Abstract
There is growing evidence that a specific region in the posterior frontolateral cortex is involved intimately in cognitive control processes. This region, located in the vicinity of the junction of the inferior frontal sulcus and the inferior precentral sulcus, was termed the inferior frontal junction (IFJ). The IFJ was shown to be involved in the updating of task representations and to be activated commonly in a within‐subject investigation of a task‐switching paradigm, the Stroop task, and a verbal n‐back task. Here, we investigate the involvement of the IFJ in cognitive control by employing a meta‐analytic approach. Two quantitative meta‐analyses of functional magnetic resonance imaging (fMRI) studies were conducted. One meta‐analysis included frontal activations from task‐switching, set‐shifting, and stimulus–response (S–R) reversal studies, the other included frontal activations from color–word Stroop studies. Results showed highly significant clustering of activations in the IFJ in both analyses. These results provide strong evidence for the consistent involvement of the IFJ in both switching and Stroop paradigms. Furthermore, they support our concept of areal specialization in the frontolateral cortex, which posits that it is not only the middorsolateral part that plays an important role in cognitive control, but also the IFJ. Finally, our results demonstrate how quantitative meta‐analyses can be used to test hypotheses about the involvement of specific brain regions in cognitive control. Hum Brain Mapp 25:22–34, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: executive functions, prefrontal cortex, meta‐analysis, task switching, set shifting, S–R reversal, Stroop task
INTRODUCTION
The ability to orchestrate our thoughts and actions in accordance with internal goals has been termed cognitive control. For example, we need to exert cognitive control when task demands change unpredictably or when we need to overcome pre‐potent response tendencies. In the neuroimaging literature, the exertion of cognitive control has been linked often to activations of middorsolateral prefrontal cortex [e.g., Braver et al.,2002; Petrides,2000]. We have collected evidence that a more posterior region in the frontolateral cortex is also involved intimately in control processes [Brass and von Cramon,2002,2004; Derrfuss et al.,2004]. Because of its anatomical location, we have termed this region the inferior frontal junction (IFJ). The IFJ is located in the vicinity of the junction of the inferior frontal sulcus (IFS) and the inferior precentral sulcus (IPrCS). Based on the results of functional imaging studies, its approximate location in Talairach coordinates [Talairach and Tournoux,1988] can be described as follows: it is located rather deep in the IPrCS and the IFS (x‐coordinate of 47 or lower), it extends in the y‐direction from −1 to 10, and it is located between the z‐coordinates 27 and 40 [Derrfuss et al.,2004].
Building on our previous work [Brass and von Cramon,2002,2004; Derrfuss et al.,2004], we have argued that the IFJ is involved in the updating of task representations. In particular, we have shown that the preparation of an abstract task rule after the presentation of a cue activates the IFJ [Brass and von Cramon,2002,2004]. Importantly, activation of the IFJ was significantly correlated with the behavioral cueing effect [Brass and von Cramon,2002] and was shown to be independent of cue encoding processes [Brass and von Cramon,2004]. In a recent within‐session within‐subject functional magnetic resonance imaging (fMRI) investigation [Derrfuss et al.,2004], we found common activation of the IFJ in a task‐switching paradigm, the Stroop task, and a verbal n‐back task. IFJ activity was also found in several other studies employing switching tasks [e.g., Dove et al.,2000; Pollmann et al.,2000; Ruge et al.,2005], the Stroop task [e.g., Mead et al.,2002; Milham et al.,2001; Zysset et al.,2001], or the n‐back task [e.g., Braver et al.,1997; Jonides et al.,1997; Ragland et al.,2002]. However, the consistency of IFJ involvement in cognitive control has received little attention. One reason for this may be that activations of the IFJ have been attributed to Brodmann areas (BA) 6, 44, or 9, thus obscuring the relatively small variance in the distribution of activation peaks.
Another reason for the neglect of the IFJ might be that the above‐mentioned tasks have been discussed as if they belonged to different domains of cognitive control (e.g., task switching, interference resolution, or working memory). However, the consistent involvement of the IFJ across tasks raises the question as to whether there might be processes shared by these tasks [Cabeza and Nyberg,2002]. We have argued that the updating of task representations is likely to be one of these processes [Brass and von Cramon,2002,2004; Derrfuss et al.,2004]. For example, in task‐switching paradigms participants have to switch between different tasks constantly, which requires repeated updating of the currently relevant task representation. In incongruent Stroop trials, participants have to enforce the relevant task representation (color naming) against a dominant, but irrelevant action (reading). Consequently, we suggest that an abstract cognitive process required in many control paradigms, updating of task representations, is linked intimately to a specific region in the posterior frontolateral cortex, namely the IFJ. Importantly, the IFJ is clearly located posterior to the middorsolateral prefrontal cortex and therefore should be regarded as a functional entity separate from middorsolateral prefrontal cortex.
Although there is now evidence from several studies for the consistent involvement of the IFJ in cognitive control, this consistency has not yet been investigated rigorously. Considerable variance exists in the results of functional imaging data, and to be confident about the reliable involvement of any brain region in a particular task, a quantitative effect‐location meta‐analysis should be conducted. In contrast to the traditional effect‐size meta‐analyses that aim at estimating the magnitude of a statistical effect, the aim of effect‐location meta‐analyses is to identify brain regions showing consistent involvement in the particular tasks under investigation [Fox et al.,1998]. By incorporating results obtained with different scanner equipment, task designs, participants, analysis tools, and statistical approaches, meta‐analyses have the potential to increase the validity of findings gained in single studies. In the context of effect‐location meta‐analytic approaches, models have been developed to compare empirical distributions of activation peaks to random distributions to identify above‐chance activation clusters [Chein et al.,2002; Turkeltaub et al.,2002; Wager et al.,2003]. One major advantage of these methods is that they do not rely on the assignment of activation peaks to Brodmann areas or to anatomical structures, but identify above‐threshold clusters in 3D space. The results obtained with these methods thus are less influenced by subjective choices of investigators.
Consequently, the present study aimed at investigating the consistency of IFJ involvement in cognitive control across published studies by employing a quantitative meta‐analytic approach. We chose the method developed by Turkeltaub et al. [2002] and carried out two meta‐analyses. The first meta‐analysis was carried out with frontal lobe activations (including anterior insula activations) from task‐switching, set‐shifting, and nonprobabilistic stimulus– response (S–R) reversal studies (for simplicity sometimes referred to as switching studies or switching paradigms below). In task‐switching paradigms, participants perform (at least) two tasks that correspond to (at least) two relevant stimulus dimensions or stimulus types, whereas in S–R reversal paradigms there is only one relevant stimulus dimension for which the S–R mapping is sometimes reversed. The distinction between task switching and set shifting is not so clear‐cut. Mostly, the Wisconsin Card‐Sorting Test (WCST) has been used to investigate set shifting. This task usually has additional requirements that are not essential in task‐switching paradigms (e.g., negative feedback processing associated with rule shifts or the identification of the presently correct rule by trial and error). However, there have been attempts to reduce the complexity of the WCST [e.g., Konishi et al.,2002; Rogers et al.,2000], making the WCST more similar to task‐switching paradigms. What is common to set‐shifting, S–R reversal, and task‐switching paradigms is the requirement to update the task representation after the necessity for a switch has been signaled. These paradigms were therefore combined to increase the number of studies entering the meta‐analysis. The second meta‐analysis carried out in the present study was conducted with frontal lobe activations (again including anterior insula activations) from color–word Stroop studies. The Stroop task was chosen because it is a cognitive control paradigm frequently investigated with fMRI that, like the task‐switching paradigm, was employed in our previous within‐subject investigation [Derrfuss et al.,2004] allowing a direct comparison of meta‐analytic and imaging results.1
MATERIALS AND METHODS
General Study Selection Criteria
To be considered for the meta‐analyses, articles had to be published in English‐language, peer‐reviewed journals between January 2000 and January 2004 (additionally, we included a task‐switching study from our own group that was in press at that time). This time range was chosen because there were no studies investigating the updating component in switching paradigms that were published before 2000 and satisfied our selection criteria (the two event‐related fMRI studies investigating the WCST by Konishi et al. [1998,1999] were not included because no across‐subject averaging was carried out in these studies). As we wanted the time range for study selection to be the same for switching tasks and the Stroop task, we restricted it for both paradigms to the above‐mentioned period.
To find relevant studies, we searched electronic databases (Medline/PubMed, and ISI Web of Science) and reference lists of articles found in those databases. Only studies applying fMRI, reporting coordinates in stereotaxic space, and covering at least the frontal lobes were considered. To reduce variance introduced by different methodologies, we included studies that employed subtraction designs and excluded studies that employed parametric designs. We report only activations and do not report deactivations. Furthermore, we only considered results obtained with healthy participants. To rule out the influence of low‐level perceptual or motor processes on the meta‐analytic results, we excluded results from comparisons with resting or fixation conditions. We did not include multiple subtractions from the same condition of interest (e.g., Stroop studies reporting incongruent vs. congruent and incongruent vs. neutral contrasts). Furthermore, we did not include results that were based on region‐of‐interest (ROI) analyses. Finally, given the focus of the present study, only frontal lobe and anterior insula activations entered the meta‐analysis. When these activations were reported in the Montreal Neurological Institute (MNI) reference system, they were transformed to Talairach space according to a formula proposed by Matthew Brett (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
Selection Criteria Specific to Task‐Switching, Set‐Shifting, and S–R Reversal Studies
We chose to include functional imaging studies of task switching, set shifting and nonprobabilistic S–R reversal, i.e., studies where participants had to update their task representation based on cue information [e.g., Brass and von Cramon,2004; Luks et al.,2002], feedback information [e.g., Konishi et al.,2002; Kringelbach and Rolls,2003], or prespecified task sequences [e.g., Dreher et al.,2002; Swainson et al.,2003]. To reduce variance introduced by the task domains that were investigated, we did not include studies where participants had to switch between languages [e.g., Hernandez et al.,2001; Price et al.,1999], movements [e.g., Jäncke et al.,2000; Umetsu et al.,2002], or types of information held in working memory [e.g., Garavan et al.,2000; Sylvester et al.,2003]. Note, however, that many of these studies found activations at or very close to the IFJ (Price et al. [1999]: −50, 6, 32; Jäncke et al. [2000]: −44, 0, 32; Garavan et al. [2000]: −43, 6, 25 and 45, 5, 31; and Sylvester et al. [2003] conjunction of switching and inhibition: −34, 7, 34).
Two task‐switching studies [Gurd et al.,2002; Kimberg et al.,2000] did not find frontal activations and therefore could not be considered in the present meta‐analysis (see Discussion). In the study by Gurd et al. [2002], when the threshold was lowered to z = 3.09 (P = 0.001, uncorrected) instead of z = 4.5 (P = 0.05, corrected), there were two frontal activations in the switching contrast that were located in the IFJ (−34, 0, 30 and 38, 6, 40). Two further studies were not included in the meta‐analysis. The study by Sohn et al. [2000] examined switch effects only in ROIs identified in the foreknowledge versus no foreknowledge contrast and the combined fMRI and transcranial magnetic stimulation (TMS) study by Rushworth et al. [2002] focused on the medial frontal cortex and therefore did not report lateral frontal activations. In fact, full consideration of frontal activations in the latter study showed that there was a peak located at the IFJ (−44, 4, 34; Matthew Rushworth, personal communication).
From the remaining studies, we chose the contrast that principally was suited to identify activations related to the switching component of the task. For example, in the study of Konishi et al. [2002], we chose the contrast designed to isolate activity related to the updating component and did not include the contrast designed to isolate activity related to the negative feedback component. From the mixed‐design study of Braver et al. [2003], we chose to include the results from the event‐related analysis, because most of the other switching studies had event‐related designs. From the study of Swainson et al. [2003] we included the go–switch versus go–repeat contrast and did not include the wait–switch versus wait–repeat contrast, because in the latter contrast participants had to switch to “a mode of response withholding” (p. 792), a requirement differing from all other switching studies considered for the meta‐analysis. After applying the above‐mentioned selection criteria and choosing the appropriate contrasts, 14 switching studies entered the meta‐analysis (Table I), yielding 16 contrasts with 97 activation maxima. Figure 1 shows these maxima colored in red on axial slices of an individual brain.
Table I.
Task‐switching, set‐shifting, and S‐R reversal studies included in the meta‐analysis
| Author | Year | Design | n | Task/contrast | Activ. | IFJ |
|---|---|---|---|---|---|---|
| Brass | 2004 | efMRI | 14 | TS, meaning‐switch vs. cue‐switch | 2 | −37 5 32 |
| Braver | 2003 | e/bfMRI | 13 | TS, switch vs. repeat (event‐related analysis) | 3 | — |
| DiGirolamoa | 2001 | bfMRI | 8b | TS, switch blocks vs. repeat blocks | 13 | (−46 12 34) |
| Dove | 2000 | efMRI | 16 | S‐R reversal, switch vs. repeat | 5 | −44 5 37, 40 8 36 |
| Dreher | 2002 | bfMRI | 8 | TS, switch/rep. blocks (conjunction of switch conditions) vs. pure blocks | 4f | — |
| Konishi | 2002 | efMRI | 16 | WCST variant, update vs. null change | 3f | −38 4 33 |
| Kringelbach | 2003 | efMRI | 9 | S‐R reversal, reversal vs. no reversal | 5 | — |
| Luks | 2002 | efMRI | 11 | TS, informative switch cue vs. baseline | 10d | — |
| Luks | 2002 | efMRI | 11 | TS, neutrally cued switch vs. baseline | 3d | — |
| Monchi | 2001 | efMRI | 11 | WCST variant, negative FB vs. control FB | 12f | −38 3 27, −46 5 27 |
| Monchi | 2004 | efMRI | 9c | WCST variant, negative FB vs. control FB | 9f | −44 9 33, 44 9 33 |
| Nagahama | 2001 | efMRI | 6 | WCST variant, negat. FB vs. sorting baseline | 7f | 46 7 29 |
| Nagahama | 2001 | efMRI | 6 | S‐R reversal, negative FB vs. sorting baseline | 6f | (−42 3 26) |
| Nakahara | 2002 | efMRI | 10 | WCST variant, neg. FB vs. sorting baseline | 10e, f | 34 4 35 |
| Pollmann | 2000 | efMRI | 12 | S‐R reversal, switch vs. repeat | 4 | 45 2 37 |
| Swainson | 2003 | efMRI | 12 | TS, go switch vs. go repeat | 1f | — |
As this study used an unusually low threshold of z > 1.96 (with no correction for multiple comparisons or application of a cluster threshold) and in comparison to other studies reported a very high number of activations (30), we decided to include only activations above a more conservative threshold of z > 3.09.
Young participants.
Control group.
Some of these activations were located within ROIs, but each activation was significant on a whole brain level at P < 0.001 (Tracy Luks, personal communication).
Coordinates published in online supplementary material.
Transformed from MNI to Talairach space.
efMRI, event‐related functional magnetic resonance imaging (fMRI); e/b, mixed design; bfMRI = blocked fMRI; TS, task switching; WCST, Wisconsin Card‐Sorting Test; FB, feedback; Activ., number of frontal lobe activations; IFJ, activations within IFJ limits; activations close to IFJ are in parentheses.
Figure 1.
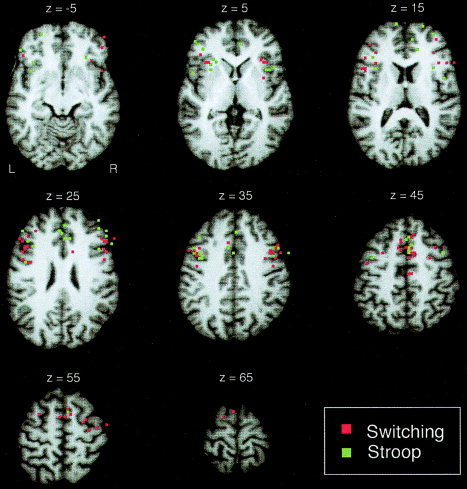
Frontal and anterior insula peaks included in the meta‐analyses (red, switching studies; green, Stroop studies) displayed on axial MR images of an individual brain in Talairach space. On each slice the peaks lying up to 4 mm above and up to 5 mm below the respective slice are shown. The variance of activation peaks makes it difficult to identify regions involved consistently in both paradigms before statistical treatment.
Selection Criteria Specific to Stroop Studies
For the Stroop meta‐analysis, we chose to include studies that employed variants of the color–word Stroop task and compared an incongruent condition with a neutral or a congruent condition. This included studies employing the color–word‐matching Stroop task [Norris et al.,2002; Zysset et al.,2001] and a study where participants had to monitor Stroop words for purple‐colored items [Banich et al.,2000]. If studies reported incongruent versus neutral and incongruent versus congruent comparisons, we chose to include the incongruent versus neutral comparison. We preferred this contrast because it has been argued [e.g., Milham et al.,2002] that incongruent and congruent trials have higher control demands compared to that for neutral trials, because in both trial types there are two dimensions conveying color information, the color of the word and word meaning, leading to increased competition relative to neutral trials. We did not include a study by Langenecker et al. [2004] because their control participants were a subset of those scanned by Mead et al. [2002], and we did not include a study by Adleman et al. [2002] because they reported only one peak for a massive frontal activation consisting of roughly 12,000 voxels in the young adult group. Altogether, 11 Stroop studies entered the meta‐analysis (Table II), yielding 11 contrasts with 64 activation maxima. Figure 1 shows these maxima colored in green on axial slices of an individual brain.2
Table II.
Stroop studies included in the meta‐analysis
| Author | Year | Design | n | Task/contrast | Activ. | IFJ |
|---|---|---|---|---|---|---|
| Banich | 2000 | bfMRI | 10 | Stroop word monitoring, I/N vs. N | 4 | (−48 10 34) |
| Banich | 2001 | bfMRI | 14 | CW Stroop, I/N vs. N | 3 | −42 10 34 |
| Fan | 2003 | efMRI | 12 | CW Stroop, I vs. C | 4c | — |
| Mead | 2002 | bfMRI | 18 | CW Stroop, I vs. N | 1 | −44 4 29 |
| Milham | 2001 | e/bfMRI | 16 | CW Stroop, I vs. N (event‐related) | 4 | −42 2 36 |
| Milham | 2002 | bfMRI | 12a | CW Stroop, I vs. C/N | 8 | (−46 14 32) |
| Milham | 2003 | efMRI | 16 | CW Stroop, I vs. oddball neutral | 9 | — |
| Norris | 2002 | SE bfMRI | 7 | CW matching Stroop, I vs. N | 6 | −38 4 33 |
| Potenza | 2003 | efMRI | 11b | CW Stroop, I vs. C | 6 | 43 7 35 |
| Steel | 2001 | bfMRI | 7 | CW Stroop, I vs. N | 14 | — |
| Zysset | 2001 | bfMRI | 9 | CW matching Stroop, I vs. N | 5 | −38 5 30 |
Young participants
Control group.
Transformed from MNI to Talairach space.
efMRI, event‐related functional magnetic resonance imaging (fMRI); bfMRI, blocked fMRI; SE, spin echo; e/b, mixed design; CW, color‐word; I, incongruent; C, congruent; N, neutral; Activ., number of frontal lobe activations; IFJ, activations within IFJ limits; activations close to IFJ are shown in parentheses.
Activation Likelihood Estimate Meta‐Analysis Method
The idea behind this meta‐analysis method is that peak coordinates reported in functional imaging studies should not be viewed as single points but rather as probability distributions around these coordinates [Turkeltaub et al.,2002]. After the transformation of activation peaks into probability distributions, a map can be created that gives for each voxel the probability that at least one of the activation maxima that entered the meta‐analysis was located in this voxel. This probability is the activation likelihood estimate (ALE). To create ALE maps, activation maxima are modeled using a 3D Gaussian probability distribution. The probability that a given maximum lies within a particular voxel is
where σ is the standard deviation (SD) for the distribution and d is the Euclidean distance of the voxel to the activation maximum. After calculating this probability for each voxel and each activation maximum, the union of these probabilities is calculated to give the ALE map [Turkeltaub et al.,2002]. We created separate ALE maps for the coordinates from the switching studies and the Stroop studies using SD = 4 mm (resulting in an full‐width half‐maximum [FWHM] of 9.4 mm). This distribution width was chosen to approximately match filter sizes commonly used in fMRI studies. To evaluate the influence of a different FWHM on potential clusters in the IFJ, we computed additional analyses with FWHM values of 7.1 mm (SD = 3 mm) and 11.8 mm (SD = 5 mm).
In a second step, the empirical ALE maps from the task‐switching and Stroop studies were compared to ALE maps for randomly distributed activation maxima. For each paradigm, the same number of activations as included in the empirical ALE map was distributed 1,000 times over a brain volume mask. As we had entered only frontal lobe coordinates in Talairach space for the empirical ALE maps, we restricted the brain volume mask to the following minimum and maximum coordinates: xmin = −62; xmax = 62; ymin = −10; ymax = 70; zmin = −25; and zmax = 75. Histograms of the thousand sets of randomly distributed maxima were averaged to obtain a histogram representing the noise distribution of ALE values. This histogram served as a null hypothesis against which the significance of the empirical ALE values was tested. As in the study by Turkeltaub et al. [2002], a conservative ALE threshold corresponding to an α level of 0.01% was chosen to reduce the probability of type I errors and to identify only the regions activated most consistently in the two tasks. The null hypothesis of random distribution was rejected for those voxels that exceeded this threshold.
RESULTS
The results of the meta‐analyses are summarized in Table III for the switching paradigms and in Table IV for the Stroop task. For the switching paradigms, five significant above‐threshold clusters with eight local maxima were identified. The maxima were located in the IFJ, the inferior frontal gyrus, the inferior frontal sulcus, the medial superior frontal gyrus, the anterior cingulate cortex (ACC)/pre‐supplementary motor area (pre‐SMA), and the insula. The maximum ALE value was 0.028 (located in the ACC/pre‐SMA). For the Stroop task, there were five significant above‐threshold cluster with five local maxima. The maxima were located in the IFJ, the ACC/pre‐SMA, the ACC/medial superior frontal gyrus (SFG), and the insula. The maximum ALE value was 0.022 (located in the IFJ). The maxima in the IFJ were very similar in both tasks. The switching maximum was located at −40, 4, 30 and the Stroop maximum was located at −40, 4, 32. Figure 2A and 2B depict these maxima, and Figure 3A depicts the overlap of significant voxels. Figure 3B shows the similarity of the overlap in the present meta‐analysis and the overlap we found for a task‐switching paradigm and the Stroop task in a functional imaging within‐subject investigation. The only other regions that showed some small overlap of significant voxels were the ACC/pre‐SMA and the ACC/medial SFG.
Table III.
Meta‐analysis of frontal lobe and anterior insula activations in task‐switching, set‐shifting, and nonprobabilistic S–R reversal studies
| Region | BA | Lat. | x | y | z | ALE | Volume (mm3) |
|---|---|---|---|---|---|---|---|
| Inferior frontal junction | 6/8/44 | L | −40 | 4 | 30 | 0.024 | 3,032 |
| Inferior frontal gyrus | 44/45 | L | −48 | 14 | 18 | 0.021 | SC |
| Inferior frontal junction | 6/8/44 | R | 44 | 10 | 34 | 0.022 | 1,700 |
| Inferior frontal sulcus | 46/45 | R | 46 | 28 | 24 | 0.017 | 268 |
| ACC/pre‐SMA | 32/6 | B | 4 | 8 | 48 | 0.028 | 2,659 |
| Superior frontal gyrus (med.) | 8 | B | 4 | 28 | 42 | 0.020 | SC |
| ACC/SFG (med.) | 32/8 | B | −8 | 20 | 42 | 0.016 | SC |
| Insula | — | R | 32 | 22 | 2 | 0.018 | 215 |
Clusters above an ALE threshold of 0.0133 (P < 0.0001) and a minimum size of 10 mm3 are listed; minimum peak distance is 5 mm. Coordinates are in Talairach space.
ACC, anterior cingulate cortex; pre‐SMA, presupplementary motor area; med., medial; SFG, superior frontal gyrus, BA, approximate Brodmann area; Lat., lateralization; B, bilateral; ALE, activation likelihood estimate; SC, same cluster.
Table IV.
Meta‐analysis of frontal lobe and anterior insula activations in color–word Stroop studies
| Region | BA | Lat. | x | y | z | ALE | Volume (mm3) |
|---|---|---|---|---|---|---|---|
| Inferior frontal junction | 6/8/44 | L | −40 | 4 | 32 | 0.022 | 1,250 |
| ACC/pre‐SMA | 32/6 | B | 2 | 14 | 42 | 0.019 | 797 |
| ACC/SFG (med.) | 32/9 | L | −2 | 36 | 26 | 0.015 | 199 |
| Insula | — | L | −26 | 22 | 6 | 0.014 | 133 |
| — | R | 36 | 12 | 6 | 0.013 | 74 |
Clusters above an ALE threshold of 0.0116 (P < 0.0001) and a minimum size of 10 mm3 are listed; minimum peak distance is 5 mm. Coordinates are in Talairach space.
ACC, anterior cingulate cortex; pre‐SMA, presupplementary motor area; SFG, superior frontal gyrus; med., medial; BA, approximate Brodmann area; Lat., lateralization; B, bilateral; ALE, activation likelihood estimate.
Figure 2.
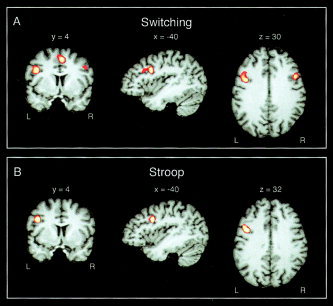
Results of the quantitative meta‐analyses. Displayed are above‐threshold voxels at the IFJ peak coordinates for switching (A) and Stroop studies (B). Results are shown on an individual brain in Talairach space and were interpolated to millimeter resolution for display purposes. Only frontal coordinates entered the meta‐analyses.
Figure 3.
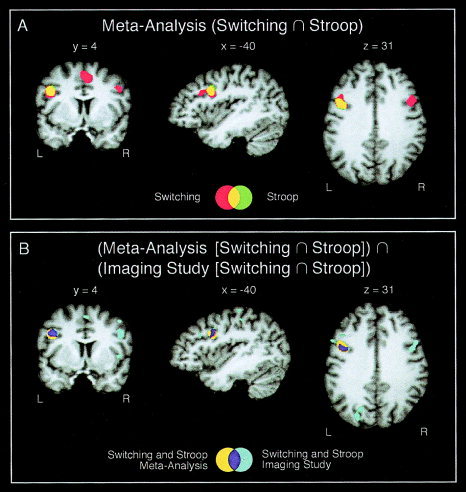
A: Overlap analysis at the IFJ for switch and Stroop meta‐analyses. B: Overlap analysis for the meta‐analytic results and the results from a functional imaging study. From the imaging study, the overlap from the switch vs. null event contrast and from the Stroop incongruent vs. neutral contrast are shown. These results, with the inclusion of an n‐back task, are reported in detail in Derrfuss et al. [2004]. Results are shown on an individual brain in Talairach space and were interpolated to millimeter resolution for display purposes. Only frontal coordinates entered the meta‐analyses.
When the analyses were repeated with different FWHM values, the peak locations in the IFJ were found to be very stable, showing only minor changes across analyses. Employing an FWHM of 7.1 mm, the IFJ peak in the switching meta‐analysis and in the Stroop meta‐analysis was located at −38, 4, 32. With an FWHM of 11.8 mm, the IFJ peaks were both located at −42, 6, 32. The effects of FWHM values are not the focus of the present study and therefore are not reported here in detail. Briefly, decreasing the FWHM led to smaller clusters of significant voxels for both meta‐analyses, and for the switching meta‐analysis led to an increase in the number of discrete above‐threshold clusters. Increasing the FWHM led to the opposite effect.
DISCUSSION
The present study, which employed the meta‐analytic approach developed by Turkeltaub et al. [2002], provides evidence for the consistent involvement of the IFJ in cognitive control. Quantitative meta‐analyses conducted separately for functional imaging studies of switching paradigms and the Stroop task identified local IFJ maxima that were nearly identical in both analyses (switching: −40, 4, 30; Stroop: −40, 4, 32). Furthermore, the above‐threshold voxels located at the IFJ overlapped to a large degree in both meta‐analyses and were very similar to the overlap found in a previous functional imaging within‐subject investigation [Derrfuss et al.,2004]. The present results support our concept of areal specialization in the frontolateral cortex, which posits that the IFJ is a functional entity separable from middorsolateral prefrontal cortex. Below, we discuss the functional role of the IFJ in cognitive control and the potential contribution of quantitative meta‐analyses to the interpretation of functional imaging studies.
Functional Role of the IFJ
We have provided evidence for the consistent involvement of the IFJ in cognitive control in a series of investigations: first, in functional imaging experiments designed to isolate the functional role of the IFJ [Brass and von Cramon,2002,2004], second, in a functional imaging within‐subject comparison of a task‐switching paradigm, the Stroop task, and the n‐back task [Derrfuss et al.,2004], and third, in the present study employing a meta‐analytic approach. Why is the IFJ consistently involved in the investigated tasks? Based on our experiments aiming at isolating the functional role of the IFJ, we have argued that the IFJ is involved in the updating of task representations. For example, in switching paradigms, participants have to constantly update the currently relevant task representation, and in the Stroop task participants have to enforce the relevant but nondominant task representation against a dominant action. Furthermore, IFJ activation was found in several other cognitive control paradigms that can also be argued to require the updating of task representations. For example, IFJ activations were found in studies investigating S–R compatibility [Bunge et al.,2002; Dassonville et al.,2001; Weissman et al.,2002], switching between movement sequences [Jäncke et al.,2000], in studies investigating the updating of task representations at the beginning of short task sequences/task blocks [Dreher and Berman,2002; Konishi et al.,2001], in a study investigating set shifting in the context of the paper‐scissors‐stone game [Omori et al.,1999], and in a study investigating abstract rule representation [Bunge et al.,2003]. Although we acknowledge that there are some important differences between these paradigms, we suggest that they have at least one process in common, the updating of task representations, and that it is this process that is related closely to activations of the IFJ.
Note, however, that the presence of an IFJ activation crucially depends on the control condition used. There are control conditions that might require task‐updating processes themselves, which might prevent the finding of a significant IFJ activation. This might be the case, for example, in contrasts of switch and repetition trials where unpredictable task sequences or an equal number of switch and repetition trials are employed [e.g., Braver et al.,2003; Luks et al.,2002]. In this case, contrasting the switch and repetition trials separately with a low‐level baseline or carrying out a time‐course analysis can inform about the possibility of similar activation of the IFJ in both trial types. Following this line of argumentation, IFJ activations should be found more frequently when there are relatively more repetition trials than switch trials. This is because such designs presumably reduce the degree of control necessary in repetition trials. Indeed, results from studies employing variants of the WCST (where participants usually switch after six to eight repetitions) or studies employing designs with unequal switch/repetition probabilities, such as the studies of Dove et al. [2000] and Pollmann et al. [2000], provide evidence for this assumption.
Contribution of Quantitative Meta‐Analyses to the Interpretation of Functional Imaging Studies
In the following section, we discuss some of the strengths and limitations of the ALE meta‐analysis method and issues pertaining to effect‐location meta‐analyses in general. One major strength of the ALE meta‐analysis technique is that it is not descriptive but provides a quantitative measure of the activation likelihood of a given voxel. This method can thus identify the regions activated most consistently in the paradigms under investigation on a quantitative basis. Consider the distribution of peaks displayed in Figure 1. Just by looking at this distribution it would be difficult to identify the areas activated most consistently in switching and Stroop studies; however, these areas can be identified by applying statistical methods. A second major advantage of the technique employed here is that it carries out the meta‐analysis in 3D space, and thus goes beyond other quantitative meta‐analysis techniques that rely on the assignment of activation peaks to Brodmann areas [e.g., Jonides et al.,2002]. This is a very important feature especially to identify clustering in a region such as IFJ, whose activations have been attributed to a number of different Brodmann areas.
The ALE meta‐analysis technique also has its limitations. Clearly, the width of the Gaussian distribution representing an activation peak has a potential influence on the results and was therefore evaluated in the present meta‐analyses. We ran the analyses with three different FWHM values between 7.1 and 11.8 mm and have shown that the peaks in the IFJ were very consistent regardless of the particular FWHM. There were some changes in other regions, indicating the importance of repeating the analyses with different FWHM values. The results also depend on the particular significance threshold applied. Similar to Turkeltaub et al. [2002], we chose a conservative threshold of α = 0.01%. This was done to identify only those regions involved most consistently in the switching paradigms and the Stroop task. At the cost of an increased type I error, less conservative thresholds will identify additional regions. Given that at present there are only few experiences with the ALE meta‐analysis method, it seems advisable to choose a conservative threshold. In their original article, Turkeltaub et al. [2002] suggest other possible improvements of their method. This includes weighting studies depending on the number of participants and the number of activations, changing the shape of the Gaussian distribution in cortical sulci, or creating noise distributions that incorporate the reduced likelihood of finding activation peaks in the white matter. Considering the very consistent involvement of the IFJ in switching paradigms and in the Stroop task, we would expect only minor changes of the IFJ peak locations if these modifications were incorporated into the meta‐analysis method.
An important issue to consider that applies to meta‐analyses in general is the careful choice of selection criteria. Clearly, meta‐analytic results crucially depend on these criteria. For example, the scope of paradigms included in the meta‐analysis can strongly influence the results. In a recent meta‐analysis investigating the shifting of attention [Wager et al.,2004], for example, no IFJ peak was found. This difference in results is due most likely to differences in the paradigms considered for the meta‐analyses. Wager et al. [2004] included studies investigating the shifting of locations, objects, attributes, tasks, and rules, and combined them in one meta‐analysis. Our choice of paradigms was motivated by our interest in the updating of task representations. Although our choice of paradigms and contrasts was motivated theoretically by our interest in a specific process, this of course does introduce an a priori bias for a specific region. We therefore included only studies investigating the shifting of tasks and rules. Furthermore, we included set‐shifting studies employing variants of the WCST. This task was not considered in the study by Wager et al. [2004].
A related issue is the treatment of contrasts that do not find any differences between two conditions and thus cannot be included in an effect‐location meta‐analysis. Consider the present meta‐analysis. If there had been a high percentage of contrasts not showing any activations in the frontal lobe, one would have doubted the consistent involvement of the frontal lobes in the first place. However, this assumption could not have been tested with an effect‐location meta‐analysis: If only 10% of the studies under consideration had found frontal lobe activations, the effect‐location meta‐analysis would have simply shown the regions most consistently involved in these 10% of studies. It is important, therefore, to report the number of studies that did and did not find activations in the particular region of the brain that was investigated. In the present study, as there were only 2 of 18 switching studies and no Stroop studies without frontal activations, we considered it justified to view the frontal lobe as being involved consistently in these paradigms. Importantly, as there might be contrasts with no differences anywhere in the brain, this problem also applies to whole‐brain meta‐analyses and not only to meta‐analyses that are restricted to a particular part of the brain. This problem differs from the so‐called file‐drawer problem of traditional effect‐size meta‐analyses, where studies yielding null effects could in principle be included in the meta‐analysis but are not available to the investigator.
Another issue to consider is the absence of above‐threshold clusters in certain areas. For example, we expected the involvement of the middorsolateral prefrontal cortex in the meta‐analysis of switching paradigms, and a peak in the inferior frontal sulcus anterior to the IFJ in the meta‐analysis of the Stroop task. Regarding the switching paradigms, individual studies found activation peaks in the middorsolateral prefrontal cortex [e.g., Dreher et al.,2002; Luks et al.,2002; Monchi et al.,2001,2004; Nakahara et al.,2002] but these were not close enough to each other to create an ALE that survived the threshold. Larger anatomical variance in more anterior prefrontal areas together with differences in number of participants, tasks, and statistical analysis methods between studies are likely to have contributed to this result. Regarding the Stroop studies, a peak in the IFS (−46, 12, 34) was identified only when we applied an FWHM of 7.1 mm. Therefore, the fact that some regions were not found to be significant in the meta‐analyses should be treated with caution. However, we can conclude with some confidence that the regions found significant in the present analyses do play a role in the Stroop task and switching paradigms because these regions were significant at a conservative threshold, when different FWHM values were used, and despite the above‐mentioned differences between studies.
CONCLUSIONS
The present study provides evidence for a functional specialization of the frontolateral cortex for cognitive control beyond a ventrolateral–dorsolateral dichotomy. By employing a quantitative meta‐analytic approach, we were able to show that the IFJ in the posterior frontolateral cortex is involved consistently in switching and Stroop studies. This suggests that there is a cognitive process intimately related to IFJ activations that is common to both paradigms. We propose that this process is likely the updating of task representations. There is now considerable evidence showing that the IFJ can be separated functionally from middorsolateral prefrontal cortex; however, the question arises as to whether the IFJ might also be separable structurally from other cortical areas. There is preliminary evidence that this might indeed be the case [Amunts et al.,2004].
Acknowledgements
We thank P. Turkeltaub and the Center for the Study of Learning at Georgetown University Medical Center for providing the ALE meta‐analysis software (online at http://csl.georgetown.edu/software/) and for their helpful support. We also thank S. Liebig for his help in preparing the figures.
Table IA.
Overview of coordinates included in the switching meta‐analysis
| Reference/coordinates | Contrast |
|---|---|
| Brass and von Cramon,2004 −37, 5, 32 55, 20, 18 | Meaning‐switch vs. cue‐switch |
| Braver et al.,2003 −16, 3, 63 −40, 30, 0 −46, 15, 21 | Switch vs. repeat (event‐related analysis) |
| DiGirolamo et al.,2001 −2, 16, 48 −28, 10, 44 −46, 12, 34 −40, 16, 32 46, 52, −2 46, 20, 10 46, 26, 24 48, 34, 24 4, 12, 54 48, 14, 28 4, −16, 42 0, −16, 42 0, 26, 44 | Switch blocks vs. repeat blocks |
| Dove et al.,2000 −44, 5, 37 −36, 20, 13 40, 8, 36 28, 23, 8 −8, 11, 47 | Switch vs. repeat |
| Dreher et al.,2002, a −40, 21, 28 −28, 6, 51 48, 17, 36 59, 29, 28 | Switch/repeat blocks (conjunction of switch conditions) vs. pure blocks |
| Konishi et al.,2002, a −48, 13, 18 −40, 41, 11 −38, 4, 33 | Update vs. null change |
| Kringelbach and Rolls,2003 −46, 29, −8 −46, 16, 14 −8, 24, 47 42, 40, −9 38, 20, 14 | Reversal vs. no reversal |
| Luks et al.,2002 −4, 3, 53 0, 10, 47 −44, 22, 21 −32, 40, 26 −44, −6, 39 −34, 32, 16 4, 8, 49 30, 34, 15 44, −5, 39 48, −5, 52 | Informative switch cue vs. baseline |
| Luks et al.,2002 −46, 26, 28 −48, 28, 17 50, 38, 24 | Neutrally cued switch vs. baseline |
| Monchi et al.,2001, a −38, 3, 27 −46, 5, 27 −44, 17, 27 −50, 30, 20 48, 15, 25 41, 13, 36 42, 30, 24 | Negative vs. control feedback |
| Monchi et al.,2001, a 32, 14, 49 34, 21, 1 −8, 21, 38 3, 27, 41 4, 16, 45 | Negative vs. control feedback |
| Monchi et al.,2004, a −42, 32, 24 34, 21, 3 −8, 21, 41 −44, 9, 33 48, 27, 28 32, 21, 3 6, 29, 41 44, 9, 33 24, 4, 44 | Negative vs. control feedback |
| Nagahama et al.,2001, a −53, 11, 33 46, 7, 29 34, 49, 12 22, −3, 55 36, −9, 59 2, 4, 48 6, 8, 44 | Negative feedback vs. sorting baseline (WCST) |
| Nagahama et al.,2001, a −42, 3, 26 53, 9, 33 8, 5, 51 6, 6, 44 28, −11, 56 26, −1, 55 | Negative feedback vs. sorting baseline (S‐R reversal) |
| Nakahara et al.,2002, a −51, 14, 18 −48, −2, 46 −32, 20, 5 46, 17, 21 34, 4, 35 30, 25, −1 42, 12, −2 −4, 9, 60 −10, 14, 45 8, 30, 46 | Negative feedback vs. sorting baseline |
| Pollmann et al.,2000 45, 2, 37 45, 16, 37 31, 2, 3 0, 2, 56 | Switch vs. repeat |
| Swainson et al.,2003, a 9, 16, 27 | Go switch vs. go repeat |
Coordinates were transformed from MNI to Talairach space; these coordinates will differ slightly from the originally published coordinates.
Table IIA.
Overview of coordinates included in the Stroop meta‐ analysis
| Reference/coordinates | Contrast |
|---|---|
| Banich et al.,2000 −48, 10, 34 −42, 28, 20 50, 16, 34 54, 24, 26 | Incongruent/neutral vs. neutral |
| Banich et al.,2001 −42, 10, 34 −34, 22, −2 2, 16, 42 | Incongruent/neutral vs. neutral |
| Fan et al.,2003, a −50, 35, −8 −26, 54, −4 −38, 28, 12 −4, 38, 26 | Incongruent vs. congruent |
| Mead et al.,2002 −44, 4, 29 | Incongruent vs. neutral |
| Milham et al.,2001 −42, 2, 36 −34, 20, 24 40, 8, 42 0, 10, 44 | Incongruent vs. neutral (event‐related analysis) |
| Milham et al.,2002 −48, 44, 8 −42, 28, 32 −46, 14, 32 −40, 4, 46 42, 16, 6 4, 10, 54 2, 32, 34 4, 18, 40 | Incongruent vs. congruent/neutral |
| Milham et al.,2003 −34, 14, 18 −46, 40, 24 32, 16, 16 48, 38, 24 50, 34, 26 40, 50, 26 44, 2, 16 8, 30, 28 60, 10, 32 | Incongruent vs. oddball neutral |
| Norris et al.,2002 −38, 4, 33 −43, 41, 4 −11, 67, 13 39, 17, 21 19, 62, 11 −1, 25, 43 | Incongruent vs. neutral |
| Potenza et al.,2003 −28, 11, 5 1, 11, 42 43, 7, 35 39, 41, 23 36, 13, 5 2, 31, 23 | Incongruent vs. congruent |
| Steel et al.,2001 −3, 36, 26 −3, 8, 31 −9, 14, 31 −38, 11, −2 −29, 19, 4 −23, 22, 9 −26, 25, 4 −49, 8, 9 6, 47, 15 12, 36, 15 3, 42, 9 0, 44, 20 35, 11, 9 26, 28, −2 | Incongruent vs. neutral |
| Zysset et al.,2001 −38, 5, 30 −38, 35, 5 44, 15, 36 31, 53, 15 1, 26, 42 | Incongruent vs. neutral |
Coordinates were transformed from MNI to Talairach space; these coordinates will differ slightly from the originally published coordinates.
Footnotes
Similar to our previous imaging study [Derrfuss et al.,2004], we had planned to include the verbal n‐back task in the present study; however, only four studies employing the verbal n‐back task satisfied our selection criteria, which we did not consider sufficient for carrying out a quantitative meta‐analysis.
This issue of Human Brain Mapping includes another Stroop meta‐analysis from our group employing a method that allows to identify dominant activation networks using replicator dynamics [Neumann et al.,2005]. This method was not employed in the present study because we included only frontal lobe coordinates and therefore expected a much lower number of activation clusters than that found in our previous study. Another important difference between the present study and the study by Neumann et al. [2005] is that we carefully selected studies according to a number of criteria for the present study, whereas we included all Stroop studies found in the BrainMap DBJ database in the study by Neumann et al. [2005]. The IFJ was found to be significant in both Stroop meta‐analyses.
REFERENCES
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL (2002): A developmental fMRI study of the Stroop color–word task. Neuroimage 16: 61–75. [DOI] [PubMed] [Google Scholar]
- Amunts K, Palomero‐Gallagher N, Brass M, Derrfuss J, Zilles K, von Cramon DY (2004): A receptor‐ and cytoarchitectonic correlate of the functionally defined inferior frontal junction area. Poster presented at the 10th Annual Meeting of the Organization for Human Brain Mapping. Online at http://www.meetingassistant.com/ohbm/view_abstract_no.php?abstractno=951.334001. Accession date: 15 March 2005.
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Barad V, Gullett D, Shah C, Brown C (2000): Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Brain Res Cogn Brain Res 10: 1–9. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Jacobson BL, Webb A, Wszalek T, Cohen NJ, Kramer AF (2001): Attentional selection and the processing of task‐irrelevant information: insights from fMRI examinations of the Stroop task. Prog Brain Res 134: 459–470. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2002): The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2004): Decomposing components of task preparation with functional MRI. J Cogn Neurosci 16: 609–620. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Barch DM (2002): The role of prefrontal cortex in normal and disordered cognitive control: a cognitive neuroscience perspective In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; p 428–447. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC (1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI (2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD (2002): Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Kahn I, Wallis J, Miller E, Wagner A (2003): Neural circuits subserving the retrieval and maintenance of abstract rules. J Neurophysiol 90: 3419–3428. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2002): Seeing the forest through the trees: the cross‐function approach to imaging cognition In: Zani A, Proverbio AM, editors. The cognitive electrophysiology of mind and brain. San Diego: Academic Press; p 41–68. [Google Scholar]
- Chein JM, Fissell K, Jacobs S, Fiez JA (2002): Functional heterogeneity within Broca's area during verbal working memory. Physiol Behav 77: 635–639. [DOI] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J (2001): The effect of stimulus‐response compatibility on cortical motor activation. Neuroimage 13: 1–14. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY (2004): Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 23: 604–612. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E (2001): General and task‐specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task‐switching. Neuroreport 12: 2065–2071. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: an event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher J, Berman K (2002): Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA 99: 14595–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J (2002): The roles of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003): Cognitive and brain consequences of conflict. Neuroimage 18: 42–57. [DOI] [PubMed] [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL (1998): Beyond the single study: function/location metanalysis in cognitive neuroimaging. Curr Opin Neurobiol 8: 178–187. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Li SJ, Stein EA (2000): A parametric manipulation of central executive functioning. Cereb Cortex 10: 585–592. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR (2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain 125: 1024–1038. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S (2001): Language switching and language representation in Spanish‐English bilinguals: an fMRI study. Neuroimage 14: 510–520. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Himmelbach M, Shah NJ, Zilles K (2000): The effect of switching between sequential and repetitive movements on cortical activation. Neuroimage 12: 528–537. [DOI] [PubMed] [Google Scholar]
- Jonides J, Badre D, Curtis C, Thompson‐Schill SL, Smith EE ( 2002): Mechanisms of conflict resolution in the prefrontal cortex In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; p 233–245. [Google Scholar]
- Jonides J, Schumacher EH, Smith EE (1997): Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci 9: 462–475. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M (2000): Modulation of task‐related neural activity in task‐switching: an fMRI study. Brain Res Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Konishi S, Donaldson DI, Buckner RL (2001): Transient activation during block transition. Neuroimage 13: 364–374. [DOI] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y (2002): Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA 99: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y (1999): Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex 9: 745–753. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y (1998): Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci 1: 80–84. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET (2003): Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage 20: 1371–1383. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM (2004): fMRI of healthy older adults during Stroop interference. Neuroimage 21: 192–200. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL (2002): Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802. [PubMed] [Google Scholar]
- Mead LA, Mayer AR, Bobholz JA, Woodley SJ, Cunningham JM, Hammeke TA, Rao SM (2002): Neural basis of the Stroop interference task: response competition or selective attention? J Int Neuropsychol Soc 8: 735–742. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V (2003): Competition for priority in processing increases prefrontal cortex's involvement in top‐down control: an event‐related fMRI study of the stroop task. Brain Res Cogn Brain Res 17: 212–222. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF (2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ (2002): Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004): Neural bases of set‐shifting deficits in Parkinson's disease. J Neurosci 24: 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001): Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H (2001): Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 11: 85–92. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Hayashi T, Konishi S, Miyashita Y (2002): Functional MRI of macaque monkeys performing a cognitive set‐shifting task. Science 295: 1532–1536. [DOI] [PubMed] [Google Scholar]
- Neumann J, Lohmann G, Derrfuss J, von Cramon DY (2005): The meta‐analysis of functional imaging data using replicator dynamics. Hum Brain Mapp 25: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris DG, Zysset S, Mildner T, Wiggins CJ (2002): An investigation of the value of spin‐echo‐based fMRI using a Stroop color–word matching task and EPI at 3 T. Neuroimage 15: 719–726. [DOI] [PubMed] [Google Scholar]
- Omori M, Yamada H, Murata T, Sadato N, Tanaka M, Ishii Y, Isaki K, Yonekura Y (1999): Neuronal substrates participating in attentional set‐shifting of rules for visually guided motor selection: a functional magnetic resonance imaging investigation. Neurosci Res 33: 317–323. [DOI] [PubMed] [Google Scholar]
- Petrides M (2000): Mapping prefrontal cortical systems for the control of cognition In: Toga AW, Mazziotta JC, editors. Brain mapping: the systems. San Diego: Academic Press; p 159–176. [Google Scholar]
- Pollmann S, Dove A, von Cramon DY, Wiggins CJ (2000): Event‐related fMRI: comparison of conditions with varying BOLD overlap. Hum Brain Mapp 9: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC (2003): An fMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry 160: 1990–1994. [DOI] [PubMed] [Google Scholar]
- Price CJ, Green DW, von Studnitz R (1999): A functional imaging study of translation and language switching. Brain 122: 2221–2235. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning‐Dixon F, Turner T, Schroeder L, Chan R, Gur RE (2002): Working memory for complex figures: an fMRI comparison of letter and fractal n‐back tasks. Neuropsychology 16: 370–379. [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW (2000): Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Koch I, Rubin O, Meiran N, von Cramon DY (2005): Advance preparation and stimulus‐induced interference in cued task switching: further insights from BOLD fMRI. Neuropsychologia 43: 340–355. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002): Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel C, Haworth EJ, Peters E, Hemsley DR, Sharma T, Gray JA, Pickering A, Gregory L, Simmons A, Bullmore ET, Williams SC (2001): Neuroimaging correlates of negative priming. Neuroreport 12: 3619–3624. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR (2003): Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task‐switching. J Cogn Neurosci 15: 785–799. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J (2003): Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41: 357–370. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Umetsu A, Okuda J, Fujii T, Tsukiura T, Nagasaka T, Yanagawa I, Sugiura M, Inoue K, Kawashima R, Suzuki K, Tabuchi M, Murata T, Mugikura S, Higano S, Takahashi S, Fukuda H, Yamadori A (2002): Brain activation during the fist‐edge‐palm test: a functional MRI study. Neuroimage 17: 385–392. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: a meta‐analysis. Neuroimage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF (2003): Valence, gender, and lateralization of functional brain anatomy in emotion: a meta‐analysis of findings from neuroimaging. Neuroimage 19: 513–531. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG (2002): A role for top‐down attentional orienting during interference between global and local aspects of hierarchical stimuli. Neuroimage 17: 1266–1276. [DOI] [PubMed] [Google Scholar]
- Zysset S, Muller K, Lohmann G, von Cramon DY (2001): Color–word matching Stroop task: separating interference and response conflict. Neuroimage 13: 29–36. [DOI] [PubMed] [Google Scholar]


