Abstract
Over the past two decades neuroimaging data have accumulated showing that the cerebellum, traditionally viewed only as a motor structure, is also active in a wide variety of sensory and cognitive tasks. We have proposed that instead of explicit involvement in any particular motor, sensory, or cognitive task, the cerebellum performs a much more fundamental computation involving the active acquisition of sensory data. We carried out an activation likelihood estimate (ALE) meta‐analysis to determine whether neuroimaging results obtained during a wide range of auditory tasks support this proposal. Specifically, we analyzed the coordinates of 231 activation foci obtained in 15 different auditory studies selected through an extensive search of the positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) literature. The studies selected represent a wide variety of purely auditory tasks using highly controlled synthesized acoustic stimuli. The results clearly revealed that in addition to temporal auditory areas of cerebral cortex, specific regions in the cerebellum are activated consistently across studies regardless of the particular auditory task involved. In particular, one area in left lateral crus I area showed the greatest volume and ALE peak value among the extratemporal regions. A subanalysis was carried out that ruled out the specific association of this cerebellar cluster with attentional demand. The results are consistent with the hypothesis that the cerebellum may play a role in purely sensory auditory processing, and are discussed in light of the broader idea of the cerebellum subserving a fundamental sensory function. Hum Brain Mapp 25:118–128, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: cerebellum, PET, fMRI, ALE, meta‐analysis, auditory function
INTRODUCTION
A growing body of evidence suggests that the cerebellum, long believed to be involved primarily in motor control, may actually contribute to a much wider range of behaviors [Ackermann and Hertrich,2000; Bower,2002; Desmond,2001; Desmond and Fiez,1998; Fiez,1996; Houk,1997; Justus and Ivry,2001; Marien et al.,2001; Rapoport et al.,2000; Saab and Willis,2003; Schmahmann,1997,2000,2004; Silveri and Misciagna,2000]. Based largely on recent advancements in neuroimaging techniques, there are in particular a growing number of suggestions about cerebellar involvement in a wide variety of cognitive and perceptual activities, including temporal processing [Ivry and Keele,1989; Jueptner et al.,1995; Nichelli et al.,1996; Pastor et al.,2004], language production and comprehension [Desmond et al.,1997; Justus,2004; Petersen et al.,1989; Silveri et al.,1994; Xiang et al.,2003], spatial reasoning [Bracke‐Tolmitt et al.,1989; Parsons et al.,1995], visual perception of motion, speed, and direction [Ivry and Diener,1991; Thier et al.,1999], visual attention [Allen et al.,1997], color discrimination [Claeys et al.,2003], tactile and proprioceptive information processing [Blakemore et al.,1998; Jueptner et al.,1997; Seitz et al.,1991], olfaction [Ferdon and Murphy,2003; Sobel et al.,1998], nociception [Helmchen et al.,2004; Saab and Willis,2001,2002], as well as sensory and cognitive states related to thirst [Parsons et al.,2000a], affect [Levisohn et al.,2000; Schmahmann and Schermann,1998], and the perception of music [Parsons,2001]. As a result, within the past 10 years, speculations regarding cerebellar function have arguably undergone the largest expansion seen for any brain structure in the last 100 years.
Based on our previous studies of tactile/sensory receiving areas of the rat cerebellum [Bower and Woolston,1983; Bower and Kassell,1990; Gundappa‐Sulur et al.,1999; Hartmann and Bower,2001], we have proposed what we consider a more fundamental and potentially unifying hypothesis: that the cerebellum is actually performing a sensory rather than a motor or specific cognitive function [Bower,1997,2002; Bower and Kassel,1990]. Specifically, we suggest that the cerebellum is involved in regulating the acquisition of sensory data across all modalities, evaluating those data in very close to real‐time, and then rapidly influencing the structures acquiring the data to assure that the highest possible quality sensory data are obtained for use by the rest of the nervous system. In this view, cerebellar computation provides a supporting role for a wide range of sensory, motor, and cognitive tasks and should be especially active for tasks that are computationally more difficult and therefore require a finer level of sensory data control [Bower,1997,2002]. In our previous human somatosensory neuroimaging experiments, we have demonstrated the predicted strong correlation between the level of cerebellar activation and the use of somatosensory (tactile) information in a sensory discrimination task [Gao et al.,1996]. These studies showed almost no activation in the lateral cerebellum with fine finger movements, unless those movements required a task involving tactile sensory data discrimination. We have also reported similar results in other cerebellum‐related structures [Liu et al.,2000; Pu et al.,1998].
Although these results are consistent with our hypothesis, the fact that the somatosensory system, like most other sensory systems (e.g., vision, taste, and olfaction), uses body movements to control sensory data acquisition makes it harder to distinguish between sensory and motor function. For this reason, we have begun to explore the possible role of the cerebellum in the sensory performance of the auditory system, where fine sensory perception is possible without any overt body movements [Parsons et al.,2000b]. Based on our sensory data acquisition hypothesis [Bower,1997,2002; Bower and Kassel,1990], we have specifically predicted that auditory perceptual tasks in the absence of any overt movements should generate specific and reproducible foci of activation within the cerebellum. In fact, it has been known since the 1940s [Snider and Stowell,1944] that the cerebellum responds to acoustic stimuli, interestingly, at shorter latencies than the auditory cortex does. Despite having been confirmed repeatedly across different animal species [Aitikin and Boyd,1975; Huang and Liu,1990; Sun et al.,1983; Wolfe and Kos,1975; Xi et al.,1994], we know of no positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) studies that have tested specifically for a purely sensory role for the cerebellum in auditory processing. Instead, most studies have focused on higher‐level aspects of auditory processing, such as the timing and duration of sounds for speech perception [Mathiak et al.,2002,2004], processing of stimuli presentation rate [Ackermann et al.,2001; Pastor et al.,2002], or perceptual timing in general [Jueptner et al.,1995; Mangels et al.,1998; Nichelli et al.,1996]. Again, our hypothesis posits that cerebellar involvement in audition is more fundamental, operating at the level of the basic mechanisms of active auditory sensory data acquisition.
The absence of any study that tests specifically for a general auditory function for the cerebellum makes this problem an ideal application for a cross‐study meta‐analysis. Accordingly, we have used the activation likelihood estimate (ALE) meta‐analytic procedure developed by Turkeltaub et al. [2002] to determine whether consistent patterns of cerebellar activation are associated with the presentation of auditory stimuli in humans, irrespective of the specifics of the associated sensory task and in the absence of variations in emotional or cognitive state or explicit motor performance. This method combines the coordinates of activation maxima from multiple studies into an ALE map for the brain, revealing interstudy consistencies that may not be immediately evident by simple visual comparison of individual reports. Specifically, we analyzed the coordinates of 231 activation foci elicited by a variety of auditory tasks and stimuli in 15 different studies chosen to normalize across cognitive, emotion, and motor task components. The results revealed several consistently activated regions of the cerebellum including a large but previously unrecognized auditory activation area in the anterior lateral cerebellar cortex, which seems activated consistently in response to auditory stimuli alone. The results are interpreted in the context of current theories of cerebellar function.
MATERIALS AND METHODS
Literature Search
We used three methods to locate studies for this meta‐analysis: (1) Medline and Web of Science databases were searched through September 2004 using the keywords positron emission tomography and functional magnetic resonance imaging (including acronyms and synonyms such as PET, fMRI, regional cerebral blood flow, BOLD, etc.) cross‐referenced with audit*, hear*, listen*, sound*, acoustic, tone*, click*, and noise, where * indicates a wild‐card; (2) we searched Web of Science database for articles that cited the works selected with the previous method (Cited Reference Search); and (3) we reviewed the reference lists of all the selected articles. Several hundred articles were obtained and reviewed as a result of this procedure.
Inclusion Criteria
Of the identified articles, we included studies in this meta‐analysis if they fulfilled six specific criteria. First, studies had to be published in a peer‐reviewed journal. Second, they had to report the coordinates of activation maxima in standardized stereotaxic space. Third, the imaged brain volume in a study had to include both the cerebrum and cerebellum entirely; all brain areas in the acquired images had to be analyzed for activations, not just regions of interest, because the analysis carried out here was probabilistic in nature, and it was therefore important that all brain areas had equal chance to be represented. Fourth, subjects had to be reported to be healthy individuals with no reported neurological or psychiatric history (we excluded studies that specifically indicated that the subjects had musical training). Fifth, studies had to contain at least one contrast involving low‐level, non‐cognitively nor emotionally connotated, aspects of auditory processing. Accordingly, we excluded studies that used human vocal sounds, environmental sounds, machinery noises, musical instruments, and music to avoid stimuli that might elicit speech analysis, imagery, or emotional responses and therefore contaminate the meta‐analysis with nonsensory elements. The sixth criterion was that contrasts in a study had to either exclude motor tasks (button press, finger lifting, etc.) or control for them by subtraction between conditions. For each article evaluated, the printed text was the primary source of information about the study. When the information contained in an article was not sufficient, we contacted the authors for clarification and in several cases were provided additional data not included in the articles [e.g., Dittmann‐Balcar et al.,2001; Lockwood et al.,1999; Zatorre et al.,2002a].
Of several hundred articles evaluated, 17 articles satisfied our criteria. Despite the relatively small size of our sample, further selection was carried out to avoid bias generated by the overrepresentation of particular experiments, tasks, or particular experimental groups. For example, the 17 articles included three reports from the same research group on the perception of sound movement [Griffiths et al.,1994,1998,2000]. To avoid bias in our sample, we elected to include only the latest study because (as also stressed by the authors) it confirmed the previous findings by means of improved protocol and analysis. We decided not to exclude an article by this group addressing a similar theoretical issue (perception of sounds rotating around the head) [Griffiths and Green,1999] because tasks, stimuli, and imaging modality differed from the other articles.
From the remaining 15 studies, we selected sets of coordinates for the meta‐analysis by choosing only those contrasts that met the fifth and sixth inclusion criteria. This yielded 37 contrasts that were also filtered further to avoid overrepresentation. Specifically, from Lockwood et al. [1999] we selected two of eight contrasts, specifically, those at 70 dB HL, which was an average intensity level falling in the intensity level range used in the other included studies. We did not include the contrasts at 30, 50, and 90 dB hearing level (HL) because the inclusion of conditions differing only for slight variations of one parameter would have inflated the weight of this study in the meta‐analysis. Furthermore, the perception of differences in sound intensity was addressed with more specificity in another selected article [Belin et al.,1998] using an intensity discrimination paradigm. Again, to avoid overrepresenting data from similar tasks, we decided not to include the secondary contrasts from Griffiths and Green [1999] and Griffiths et al. [2000] that subanalyzed minor differences in the perception of clockwise and anticlockwise sound rotation around the head (9 foci total), and sound movement to the left and to the right (43 foci total), respectively.
In summary, our final pool of studies for the meta‐analysis consisted of 15 articles spanning July 1997 to August 2004. There were 27 selected contrasts encompassing a wide variety of passive and active auditory tasks and highly controlled synthesized stimuli, for a total of 231 foci. Table I shows the list of selected articles and contrasts and provides a summary of the main parameters of interest. These articles have been coded and input into the BrainMap database (online at http://www.brainmap.org) where they are available for further reference.
Table I.
Summary of studies included in the meta‐analysis
| Article and contrasts | Imaging modality | n | Filter (mm) | Stimuli | Stimuli presentation | Foci |
|---|---|---|---|---|---|---|
| Passive listening | ||||||
| Ackermann et al.,2001 | fMRI | 8 | 10 | Clicks | Binaural | |
| Main effecta | 6 | |||||
| 1st‐order effecta | 2 | |||||
| 2nd‐order effect | 3 | |||||
| Griffiths and Green,1999 | PET | 6 | 16 | Broadband noise | Binaural | |
| Combined sounds vs. resta | 5 | |||||
| Rotating vs. coherent | 4 | |||||
| Griffiths et al.,2000 | fMRI | 4 | 8 | Pure tones | Binaural | |
| Movement vs. stationarya | 22 | |||||
| Lockwood et al.,1999 | PET | 12 | 10 | Pure tones | Monaural R | |
| 0.5 kHz, 70 dB HL vs. resta | 21 | |||||
| 4.0 kHz, 70 dB HL vs. resta | 13 | |||||
| Ortuño et al.,2002 | PET | 10 | 12 | Clicks | Binaural | |
| Main effect listeninga | 9 | |||||
| Pastor et al.,2002 | PET | 9 | 10 | Clicks | Monaural R | |
| All frequencies vs. rest | 5 | |||||
| 40 Hz vs. groupeda | 2 | |||||
| Rao et al.,1997 | fMRI | 13 | 8 | Pure tones | Binaural | |
| Listening vs. rest | 3 | |||||
| Reyes et al.,2004 | PET | 9 | 11 × 12 × 14 | AM tones, pure tones | Monaural R | |
| Pure tones vs. rest | 4 | |||||
| 40 Hz AM vs. rest | 4 | |||||
| 40 Hz AM vs. pure tone | 2 | |||||
| Sevostianov et al.,2002 | PET | 18 | 10 | Pure tones | Monaural R/L | |
| Ignore deviant vs. ignore standarda | 16 | |||||
| Thivard et al.,2000 | PET | 8 | 15 | Complex sounds | Binaural | |
| Stationary vs. rest | 6 | |||||
| SM vs. rest | 6 | |||||
| SM vs. stationary | 4 | |||||
| Active listening | ||||||
| Belin et al.,1998 | PET | 7 | 12 | Complex tones | Binaural | |
| Intensity discr. vs. standard soundsa | 6 | |||||
| Belin et al.,2002 | PET | 7 | 12 | Complex tones | Binaural | |
| Duration discr. vs. standard soundsa | 19 | |||||
| Poeppel et al.,2004 | PET | 10 | 15 × 15 × 9 | FM pure tones | Free field | |
| FM sweeps vs. resta | 34 | |||||
| van Dijk and Backes,2003 | fMRI | 8 | 8 | Tone pulses, noise bursts | Monaural R | |
| Simultaneous masking vs. resta | 13 | |||||
| Backward masking vs. rest | 9 | |||||
| Simultaneous vs. backwarda | 5 | |||||
| Backward vs. simultaneous | 3 | |||||
| Vouloumanos et al.,2001 | fMRI | 15 | 8 | Complex sounds, pure tones | Binaural | |
| Complex nonspeech vs. simple tones | 5 | |||||
| Total | 231 |
Contrasts containing cerebellar activations in indicated study.
AM, amplitude modulated; FM, frequency modulated; HL, hearing level; SM, spectral motion.
Procedure
Before analysis, the coordinates from studies that used the Montreal Neurological Institute (MNI) templates were transformed to Talairach coordinate space [Brett,1999]. Once all the coordinates were in Talairach space, they were imported into a Java‐based version of ALE software developed at the Research Imaging Center (online at http://www.brainmap.org/ale) and analyzed with a fully automated procedure. The Talairach space was divided into 2 mm × 2 mm × 2 mm voxels and a whole‐brain ALE map created by modeling the foci as localization probability distributions centered at the given coordinates, and for each voxel calculating the probability each focus was located within that particular voxel using a 3D Gaussian function of 12 mm full‐width half‐maximum (FWHM), computing the ALE value as the union of these probabilities, and then assessing statistical significance using the threshold determined by a permutation test of randomly generated sets of foci. Detailed description of the ALE method and the statistical test employed can be found in Turkeltaub et al. [2002] and Laird et al. [2005a,b]. For purposes of visualization, the ALE map was imported into AFNI software [Cox,1996] and masked using an International Consortium for Brain Mapping (ICBM) template normalized to Talairach space [Kochunov et al.,2002].
With the same procedure, a second ALE map was generated to subanalyze the 10 studies containing only passive listening tasks (see Table I). This subsample was obtained by removing the five active listening discriminative studies from our total sample [i.e., Belin et al.,1998,2002; Poeppel et al.,2004; van Dijk and Backes,2003; Vouloumanos et al.,2001] and consisted of 19 contrasts for 137 foci in total.
RESULTS
The ALE meta‐analysis carried out on all 15 articles revealed 11 clusters of significant likelihood for activation found in bilateral auditory cortex, bilateral cerebellum, right prefrontal association cortex, right anterior insula, and right parietal association cortex. Figure 1 shows these locations in horizontal sections selected at the z‐axis value of each cluster's ALE maximum. The coordinates and ALE value for each of the local maxima, as well as the volume of the corresponding cluster, are reported in Table II. As can be seen, the highest probabilities for activation in the cerebral cortex were found in the primary and secondary auditory cortex, and in addition above threshold activation regions were also found in the left transverse temporal gyrus and in the right superior temporal gyrus. Within the temporal lobe, an additional peak was identified more anterior, in the higher‐order auditory cortex of the left superior temporal gyrus.
Figure 1.

Brain regions showing significant likelihood for activation in response to auditory processing across all studies. A: Right inferior parietal lobule. B: Right middle frontal gyrus. C: Left transverse temporal gyrus (AI) and right superior temporal gyrus (AII). D: Right anterior insula. E: Left superior temporal gyrus (higher‐order auditory cortex). F: Left cerebellum‐lobule V. G: Right cerebellum‐lobule V. H: Left cerebellum‐lateral crus I, and right cerebellum‐posterior crus II. I: Right cerebellum‐posterior crus I.
Table II.
Locations of significant ALE maxima
| Region | BA | Coordinates | Volume (mm3) | ALE (×10−3)* | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Auditory cortex | ||||||
| Left transverse temporal gyrus | 41 | −40 | −26 | 10 | 11032 | 27.20 |
| Right superior temporal gyrus | 42 | 60 | −20 | 10 | 9608 | 20.32 |
| Left superior temporal gyrus | 22 | −50 | 2 | −6 | 768 | 11.66 |
| Cerebellum | ||||||
| Left cerebellum, crus I | −44 | −50 | −32 | 1048 | 10.33 | |
| Right cerebellum, lobule V | 28 | −38 | −28 | 336 | 10.26 | |
| Right cerebellum, crus I | 26 | −76 | −36 | 312 | 10.00 | |
| Left cerebellum, lobule V | −20 | −50 | −22 | 184 | 8.99 | |
| Right cerebellum, crus II | 4 | −82 | −32 | 152 | 7.88 | |
| Right hemisphere | ||||||
| Right middle frontal gyrus | 10 | 34 | 38 | 12 | 416 | 10.00 |
| Right anterior insula | 36 | 18 | 4 | 1024 | 9.89 | |
| Right inferior parietal lobule | 40 | 48 | −46 | 46 | 528 | 9.72 |
Cerebellar ALE Patterns
As also summarized in Figure 1, five peaks are seen clearly in the cerebellum: lateral crus I area of the left hemisphere, bilateral hemispheric lobule V, and posterior crus I and II areas in the right hemisphere. As shown in Figure 2A, all peaks found in the cerebellum were hemispheric, with no consistent pattern of activations in the vermal or paravermal regions. Furthermore, the ALE cerebellar clusters seemed contained within a restricted range along the z‐axis (between z = −22 and z = −36). Among the cerebellar areas, the largest volume was for a region extending between crus I and lobule VI (Fig. 2B). Overall, the extent of this cluster was exceeded only by the two main regions in bilateral primary and secondary auditory cortex (see Table II). This cerebellar activation showed some lateralized symmetry with the maximum in the right crus I, although the right peak was smaller in volume and more posterior than that on the left. The two peaks in the left and right cerebellar lobule V showed a higher degree of symmetry, whereas the cerebellar cluster in the medial posterior crus II area with the smallest volume and ALE value was only found on the right side.
Figure 2.
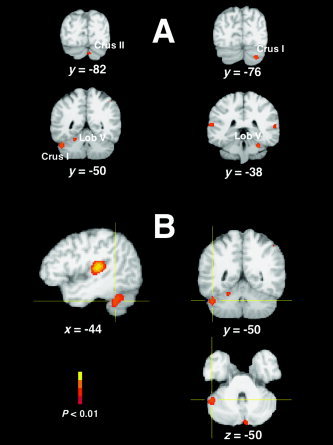
A: Location of cerebellar ALE clusters (coronal sections). All the areas consistently activated during auditory tasks are hemispheric and concentrated in a restricted range along the z‐axis. B: The largest cerebellar ALE cluster (maximum at x, y, z = −44, −50, −32) extends from crus I (x, y, z = −48, −60, −38) to lobule VI (x, y, z = −40, −46, −24). The sagittal view (left image) shows the extension of this cluster compared to the largest temporal cluster.
Right Cerebral Hemispheric Maxima and Subanalysis of Passive Listening Studies
Finally, although the primary focus of this study was patterns of cerebellar activation, the meta‐analysis also revealed three consistently lateralized peaks in the right cerebral hemisphere comparable by volume and ALE value with the smaller clusters seen in the cerebellum. One cluster was seen in the inferior parietal lobule, and the other two anteriorly in the middle frontal gyrus and anterior insula (Fig. 1A,B,D, respectively). A subanalysis of the data (Fig. 3A) indicated that the right cerebral hemispheric clusters were no longer present when the five active listening studies were removed from the sample and only the 10 passive listening studies were analyzed. As shown in Figure 3B, removing these studies had a much less profound effect on the largest cerebellar cluster found in lateral crus I, resulting in a minor modification of its shape and a slight reduction of the peak ALE value.
Figure 3.

A: Comparison of the right hemispheric areas presumed to be related to attention when meta‐analysis was carried out including (left) or excluding (right) tasks involving auditory discrimination. B: Comparison of the largest region of cerebellar activation when meta‐analysis was carried out including (left) or excluding (right) tasks involving auditory discrimination. Note slight change in the ALE maximum.
DISCUSSION
The primary aim of this study was to determine whether auditory processing tasks alone, independent of the particular task design or auditory stimulus, were sufficient to consistently activate neuroimaging signals in the human cerebellum. Our ALE meta‐analysis revealed that five cerebellar areas show a significant likelihood for activation across the range of tasks represented in this analysis. We first consider the plausibility of the overall analysis by examining the results obtained in auditory regions of cerebral cortex. We then address the cerebellar results in the context of anatomical and physiological evidence supporting its possible general role in auditory function, and more generally in sensory processes.
Auditory Cortex
As would be expected, the primary auditory cortices showed the greatest likelihood for activation in this analysis. As also expected, the wide variety of tasks, contrasts, and stimuli chosen for this meta‐analysis resulted in a considerable extension of the ALE clusters over a large region of auditory cortex. Although these clusters covered the most part of primary and secondary auditory cortex, the left hemispheric cluster was somewhat larger and peaks more medial than on the right. The lateralization of auditory cortical functions, observed in numerous studies before, continues to be an active area of investigation [Lauter et al.,1985; Poeppel et al.,2004; Tervaniemi and Hugdahl,2003; Woldorff et al.,1999; Zatorre and Belin,2001; Zatorre et al.,2002b], and might be an interesting subject for its own meta‐analysis, but is outside the focus of the current work.
Beyond the perhaps not‐too‐surprising preservation of primary auditory responses, closer examination of cortical activation regions also showed several more focused and lateralized patterns of consistent activation in the frontal and parietal regions of the right hemisphere (Fig. 1A,B,D). As discussed in more detail below, these activations have been associated previously with tasks involving increased attentional demand [Pardo et al.,1991; Paus et al.,1997; Sturm et al.,2004; Zatorre et al.,1999]. The most important point for the current report is that the presence of both larger and smaller scale cortical auditory activation patterns suggests that the patterns of cerebellar activity emerging from the meta‐analysis could also be considered generally characteristic of the brain's response to an auditory input irrespective of the particular tasks involved.
Response Patterns in the Cerebellum
The primary purpose of this study was to determine whether auditory processing studies in general consistently activated the cerebellum in the absence of specific cognitive, emotional, or motor elements. Our hypothesis suggesting a role for the cerebellum in the control of sensory data acquisition [Bower,1997,2002; Bower and Kassel,1990; Bower and Parsons,2003], predicts that this should be the case.
The basic results presented here support this conjecture. Overall, the presence of cerebellar activations across the selected studies was constant as it was observed in 11 of 15 articles (73.3%) and in 13 of 27 contrasts (48.1%), for a total of 39 of 239 foci (16.0%). Furthermore, the ALE meta‐analysis revealed that 5 of 11 total areas consistently activated during auditory processing were located in the cerebellum, and that the cluster with the largest volume after the auditory cortex itself was located in the left lateral crus I of the cerebellum (Table II). Moreover, its maximum showed the fourth highest ALE value after the three temporal peaks. The ALE meta‐analysis indicated a good level of concordance for the location of cerebellar foci across studies, even though these studies differed greatly in tasks, contrasts, and stimuli, ranging from passive listening to pure tones [Lockwood et al.,1999; Sevostianov et al.,2002] or clicks [Ackermann et al.,2001; Ortuño et al.,2002; Pastor et al.,2002] to various types of active auditory discrimination [Belin et al.,1998,2002; Poeppel et al.,2004] and masking tasks [van Dijk and Backes,2003]. Because imaging studies explicitly manipulating cognitive, emotion, or motor confounds were specifically excluded from this study, cerebellar activation also did not depend on variations in these conditions. Although the relatively few neuroimaging studies intended specifically to examine cerebellar auditory responses have designed their tasks to focus on one or another hypothesized specific function [e.g., timing: Ackermann et al.,2001; Pastor et al.,2002; speech processing: Mathiak et al.,2002;2004], our data suggest that the revealed cerebellar activations are more likely related to the general processing of auditory stimuli. This is the kind of result that can be revealed most clearly by a meta‐analysis of multiple published studies.
Anatomical and Physiological Support for the Cerebellar Imaging Results
Although imaging data clearly can be used to infer function, it is important that functional interpretations also have a solid basis in the anatomy and physiology of the neural circuits under study. Physiologically, it was shown 60 years ago in cats that auditory stimuli alone induce strong short latency responses in the cerebellum [Snider and Stowell,1944]. Subsequent physiological studies have confirmed the same result across different animal species [Aitikin and Boyd,1975; Huang and Liu,1990; Sun et al.,1983; Wolfe and Kos,1975; Xi et al.,1994]. It has also been demonstrated anatomically that the cochlear nucleus, the first relay nucleus along the auditory pathway, sends efferents directly to the cerebellum [Huang et al.,1982] and together with the superior olive directly receives retrograde cerebellifugal projections [Gacek,1973; Rossi et al.,1967]. Although the functional significance of this direct cerebellar projection close to the auditory periphery has been little considered, it has been shown that the cerebellum can exert an inhibitory effect on the auditory nerve action potentials and on cochlear microphonics [Velluti and Crispino,1979]. Furthermore, central structures such as the inferior colliculus, the medial geniculate body, and the auditory cortex have also been shown to communicate indirectly with the cerebellum via the dorsolateral pontine nuclei [Aitkin and Boyd,1978; Huffman and Henson,1990], providing abundant support for a cerebellar role in primary auditory processing both centrally and peripherally.
The Cerebellum and Attention
As described, our meta‐analysis suggests that the cerebellum is just as likely to be activated by general auditory tasks as is the auditory cortex, and therefore may very well provide as generalized a function. Accordingly, we suggest that cerebellar imaging tasks built around hypotheses for cerebellar involvement in very specific sensory or cognitive tasks (for example, perceptual timing [Ivry and Keele,1989]) should control for such a generalized function. Of the many new cognitive theories for cerebellar function, perhaps the most difficult to control in this way are those proposing a cerebellar role in attention [Akshoomoff and Courchesne,1992; Allen et al.,1997; Gottwald et al.,2003; Le et al.,1998].
The presence in our ALE map of activation clusters in the right frontal and parietal lobe of the cerebral cortex provided a means to test the specific association of the cerebellar activation regions with the control of attentional mechanisms. Specifically, these right hemispheric cortical regions have been associated previously with a network proposed to be involved in the control of attentional mechanisms [Pardo et al.,1991; Paus et al.,1997; Sturm et al.,2004; Zatorre et al.,1999]. Consistent with this suggestion, when we removed the five studies from our pool that were based on active listening discriminative tasks [i.e., Belin et al.,1998,2002; Poeppel et al.,2004; van Dijk and Backes,2003; Vouloumanos et al.,2001], these regions no longer appeared in our ALE map (Fig. 3A). As shown in Figure 3B, removing these studies modified the shape only slightly and slightly reduced the peak ALE value for the large lateral cluster in crus I and had a similar effect on activations in auditory cortex. This result suggests, once again, that at least this region of the cerebellum is associated with some general auditory function rather than any particular cognitive activity such as attention. Although the parameter of interest of the maps shown in this study is probability of occurrence across studies rather than magnitude, the increased local likelihood of activation with discriminative tasks is reminiscent of the results of our original somatosensory imaging studies in which activation of the lateral cerebellar nuclei was greatest when the sensory information was being used for discrimination [Gao et al.,1996]. We interpret both results to support the hypothesis that the involvement of the cerebellum in sensory processing may very well be ramped up or down depending on the demand for sensory data [Bower,2002]; however, this conclusion must be qualified by the relatively small number of studies available for analysis. The results of this meta‐analysis suggest that levels of cerebellar activation might very well scale with increased sensory demand, a prediction which could be tested using imaging techniques.
CONCLUSIONS
In conclusion, this meta‐analysis supports a general role for the cerebellum in auditory sensory processing, consistent with our larger hypothesis for its involvement in the control of sensory data acquisition in general [Bower,1997,2002; Bower and Parsons,2003]. Although the data presented here do not prove the case, the analysis at least demonstrates, once again, that consistent cerebellar responses can be obtained in the absence of motor behavior and in the presence of pure sensory stimulation. Further, we would predict that human neuroimaging studies should consistently find cerebellar activation patterns, especially in the lateral regions of crus I, regardless of the nature of the auditory task involved. Our theory predicts that the amplitude of those responses should scale with the degree of complexity of the auditory task, and therefore the demand for high‐quality sensory data. Unfortunately, the literature on auditory activation patterns in the cerebellum remains too sparse to test this prediction using meta‐analytical techniques. Finally, we would predict that disruption or removal of this region of the cerebellum is likely to interfere with fundamental aspects of auditory processing (e.g., pitch perception or sound localization). Such a general role for the cerebellum in primary audition is supported phylogenetically by the close evolutionary association of the cerebellum with the lateral line and the auditory portion of the hindbrain [Devor,2000; Paulin,1993]. Based on the evolutionary history of the cerebellum and the data shown here, it seems reasonable to suggest that further analysis of the cerebellar role in audition, even in humans, will provide an important new perspective on the general function of this large and important brain structure.
Acknowledgements
This work was supported by a grant from the Computational and Integrative Biology program at the National Science Foundation. We thank L. Parsons for continuing conversations on the role of the cerebellum in sensory processing in general; and M. Gonzales, N. Ortiz, and B. Rowe for their ongoing excellent administrative support for our projects.
REFERENCES
- Ackermann H, Hertrich I (2000): The contribution of the cerebellum to speech processing. J Neurolinguistics 13: 95–116. [Google Scholar]
- Ackermann H, Riecker A, Mathiak K, Erb M, Grodd W, Wildgruber Dirk (2001): Rate‐dependent activation of a prefrontal‐insular‐cerebellar network during passive listening to trains of click stimuli: an fMRI study. Neuroreport 12: 4087–4092. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Boyd J (1975): Responses of single units in cerebellar vermis of the cat to monaural and binaural stimuli. J Neurophysiol 38: 418–429. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Boyd J (1978): Acoustic input to the lateral pontine nuclei. Hear Res 1: 67–77. [DOI] [PubMed] [Google Scholar]
- Akshoomoff NA, Courchesne E (1992): A new role for the cerebellum in cognitive operations. Behav Neurosci 106: 731–738. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E (1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Belin P, McAdams S, Smith B, Savel S, Thivard L, Samson S, Samson Y (1998). The functional anatomy of sound intensity discrimination. J Neurosci 18: 6388–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, McAdams S, Thivard L, Smith B, Savel S, Zilbovicius M, Samson S, Samson Y (2002). The neuroanatomical substrate of sound duration discrimination. Neuropsychologia 40: 1956–1964. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD (1998): Central cancellation of self‐produced tickle sensation. Nat Neurosci 1: 635–640. [DOI] [PubMed] [Google Scholar]
- Bower JM (1997): Control of sensory data acquisition In: Schmahmann JD, editor. The cerebellum and cognition. San Diego: Academic Press; p 489–513. [Google Scholar]
- Bower JM (2002): The organization of cerebellar cortical circuitry revisited: implications for function. Ann N Y Acad Sci 978: 135–155. [DOI] [PubMed] [Google Scholar]
- Bower JM, Woolston DC (1983): Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol 49: 745–766. [DOI] [PubMed] [Google Scholar]
- Bower JM, Kassell J (1990): Variability in tactile projection patterns to cerebellar folia crus IIA of the Norway rat. J Comp Neurol 302: 768–778. [DOI] [PubMed] [Google Scholar]
- Bower JM, Parsons LM (2003): Rethinking the lesser brain. Sci Am 289: 50–57. [DOI] [PubMed] [Google Scholar]
- Bracke‐Tolmitt R, Linden A, Canavan AGM, Rockstroh B, Scholz E, Wessel K, Diener HC (1989): The cerebellum contributes to mental skills. Behav Neurosci 103: 442–446. [Google Scholar]
- Brett M (1999): The MNI brain and the Talairach atlas, Cambridge Imagers. Online at http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html.
- Claeys KG, Orban GA, Dupont P, Sunaert S, Van Hecke P, De Schutter E (2003): Involvement of multiple functionally distinct cerebellar regions in visual discrimination: a human functional imaging study. Neuroimage 20: 840–854. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance imaging neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Desmond JE (2001): Cerebellar involvement in cognitive function: evidence from neuroimaging. Int Rev Psychiatry 13: 283–294. [Google Scholar]
- Desmond JE, Fiez JA (1998): Neuroimaging studies of the cerebellum: language, learning, and memory. Trends Cog Sci 2: 355–362. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner BD, Ginier BL, Glover GH (1997): Lobular patterns of cerebellar activation in verbal working‐memory and finger‐tapping tasks as revealed by functional MRI. J Neurosci 17: 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A (2000): Is the cerebellum like cerebellar‐like structures? Brain Res Brain Res Rev 34: 149–156. [DOI] [PubMed] [Google Scholar]
- Dittmann‐Balcar A, Jüptner M, Jentzen W, Schall U (2001): Dorsolateral prefrontal cortex activation during automatic auditory duration‐mismatch processing in humans: a positron emission tomography study. Neurosci Lett 308: 119–122. [DOI] [PubMed] [Google Scholar]
- Ferdon S, Murphy C (2003): The cerebellum and olfaction in the aging brain: a functional magnetic resonance imaging study. Neuroimage 20: 12–21. [DOI] [PubMed] [Google Scholar]
- Fiez JA (1996): Cerebellar contributions to cognition. Neuron 16: 13–15. [DOI] [PubMed] [Google Scholar]
- Gacek RR (1973): A cerebellocochlear nucleus pathway in the cat. Exp Neurol 41: 101–111. [DOI] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT (1996): Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 272: 545–547. [DOI] [PubMed] [Google Scholar]
- Gottwald B, Mihajlovic Z, Wilde B, Mehdorn HM (2003): Does the cerebellum contribute to specific aspects of attention? 41: 1452–1460. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Green GGR (1999): Cortical activation during perception of a rotating wide‐field acoustic stimulus. Neuroimage 10: 84–90. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Bench CJ, Frackowiak RSJ (1994): Human cortical areas selectively activated by apparent sound movement. Curr Biol 4: 892–895. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Rees G, Rees A, Green GGR, Witton C, Rowe D, Büchel C, Turner R, Frackowiak RSJ (1998): Right parietal cortex is involved in the perception of sound movement. Nat Neurosci 1: 74–79. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Green GGR, Rees A, Rees G (2000): Human brain areas involved in the analysis of auditory movement. Hum Brain Mapp 9: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundappa‐Sulur G, De Schutter E, Bower JM (1999): Ascending granule cell axon: an important component of cerebellar cortical circuitry. J Comp Neurol 408: 580–596. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Bower JM (2001): Tactile responses in the granule cell layer of cerebellar folium Crus IIa of freely behaving rats. J Neurosci 21: 3549–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen C, Mohr C, Erdmann C, Binkofski F (2004): Cerebellar neural responses related to actively and passively applied noxious thermal stimulation in human subjects: a parametric fMRI study. Neurosci Lett 361: 237–240. [DOI] [PubMed] [Google Scholar]
- Houk JC (1997): On the role of the cerebellum and basal ganglia in cognitive signal processing. Prog Brain Res 114: 543–552. [DOI] [PubMed] [Google Scholar]
- Huang CM, Liu G (1990): Organization of the auditory area in the posterior cerebellar vermis of the cat. Exp Brain Res 81: 377–383. [DOI] [PubMed] [Google Scholar]
- Huang CM, Liu G, Huang R (1982): Projections from the cochlear nucleus to the cerebellum. Brain Res 244: 1–8. [DOI] [PubMed] [Google Scholar]
- Huffmann RF, Henson OW (1990): The descending auditory pathway and the acousticomotor systems: connections with the inferior colliculus. Brain Res Brain Res Rev 15: 295–323. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW (1989): Timing functions of the cerebellum. J Cogn Neurosci 1: 136–152. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Diener HC (1991): Impaired velocity perception in patients with lesions of the cerebellum. J Cogn Neurosci 3: 355–366. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Rijntjes M, Weiller C, Faiss JH, Timmann D, Mueller SP, Diener HC (1995): Localization of a cerebellar timing process using PET. Neurology 45: 1540–1545. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Ottinger S, Fellows SJ, Adamschewski J, Flerich L, Muller SP, Diener HC, Thilmann AF, Weiller C (1997): The relevance of sensory input for the cerebellar control of movements. Neuroimage 5: 41–48. [DOI] [PubMed] [Google Scholar]
- Justus T (2004): The cerebellum and English grammatical morphology: evidence for production, comprehension, and grammaticality judgments. J Cogn Neurosci 16: 1115–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus T, Ivry RB (2001): The cognitive neuropsychology of the cerebellum. Int Rev Psychiatry 13: 276–282. [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox PT (2002). An optimized individual target brain in the Talairach coordinate system. Neuroimage 17: 922–927. [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005a): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT (2005b): A comparison of label‐based meta‐analysis and activation likelihood estimation in the Stroop task. Hum Brain Mapp 25: 6–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter J, Herscovitch P, Formby C, Raichle ME (1985): Tonotopic organization in human auditory cortex revealed by PET. Hear Res 20: 199–205. [DOI] [PubMed] [Google Scholar]
- Le TH, Pardo JV, Hu X (1998): 4 T‐fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contributions. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin‐Golomb A, Schmahmann JD (2000): Neuropsychological consequences of cerebellar tumor resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 123: 1041–1050. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pu Y, Gao JH, Parsons LM, Xiong J, Liotti M, Bower JM, Fox PT (2000): The human red nucleus and lateral cerebellum in cooperative roles supporting sensory discrimination. Hum Brain Mapp 10: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Coad ML, Arnold SA, Wack DS, Murphy BW, Burkard RF (1999): The functional anatomy of the normal human auditory system: responses to 0.5 and 4.0 kHz tones at varied intensities. Cereb Cortex 9: 65–76. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N (1998): Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Brain Res Cogn Brain Res 7: 15–39. [DOI] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP (2001): The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang 79: 580–600. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H (2002): Cerebellum and speech perception: a functional magnetic resonance imaging study. J Cogn Neurosci 14: 902–912. [DOI] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H (2004): Discrimination of temporal information at the cerebellum: functional magnetic resonance imaging of nonverbal auditory memory. Neuroimage 21: 154–162. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Alway D, Grafman J (1996): Perceptual timing in cerebellar degeneration. Neuropsychologia 34: 863–871. [DOI] [PubMed] [Google Scholar]
- Ortuño F, Ojeda N, Arbizu J., López P, Martí‐Clement JM, Peñuelas I, Cervera S (2002): Sustained attention in a counting task: normal performance and functional neuroanatomy. Neuroimage 17: 411–420. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Parsons LM (2001): Exploring the functional neuroanatomy of music performance, perception, and comprehension. Ann N Y Acad Sci 930: 211–231. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Fox PT, Downs JH, Glass T, Hirsch TB, Martin CC, Jerabek PA, Lancaster JL (1995). Use of implicit motor imagery for visual shape discrimination as revealed by PET. Nature 375: 54–58. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Denton D, Egan G, McKinley M, Shade R, Lancaster J, Fox PT (2000a): Neuroimaging evidence implicating cerebellum in support of sensory/cognitive processes associated with thirst. Proc Natl Acad Sci USA 97: 2332–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LM, Schmahmann JD, Grill SE, Bower JM (2000b): Neurological evidence implicating the cerebellum in fine auditory discriminations. Proc Soc Neurosci 26: 2005. [Google Scholar]
- Pastor MA, Artieda J, Arbizu J, Marti‐Clement JM, Peñuelas I, Masdeu JC (2002): Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci 22: 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor MA, Day BL, Macaluso E, Friston KJ, Frackowiak RS (2004): The functional neuroanatomy of temporal discrimination. J Neurosci 24: 2585–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin MG (1993): The role of the cerebellum in motor control and perception. Brain Behav Evol 41: 39–50. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC (1997): Time‐related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. J Cogn Neurosci 9: 392–408. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1989): Positron emission tomographic studies of the processing of single words. J Cogn Neurosci 1989: 153–170. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR (2004): Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia 42: 183–200. [DOI] [PubMed] [Google Scholar]
- Pu Y, Liu YJ, Gao JH, Parsons LM, Xiong J, Liotti M, Bower JM, Qin YL, Fox PT (1998): Implication of human inferior olive in sensory discrimination: an fMRI study. Proc Soc Neurosci 24: 1150. [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR (1997): Distributed neural systems underlying the timing of movements. J Neurosci 17: 5528–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, van Reekum R, Mayberg H (2000): The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 12: 193–198. [DOI] [PubMed] [Google Scholar]
- Reyes SA, Salvi RJ, Burkard RF, Coad ML, Wack DS, Galantowicz PJ, Lockwood AH (2004): PET imaging of the 40 Hz auditory steady state response. Hear Res 194: 73–80. [DOI] [PubMed] [Google Scholar]
- Rossi G, Cortesina G, Robecchi MG (1967): Cerebellifugal fibres to the cochlear nuclei and superior olivary complex. Acta Otolaryngol 63: 166–171. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD (2001): Nociceptive visceral stimulation modulates the activity of cerebellar Purkinje cells. Exp Brain Res 140: 122–126. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD (2002): Cerebellar stimulation modulates the intensity of a visceral nociceptive reflex in the rat. Exp Brain Res 146: 117–121. [DOI] [PubMed] [Google Scholar]
- Saab CY, Willis WD (2003): The cerebellum: organization, functions and its role in nociception. Brain Res Brain Res Rev 42: 85–95. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, editor. 1997. The cerebellum and cognition. San Diego: Academic Press; 665 p. [Google Scholar]
- Schmahmann JD (2000): The role of the cerebellum in affect and psychosis. J Neurolinguist 13: 189–214. [Google Scholar]
- Schmahmann JD (2004): Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16: 367–378. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998): The cerebellar cognitive affective syndrome. Brain 121: 561–579. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC (2000): MRI atlas of the human cerebellum. San Diego: Academic Press; 167 p. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE, Bohm C, Greitz T, Stoneelander S (1991): Somatosensory discrimination of shape: tactile exploration and cerebral activation. Eur J Neurosci 3: 481–492. [DOI] [PubMed] [Google Scholar]
- Sevostianov A, Fromm S, Nechaev V, Horwitz B, Braun A (2002): Effect of attention on central auditory processing: an fMRI study. Int J Neurosci 112: 587–606. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Leggio MG, Molinari M (1994): The cerebellum contributes to linguistic production: a case of agrammatic speech following a right cerebellar lesion. Neurology 44: 2047–2050. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Misciagna S (2000): Language, memory, and the cerebellum. J Neurolinguist 13: 129–143. [Google Scholar]
- Snider RS, Stowell A (1944): Receiving areas of the tactile, auditory and visual systems in the cerebellum. J Neurophysiol 7: 331–357. [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JDE, Sullivan EV (1998): Odorant‐induced and sniff‐induced activation in the cerebellum of the human. J Neurosci 18: 8990–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, Kemna S, Herzog H, Willmes K (2004): Network for auditory intrinsic alertness: a PET study. Neuropsychologia 42: 563–568. [DOI] [PubMed] [Google Scholar]
- Sun X, Jen PH, Kamada T (1983): Mapping of the auditory area in cerebellar vermis and hemispheres of the mustache bat. Brain Res 271: 162–165. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 122 p. [Google Scholar]
- Tervaniemi M, Hugdahl K (2003): Lateralization of auditory‐cortex functions. Brain Res Brain Res Rev 43: 231–246 [DOI] [PubMed] [Google Scholar]
- Thier PT, Haarmeier S, Treue S, Barash S (1999): Absence of a common functional denominator of visual disturbances in cerebellar disease. Brain 122: 2133–2146. [DOI] [PubMed] [Google Scholar]
- Thivard L, Belin P, Zilbovicius M, Poline JB, Samson Y (2000): A cortical region sensitive to auditory spectral motion. Neuroreport 11: 2969–2972. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16: 765–780 [DOI] [PubMed] [Google Scholar]
- van Dijk P, Backes WH (2003): Brain activity during auditory backward and simultaneous masking tasks. Hear Res 181: 8–14. [DOI] [PubMed] [Google Scholar]
- Velluti R, Crispino L (1979): Cerebellar actions on cochlear microphonics and on auditory nerve action potentials. Brain Res Bull 4: 621–624. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Kiehl KA, Werker JF, Liddle PF (2001): Detection of sounds in the auditory stream: event‐related fMRI evidence for differential activation to speech and nonspeech. J Cogn Neurosci 13: 994–1005. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Tempelmann C, Fell J, Tegeler C, Gaschler‐Markefski B, Hinrichs H, Heinz HJ, Scheich H (1999): Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum Brain Mapp 7: 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JW, Kos CM (1975): Cerebellar inhibition of auditory function. Trans Am Acad Ophthalmol Otolaryngol 80: 143–147. [PubMed] [Google Scholar]
- Xi MC, Woody CD, Gruen E (1994): Identification of short latency auditory responsive neurons in the cat dentate nucleus. Neuroreport 5: 1567–1570. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH (2003): Involvement of the cerebellum in semantic discrimination: an fMRI study. Hum Brain Mapp 18: 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P (2001): Spectral and temporal processing in human auditory cortex. Cereb Cortex 11: 946–953. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Mondor TA, Evans AC (1999): Auditory attention to space and frequency activates similar cerebral systems. Neuroimage 10: 544–554. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Bouffard M, Ahad P, Belin P (2002a): Where is “where” in the human auditory cortex? Nat Neurosci 5: 905–909. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P, Penhune V (2002b): Structure and function of auditory cortex: music and speech. Trends Cogn Sci 6: 37–46. [DOI] [PubMed] [Google Scholar]


