐intensity cycling) between blood pressure and insular rCBF distributions were found [Williamson et al.,1999].
None of these studies, however, allowed for an absolute quantification of rCBF in the insular regions. In this study, a correlation between absolute blood flow in the insula and heart rate was established. This included increases in flow and heart rate during handgrip exercise (SPM analysis; see Table II) and, probably more interestingly, also when pooling all image data from all healthy subjects (Fig. 2). As the analysis included all rest scans and all exercise scans, with each subject having their distinct handgrip performance, different rest heart rates, and individual cardiovascular responses, this correlation may point to a fundamental coupling between heart rate and insular activation. Initial heart rate elevations up to a rate of about 100 bpm are due primarily to vagal withdrawal of parasympathetic activity [Robinson et al.,1966], which could well explain our findings.
We did not find a relation between insular flow and blood pressure, but this is not in contradiction to the central command hypothesis, as central command influence on muscle sympathetic nervous activity (causing vasoconstriction) is evident only during intense motor effort and is dominated normally by muscle afferent reflexes [Victor et al.,1995]. In contrast, skin sympathetic outflow is controlled mainly by central command even at low exercise intensity [Vissing et al.,1991].
Whether the observed insular activation represents a feed‐forward or feedback signal cannot be determined from the first part of our study. Evidence for a feed‐forward mechanism is given by the second part of the study in paraplegic subjects with a complete neural lesion of the spinal cord. These subjects lack sensory feedback from the legs such as afferent signals from chemoreceptors, muscle spindles, tendons, and joints. Brain regions activated during both handgrip (feed‐forward and feedback signals) and attempted foot lifting (feed‐forward signal only) can be considered to be involved in high‐level motor control, such as initiation or coordination of movement (e.g., the cerebellum and SMA). These regions, however, may also accompany movement without being related directly to execution of movement, such as when contractions are attempted. Because we have independent data in the form of increases in cardiovascular variables, we conclude that the insula is involved in feed‐forward regulation of the cardiovascular system.
Our observation of increases in heart rate and blood pressure during handgrip as well as during attempted foot lifting in paraplegic subjects is in contradiction to results reported previously [Hobbs and Gandevia,1985]. Briefly, Hobbs and Gandevia report no changes in heart rate or blood pressure in paraplegic subjects who attempted to contract paralyzed leg muscles. They conclude that pathways descending to and arising from the spinal cord below the lesion are required to generate a cardiovascular response, and consequently they reject the possibility of a feed‐forward mechanism. A possible reason for the difference could be that the subjects included in our study had been paraplegic for 5 years or more, whereas Hobbs and Gandevia [1985] studied paraplegics within the first weeks and months after spinal cord injury. One would naively expect the opposite finding, however, because adaptation presumably becomes more prominent with time. There is evidence of plasticity in the motor cortex within a few weeks in normal subjects [Karni et al.,1995] and after limb amputation [Kew et al.,1994], but the paraplegic subjects in this study showed no evidence for non‐normal placement of the hand or foot areas. If the spinal cord is needed for the generation of a cardiovascular response as suggested by Hobbs and Gandevia [1985], this requirement might be lost after adaptation to lacking afferent signal from the lower limbs.
Others have pointed out that the pattern of brain activation during handgrip in paraplegics is “strikingly different from healthy subjects” [Bruehlmeier et al.,1998]. Abnormalities include generally greater rCBF changes and activation of the ipsilateral sensory‐motor cortex and the contralateral cerebellum. Spinal cord injury and cerebral deafferentation seem to cause a global reduction in glucose metabolism, whereas brain regions involved in attention and initiation of movement show increased glucose metabolism [Roelcke et al.,1997]. No similar effects on rCBF were observed in this study.
In cognitive, nonmotor tasks, the insula is not a site of typical activation in cognitive tasks [Table II in Cabeza and Nyberg,2000], but the central part of the insula is involved in tasks requiring attentional control [Coull and Nobre,1998; Hopfinger et al.,2000], whereas the anterior and posterior parts of the insula are often found activated as hypothesized members of neural networks relating to working memory [Braver et al.,1997], emotion [Damasio,2003], and pain [Peyron et al.,2000].
The data from this study show activation in the insula during rhythmic handgrip and rCBF in the insula across all conditions and subjects is correlated with heart rate. Paraplegic subjects show activation in the insula in situations that are characterized by feed‐forward control alone (attempted foot lifting) and in situations with combined feed‐forward and feedback control (rhythmic handgrip).
Acknowledgements
We thank the John and Birthe Meyer Foundation for donating the scanners and the cyclotron. We also thank the staff at the PET and Cyclotron Unit and at the MR Unit for their help.
REFERENCES
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law I (1996): Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study. J Cereb Blood Flow Metab 16: 794–803. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC (1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A (1998): How does the human brain deal with a spinal cord injury? Eur J Neurosci 10: 3918–3922. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB (1990): Role of the cerebral cortex in autonomic function In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. New York: Oxford University Press; p 208–223. [Google Scholar]
- Colebatch JG, Deiber MP, Passingham RE, Friston KJ, Frackowiak RS (1991): Regional cerebral blood flow during voluntary arm and hand movements in human subjects. J Neurophysiol 65: 1392–1401. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC (1998): Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18: 7426–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A (2003): Looking for Spinoza: joy, sorrow and the feeling brain. Orlando: Harcourt; p 83–126. [Google Scholar]
- Decety J, Jeannerod M, Durozard D, Baverel G (1993): Central activation of autonomic effectors during mental simulation of motor actions in man. J Physiol (Lond) 461: 549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Ibáñez V, Sadato N, Hallett M (1996): Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 75: 233–247. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA (1989): Noninvasive functional brain mapping by change‐distribution analysis of averaged PET images of H2 15O tissue activity. J Nucl Med 30: 141–149. [PubMed] [Google Scholar]
- Fox PT, Raichle ME (1986): Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC (1997): Human brain function. San Diego: Academic Press; 528 p. [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991): Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11: 690–699. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Mazziotta JC (1993): Within‐arm somatotopy in human motor areas determined by positron emission tomography imaging of cerebral blood flow. Exp Brain Res 95: 172–176. [DOI] [PubMed] [Google Scholar]
- Hobbs SF, Gandevia SC (1985): Cardiovascular responses and the sense of effort during attempts to contract paralysed muscles: role of the spinal cord. Neurosci Lett 57: 85–90. [DOI] [PubMed] [Google Scholar]
- Holm S, Law I, Paulson OB (1996): 3D PET activation studies with an H215O bolus injection. Count rate performance and dose optimization In: Myers R, Cunningham V, Bailey D, Jones T, editors. Quantification of brain function using PET. San Diego: Academic Press; p 93–97. [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3: 284–291. [DOI] [PubMed] [Google Scholar]
- Ibáñez V, Deiber MP, Sadato N, Toro C, Grissom J, Woods RP, Mazziotta JC, Hallett M (1995): Effects of stimulus rate on regional cerebral blood flow after median nerve stimulation. Brain 118: 1339–1351. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Kew JJ, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S, Frackowiak RS, Brooks DJ (1994): Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J Neurophysiol 72: 2517–2524. [DOI] [PubMed] [Google Scholar]
- Kjær M, Secher NH (1992): Neural influence on cardiovascular and endocrine responses to static exercise in humans. Sports Med 13: 303–319. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J (1913): The regulation of respiration and circulation during the initial stages of muscular work. J Physiol 47: 112–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Mitchell JH, Mizuno M, Rube N, Saltin B, Secher NH (1985): Partial neuromuscular blockade and cardiovascular responses to static exercise in man. J Physiol 359: 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Huang SC, Phelps ME, Carson RE, MacDonald NS, Mahoney K (1985): A noninvasive positron computed tomography technique using oxygen‐15‐labeled water for the evaluation of neurobehavioral task batteries. J Cereb Blood Flow Metab 5: 70–78. [DOI] [PubMed] [Google Scholar]
- Meyer E (1989): Simultaneous correction for tracer arrival delay and dispersion in CBF measurements by the H215O autoradiographic method and dynamic PET. J Nucl Med 30: 1069–1078. [PubMed] [Google Scholar]
- Nowak M, Olsen KS, Law I, Holm S, Paulson OB, Secher NH (1999): Command‐related distribution of regional cerebral blood flow during attempted handgrip. J Appl Physiol 86: 819–824. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC (1992): Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L (2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Clin Neurophysiol 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Robinson BF, Epstein SE, Beiser GD, Braunwald E (1966): Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res 19: 400–411. [DOI] [PubMed] [Google Scholar]
- Roelcke U, Curt A, Otte A, Missimer J, Maguire RP, Dietz V, Leenders KL (1997): Influence of spinal cord injury on cerebral sensorimotor systems: a PET study. J Neurol Neurosurg Psychiatry 62: 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibáñez V, Deiber M, Hallett M (1996a): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Ibáñez V, Deiber MP, Campbell G, Leonardo M, Hallett M (1996b): Frequency‐dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab 16: 23–33. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Georg Thieme Verlag; 122 p. [Google Scholar]
- Victor RG, Secher NH, Lyson T, Mitchell JH (1995): Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res 76: 127–131. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG (1991): Stimulation of skin sympathetic nerve discharge by central command. Differential control of sympathetic outflow to skin and skeletal muscle during static exercise. Circ Res 69: 228–238. [DOI] [PubMed] [Google Scholar]
- Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, Muller S, Diener HC, Thilmann AF (1996): Brain representation of active and passive movements. Neuroimage 4: 105–110. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH (1999): Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol 87: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, McColl R, Mathews D, Winchester P, Friberg L, Mitchell JH (1997): Activation of the insular cortex during dynamic exercise in humans. J Physiol 503: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JW, Nobrega AC, Winchester PK, Zim S, Mitchell JH (1995): Instantaneous heart rate increase with dynamic exercise: central command and muscle‐heart reflex contributions. J Appl Physiol 78: 1273–1279. [DOI] [PubMed] [Google Scholar]


