Abstract
Autobiographical memory is based on interactions between episodic memory contents, associated emotions, and a sense of self‐continuity along the time axis of one's life. The functional neuroanatomy subserving autobiographical memory is known to include prefrontal, medial and lateral temporal, as well as retrosplenial brain areas; however, whether gender differences exist in neural correlates of autobiographical memory remains to be clarified. We reanalyzed data from a previous functional magnetic resonance imaging (fMRI) experiment to investigate gender‐related differences in the neural bases of autobiographical memories with differential remoteness and emotional valence. On the behavioral level, there were no significant gender differences in memory performance or emotional intensity of memories. Activations common to males and females during autobiographical memory retrieval were observed in a bilateral network of brain areas comprising medial and lateral temporal regions, including hippocampal and parahippocampal structures, posterior cingulate, as well as prefrontal cortex. In males (relative to females), all types of autobiographical memories investigated were associated with differential activation of the left parahippocampal gyrus. By contrast, right dorsolateral prefrontal cortex was activated differentially by females. In addition, the right insula was activated differentially in females during remote and negative memory retrieval. The data show gender‐related differential neural activations within the network subserving autobiographical memory in both genders. We suggest that the differential activations may reflect gender‐specific cognitive strategies during access to autobiographical memories that do not necessarily affect the behavioral level of memory performance and emotionality. Hum Brain Mapping 24:313–324, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: fMRI, mental recall, prefrontal cortex, parahippocampal gyrus, cognition, emotions
INTRODUCTION
Autobiographical memory can best be defined as a complex subsystem of episodic memory that particularly implies emotion processing and provides a direct link to awareness of the time course of one's life [Fink et al.,1996; Tulving,1983; Tulving and Markowitsch,1998]. The complex neural mechanisms subserving autobiographical memory have been assessed with functional neuroimaging techniques by few investigators. Using positron emission tomography (PET), Fink et al. [1996] demonstrated that emotional autobiographical memory (relative to a resting baseline) activates a preponderantly right hemispheric network of prefrontal and temporal, as well as posterior cingulate/retrosplenial brain regions. In a functional magnetic resonance imaging (fMRI) study [Piefke et al.,2003], similar neural structures were implicated in recent and remote autobiographical memories with positive or negative emotional valence, albeit with a stronger left hemisphere preponderance. Functional neuroimaging data on autobiographical memory reported by other investigators are in accordance with these findings [Maguire et al.,2001a,b], although no consensus has been achieved as to the issue of hemispheric lateralization of episodic autobiographical and nonautobiographical memory processing [Lee et al.,2003; Piefke et al.,2003].
In addition, the interesting question remains to be clarified whether gender differences occur with regard to the functional neuroanatomy subserving autobiographical memory as has been reported for autobiographical memory performance (e.g., females' autobiographical recollections were reportedly more detailed and emotionally intense than were males' personal memories) [Davis,1999; Fujita et al.,1991]. Two hypotheses have been proposed that may account for such behavioral gender differences in the memory domain. The “affect intensity hypothesis” suggests that females have superior memory abilities as they experience and remember life events emotionally more intensely than males and that women may thus encode life events more deeply than males do [Fujita et al.,1991]. By contrast, the “cognitive style hypothesis” claims that females differ from males with respect to their way of encoding, rehearsing, and thinking about emotional experiences, and regarding their strategies of generating responses during experimental memory tasks [Seidlitz and Diener,1998]. According to the first model, superior memory abilities of females should be eliminated when affect intensity of personal recollections is experimentally controlled for. Furthermore, the affect intensity hypothesis predicts that females should present with similar neural activation patterns as males but exhibit stronger activations [Canli et al.,2002], especially in those brain areas known to be involved in emotion processing [Dolan,2000]. Again, there should be no differences in the strength of activations when the affect intensity of memories is controlled. By contrast, the cognitive style hypothesis expects that gender‐specific differences in memory performance will persist even if affect intensity of autobiographical recall is experimentally controlled. Moreover, this view suggests that males and females may show qualitative rather than quantitative differences in brain activation patterns associated with emotional memory processing.
Differential performance of males and females in memory processing, although in the nonautobiographical domain, has also been observed repeatedly in verbal and spatial memory. It was found that males outperformed females in spatial memory tasks [Sandstrom et al.,1998; Vecchi and Girelli,1998], whereas females demonstrated superior memory abilities in the verbal domain [Halpern,1992; Mekarski et al.,1996; Vogel,1990]. These findings may suggest that gender differences in the neural mechanisms subserving autobiographical memory can be expected specifically within brain areas subserving spatial (e.g., hippocampus and parahippocampal gyrus) and verbal memory (e.g., left hemisphere inferolateral frontal and temporal cortices).
Functional neuroimaging research has not yet explicitly addressed gender‐related distinctiveness in neural mechanisms subserving autobiographical memory. However, a considerable number of studies have reported gender differences in the neural substrates of various aspects of cognition [Fitch and Bimonte,2002; Paulson et al.,1998] and emotion processing [Cahill et al.,2001; Killgore et al.,2001; Lane et al.,1997]. For example, Cahill et al. [2001] investigated gender‐specific amygdala responses to emotionally arousing video clips and subsequent memory for the respective stimuli using 18F‐fluorodeoxyglucose (18FDG) PET. They reported a gender‐related differential lateralization of amygdala involvement in memory storage of negatively valenced film clips. Convergent results were reported by Canli et al. [2002] using negatively valenced and neutral pictures as stimulus materials. Gender specificities in the interpretation of experimental stimulus materials resulting from social learning and sensory processing [Fitch and Bimonte,2002; Fujita et al.,1991; Paulson et al.,1998] may also have contributed to the gender differences in neural activations reported [Adinoff et al.,2003]. The issue of whether gender differences can be expected for amygdala function in emotional memory thus remains unclear.
Based on the previous studies on gender differences in memory, cognition, and emotion processing, the present study is guided by the hypothesis that gender‐related differences in the functional neuroanatomy of emotional autobiographical memory may occur primarily in parahippocampal or hippocampal regions subserving spatial memory as well as left hemisphere inferolateral frontal and temporal brain areas engaged in verbal aspects of memory processing. We directly assessed the issue of gender differences in neural mechanisms underlying retrieval of distinct types of emotional autobiographical memory by reanalyzing data from a previous fMRI experiment [Piefke et al.,2003]. In that study, which included 10 males and 10 females, we measured changes in the regional blood oxygenation level‐dependent (BOLD) signals associated with four experimental memory conditions of interest (recent and remote autobiographical memory retrieval with positive or negative emotional valence) and a low‐level “baseline,” applying a factorial blocked design with the factors TIME (recent and remote memories) and EMOTION (positive and negative tone). A more detailed description of the main effects of these factors has been published previously by Piefke et al. [2003]. A second level analysis was carried out in the present study to analyze data for gender differences in neural mechanisms underlying emotional autobiographical memory.
SUBJECTS AND METHODS
Subjects
Age‐matched, right‐handed subjects (10 men, mean age ± SD = 26.80 ± 3.39 years; 10 women, mean age ± SD = 25.50 ± 2.55 years) with no history of psychiatric or neurologic disorders were enrolled in the experiment. Participants were all students at the University of Bielefeld. Most were studying psychology (n = 14), although six had other fields of study: biology (n = 2); law (n = 3); and social pedagogy (n = 1). The study was accomplished in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all subjects before participation, and the study was approved by the local ethics committee.
Experimental Design
The experimental design of the present study has been described previously in detail by Piefke et al. [2003]. In the present study, we summarize the study design referring particularly to those aspects that are relevant in respect of the analysis of gender differences in emotional autobiographical memory. In short, individual stimuli were acquired using a semistructured autobiographical interview. Participants reported both childhood and recent memories with positive or negative emotional valence. They were required to provide detailed context information for each remembered episode to allow for the preparation of verbal stimuli describing specific situations of the respective episodes. This material was used during fMRI measurements to trigger the associated autobiographical memories.
Individual autobiographical materials were analyzed for spatial and temporal context information that builds up the main characteristics of episodic nonautobiographical and autobiographical memory. Each stimulus sentence of each subject (20 subjects with 240 stimuli each, leading to 4,800 total stimulus sentences for the group of participants) was judged independently (by two raters who were not involved in the study) as to whether it contained spatial context information, temporal context information, both types of context information, or neither type. The stimulus sets of the first five subjects were analyzed using a priori fixed rating criteria. Based on these pilot ratings, criteria were revised and complemented, resulting in a catalogue of standard criteria used for the rating of all stimulus sets. The interrater coefficient (Cohen's κ) was r = 0.80, indicating a satisfactory consensus between the two raters.
For visual presentation of the verbal stimulus material during the MR experiment, a mirror construction was used to reflect the stimulus display. Six individual trials (i.e., stimuli, that each triggered different autobiographical episodes) of one of four memory conditions (recent positive, recent negative, remote positive, and remote negative memories) were blocked together. Each trial consisted of a stimulus on time (SOT) of 4.3 s and a 1‐s interstimulus interval (ISI). Each block of trials thus had a duration of 31.8 s. Memory conditions were separated from each other by low‐level baselines (each lasting 16 s), during which the instructions for the next block of trials were presented. Instructions were as follows: “Please remember the events and situations of your personal life history specified in the displayed sentences as vividly and emotionally as possible.”
Four whole brain volumes were acquired per baseline, and eight whole brain volumes were acquired per block of trials for each memory condition. A total of five experimental runs each consisting of nine baselines and eight memory blocks were carried out, leading to the acquisition of a total of 500 volumes per subject (100 volume images per run). This scanning paradigm resulted in two repeats per condition per experimental run, leading to 10 repeats per condition per subject. The order of the memory conditions was counterbalanced across runs and individuals. There was no repeat of the individual stimulus sentences (240 stimuli for each subject) neither within nor across the experimental conditions to avoid any habituation effects.
To control for the subjects' alertness during a block of trials, we included a subordinate reaction time task into the autobiographical memory task. Subjects were instructed to press a button on a keypad as fast as possible upon detection of a checkerboard presented one to four times during the ISI of each block of trials for 500 ms. The number of times a checkerboard was presented was kept identical across all conditions.
To assess successful recognition and correct association of each stimulus with the respective personal past episode during the fMRI measurement, subjects retrospectively indicated for each of the individual stimuli: (1) whether they recognized the associated context during scanning; (2) whether it triggered a positive or a negative emotional response associated with the memory; and (3) whether it was associated with a recent or a remote memory.
In addition, participants completed a questionnaire on characteristic features of autobiographical memory for each experimental memory condition. On a rating scale ranging from 0 to 5 (0, not at all; 1, scarcely; 2, slightly; 3, fairly; 4, intense; and 5, highly intense), subjects retrospectively rated intensity of the memory characteristics retrieved during the fMRI measurement with regard to the following items: picture‐likeness; scene‐likeness; coloredness; emotionality; re‐experience; vividness; richness of details; role of language; olfactory perceptions; temperature; perceptions of touch; acoustical perceptions; and gustatory perceptions.
For image processing and all statistical calculations, the statistical parametric mapping software SPM99 (Wellcome Department of Imaging Neuroscience, London, UK; online at http://www.fil.ion.ucl.ac.uk) was used. After image preprocessing (realignment, coregistration, normalization, and smoothing), functional MR data analysis was carried out by modeling the experimental memory conditions and the baseline by means of reference waveforms that correspond to boxcar functions convolved with a hemodynamic response function [Friston et al.,1995a,b]. Accordingly, a design matrix that comprised contrasts modeling alternating intervals of “activation” (referring to the four different memory conditions) and “baseline” was defined. Specific effects were assessed by applying appropriate linear contrasts to the parameter estimates of the four experimental memory conditions and the baselines resulting in t statistics for each voxel. These formed SPMs (SPMt) of differences between both memory conditions and between memory conditions and baseline. SPMt statistics were interpreted in light of the theory of probabilistic behavior of Gaussian random fields.
Gender‐specific differences in neural substrates of emotional autobiographical memory retrieval were assessed by second‐level analysis constituting a random‐effects model. For each of the simple effects (recent, remote, positive, and negative autobiographical memories relative to baseline), the individual contrast images of each subject describing the corresponding effect were entered into a second‐level analysis based on a two‐sample t test (two groups with 10 subjects each). In addition, the gender‐specific differential activations for the contrast “all memory conditions versus baseline” were assessed. Due to the strict character of the analysis carried out (second‐level analysis based on a random‐effects model) and the predicted small effect size, a height threshold of P < 0.001, uncorrected for multiple comparisons (corresponding to t = 3.61) and an extent threshold of 10 voxels were applied. In an explorative analysis such as the present one, the use of an uncorrected threshold is well justified to exclude that representative group‐related differential activations associated with the experimental tasks are erased by statistical correction employing the family‐wise error (FWE) or false‐discovery rate (FDR). Uncorrected statistical thresholds have been applied to second‐level analyses of fMRI data by several studies published previously [e.g., Buccino et al.,2004; Gottfried et al.,2003; Thiel et al.,2002; Wicker et al.,2003; Zafiris et al.,2004] that suggest this thresholding method to represent an appropriate approach. We did not accomplish small volume corrections (SVC), as no straightforward anatomic hypotheses are available regarding gender‐related differences in autobiographical memory that would allow for a priori definition of regions of interest (ROIs).
To show the neural network of brain regions activated by the conjoint memory conditions relative to baseline across males and females, the statistical threshold was set to P < 0.05 (corrected for multiple comparisons), with no extent threshold [see Piefke et al.,2003].
Gender‐specific differences in the postscanning behavioral data (test of stimulus recognition, rating questionnaire on imagery, emotional intensity of the memories retrieved, etc.) were assessed using a two‐sample t test.
RESULTS
Behavioral Data
Reaction times and error rates in the subordinate alertness task during the fMRI experiment
Across males and females, statistical analysis of the subjects' reaction times and error rates in the subordinate alertness task did not reveal any significant differences between the four memory conditions, suggesting that the overall level of attention was the same for all memory conditions. The analysis of Gender × Condition interactions did not show a significant gender effect on reaction times and error rates for any of the memory types.
Postscanning debriefing procedures
Postscanning recognition of stimulus sentences and their correct assignment to the respective personal episodes of the subjects' past was 97.6% across genders and the five experimental runs, without statistically significant differences (P < 0.05, corrected for multiple comparisons) between the four memory conditions (childhood positive = 96.8 ± 5.6%; childhood negative = 96.6 ± 5.5%; recent positive = 98.3 ± 4.1%; and recent negative = 98.7 ± 3.1%). There was a tendency toward a higher percentage of recognition for recent relative to remote memories. As a Kolmogorov–Smirnov test revealed a normal distribution of the data, gender differences in the percentage of correct stimulus recognition were assessed using a t test. This demonstrated that there were no significant gender differences in recognition performance for any of the experimental memory conditions, even at more liberal statistical threshold of P < 0.001 (uncorrected for multiple comparisons).
Mean ratings (± SD) of recent, remote, positive, and negative memories given by the subjects (irrespective of gender) on the postscanning questionnaire concerned with characteristic features of autobiographical memory are displayed in Tables I, II, III. The data resulting from this questionnaire showed a normal distribution. An analysis of variance (ANOVA) with repeated measures on one factor (interaction Factor × Group; degrees of freedom [df] = 1) was thus applied. To keep the significance level for multiple comparisons between items at P < 0.05, single comparisons' α levels were adjusted according to the Bonferroni inequality. For all 20 subjects (irrespective of gender), significantly higher ratings for recent relative to remote memories were obtained for the items picture‐likeness (F = 10.7; P = 0.004), emotionality (F = 12.0; P = 0.003), re‐experience (F = 17.3; P = 0.001), and richness of details (F = 13.6; P = 0.002). By contrast, no statistically significant differences in the subjects' ratings were observed for positive versus negative memories. Table II and III show a comparison of mean ratings given by males (n = 10) and females (n = 10) on the postscanning questionnaire for recent and remote (Table II) as well as positive and negative (Table III) memories. A t test did not reveal statistically significant gender differences in the ratings of memories for any items included in the questionnaire, even at a more liberal statistical threshold of P < 0.001 (uncorrected for multiple comparisons).
Table I.
Mean rating of recent, remote, positive, and negative personal memories across males and females
| Item | Remote | Recent | P (recent vs. remote) | Positive | Negative | P (positive vs. negative) |
|---|---|---|---|---|---|---|
| Picture‐likeness | 3.55 ± 0.83 | 4.15 ± 0.93 | 0.004* | 3.90 ± 0.58 | 3.75 ± 0.72 | 0.267 |
| Scene‐likeness | 2.90 ± 1.41 | 3.40 ± 1.57 | 0.034 | 3.10 ± 1.29 | 3.00 ± 1.30 | 0.494 |
| Vividness | 2.85 ± 0.99 | 3.40 ± 1.10 | 0.066 | 3.25 ± 0.79 | 3.25 ± 0.97 | 1.000 |
| Coloredness | 3.40 ± 1.35 | 3.20 ± 1.47 | 0.340 | 3.40 ± 1.50 | 3.05 ± 1.50 | 0.069 |
| Language | 2.15 ± 1.53 | 2.65 ± 1.63 | 0.013 | 2.40 ± 1.67 | 2.55 ± 1.82 | 0.545 |
| Emotionality | 2.85 ± 1.35 | 3.60 ± 1.23 | 0.003* | 3.35 ± 1.04 | 3.55 ± 1.28 | 0.214 |
| Re‐experience | 3.20 ± 1.01 | 4.10 ± 0.91 | 0.001* | 3.60 ± 0.88 | 3.95 ± 0.76 | 0.015 |
| Details | 2.60 ± 1.19 | 3.35 ± 1.23 | 0.002* | 3.10 ± 1.21 | 3.10 ± 1.07 | 1.000 |
| Olfactory | 0.65 ± 0.88 | 0.70 ± 0.80 | 0.754 | 1.05 ± 1.28 | 0.70 ± 0.92 | 0.049 |
| Temperature | 1.40 ± 1.31 | 1.85 ± 1.63 | 0.045 | 1.45 ± 1.57 | 1.63 ± 1.74 | 0.250 |
| Touch | 1.70 ± 1.56 | 2.00 ± 1.89 | 0.092 | 2.00 ± 1.72 | 1.80 ± 1.67 | 0.297 |
| Acoustical | 2.00 ± 1.49 | 2.45 ± 1.67 | 0.017 | 2.15 ± 1.57 | 2.20 ± 1.58 | 0.666 |
| Gustatory | 0.80 ± 1.28 | 0.85 ± 1.09 | 0.777 | 1.00 ± 1.30 | 0.55 ± 0.89 | 0.070 |
Mean ratings ± standard deviation given by the subjects irrespective of gender (n = 20) on items (picture‐likeness, scene‐likeness, coloredness, emotionality, re‐experience, vividness, richness of details, role of language, olfactory perceptions, temperature, perceptions of touch, acoustical perceptions, gustatory perceptions) included into the postscanning debriefing questionnaire on characteristics of the autobiographical episodes retrieved during fMRI measurement. Subjects rated their memories on a five‐point rating scale (0, not at all; 1, scarcely; 2, slightly; 3, fairly; 4, intense; and 5, highly intense). For the contrast between recent and remote memories (irrespective of emotional valence), statistically significant differential ratings were observed for the items picture‐likeness, emotionality, reexperience, and details whereas positive and negative memories (irrespective of remoteness of memories) did not obtain significant differential ratings on any items.
P < 0.05, for multiple (13) comparisons (corrected α = 0.004).
Table II.
Male and female mean rating of recent and remote memories irrespective of emotional valence
| Item | Males | Females | P Time × Gender | ||||
|---|---|---|---|---|---|---|---|
| Recent | Remote | P | Recent | Remote | P | ||
| Picture‐like | 4.40 ± 0.70 | 13.60 ± 0.70 | 0.022 | 3.90 ± 1.10 | 3.50 ± 0.97 | 0.104 | 0.288 |
| Scene‐like | 3.40 ± 1.65 | 2.60 ± 1.58 | 0.070 | 3.40 ± 1.58 | 3.20 ± 1.23 | 0.343 | 0.187 |
| Vividness | 3.80 ± 1.03 | 3.10 ± 1.20 | 0.153 | 3.00 ± 1.05 | 2.60 ± 0.70 | 0.269 | 0.600 |
| Colored | 3.00 ± 1.25 | 3.30 ± 0.95 | 0.468 | 3.40 ± 1.71 | 3.50 ± 1.72 | 0.343 | 0.630 |
| Language | 3.20 ± 1.48 | 2.30 ± 1.42 | 0.019 | 2.10 ± 1.66 | 2.00 ± 1.70 | 0.591 | 0.040 |
| Emotionality | 3.60 ± 1.58 | 2.80 ± 1.62 | 0.053 | 3.60 ± 0.84 | 2.90 ± 1.10 | 0.025 | 0.824 |
| Re‐experience | 4.00 ± 0.94 | 3.10 ± 1.20 | 0.054 | 4.20 ± 0.92 | 3.30 ± 0.82 | 0.001* | 1.000 |
| Details | 3.60 ± 1.17 | 2.70 ± 1.34 | 0.019 | 3.10 ± 1.29 | 2.50 ± 1.08 | 0.051 | 0.476 |
| Olfactory | 0.60 ± 0.84 | 0.50 ± 0.71 | 0.591 | 0.80 ± 0.79 | 0.80 ± 1.03 | 1.000 | 0.754 |
| Temperature | 1.40 ± 0.71 | 1.20 ± 1.40 | 0.591 | 2.30 ± 1.49 | 1.60 ± 1.26 | 0.010 | 0.247 |
| Touch | 1.70 ± 2.16 | 1.40 ± 1.71 | 0.193 | 2.30 ± 1.64 | 2.00 ± 1.41 | 0.279 | 1.000 |
| Acoustical | 2.90 ± 1.60 | 2.60 ± 1.43 | 0.081 | 2.00 ± 1.70 | 1.40 ± 1.35 | 0.081 | 0.391 |
| Gustatory | 1.00 ± 1.41 | 1.00 ± 1.49 | 1.000 | 0.70 ± 0.67 | .60 ± 1.07 | 0.678 | 0.777 |
Mean ratings ± standard deviation for recent and remote memories (irrespective of emotional valence) given by males (n = 10) and females (n = 10) on the items included into the postscanning debriefing questionnaire (see legend to Table I). The data show that there were no statistically significant gender‐related differences in the postscanning ratings of recent and remote autobiographical memories. Due to the loss of statistical power, three items rated significantly higher for recent relative to remote memories in the statistical analyses across all 20 subjects (picture‐likeness, emotionality, and details) were nonsignificant with respect to the factor TIME when ANOVA was calculated for males and females separately. The item reexperience was rated differentially for recent vs. remote memories (statistically significant at P < 0.05, corrected for multiple comparisons) by females but not by males; however, this result depends on the relatively higher standard deviation in ratings for this item in the male group.
P < 0.05, for multiple (13) comparisons (corrected α = 0.004).
Table III.
Male and female mean rating of positive and negative memories irrespective of emotional valence
| Item | Males | Females | P Emotion × Gender | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | P | Positive | Negative | P | ||
| Picture‐like | 4.10 ± 0.57 | 3.70 ± 0.67 | 0.037 | 3.70 ± 1.06 | 3.80 ± 0.79 | 0.591 | 0.054 |
| Scene‐like | 3.10 ± 1.45 | 2.80 ± 1.40 | 0.297 | 3.10 ± 1.20 | 3.20 ± 1.23 | 0.343 | 0.169 |
| Vividness | 3.40 ± 0.84 | 3.50 ± 0.85 | 0.343 | 3.10 ± 0.74 | 3.00 ± 1.05 | 0.780 | 0.588 |
| Colored | 3.20 ± 1.23 | 2.70 ± 1.35 | 0.138 | 3.60 ± 1.78 | 3.40 ± 1.71 | 0.343 | 0.424 |
| Language | 2.70 ± 1.70 | 3.00 ± 1.94 | 0.560 | 2.10 ± 1.66 | 2.10 ± 1.66 | — | 0.552 |
| Emotionality | 3.40 ± 1.26 | 3.60 ± 1.58 | 0.443 | 3.30 ± 0.82 | 3.50 ± 0.97 | 0.343 | 1.000 |
| Re‐experience | 3.50 ± 0.85 | 3.90 ± 0.74 | 0.104 | 3.70 ± 0.95 | 4.00 ± 0.82 | 0.081 | 0.714 |
| Details | 3.40 ± 1.26 | 3.40 ± 1.07 | 1.000 | 2.80 ± 1.14 | 2.80 ± 1.03 | 1.000 | 1.000 |
| Olfactory | 0.90 ± 1.29 | 0.60 ± 0.97 | 0.193 | 1.20 ± 1.32 | 0.80 ± 0.92 | 0.168 | 0.773 |
| Temperature | 1.10 ± 1.45 | 1.56 ± 2.01 | 0.065 | 1.80 ± 1.69 | 1.70 ± 1.57 | 0.780 | 0.100 |
| Touch | 1.70 ± 2.06 | 1.30 ± 1.70 | 0.223 | 2.30 ± 1.34 | 2.30 ± 1.57 | 1.000 | 0.295 |
| Acoustical | 2.70 ± 1.57 | 2.60 ± 1.58 | 0.591 | 1.60 ± 1.43 | 1.80 ± 1.55 | 0.168 | 0.196 |
| Gustatory | 1.30 ± 1.57 | 0.50 ± 0.71 | 0.087 | 0.70 ± 0.95 | 0.60 ± 1.07 | 0.591 | 0.140 |
Mean ratings ± standard deviation for positive and negative memories (irrespective of the remoteness of memories) given by males (n = 10) and females (n = 10) on the items included into the postscanning debriefing questionnaire (see legend to Table I). The data show that there were no statistically significant gender‐related differences in the postscanning ratings of positive and negative autobiographical memories.
For the analysis of gender‐related differences in spatial and temporal context information included in all 4,800 stimulus sentences, a two‐sample t test was applied. Males and females did not differ from each other with respect to spatial and temporal aspects of the autobiographical materials provided during the prescanning interview, even at an uncorrected statistical threshold of P < 0.001.
Neuroimaging Data
Brain activity associated with the conjoint memory conditions relative to baseline across males and females
Across genders, significant increases in neural activity (P < 0.05, corrected for multiple comparisons) related to all memory conditions (irrespective of remoteness and emotional tone) relative to baseline were observed bilaterally in the posterior cingulate/retrosplenial cortex, medial and lateral temporal cortex, temporal‐occipital cortex, and dorsal‐occipital cortex extending into the fusiform gyrus, the parahippocampal and hippocampal regions, and bilaterally although predominantly left hemispheric in the ventrolateral and dorsolateral prefrontal cortex (see Table IV). Further areas of significant activation were observed in the premotor areas, right cerebellum, and left superior parietal cortex.
Table IV.
Relative increases in brain activity common to all experimental memory conditions across males and females
| Brain region | Side | Coordinates | t | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Superior frontal gyrus | Left | −10 | +62 | +28 | 7.2 |
| Inferior frontal gyrus | Left | −50 | +32 | −10 | 11.8 |
| Ventral premotor cortex | Right | +40 | +18 | +20 | 7.0 |
| Left | −46 | +22 | +20 | 14.4 | |
| Dorsal premotor cortex | Left | −42 | +6 | +52 | 10.6 |
| Medial premotor cortex | Left | −2 | +12 | +56 | 11.7 |
| Temporal pole | Right | +54 | +16 | −32 | 8.0 |
| Left | −52 | +14 | −34 | 11.3 | |
| Middle temporal gyrus | Right | +60 | 0 | −24 | 6.0 |
| Left | −56 | +2 | −24 | 12.35 | |
| Hippocampus | Left | −24 | −20 | −18 | 8.6 |
| Superior parietal cortex | Left | −30 | −54 | +48 | 5.6 |
| Retrosplenial cortex | Left | −6 | −56 | +4 | 12.0 |
| Fusiform gyrus | Right | +36 | −64 | −24 | 16.2 |
| Left | −28 | −66 | −18 | 13.0 | |
| Extrastriate cortex | Right | +46 | −82 | +2 | 9.7 |
| Left | −42 | −78 | +4 | 9.7 | |
| Right cerebellum | Right | +14 | −82 | −42 | 10.7 |
Joint effect of all memory conditions vs. baseline (CP + CN + RP + RN > BL). All activations significant at P < 0.05, corrected for multiple comparisons across the whole brain volume. Brain regions showing relative significant BOLD signal increases (across all 20 subjects, irrespective of gender) associated with all memory conditions vs baseline. For each region of activation, the coordinates in standard stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest t‐value. Coordinates (in mm): x, distance to right (+) or left (−) of midsagittal plane; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; and z, distance above (+) or below (−) the intercommissural (AC‐PC) plane.
CP, positive childhood events; CN, negative childhood events; RP, positive recent events; RN, negative recent events; BL, baseline. From Piefke et al., Brain,2003. Reproduced by permission of Oxford University Press.
Gender differences in brain activity associated with all memory conditions relative to baseline
Analysis of gender‐specific differences in neural activations associated with combined memory conditions showed that males (relative to females) differentially activated left parahippocampal gyrus (P < 0.001, uncorrected), whereas females (relative to males) differentially activated the right insula and the right dorsolateral prefrontal cortex (P < 0.001, uncorrected). Figure 1 and Table V display the brain regions with gender‐related differential activations during autobiographical memory retrieval (irrespective of remoteness and emotional valence of memories).
Figure 1.
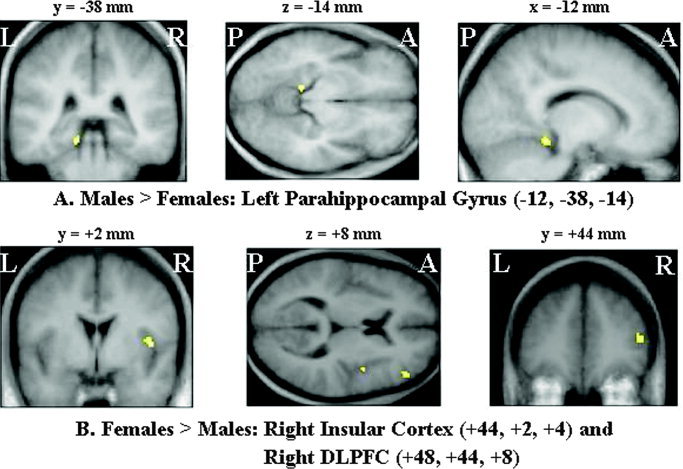
Gender‐specific relative increases in neural activity (for 10 males and 10 females) associated with the joint experimental memory conditions versus baseline. The local maxima of areas of statistically significant relative increases in neural activity (P < 0.001, uncorrected) are superimposed on MRI sections of the male and the female group mean 3‐D structural image normalized into the standard stereotactic space defined by Talairach and Tournoux [1988] to depict the functional anatomy of the activations and their relationship to the underlying structural anatomy. In males (relative to females), there is increased neural activity in the left parahippocampal gyrus (A). By contrast, females exhibit greater activation in the right dorsolateral prefrontal cortex (DLPFC) and insular cortex than do males (B). R, right; L, left; A, anterior; P, posterior.
Table V.
Gender‐specific relative increases in brain activity associated with conjoint experimental memory conditions and simple effect of each type of memory
| Activations vs. baseline | Region | Side | Coordinates | t | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Males > females | ||||||
| CP + CN + RP + RN | Parahippocampal gyrus | Left | −12 | −38 | −14 | 5.46 |
| CP + CN | Parahippocampal gyrus | Left | −14 | −36 | −16 | 4.14 |
| RP + RN | Parahippocampal gyrus | Left | −14 | −38 | −14 | 5.12 |
| RP + CP | Parahippocampal gyrus | Left | −12 | −38 | −16 | 5.65 |
| RN + CN | Parahippocampal gyrus | Left | −14 | −38 | −14 | 3.74 |
| Female > males | ||||||
| CP + CN + RP + RN | DLPFC | Right | +48 | +44 | +8 | 4.78 |
| Insular cortex | Right | +44 | +2 | +4 | 5.37 | |
| CP + CN | DLPFC | Right | +48 | +44 | +8 | 4.72 |
| Insular cortex | Right | +44 | +2 | +4 | 4.84 | |
| RP + RN | DLPFC | Right | +48 | +42 | +6 | 4.09 |
| RP + CP | DLPFC | Right | +46 | +44 | +10 | 4.30 |
| RN + CN | DLPFC | Right | +48 | +44 | +8 | 4.97 |
| Insular cortex | Right | +44 | +2 | +4 | 4.48 | |
All activations significant at P < 0.001, uncorrected. Extent threshold 10 voxels. Brain regions showing significant gender‐specific relative BOLD signal increases associated with all memory conditions vs. baseline (CP + CN + RP + RN > BL) and the simple effect of each memory type (recent, remote, positive, and negative) vs. baseline. For each region of gender‐related activation, coordinates in standard stereotactic space are given referring to the maximally activated focus within an area of activation as indicated by the highest t‐value. Corodinates (in mm): x, distance to right (+) or left (−) of midsagittal plane; y, distance anterior (+) or posterior (−) to vertical plane through the anterior commissure; and z, distance above (+) or below (−) the intercommissural (AC‐PC) plane.
CP, positive childhood events; CN, negative childhood events; RP, positive recent events; RN, negative recent events; BL, baseline; DLPFC, dorsolateral prefrontal cortex.
Gender differences in brain activity associated with the simple effects of memory type relative to baseline
In males (relative to females), the observed gender‐related differential activity in the left parahippocampal gyrus was contained in each type of memory (compared to baseline). Correspondingly, differential right dorsolateral prefrontal cortex activation was observed in all types of autobiographical memory in females (relative to males), whereas the right insular cortex was activated differentially mainly in the negative and the remote memory conditions (see Table V). Although this finding suggests an interaction of the two experimental factors of interest (TIME; EMOTION) for the insular cortex, appropriate statistical tests showed that the interaction was not significant. However, we found several small significant activations (P < 0.001, uncorrected for multiple comparisons; extent threshold 10 voxels) related to interactions between the group variable (GENDER) and the two experimental factors (TIME; EMOTION) and to the main effects of TIME and EMOTION, which were located in brain regions within the memory network activated in both genders. These relative changes in neural activity related to the complex contrasts (interactions; main effects) cannot be interpreted easily with reference to the group variable GENDER. Indeed, they are not indicative of basal differences in the neural networks supporting autobiographical memory in males and females. We therefore do not refer to the complex contrasts here, but rather focus on the simple effects of both the conjoint memory conditions and each memory type (compared to baseline), which provide a more transparent view on putative gender‐related differences in the neural mechanisms of autobiographical memory.
DISCUSSION
Our data show evidence for both common and differential neural mechanisms subserving emotional autobiographical memory retrieval in males and females. Across the genders, activations were observed bilaterally in the posterior cingulate cortex, and medial and lateral temporal areas extending into the parahippocampal and hippocampal regions. Furthermore, bilateral although predominantly left hemispheric activations in the ventrolateral and dorsolateral prefrontal cortices were common to both males and females. These brain areas are known to be part of the neural network supporting episodic nonautobiographical and autobiographical memory retrieval [see e.g., Nadel and Moscovitch,1997; Squire,1992; Tulving and Markowitsch,1998]. More importantly, with regard to the purpose of the study, we observed gender‐related differential activations in the left parahippocampal region (with males showing more activation than females) and in the right dorsolateral prefrontal cortex (with females showing greater activation than males) in all memory conditions. In addition, females showed differential activation during the retrieval of remote and negative memories in the right insula. To clarify the issue of whether the gender‐related differences in relative BOLD signal change associated with the memory tasks reflected activation rather than deactivation of the respective brain areas, the conjoint memory conditions were compared to the baseline for males and females, separately. Brain areas with gender‐related differential activity were included into the network showing activation in this comparison for both males (left parahippocampal gyrus) and females (right dorsolateral prefrontal cortex and right insula), although the gender‐specific peak coordinates were located peripherally in the activated network. Inclusion into this network suggests that the gender‐related differential neural activity observed in the present study reflected activations, but not deactivations.
We suggest that these gender‐related differences in the neural mechanisms underlying emotional autobiographical memory retrieval are likely to reflect the use of gender‐specific cognitive strategies when assessing autobiographical memories.
A considerable number of studies indicated that there may exist gender differences in the hemispheric lateralization of cognitive functions. Specifically, estrogen is supposed to modulate functional hemispheric lateralization [McEwen et al.,1998; Williams,1998; Wisniewski,1998] yielding differences in neuropsychologic task performance between males and females and variation in cognitive performance across the menstrual cycle in females. In the present study, we did not control for different phases of the menstrual cycle and blood level concentrations of 17β‐estradiol and testosterone. Our data thus cannot contribute to the long‐standing debate on the issue of whether and how estrogen may influence brain functions. Males differentially relied on a left hemisphere area (parahippocampal gyrus) during emotional autobiographical memory retrieval, whereas females showed differential right hemisphere activations of the dorsolateral prefrontal cortex and the insula. Our data are thus consistent with the notion of gender‐related differences in the hemispheric lateralization of brain functions. Nevertheless, some caveats should be considered when assessing gender differences in functional neuroimaging data. Grabowski et al. [2003] pooled PET data from five experiments on blood flow correlates of naming concrete entities to reanalyze the data for effects of gender. They reported a differential hemispheric lateralization in males and females, which is in line with that observed in the present study; however, the authors emphasized that gender‐specific activations such as these can be interpreted in at least three ways. They may actually indicate stronger left lateralization in males and stronger right lateralization in females. They may also reflect a gender‐related differential magnitude of increases or decreases in neural activity. Finally, it has to considered that they mirror gender differences in the degree of effort or engagement in the task.
Moreover, the functional and behavioral significance of gender‐specific differences in the brain remains to be clarified. The “dual‐function hypothesis” proposed by De Vries [2004] should be considered in this context. In brief, this hypothesis suggests that neuronal gender differences can be assumed to serve at least two functions. They may induce gender‐related differences in cognitive processing and overt behavior, but may also prevent emergence of gender differences in cognitive functions and behavior in that they compensate for distinct physiologic conditions depending, for example, on gender‐related differential gonadal hormone levels [see also De Vries and Boyle,1998].
As in the study by Grabowski et al. [2003] and previous functional neuroimaging studies on distinct aspects of language processing [Jaeger et al.,1998; Kansaku et al.,2000; Pugh et al.,1996], we observed no gender differences at the behavioral level of task performance. Neither the subordinate reaction time task included in the autobiographical memory experiment nor the postscanning debriefing revealed any gender differences in memory performance or emotional intensity of memories. The percentage of recognition of the autobiographical stimulus sentences, the correct assignment to the respective personal past episodes, and the ratings of the memories retrieved during the fMRI measurement did not differ significantly between genders. The gender‐specific neural activations associated with autobiographical memory retrieval thus cannot be ascribed to differential task performance, suggesting that males and females may resort to differential but comparably effective cognitive strategies when assessing memories of their personal past. For further clarification of this issue, a qualitative comparison between the retrieval strategies employed by the male and female subjects would be required. The design of the present study does not allow for such an analysis and, thus, we cannot provide additional evidence for our hypothesis of equally effective retrieval strategies in males and females.
Our findings are in good accordance with a previous 18FDG PET study on nonautobiographical emotional memory by Cahill et al. [2001], who reported that ratings of emotional reactions to emotional and neutral film clips were highly similar across males and females, despite gender‐specific differential memory‐related activations observed.
The present results as well as previous functional neuroimaging data on neural mechanisms of memory and language processing may suggest gender‐specific patterns of brain function to be neurobiologically rather than socially determined [e.g., Killgore and Yurgelun‐Todd,2001], as differential activation patterns observed had no correlate on the behavioral level of task performance [Cahill et al.,2001; Grabowski et al.,2003; Jaeger et al.,1998; Kansaku et al.,2000; Pugh et al.,1996]. It is reasonable to assume that neurobiological gender differences may include differential hemispheric lateralization and differential involvement of cortical and subcortical pathways in cognitive and emotional processing that do not necessarily become manifest in behavior and experimentally measurable aspects of cognitive and emotional processing. However, it has been shown that the development of autobiographical memory is likely genetically, neurobiologically, and socially determined [Davis,1999]. In addition, it has been shown that brain plasticity and persistent developmental interactions among neurophysiologic and environmental factors are likely to have induced genetically determined global differences between the functional organization of the male and female central nervous system [Buss,2003]. Although environmental factors may also induce changes in neuronal structure and functional brain development [Grossman and Wood,1993], socially determined differences in brain structure and function between males and females should also be mirrored on the performance level because influences of the social environment directly address the behavior of individuals. Neuroimaging evidence for gender‐related differential neural responses during emotional memory and language processing that have no behavioral correlate therefore suggests that these gender‐specific neural mechanisms may depend on some kind of neurobiologically (rather than socially) determined sexually dimorphic functional organization of the human brain.
Contrary to some previous reports of superior emotional memory ability in females [Davis,1999; Fujita et al.,1991], the current behavioral data do not indicate that females have emotionally more intense memories than males do. Our finding of gender‐related differential neural activation patterns associated with comparable performance and emotional intensity of autobiographical memory retrieval may thus support the cognitive style hypothesis rather than the affective intensity hypothesis, given that the use of distinct cognitive memory styles by males and females does not necessarily become manifest in behavioral aspects of autobiographical memory. In support of this hypothesis, the three brain areas exhibiting gender‐related activations in the present study have been implicated in memory processing and cognitive strategies rather than in emotion [Epstein et al.,2002; Leube et al.,2001; Rossi et al.,2001]. The dorsolateral prefrontal cortex was suggested to be involved in monitoring and manipulation of information in working memory [Veltman et al.,2003], in motor response selection [Buccino et al.,2004], and in episodic memory encoding and retrieval strategies [for review, see Lee et al.,2003]. In addition, the dorsolateral prefrontal cortex has been implicated in temporal order memory by lesion studies in animals [Petrides,1991], neuropsychologic assessment of patients with frontal brain damage [Milner et al.,1991; Shimamura et al.,1990], and in recent functional neuroimaging studies [Cabeza et al.,2000; Suzuki et al.,2002]. Suzuki et al. [2002] demonstrated that right dorsolateral prefrontal cortex is engaged primarily in temporal context memory and serial ordering between distinct episodes whereas left dorsolateral prefrontal cortex mediates recall of temporal context within a single episode. Our finding of gender‐related differential right dorsolateral prefrontal cortex activations in females (relative to males) during the retrieval of all types of emotional autobiographical memory may thus indicate that females rely more strongly than males do on serial ordering of personal past events when recollecting emotionally laden autobiographical experiences. One can assume that females (compared to males) are more engaged in the temporal sequencing of events, presumably yielding higher monitoring demands on working memory, which have also been associated with dorsolateral prefrontal cortex function [e.g., Veltman et al.,2003].
The parahippocampal gyrus, which was differentially activated in males (compared to females) in the present study has been reported primarily to support spatial learning and spatial navigation [Maguire et al.,1996; Malkova and Mishkin,2003], as well as spatial context memory [Tsukiura et al.,2002], although it may also play a more general role in memory function [Cabeza et al.,2002; Strange et al.,2002]. In particular, Epstein and Kanwisher [1998] suggested the “parahippocampal place area” to be involved in processing of scenes and landmarks [see also Weiss et al.,2000]. Given that autobiographical memory is likely to depend extensively on spatial cognition (e.g., during scene memory), the observed gender‐related activations of the parahippocampal gyrus in males may indicate that males rely more comprehensively than females do on spatial cognition when assessing memories of their personal past. Our data also largely comply with the hypothesis that males have better spatial memory abilities than females do, as suggested by behavioral data [Sandstrom et al.,1998; Vecchi and Girelli,1998]. Interestingly, a recent study showed evidence that males' superior spatial memory performance is highly selective. Postma et al. [2004] demonstrated that gender differences in spatial memory processing with more accurate male performance occurred only for precise metric positional information in a wayfinding and an object location memory task whereas no gender‐related differential performance was observed in a memory task requiring the processing of topological information (object‐to‐position assignment). Based on these findings and our current data, one can speculate that males may rely preponderantly on spatial context memory when “navigating” through their personal past whereas females primarily depend on temporal context memory, i.e., strategies of generating temporal links between separate episodes during autobiographical recollection. Given the gender‐related hemispheric lateralization of activations observed in the present study (left in males, right in females), this presumption is in good accordance with the finding that the right hemisphere may be engaged specifically in the processing of temporal information [Nenadic et al.,2003; Numminen et al.,2004]. In addition, some evidence supporting our hypothesis comes from a neuropsychologic experiment on temporal order memory using the Rey Auditory Verbal Learning Test (AVLT), which demonstrated that incidental memory for the temporal order of items was sensitive to both age and gender of subjects [Vakil and Blachstein,1994].
The insular cortex, which was activated differentially in females during the processing of remote and negative autobiographical memories, has not only been implicated in memory and cognition, but also in emotion processing. For example, previous functional neuroimaging studies suggest that the insula is involved predominantly in negative emotional states such as pain and distress [Derbyshire et al.,1997; Iadarola et al.,1998], anger and disgust [Phillips et al.,1997], as well as hunger and thirst [Tataranni et al.,1999]. The insular cortex was reported to subserve rather cognitive aspects of emotion processing, particularly when some kind of reflection on personal distress or disadvantage is required [Carr et al.,2003; Lévesque et al.,2003; for review, see Wager et al.,2003]. In an fMRI study on neural representations of emotional states that may guide human economic decision making, for example, Sanfey et al. [2003] implicated the (anterior) insula in representation and evaluation of specific negative emotional states [see also Calder et al.,2001]. This hypothesis is in line with our present finding of insular cortex activation during the recollection of negatively valenced childhood memories, a task that is likely to involve evaluative processing of early negative experiences.
We conclude that our current and previous studies support the hypothesis that gender differences in emotional memory depend on differential cognitive styles of encoding, rehearsing, and thinking about emotionally laden personal experiences in males and females [Killgore et al.,2001; Killgore and Yurgelun‐Todd,2001; Seidlitz and Diener,1998]. Specifically, males may differentially draw upon spatial context memory during autobiographical remembering whereas females may differentially use strategies of temporal context memory to assess their personal past. Such gender‐related differences in brain function do not necessarily affect the behavioral level of emotional memory performance and are therefore likely to be neurobiologically rather than socially determined.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (to H.J.M. and G.R.F.). We are grateful to our colleagues from the MRI and Cognitive Neurology groups. We thank Oxford University Press for the permission to reprint Table IV, which was originally published as Table 1 in Piefke et al. [2003]: Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory, Brain 126:650–668.
REFERENCES
- Adinoff B, Devous MD, Best SE, Chandler P, Alexander D, Payne K, Harris TS, Williams MJ (2003): Gender differences in limbic responsiveness, by SPECT, following a pharmacologic challenge in healthy subjects. Neuroimage 18: 697–706. [DOI] [PubMed] [Google Scholar]
- Buss DM (2003): Evolutionary psychology. Boston: Pearson; 480 p. [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G (2004): Neural circuits underlying imitation learning of hand actions: an event‐related fMRI study. Neuron 42: 323–334. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L (2000): Age‐related differences in neural activity during item and temporal‐order memory retrieval: a positron emission tomography study. J Cogn Neurosci 12: 197–206. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L (2002): Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage 16: 317–330. [DOI] [PubMed] [Google Scholar]
- Cahill LR, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT (2001): Sex‐related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem 75: 1–9. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW (2001): Neuropsychology of fear and loathing. Nat Rev Neurosci 2: 352–363. [DOI] [PubMed] [Google Scholar]
- Canli T, Desmond JE, Zhao Z, Gabrieli JD (2002): Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA 99: 10789–10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta J, Lenzi GL (2003): Neural mechanisms of empathy in humans: a relay from systems for imitation to limbic areas. Proc Natl Acad Sci USA 100: 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ (1999): Gender differences in autobiographical memory for childhood emotional experiences. J Pers Soc Psychol 76: 498–510. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Jones AK, Gyulai F, Clark S, Townsend D, Firestone LL (1997): Pain processing during three levels of noxious stimulation produces differential patterns of central activity. Pain 73: 431–445. [DOI] [PubMed] [Google Scholar]
- De Vries GJ (2004): Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145: 1063–1068. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA (1998): Double duty for sex differences in the brain. Behav Brain Res 92: 205–213. [DOI] [PubMed] [Google Scholar]
- Dolan RJ (2000): Emotional processing in the human brain revealed through functional neuroimaging In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; p 1115–1131. [Google Scholar]
- Epstein CM, Sekino M, Yamaguchi K, Kamiya S, Ueno S (2002): Asymmetries of prefrontal cortex in human episodic memory effects: effects of transcranial magnetic stimulation on learning abstract patterns. Neurosci Lett 320: 5–8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N (1998): A cortical representation of the local visual environment. Nature 389: 598–601. [DOI] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD (1996): Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci 16: 4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Bimonte HA (2002): Hormones, brain, and behavior: putative biological contributions to cognitive sex differences In: McGillycuddy‐De Lisi A, De Lisi R. editors. Biology, society, and behavior: the development of sex differences in cognition Vol 21 Westport, CT: Ablex; p 55–91. [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Holmes A, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Fujita F, Diener E, Sanvik E (1991): Gender differences in negative affect and well‐being: the case for emotional intensity. J Pers Soc Psychol 61: 427–434. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2003): Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104–1107. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Eichhorn GR, Tranel D (2003): Effects of gender on blood flow correlates of naming concrete entities. Neuroimage 20: 940–954. [DOI] [PubMed] [Google Scholar]
- Grossman M, Wood W (1993): Sex differences in intensity of emotional experience: a social role interpretation. J Pers Soc Psychol 65: 1010–1022. [DOI] [PubMed] [Google Scholar]
- Halpern DF (1992): Sex differences in cognitive abilities. Hillsdale, NJ: Erlbaum; 308 p. [Google Scholar]
- Iadarola MJ, Berman KF, Zeffiro TA, Byas‐Smith MG, Gracely RH, Max MB, Bennet GJ (1998): Neural activation during acute capsaicin‐evoked pain and allodynia assessed with PET. Brain 121: 931–947. [DOI] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, Van Valin RD, Kemmerer DL, Murphy BW, Wack DS (1998): Sex differences in brain regions activated by grammatical and reading tasks. Neuroreport 9: 2803–2807. [DOI] [PubMed] [Google Scholar]
- Kansaku K, Yamaura A, Kitazawa S (2000): Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex 10: 866–872. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun‐Todd DA (2001): Sex‐specific developmental changes in amygdala responses to affective faces. Neuroreport 12: 427–433. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun‐Todd DA (2001): Sex differences in amygdala activation during the perception of facial affect. Neuroreport 12: 2543–2547. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Lee ACH, Robbins TW, Owen AM (2003): Episodic memory meets working memory in the frontal lobe: functional neuroimaging studies of encoding and retrieval. Crit Rev Neurobiol 14: 165–197. [PubMed] [Google Scholar]
- Leube DT, Erb M, Grodd W, Bartels M, Kircher TT (2001): Differential activation in parahippocampal and prefrontal cortex during word and face encoding tasks. Neuroreport 12: 2773–2777. [DOI] [PubMed] [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M (2003): Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53: 502–510. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD (1996): Learning to find your way: a role for the human hippocampal formation. Proc R Soc Lond B Biol Sci 263: 1745–1750. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Henson RNA, Mummery CJ, Frith CD (2001a): Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport 12: 441–444. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha‐Khadem F, Mishkin M (2001b): The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain 124: 1156–1170. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M (2003): One‐trial memory for object‐place associations after separate lesions of hippocampus and posterior parahippocampal regions in the monkey. J Neurosci 23: 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG (1998): Clinically relevant basic science studies on gender differences and sex hormone effects. Psychopharmacol Bull 34: 251–259. [PubMed] [Google Scholar]
- Mekarski JE, Cutmore TR, Suboski W (1996): Gender differences during processing of the Stroop task. Percept Mot Skills 83: 563–568. [DOI] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G (1991): Frontal‐lobe contribution to recency judgements. Neuropsychologia 29: 601–618. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M (1997): Memory consolidation, retrograde amnesia, and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Gaser C, Volz HP, Rammsayer T, Hager F, Sauer H (2003): Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res 148: 238–246. [DOI] [PubMed] [Google Scholar]
- Numminen J, Schurmann M, Hiltunen J, Joensuu R, Jousmaki V, Koskinen SK, Salmelin R, Hari R (2004): Cortical activation during a spatiotemporal tactile comparison task. Neuroimage 22: 815–821. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL (1998): Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation. Pain 76: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. (1991): Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc R Soc Lond B Biol Sci 246: 299–306. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS (1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–498. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR (2003): Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain 126: 650–668. [DOI] [PubMed] [Google Scholar]
- Postma A, Jager G, Kessels RPC, Koppeschaar HPF, van Honk J (2004): Sex differences for selective forms of spatial memory. Brain Cogn 54: 24–34. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher RM, Shankweiler DP, Katz L, Gore JC (1996): Cerebral organization of component processes in reading. Brain 119: 1221–1238. [DOI] [PubMed] [Google Scholar]
- Rossi S, Cappa SF, Babiloni C, Pasqualetti P, Miniussi C, Carducci F, Babiloni F, Rossini PM (2001): Prefrontal cortex in long‐term memory: an “interference” approach using magnetic stimulation. Nat Neurosci 4: 948–952. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA (1998): Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res 6: 351–360. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (2003): The neural basis of economic decision‐making in the ultimatum game. Science 300: 1755–1758. [DOI] [PubMed] [Google Scholar]
- Seidlitz L, Diener E (1998): Sex differences in the recall of affective experiences. J Pers Soc Psychol 74: 262–271. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR (1990): Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia 28: 803–813. [DOI] [PubMed] [Google Scholar]
- Squire LR (1992): Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231. [DOI] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ (2002): Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci 22: 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Fujii T, Tsukiura T, Okuda J, Umetsu A, Nagasaka T, Mugikura S, Yanagawa I, Takahashi S, Yamadori A (2002): Neural basis of temporal context memory: a functional MRI study. Neuroimage 17: 1790–1796. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotactic atlas of the human brain. New York: Thieme; 122 p. [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96: 4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ (2002): Cholinergic modulation of experience‐dependent plasticity in human auditory cortex. Neuron 35: 567–574. [DOI] [PubMed] [Google Scholar]
- Tsukiura T, Fujii T, Takahashi T, Xiao R, Sugiura M, Okuda J, Iijima T, Yamadori A (2002): Medial temporal lobe activation during context‐dependent relational processes in episodic retrieval: an fMRI study. Hum Brain Mapp 17: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1983): Elements of episodic memory. Oxford: Clarendon Press; 351 p. [Google Scholar]
- Tulving E, Markowitsch HJ (1998): Episodic and declarative memory: role of the hippocampus. Hippocampus 8: 198–204. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H (1994): A supplementary measure in the Rey AVLT for assessing incidental learning of temporal order. J Clin Psychol 50: 240–245. [DOI] [PubMed] [Google Scholar]
- Vecchi T, Girelli L (1998): Gender differences in visuo‐spatial processing: the importance of distinguishing between passive storage and active manipulation. Acta Psychol 99: 1–16. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Serge A, Rombouts RB, Dolan RJ (2003): Maintenance versus manipulation in verbal working memory revisited: an fMRI study. Neuroimage 18: 247–256. [DOI] [PubMed] [Google Scholar]
- Vogel SA (1990): Gender differences in intelligence, language, visual‐motor abilities, and academic achievement in students with learning disabilities: a review of the literature. J Learn Disabil 23: 44–52. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF (2003): Valence, gender, and lateralization of functional brain anatomy in emotion: a meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Wunderlich G, Tellmann L, Halligan PW, Freund HJ, Zilles K, Fink GR (2000): Neural consequences of acting in near versus far space: a physiological basis for clinical dissociations. Brain 123: 2531–2541. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003): Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40: 655–664. [DOI] [PubMed] [Google Scholar]
- Williams CL (1998): Estrogen effects on cognition across the lifespan. Horm Behav 34: 80–84. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB (1998): Sexually dimorphic patterns of cortical asymmetry, and the role for sex steroid hormones in determining cortical patterns of lateralization. Psychoneuroendocrinology 23: 519–547. [DOI] [PubMed] [Google Scholar]
- Zafiris O, Kircheis G, Rood HA, Boers F, Häussinger D, Zilles K (2004): Neural mechanisms underlying impaired visual judgement in the dysmetabolic brain: an fMRI study. Neuroimage 22: 541–552. [DOI] [PubMed] [Google Scholar]


