Abstract
Previous studies exploring the neural substrates of executive functioning used task‐specific analyses, which might not be the most appropriate approach due to the difficulty of precisely isolating executive functions. Consequently, the aim of this study was to use positron emission tomography (PET) to reexamine by conjunction and interaction paradigms the cerebral areas associated with three executive processes (updating, shifting, and inhibition). Three conjunction analyses allowed us to isolate the cerebral areas common to tasks selected to tap into the same executive process. A global conjunction analysis demonstrated that foci of activation common to all tasks were observed in the right intraparietal sulcus, the left superior parietal gyrus, and at a lower statistical threshold, the left lateral prefrontal cortex. These regions thus seem to play a general role in executive functioning. The right intraparietal sulcus seems to play a role in selective attention to relevant stimuli and in suppression of irrelevant information. The left superior parietal region is involved in amodal switching/integration processes. One hypothesis regarding the functional role of the lateral prefrontal cortex is that monitoring and temporal organization of cognitive processes are necessary to carry out ongoing tasks. Finally, interaction analyses showed that specific prefrontal cerebral areas were associated with each executive process. The results of this neuroimaging study are in agreement with cognitive studies demonstrating that executive functioning is characterized by both unity and diversity of processes. Hum. Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: executive functions, positron emission tomography (PET), parietal, prefrontal
INTRODUCTION
Executive functioning encompasses a series of high‐level processes, the main aim of which is to facilitate adaptation to new or complex situations, when highly practiced cognitive abilities no longer suffice. The neural substrates of executive functioning were assumed originally to be located in the frontal lobes, because patients with lesions in the anterior part of the brain frequently demonstrated impaired performance in a wide range of tasks assessing executive functioning [e.g., Burgess and Shallice, 1996a, b; Owen et al., 1990; Shallice, 1982]. More recently, neuroimaging studies have suggested that executive functioning relies on a distributed cerebral network encompassing frontal and posterior associative cortices [Collette and Van der Linden, 2002]. These data were obtained using various paradigms such as dual‐task coordination [D'Esposito et al., 1995], task switching [Evans et al., 1996; Lauber et al., 1997], working memory updating [Salmon et al., 1996; Van der Linden et al., 1999], online manipulation of items [Collette et al., 1999; Garavan et al., 2000], inhibition of information [Collette et al., 2001; Taylor et al., 1997], and response sequencing, monitoring, and manipulation [Owen et al., 1996].
Although the importance of the prefrontal areas to executive processes is clearly established, no consensus has been reached regarding the fractionation of functions within those regions. Indeed, some authors have suggested that the lateral frontal cortex is organized according to the nature of the material being processed, with dorsolateral frontal regions being principally responsible for spatial information, whereas the ventrolateral frontal regions are related to nonspatial information [Goldman‐Rakic, 1995, 1996]. Other data support the hypothesis that memory processes within the dorsolateral and ventrolateral frontal cortex are organized according to the type of processing required. According to Owen [2000], a general role for the ventrolateral frontal cortex in memory would be to trigger active low‐level encoding and retrieval strategies (such as those involved in the forward span task, requiring a relatively straightforward mapping of stimulus to response). In contrast, the dorsolateral frontal cortex would be activated in memory situations (such as free recall or backward digit span) that require monitoring of the responses produced and information assimilated. More generally, Duncan and Owen [2000] proposed that a specific frontal lobe network including the mid‐dorsolateral, mid‐ventrolateral, and dorsal anterior cingulate cortex is associated consistently with a broad range of tasks requiring, among other processes, response selection, working memory maintenance, and stimulus retrieval, whereas much of the remainder of the frontal cortex, including most of the medial and orbital regions, is largely insensitive to these task demands. In support of this hypothesis, Collette and Van der Linden [2002] showed that some prefrontal areas (BA 9/46, BA 10 and anterior cingulate gyrus) are systematically activated by a wide range of executive tasks, suggesting that they are involved in general executive processes. Other frontal (Brodmann's area [BA] 6, 8, 44, 45, and 47) and parietal regions (BA 7 and 40) are also activated during the performance of executive tasks. Because these regions were involved less systematically in the different executive processes explored in that review, it was hypothesized that they have more specific functions; however, the distinction between prefrontal areas that underlie general and specific executive processes remains elusive at this time and has yet to be confirmed empirically. Finally, in a recent metaanalysis, Wager and Smith [2003] showed that different executive processes (continuous updating, memory for temporal order, manipulation of information in working memory, and selective attention) are associated with specific cerebral areas. For example, manipulation of information (including dual task requirements or mental operations of switching and inhibition) most frequently activates the right inferior prefrontal cortex (BA 10 and 47). The superior frontal cortex (BA 6, 8, and 9) responds most when working memory must be updated continuously and when memory for temporal order must be maintained. Selective attention to features of a stimulus to be stored in working memory activates the medial prefrontal cortex (BA 32) in storage tasks. The posterior parietal cortex (BA 7) is involved in these three executive processes and is also associated with basic control over the focus of attention. These results show that executive functions may be fractionated into different component processes and that these components are associated with specific cerebral areas.
Wager and Smith's [2003] interpretation of the functional role of these areas was based on a metaanalysis and this interpretation must be confirmed using specific experimental designs. Moreover, the studies included in their metaanalysis suffered from some limitations. Indeed, a potential drawback of many executive neuroimaging studies is the use of task‐specific analyses (or subtraction design), where the specificity of active versus baseline differences for the cognitive function under study is often questionable [Friston et al., 1996; Sidtis et al., 1999]. Indeed, the multicompound aspect of executive tasks leads to major difficulties in finding experimental and control tasks that enable the isolation of one specific executive process. For example, some authors have used the Wisconsin Card‐Sorting Test [Milner, 1963] to determine the neural substrates of inhibitory processes [e.g., Konishi et al., 1999], whereas others consider that this task explores shifting abilities [Nagahama et al., 1996]. Moreover, these neuroimaging studies were not designed to determine whether different tasks assessing a single executive process generate increased cerebral activity in similar prefrontal areas. Evidence against this hypothesis comes from the comparison of the studies of Salmon et al. [1996] and Van der Linden et al. [1999], both of which use the running span task to explore the neural substrates of the updating process. The results of these studies demonstrated that updating was associated with bilateral increases in activity in dorsolateral prefrontal and parietal regions when a recognition procedure was used [Salmon et al., 1996]. With a recall procedure, the most significant increase of regional cerebral blood flow (rCBF) specifically occurred in the left frontopolar cortex (BA 10), extending to the left middle frontal cortex [Van der Linden et al., 1999].
The aim of the present study was to explore the unity and diversity of the neural substrate of executive functioning by taking into account the methodological limitations listed above. More specifically, we were interested in determining cerebral areas that are specific to various executive processes and the ones that they have in common. We capitalized on a cognitive study by Miyake et al. [2000] that used latent variable analysis to determine to what extent different executive functions can be considered unitary (in the sense that they are a reflection of the same underlying mechanism or ability) or nonunitary. Miyake et al. [2000] used a set of nine executive tasks to explore the separability of three functions often described as executive functions: updating, shifting, and inhibition. The results indicated that these three functions were clearly separable at a cognitive level, although they did share some features in common. To determine the cerebral areas associated with these three executive processes, the tasks used by Miyake et al. [2000] were adapted to the positron emission tomography (PET) methodology and matched control tasks were developed. Conjunction analyses were used to isolate cerebral areas commonly activated by the different tasks. The neural substrates for updating, shifting, and inhibition were explored separately in three experiments. Moreover, a conjunction analysis was also carried out on all executive tasks to identify the cerebral areas commonly activated by the three executive functions. Interaction analyses were used to highlight the brain regions specifically associated with each executive process. Given the multicompound aspects of executive tasks (i.e., a task requiring mainly an updating of information process probably also involves other executive processes to some extent, such as inhibition or dual‐task coordination), we consider that the simultaneous use of conjunction and interaction statistical designs, instead of task‐specific analyses, should allow for a better characterization of the cerebral areas that are both common and specific to these executive processes.
SUBJECTS AND METHODS
Subjects
Thirty‐seven right‐handed European volunteers (19 males; age range, 18–34 years) gave written informed consent to take part in this study, which was approved by the University of Liège Ethics Committee. None had any past medical history or used any centrally acting medication. Twelve subjects participated in the experiment assessing the updating function, twelve others in the study of the shifting function, and the remaining thirteen in the study of inhibitory processes.
Cognitive Tasks
In the studies exploring the neural substrates of the updating and shifting functions, the experimental design was composed of three conditions, each one consisting of an experimental task and a matched control task. The inhibition function was explored using two conditions (each one of which was also composed of an experimental and a control task). The experimental updating tasks required subjects to process strings of items of unknown length, and then to recall or identify a specific set of the items most recently presented. Matched control tasks only required the temporary storage of items, without any requirement to update the presented information. The memory load of the control and experimental tasks was similar in each condition. The experimental shifting tasks required subjects to alternate between several cognitive processes or between various aspects of the items. The matched control tasks required performing each of the two processes involved in the experimental tasks in isolation. In the study exploring inhibition, the experimental tasks required subjects to suppress the influence of a prepotent but irrelevant cognitive process, whereas the control tasks necessitated similar processes to the inhibitory tasks, except for the prepotent process.
Updating function
In the first condition, subjects had to store and update lists of consonants. In the second condition, words were presented that had to be semantically processed, and in the third condition, subjects had to process sounds with different pitches.
Consonant condition.
The material consisted of the 19 French consonants with monosyllabic names. Consonants were presented one at a time on a visual display and subjects responded aloud. In the control working memory task, randomly ordered sequences of four consonants were displayed and subjects were instructed to rehearse the stimuli silently and to remember them serially to repeat the sequence aloud after presentation of each list. In the updating working memory task [adapted from Morris and Jones, 1990; see also Van der Linden et al., 1994], lists of four, six, eight, or ten consonants were presented. Subjects were not informed of the length of each list before presentation. They were asked to rehearse silently and to remember serially only the last four items. They had to repeat those four items aloud after the presentation of each list.
Word condition.
The material consisted of 36 French mono‐, bi‐, and trisyllabic words. These words belonged to six different semantic categories (fishes, birds, vegetables, fruits, clothes, and tools). Words were presented one at a time on a visual display. In the control working memory task, exemplars of different semantic categories were presented and the participants memorized only the exemplars belonging to a prespecified semantic category (this category was displayed in the top of the screen during the whole trial). They had to freely recall those items aloud after presentation of each list. In the updating working memory task, exemplars belonging to three or four different semantic categories were presented sequentially while the names of the categories remained on the top of the screen. Participants had to retain only the last exemplar presented for each category and then recall them freely at the end of the series.
Sound condition.
Three kinds of sounds were created using Praat 3.8.6.1 software [Boersma and Weenink, 2003]: low‐pitch (880 Hz), middle‐pitch (440 Hz), and high‐pitch (220 Hz) tones. These sounds were presented to subjects using earphones. The task was composed of three trials, each trial corresponding to the random presentation of 21 sounds. In the control working memory task, subjects had to signal, by pressing a response key, each occurrence of a predetermined sequence of three sounds (i.e., 440, 220, and 880 Hz), which corresponded to a memory load of three items. A target sequence was presented before the onset of each trial. The updating working memory task consisted of detecting the fourth occurrence of each kind of tone. This required a continuous updating of the number of times the low‐, medium‐ and high‐pitched tones had been presented.
Shifting function
In the first condition, subjects had to carry out arithmetic operations. In the second and third conditions, subjects had to carry out verbal and visual categorizations, respectively. The experimental design consisted in the presentation of two experimental (“shifting”) tasks and two different control tasks. Each control task required participants to perform only one of the two cognitive processes involved in the corresponding shifting task. Radiation safety rules did not allow us to carry out each control task twice.
Arithmetic operations.
The material consisted of numbers ranging from 10 to 99. Numbers were presented one at a time on a visual display and subjects responded aloud. In the first control task, subjects were instructed to add three to each visually presented number, whereas in the second control task, subjects were asked to subtract three from each number. In the shifting task, subjects had to alternate between adding three to and subtracting three from each presented number. The control tasks thus required the continuous performance of similar arithmetic operations, whereas the experimental tasks required a continuous shifting between two types of cognitive operations (addition vs. subtraction).
Verbal categorization.
The material consisted of the 64 number–letter pairs. Numbers comprised four odd and four even digits and letters corresponded to four consonants and four vowels. These number–letter pairs were presented visually one at a time in one of four quadrants of the computer screen. In the first control task, number–letter pairs were presented in the two upper quadrants of the screen. The participants had to process only the number and to decide whether it was odd or even. In the second control task, number–letter pairs were presented in the lower part of the screen and the subjects' task was to make a consonant/vowel decision on the letter. In the shifting task, number–letter pairs were presented in all four quadrants in a clockwise rotation. Subjects had to process the number (odd/even) when the pair was presented at the top of the screen and the letter (consonant/vowel) when the pair was presented at the bottom. The trials within the control tasks thus required no shifting, whereas half of the trials in the experimental tasks required shifting between the two types of categorization operations.
Visual categorization.
The material consisted of geometric figures often called Navon figures [Navon, 1977] in which the lines of a large “global” figure (e.g., a triangle) are composed of much smaller “local” figures (e.g., squares). Figures were constructed to induce a global precedence effect; namely, the local elements were positioned very close together and care was taken to ensure that the global identity constituted a good exemplar of the target figure [Wilkinson et al., 2001]. Figures were presented one at a time on a visual display. In the first control task, exemplars with plain lines were presented and the participants had to say aloud the number of lines in the global figure (i.e., one for a circle, two for a cross, three for a triangle, and four for a square). In the second control task, figures with dotted lines were presented and subjects had to say aloud the number of lines in the local figures. In the shifting tasks, Navon figures with plain or dotted lines were presented randomly. Depending on the lines in which the figure was printed (plain or dotted), participants were instructed to say out loud the number of lines in the global, overall figure (plain) or the local, smaller figures (dotted line). When the lines of the stimuli changed across successive trials, the participants thus had to shift from examining the local features to the global features or vice versa.
Inhibition function
To explore inhibitory processes, the Stroop task [Stroop, 1935] and the antisaccade task [Roberts et al., 1994] were administered. A stop‐signal task [Logan, 1994] was also included in the initial study of Miyake et al. [2000]; however, this task was not associated clearly with their inhibitory factor and consequently was not used in our study.
Stroop condition.
The material consisted of 12 words printed in six different inks. Half of the words represented the name of a color and the other half represented concrete items. Both color and concrete words were written in colored ink. Words were presented one at a time on a visual display. In the control tasks, randomly ordered sequences of concrete words were displayed and subjects were instructed to read the items as quickly as possible. In the Stroop task, words displayed represented a color name and were written in a randomly selected mismatching color (e.g., “red” written in green). Participants were instructed to verbally name the color of each stimulus as fast as possible.
Antisaccade condition.
During each trial in this condition, a fixation point was first presented in the middle of the computer screen for a variable amount of time ranging from 500 to 2,500 ms. A visual cue was then presented at the farthest point left or right of the screen for 225 ms, followed by the presentation of the target stimulus for 150 ms before it was masked by gray cross‐hatching. In the control tasks, the target stimulus was presented on the same side as the visual cue whereas in the inhibition task, it was presented on the opposite side. The visual cue was a black square, and the target stimulus consisted of an arrow inside an open square. The participants' task was to indicate the direction of the arrow (left, up, or right) with a key‐press response. Given that the arrow appeared for only 150 ms before being masked, participants were required to inhibit their reflexive response to the initial cue, because this response would make it difficult to correctly identify the position of the arrow.
Subjects were trained 5 or 6 days before the PET session, and the instructions were rehearsed 3 min before each acquisition. After the scanning session, post‐hoc questioning of the subjects indicated that they had perfectly complied with the task instructions. Each task was carried out twice during the session and was counterbalanced between subjects to control for order effects.
PET Scanning
PET was used because several tasks required a vocal response to be recorded and because the anticipated prefrontal regions of interest might be affected by functional magnetic resonance imaging (fMRI) artifacts. PET data were acquired on a Siemens CTI 951 R 16/31 scanner in 3‐D mode. The subject's head was stabilized by a thermoplastic facemask secured to the head holder (TruScan Imaging, Annapolis, MD), and a venous catheter was inserted in a left antebrachial vein. First, a 20‐min transmission scan was acquired for attenuation correction using three rotating sources of 68Ge. Next, rCBF, taken as a marker of local neuronal activity [Jueptner and Weiller, 1995], was estimated during 12 emission scans. Each scan consisted of two frames: a 30‐s background frame and a 90‐s acquisition frame. The slow intravenous water (H2 15O) infusion began 10 s before the second frame. Six mCi (222 MBq) were injected for each scan, in 5 cc saline, over a period of 20 s. The infusion was totally automated so as not to disturb the subject during the scanning period. Data were reconstructed using a Hanning filter (cutoff frequency: 0.5 cycles/pixel) and corrected for attenuation and background activity. The order of presentation of cognitive tasks was assigned randomly, with the exception that a cognitive task was not administered twice in succession and that no more than two experimental or control tasks were administered in succession.
Data Analysis
PET data were analyzed using statistical parametric mapping (SPM99; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK; online at http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (The Mathworks Inc., Natick, MA). For each subject, all scans were realigned together and then normalized to a standard PET template using the same transformations [Frackowiak et al., 1997]. Finally, PET images were smoothed using a Gaussian kernel of 16‐mm full‐width at half‐maximum (FWHM). Such data transformations allow for voxel‐by‐voxel averaging of data across subjects and for direct cross‐reference to the anatomic features in the standard stereotactic atlas [Talairach and Tournoux, 1988].
The condition and subject (block) effects were estimated according to the general linear model at each voxel, using a random‐effect analysis. In SPM99, the random‐effect analysis is a two‐step procedure applied to accommodate intra‐ and interindividual variability of PET data, and thus accounting explicitly for subject‐by‐condition interaction effects. At the first step, areas of significant change were determined at the within‐subject level using linear contrasts of condition estimates. For each individual, subtraction contrasts were computed separately. The resulting estimates (i.e., individual contrast images) fitted the within‐subject component of variance. At the second step of analysis, the residual between‐subject variance was assessed comparing individual estimates created at the first level. The resulting set of voxel values for each contrast constituted a map of the t statistic (SPM[t]), thresholded at P < 0.001. This uncorrected statistical level was used only to emphasize regional prefrontal activation in a global conjunction analysis. For other analyses, statistical inferences were obtained at the voxel or cluster level, at P < 0.05, corrected for multiple comparisons. Random‐effect analyses were used to determine the presence of statistical effects between control and experimental tasks in each subject. Random‐effect analyses can be carried out if the assumption of variance equivalence (or that the error terms are uncorrelated) is met. The hypothesis is probably valid for our data because similar results were obtained with a fixed‐effect analysis.
To determine the cerebral areas common to the three executive functions, a conjunction analysis was carried out in which the changes in cerebral activity common to the comparison of the executive and control tasks (for all eight conditions) were assessed. We also reported the results of conjunction analyses carried out separately for each of three executive processes. Finally, interaction analyses were carried out for each executive process in opposition to the two others. This was done to determine the cerebral areas specific to the processes of updating, shifting, and inhibition. Regions demonstrating interaction effects were determined as follows. First, cerebral areas with differential changes or rCBF between the executive and control tasks of two conditions were determined, i.e., (updating executive tasks − updating control tasks) − (shifting executive tasks − shifting control tasks). Second, plots of activity in these regions were computed for the different conditions. Cerebral areas were selected as demonstrating an interaction effect between two executive processes when greater activity in the executive tasks, in comparison to the control tasks, was found in one condition but not in the other.
RESULTS
Neuropsychological Performance
Numbers of correct responses and response times for the control and experimental tasks in each condition (mean ± standard deviation [SD]) were analyzed using analysis of variance (ANOVA). The results showed that performance was better on the control tasks than on the updating tasks (consonants: F[1,11] = 47.1, P < 0.0001; words: F[1,11] = 163.8, P < 0.0001; sounds: F[1,11] = 26.9, P < 0.005). The percentages of correct responses in the updating and control tasks, respectively, were 79 ± 18 versus 98.7 ± 3 (consonants), 76.3 ± 11 versus 99.6 ± 2 (words), and 66.3 ± 23 versus 87.4 ± 17 (sounds). With regard to the shifting process, the comparison of the three experimental tasks to their control counterparts demonstrated slower response times in the shifting tasks (arithmetic operations: F[1,11] = 17.50, P < 0.005; verbal categorization: F[1,11] = 17.23, P < 0.0001; visual categorization: F[1,11] = 50.41, P < 0.0001). The response times (ms) in the shifting and control tasks, respectively, were 1,404 ± 203 versus 1,308 ± 199 (arithmetic operations), 641 ± 110 versus 529 ± 67 (verbal categorization), and 1,042 ± 193 versus 711 ± 149 (visual categorization). Finally, in the study exploring inhibitory processes, slower response times were found in the Stroop and antisaccade tasks compared to their respective control tasks (Stroop: F[1,12] = 52.67, P < 0.001; antisaccade: F[1,12] = 52.67, P < 0.001). The response times (ms) in the inhibition and control conditions, respectively, were 739 ± 100 versus 682 ± 100 (Stroop) and 427 ± 75 versus 363 ± 59 (antisaccade). To assess the influence of task difficulty on cerebral activity, the different conditions were also compared in a fixed‐effect analysis with individual performance as a confounding covariate (fixed‐effect analysis). This analysis yielded results similar to those reported in the tables.
Imaging Data
Conjunction analyses between the executive functions of updating, shifting, and inhibition
To determine the common cerebral areas activated by all three executive functions, a conjunction analysis was carried out in which the changes in cerebral activity common to the comparison of the eight executive tasks with the eight matched control tasks were assessed. This analysis showed shared increases in activity during the executive tasks in the left superior parietal gyrus, near the precuneus (BA 7), and in the right intraparietal sulcus (Table I; Figs. 1, 2). Because all previous studies reported that prefrontal activity was associated with executive functioning, the statistical level was thresholded to P < 0.001, uncorrected for multiple comparisons, to examine changes in cerebral activity between executive and control tasks in frontal areas. Increased cerebral activity during the performance of all executive tasks was observed in the left middle frontal gyrus (BA 9 and BA 10/46; coordinates: x = −59, y = 11, z = 27 and x = −44, y = 45, z = 16, respectively; Z score = 4.34 and 3.69, respectively) as well as in the left inferior frontal gyrus (BA 45; coordinates: x = −59, y = 20, z = 16; Z score = 4.12).
Table I.
Conjunction analysis identifying common brain regions activated for the executive functions of updating, shifting, and inhibition
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| L superior parietal cortex (BA 7) | −18 | −69 | 51 | 5.87 |
| −14 | −62 | 51 | 5.16 | |
| R intraparietal sulcus | 38 | −43 | 39 | 5.54 |
Voxel P value < 0.05, corrected for multiple comparisons.
BA, Brodmann area.
Figure 1.
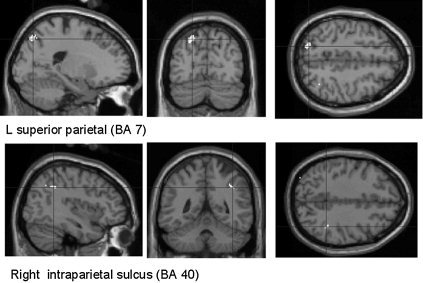
Brain activation observed in the conjunction analysis between the eight executive tasks (in comparison to their respective control tasks). Regions with significant rCBF increase are superimposed upon a T1‐weighted MRI slice normalized into a standard stereotactic space [Talairach and Tournoux, 1988]. Coronal sections are shown, respectively, 69 and 43 mm posterior to the anterior commissure and transverse sections are shown, respectively, 51 and 39 mm above the reference plane. Coordinates of all significant regions are given in Table I.
Figure 2.
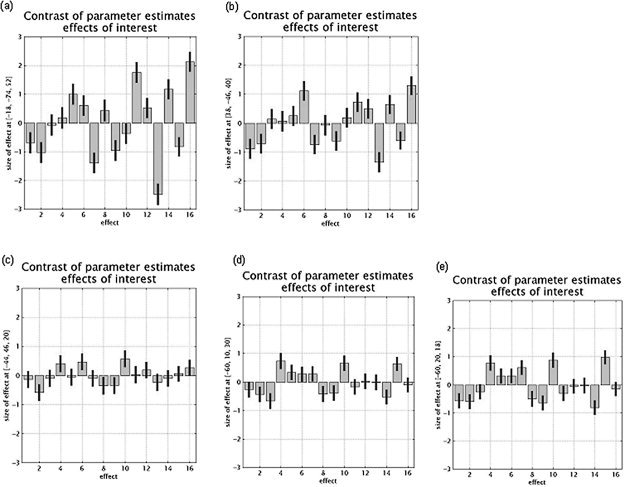
Plots of relative cerebral activity for the regions highlighted in the conjunction analysis of the three executive processes. a: Left superior parietal cortex (BA 7). b: Right intraparietal sulcus. c: Left middle frontal gyrus (BA 46). d: Left middle frontal gyrus (BA 10). e: Left inferior frontal gyrus (BA 45). The coordinates of each voxel are indicated on the y‐axis (Montreal Neurological Institute [MNI] coordinates). Cognitive tasks are represented on the x‐axis (1–3, updating control tasks; 4–6, updating experimental tasks; 7–9, shifting control tasks; 10–12, shifting experimental tasks; 13–14, inhibition control tasks; 15–16, inhibition experimental tasks).
We were also interested in determining the cerebral areas associated with each executive process. A conjunction analysis was therefore carried out in which the changes in cerebral activity common to the comparison of updating tasks to control storage tasks (for consonants, words, and sounds together) were assessed. This analysis showed increased activity in the left frontopolar cortex (BA 10), in the left (BA 9), and right middle frontal gyrus (BA 9/46) and bilaterally in the superior frontal sulcus (BA 6). Foci of cerebral activity were also found in the left inferior frontal (BA 44/45) and right lateral orbitofrontal (BA 11) areas. Increased cerebral activity was also found bilaterally in the intraparietal sulcus and in the right inferior parietal gyrus (BA 40). Finally, foci of increased cerebral activity were found in the medial and right cerebellum (Table II).
Table II.
Conjunction analysis identifying common brain regions activated by the comparison of the three updating (consonants, words, and sounds) and control tasks
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| L frontopolar cortex (BA 10) | −30 | 49 | 1 | 7.80 |
| −32 | 49 | 10 | 6.69 | |
| −34 | 39 | 11 | 5.45 | |
| L middle frontal gyrus (BA 9) | −40 | 22 | 21 | 5.56 |
| −53 | 17 | 27 | 5.94 | |
| R middle frontal gyrus (BA 9/46) | 48 | 42 | 20 | 5.51 |
| 48 | 34 | 22 | 5.60 | |
| L and R superior frontal sulcus (BA 6) | −26 | 7 | 53 | 5.28 |
| 32 | −1 | 50 | 5.26 | |
| L inferior frontal gyrus (BA 44/45) | −61 | 17 | 19 | 5.06 |
| R lateral orbitofrontal cortex (BA 11/10) | 42 | 56 | −16 | 5.25 |
| L and R intraparietal sulcus | −26 | −58 | 47 | 5.82 |
| 48 | −59 | 55 | 5.89 | |
| R inferior parietal gyrus (BA 40) | 55 | −37 | 42 | 6.68 |
| Medial cerebellum | 2 | −71 | −30 | 5.86 |
| 2 | −61 | −21 | 5.98 | |
| 4 | −63 | −12 | 6.02 | |
| R cerebellum | 51 | −50 | −27 | 5.31 |
| 18 | −67 | −19 | 5.36 | |
Voxel P < 0.05, corrected for multiple comparisons.
BA, Brodmann area.
A similar analysis was applied to tasks in the shifting condition, demonstrating the existence of significant foci of activation in the right supramarginal gyrus (BA 40), the left precuneus and the left superior parietal cortex (BA 7) (Table III).
Table III.
Conjunction analysis identifying common brain regions activated by the comparison of the three shifting (arithmetic operations, visual, and verbal categorization) and control tasks
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| R supramarginal gyrus (BA 40)a | 30 | −53 | 38 | 5.44 |
| L precuneusa | −6 | −65 | 55 | 5.25 |
| L superior parietal (BA 7)a | −48 | −54 | 54 | 4.92 |
| L superior parietal cortex (BA 7)b | −14 | −65 | 53 | 4.16 |
| R intraparietal sulcusb | 34 | −47 | 37 | 4.47 |
| L middle frontal gyrus (BA 9, 10/46)b | −59 | 7 | 27 | 2.24 |
| −48 | 42 | 18 | 3.02 | |
| L inferior frontal gyrus (BA 45)b | −60 | 20 | 16 | 2.13 |
Voxel P < 0.05, corrected for multiple comparisons.
Voxel P < 0.05, uncorrected for multiple comparisons.
BA, Brodmann area.
Finally, when the two inhibitory tasks were jointly compared to their respective control tasks, no statistically significant foci of increased cerebral activity were found at a corrected P < 0.05 threshold. However, the right inferior frontal cortex is frequently considered to be associated with inhibitory processes [e.g., Aron et al., 2004]. In fact, an increase in cerebral activity was observed in this region when the correction for multiple comparisons was not applied (Table IV).
Table IV.
Conjunction analysis identifying common brain regions activated by the comparison of the two inhibitory (Stroop and antisaccade) and control tasks
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| L superior parietal cortex (BA 7) | −20 | −73 | 48 | 3.11 |
| R intraparietal sulcus | 38 | −43 | 43 | 2.89 |
| L middle frontal gyrus (BA 9, 10/46) | −59 | 15 | 23 | 2.02 |
| −42 | 50 | 22 | 1.82 | |
| L and R inferior frontal gyrus (BA 45) | −59 | 18 | 10 | 2.71 |
| 61 | 18 | 16 | 3.74 | |
Voxel P < 0.05, uncorrected for multiple comparisons.
BA, Brodmann area.
Surprisingly, the cerebral areas described in the conjunction analysis with the three executive processes were not highlighted in the conjunction analyses for the shifting and inhibition processes. To be certain that these regions were activated by shifting and inhibitory tasks and, consequently, to reject the hypothesis that the results of the conjunction analysis with the three executive processes were mainly driven by updating tasks, the statistical level was thresholded to P < 0.05 uncorrected for multiple comparisons, to examine changes in cerebral activity in these areas related to executive and control tasks. We did in fact observe increases in cerebral activity during the execution of shifting and inhibitory tasks in the left superior parietal gyrus, the right intraparietal sulcus, and the left middle and inferior frontal gyri (see Tables III and IV).
Interaction analyses between the three executive processes
Conjunction analysis highlighted cerebral areas common to the three executive processes and to several tasks assessing each of these processes. Another question of interest concerns the cerebral regions specifically associated with each executive process. Interaction analyses were carried out to identify the cerebral areas that were activated by only one executive function, but not by the other two.
Cerebral areas that showed higher rCBF increases for updating than for shifting were the right superior frontal sulcus (BA 6), the left frontopolar cortex (BA 10), and the right inferior frontal sulcus (BA 10). When compared to inhibitory tasks, the updating function relied upon activation of the left intraparietal sulcus and frontopolar gyrus (Table V; Fig. 3).
Table V.
Interaction analyses: cerebral areas more active in the updating process than in shifting and inhibition processes
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| Updating vs. shifting | ||||
| R superior frontal sulcus (BA 6)a | 30 | 10 | 53 | 4.66 |
| L frontopolar gyrus (BA 10)a | −24 | 62 | −10 | 4.17 |
| R inferior frontal sulcus (BA 10)a | 34 | 51 | 9 | 4.22 |
| Updating vs. inhibition | ||||
| L intraparietal sulcusb | −34 | −58 | 45 | 5.17 |
| L frontopolar gyrus (BA 10)a | −26 | 62 | −3 | 3.91 |
Determined as (updating − control) − (shifting − control) and (updating − control) − (inhibition − control).
Cluster P < 0.05, corrected for multiple comparisons.
Voxel P < 0.05, corrected for multiple comparisons.
BA, Brodmann area.
Figure 3.
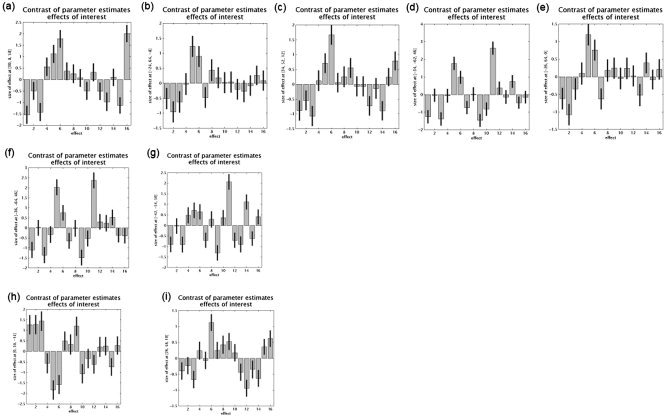
Plots of relative cerebral activity in the 16 cognitive tasks. First line: cerebral areas more active in the updating process compared to that in shifting (a–c) and inhibition (d, e) processes. Second line: cerebral areas more active in the shifting process compared to that in the inhibition (f, g) process. Third line: cerebral areas more active in the inhibition process compared to that in the updating (h) and shifting (i) processes. The coordinates of each voxel are indicated on the y‐axis (MNI coordinates); cognitive tasks are represented on the x‐axis (1–3, updating control tasks; 4–6, updating experimental tasks; 7–9, shifting control tasks; 10–12, shifting experimental tasks; 13–14, inhibition control tasks; 15–16, inhibition experimental tasks).
Cerebral areas specifically associated with the shifting processes were found only in comparison with inhibition. These areas are located in the left intraparietal sulcus (Table VI; Fig. 3).
Table VI.
Interaction analyses: cerebral areas more active in the shifting process than in updating and inhibition processes
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| Shifting vs. inhibition | ||||
| Left intraparietal sulcus | −36 | −60 | 45 | 4.37 |
| −42 | −50 | 52 | 3.53 | |
Determined as (shifting − control) − (updating − control) and (shifting − control) − (inhibition − control). No areas were more active in the shifting process compared to activation in the updating process. Cluster P value < 0.05, corrected for multiple comparisons.
Finally, when inhibition processes were contrasted to updating, they were found to be associated with activation in the right orbitofrontal gyrus (BA 11). The comparison of inhibition to shifting processes demonstrated increased cerebral activity in the right middle/superior frontal gyrus (BA 10) (Table VII; Fig. 3).
Table VII.
Interaction analyses: cerebral areas more active in the inhibition process than in updating and shifting processes
| Brain area | Stereotactic coordinates | Z score | ||
|---|---|---|---|---|
| x | y | z | ||
| Inhibition vs. updating | ||||
| R orbitofrontal gyrus (BA 11)a | 0 | 36 | −14 | 4.79 |
| Inhibition vs. shifting | ||||
| R middle/superior frontal gyrus (BA 10)b | 26 | 47 | 7 | 4.38 |
Determined as (inhibition − control) − (updating − control) and (inhibition − control) − (shifting − control).
Voxel P < 0.05, corrected for multiple comparisons.
Cluster P < 0.05, corrected for multiple comparisons.
Brodmann area.
DISCUSSION
The aim of this study was to examine the unity and diversity of the neural substrate of executive functioning. We capitalized on a study by Miyake et al. [2000] that demonstrated that the processes of updating, shifting and inhibition are clearly separable at a cognitive level. To avoid certain criticisms that could be leveled at previous studies, the cerebral areas associated with each process were evaluated using several tasks. Changes in activity between the executive and control tasks were assessed with a random‐effect model that reported activation observed in each and every subject in our sample. Conjunction analyses were carried out between pairs of experimental and control tasks. This procedure allowed us to determine the common cerebral areas activated by two or three experimental tasks for each executive process and by three executive processes simultaneously. Conversely, interaction analysis demonstrated cerebral activity specifically associated with the processes of updating, shifting and inhibition. Because at least two control and experimental tasks were used to determine the neural substrates of each executive process, the patterns of cerebral activity evidenced here by conjunction and interaction analyses are less dependent on the specific requirements of the tasks administered.
The results of this series of experiments can be summarized as follows. First, the common cerebral areas activated by all three executive functions (updating, shifting, and inhibition) are the posterior regions located in the left superior parietal gyrus and in the right intraparietal sulcus. At a lower statistical threshold, increases in rCBF were also observed in the left middle and inferior frontal gyri. In addition to these common areas, each executive process also relied on specific regions. The updating process thus depends upon a bilateral neural network including both anterior and posterior cerebral areas. Shifting processes are associated with parietal activation and, at a lower statistical threshold, with activity in left middle and inferior frontal gyri. Finally, few prefrontal areas are specifically associated with inhibitory processes when contrasted to updating and shifting processes. Activation common to the two inhibitory tasks is found only with a priori hypotheses. Common activation was found in the parietal areas, in the left middle frontal gyrus and the inferior frontal cortex bilaterally.
These neuroimaging data provide some important information concerning the unity and diversity of executive functioning. The unity is attested to by the existence of cerebral areas commonly involved in the running of several different executive processes. The description of areas activated by one of the processes but not by either of the other two is in agreement with the hypothesis that there is some degree of specificity in executive functioning. These two aspects will be discussed in the next sections.
Diversity of Executive Functioning: Cerebral Areas Specific to Each Executive Process
With regard to the updating process, conjunction analysis demonstrated foci of activity in several frontal areas: frontopolar (BA 10), superior (BA 6), middle (BA 9/46), inferior (BA 44/45), and orbitofrontal (BA 11) cortices, as well as in the intraparietal sulcus and the cerebellum. Previous studies that explored the neural substrate of updating demonstrated the involvement of a large anterior‐posterior cerebral network during the performance of updating tasks. These areas mainly comprised the dorsolateral prefrontal cortex, the frontopolar cortex, the inferior frontal gyrus, the anterior cingulate gyrus, and the superior and posterior parietal cortex [for a review, see Collette and Van der Linden, 2002]. Overall, then, the results of the present study are consistent with those of earlier studies. Moreover, we demonstrated that activity in these areas is independent of the exact requirements of the tasks (based on conjunction analysis), and that the left frontopolar gyrus (BA 10) is associated more specifically with updating than with other executive functions (as evidenced by interaction analyses). Given the great number of cerebral areas commonly associated with updating, we can suppose that various high‐level cognitive processes intervene during the performance of these tasks. More specifically, the frontopolar cortex was found previously to be associated with the evaluation and selection of internally generated information [Christoff and Gabrielli, 2000]. This is a key process for updating, because subjects must continuously compare new information with items already encoded so that they can keep in working memory only a specific set of the last items presented.
Our results suggest that a common activation of the right supramarginal gyrus, left precuneus, left superior parietal cortex and, at a lower statistical threshold, the right intraparietal sulcus and left middle and inferior frontal gyri is associated with the performance of shifting tasks. Moreover, the left intraparietal sulcus is associated specifically with shifting abilities as compared to inhibition processes (but not to updating). Activity in the intraparietal sulcus is found frequently in association with activity in the prefrontal areas in shifting tasks [Dove et al., 2000; Nagahama et al., 2001; Sohn et al., 2000]. Kimberg et al. [2000] suggested previously that the superior parietal region might be involved in the switching process. Sohn et al. [2000] attributed activation of the superior and posterior parietal cortex to endogenous goal‐directed preparation for a subtask, whereas the inferior parietal cortex may be responsible for stimulus‐driven completion during a specific subtask. In a recent metaanalysis, Wager et al. [2004] demonstrated that seven separate regions were reliably activated across a series of studies of attention shifting of various types (e.g., location shifts, rule switching, object switching, and task switching). These regions included both posterior (parietal and occipital) and anterior areas (including the dorsolateral prefrontal cortex and anterior insula). Interestingly, these regions are activated for all shifting tasks, providing evidence for a unitary set of mechanisms underlying shifting. In our study, the same cerebral areas are associated with the realization of shifting tasks, varying according to the exact switching mechanisms they require (e.g., predictable vs. unpredictable shifting; shifting between cognitive operations, response sets, or visual attributes of items). These results are also in accordance with the hypothesis that mechanisms common to various kinds of shifting tasks exist.
Another important question is why cerebral areas common to the three executive processes were not observed at a corrected statistical threshold for the shifting tasks (as for the updating tasks). The design of this study did not allow us to answer this question clearly. However, we can suggest that given the multicompound aspect of executive tasks, the executive components most involved in shifting tasks differ from those common to the three executive functions, and this different degree of involvement leads to a proportionally lower activation of the cerebral areas common to the three executive processes. In addition, in this study and in the Wager et al. [2004] study, activity in the anterior frontal regions was only apparent at a lower statistical threshold. Accordingly, previous neuroimaging studies showed more systematic activation of parietal than prefrontal areas in various shifting tasks [for a review, see Collette and Van der Linden, 2002]. Moreover, neuropsychological studies indicate that frontal patients do not systematically exhibit decreased shifting abilities, whereas a patient with parietal lesions was reported to show impairments on such tasks [Gehring and Knight, 2002]. Taken together, these data suggest that parietal areas play a more basic functional role in shifting processes than prefrontal areas.
Finally, the conjunction analysis of the inhibitory tasks evidenced only common cerebral areas already found in the conjunction of the three executive processes, as well as activity in the right inferior frontal gyrus (BA 45). Interaction analyses demonstrated that the right orbitofrontal gyrus (BA 11) and the right middle/superior frontal gyrus (BA 10) are associated more closely with inhibitory functioning than with updating and shifting processes. The interaction results are congruent with previous studies that used variants of the Stroop paradigm [Bench et al., 1993; Bush et al., 1998; George et al., 1994; Larrue et al., 1994; Pardo et al., 1990; Taylor et al., 1997]. At this time, the exact role of the regions associated with inhibitory processes is not fully understood. The right inferior frontal region highlighted in the conjunction analysis was associated with a series of tasks requiring the inhibition of irrelevant responses, task‐sets, or intrusive memories. Aron et al. [2004] proposed that this region is involved in the suppression of irrelevant responses. It must nevertheless be emphasized that activity in this region is rather weak, although these two inhibitory tasks were associated with a common factor in the Miyake et al. [2000] study. Several explanations can be proposed to explain these results. Firstly, several authors consider that the term “inhibition” has been overextended and that this concept in fact refers to several different processes [Friedman and Miyake, 2004; Nigg, 2000]. The two tasks used in this study thus may differ in their exact inhibitory requirements and consequently in the cerebral areas involved. Secondly, the cerebral areas activated by inhibitory tasks are quite heterogeneous. Recently, for example, Nelson et al. [2003] demonstrated a dissociation in the cerebral areas associated with the performance on different inhibitory tasks: the inferior frontal gyrus is involved when a subject is faced with the need to resolve interference among potentially conflicting attributes of a stimulus, whereas the anterior cingulate cortex is involved when conflicting stimulus‐response associations are presented. Moreover, even though inhibition is associated frequently with the right inferior frontal gyrus [Aron et al., 2004], activity was found in the left inferior frontal gyrus when subjects had to resolve interference in verbal working memory tasks [D'Esposito et al., 1999b; Jonides et al., 1998]. Because our experimental design comprised a verbal and a nonverbal inhibitory task, this could also explain the low level of cerebral activity found in the conjunction analysis. Finally, a few authors have proposed recently that noninhibitory mechanisms are sufficient to perform some tasks considered to be inhibitory [e.g., Mcleod et al., 2003]. In this context, the weak cerebral activity found in the conjunction analysis could be explained by the absence of inhibitory processes responsible for performance on these tasks.
In summary, these conjunction and interaction analyses demonstrated that the executive functions of updating, shifting, and inhibition are associated with specific cerebral areas, which reflect the diverse nature of executive functioning. Several areas are associated with each process, and the notions of updating, shifting, and inhibition most probably include a series of very specific cognitive processes. Further studies developing more specific tasks are thus necessary to disentangle the exact cognitive processes associated with each area.
Unity of Executive Functioning: Cerebral Areas Common to All Three Executive Processes
The common cerebral areas activated by updating, shifting, and inhibition were the left superior parietal cortex and the right intraparietal sulcus. At a lower statistical threshold, cerebral activity common to all executive tasks was also found in the left middle frontal gyrus and in the left inferior frontal gyrus. Based on our experimental design, these areas can be considered to subserve cognitive processes common to a wide range of executive tasks.
Previous studies have suggested that executive functioning is based on a network of anterior and posterior cerebral areas and is not subserved by the frontal lobes alone. Our results are in agreement with the conceptualization of executive functioning in terms of interrelationships between anterior and posterior cerebral areas [Collette and Van der Linden, 2002; D'Esposito and Grossman, 1996; Fuster, 1993; Morris, 1994a, b; Weinberger, 1993]. The results obtained in this study emphasize the critical role of the parietal areas in executive functioning, because the left superior parietal cortex and the right intraparietal sulcus were activated by all three executive processes in every subject. At this time, very few neuroimaging data clearly attribute a role to these areas in connection with executive functioning and there has been no general agreement concerning their individual function [Cohen et al., 1997; Collette et al., 1999; Garavan et al., 2000]. Although the lateral frontal gyrus has been associated frequently with executive functioning, a wide range of functions has been attributed to this area [Collette and Van der Linden, 2002]. Based on a behavioral study using latent variable analyses, Miyake et al. [2000] proposed that cognitive processes common to updating, shifting, and inhibition could relate to either the maintenance of goal and context information concerning the task to be carried out or to inhibitory abilities.
Before discussing the functional role of parietal and prefrontal areas in executive functioning, we must exclude some alternative hypotheses regarding the involvement of posterior areas in these tasks. Parietal activation during working memory or executive tasks is interpreted frequently in terms of storage function [e.g., Honey et al., 2000]. This interpretation is not consistent with our results, because the memory load for each experimental and control task was carefully equated. Moreover, our foci of activity did not correspond to the areas classically associated with storage processes [Becker et al., 1999]. Activity in these areas cannot be attributed to task difficulty, because similar results were obtained when the subjects' performance was introduced as a confounding covariate in the conjunction analysis. An interpretation of our data in terms of conscious visual mental imagery during the performance of executive tasks [Jahanshahi et al., 2000] can also be rejected. Indeed, the focus of activity reported by Jahanshahi et al. [2000] was located in the precuneus and not in the superior parietal cortex, as in the present study. Finally, it could be claimed that the greater involvement of posterior parietal areas in executive functioning is due to a default state of these regions, which systematically have a higher level of cerebral activity than do prefrontal areas. The observation of the effect size in the control tasks (see Fig. 2) for the anterior and posterior cerebral areas identified by the conjunction analysis does not agree with this hypothesis. Indeed, for the control tasks for the consonants and semantic updating, arithmetic shifting, and Stroop inhibition conditions, the effect size in prefrontal areas was greater than it was in posterior cerebral areas, whereas the reverse was observed for control tasks for sound updating, verbal categorization, and antisaccade tasks. The main involvement of parietal areas in executive tasks thus cannot be explained by a generally higher level of cerebral activity in these areas.
Role of the intraparietal sulcus
Miyake et al. [2000] proposed that a key characteristic of all executive tasks is the necessity to inhibit some information. In agreement with this proposition, the anterior intraparietal sulcus could play a role in attention to behaviorally relevant stimuli and suppression of task‐irrelevant information. Coull and Frith [1998] demonstrated that the right intraparietal sulcus was associated with both spatial and nonspatial attentional tasks and suggested that this region was involved in attentional processes (such as selective attention) that act as a lowest common denominator for many types of cognitive processes. Similarly, Wojciulik and Kanwisher [1999] proposed that the anterior intraparietal sulcus could be involved in suppression of unattended information or task‐irrelevant distractors (whatever the modality of presentation). Indeed, although no study has explored formally the role of the intraparietal sulcus in distractor suppression, most imaging studies that report parietal activity in visual attention tasks used displays that contained irrelevant stimuli, and the few studies that did not find intraparietal activity used displays without irrelevant distractors [Corbetta et al., 1995; Le et al., 1998; Rees et al., 1997]. Finally, Corbetta and Shulman [2002] and Imaruoka et al. [2003] proposed that this area is related to intentional selection of stimuli and responses. Taken together, these studies suggest that the intraparietal sulcus plays a general role in selective visual attention. Because most of the tasks used in the present study necessitated auditory linguistic processes, activity in this area could be related to amodal selective attention to behaviorally relevant information and suppression of irrelevant external stimuli.
Role of the superior parietal cortex
The other posterior cerebral area activated by the executive processes of updating, shifting, and inhibition was the left superior parietal cortex (BA 7). Task switching and feature integration would constitute a good explanation for activity in this area, because executive tasks are more complex than are the matched reference tasks, and thus the performance of such tasks often necessitated alternating between or integrating several subcomponents of the tasks. For example, updating tasks required switching between storage and processing while integrating series of stimuli for the response. Shifting tasks obviously necessitated alternating between several cognitive processes or between various aspects of the items while integrating cues and responses. Finally, inhibition tasks also required alternating between well‐learned (relatively automatic) cognitive processes and less usual (more controlled) processes. In agreement with this hypothesis, some studies have demonstrated increased activity in the superior parietal cortex during visual and verbal alternating tasks (such as alternating between the processing of letters and digits or alternately producing words belonging to different semantic categories) [Dove et al., 2000; Gurd et al., 2002] as well as during various attentional tasks that require feature integration [Corbetta et al, 1995; Le et al., 1998; Wojciulik and Kanwisher, 1999]. Switching and integration could be combined in an attentional set to maintain or actively suppress working memory representations during the accomplishment of executive tasks [Corbetta and Shulman, 2002]. Similarly, Wager and Smith [2003] proposed that this region might play a role in implementing a task‐related selection bias established by the anterior and dorsolateral prefrontal areas.
Role of the prefrontal areas
Many functions have been attributed to the middle and inferior lateral prefrontal cortex: manipulation of information [Collette et al., 1999; D'Esposito et al., 1999a; Postle et al., 1999], dual task coordination [D'Esposito et al., 1995], shifting processes [Kübler et al., 2003; Nagahama et al., 2001; Rogers et al., 2000], and inhibition [Chee et al., 2000; Collette et al., 2001]. The systematic activation of these areas by such a wide range of various executive tasks suggests that they are involved in general executive processes. According to Owen [2000], the ventrolateral prefrontal cortex selects and the dorsolateral prefrontal cortex monitors information during executive tasks. Recently, Smith et al. [2002; see also Wager and Smith, 2003] proposed that one function of the dorsolateral prefrontal cortex could be temporal coding of representations to be processed. Indeed, all tasks that require the reordering of a temporal sequence or maintaining memory for temporal order routinely activate this region. In that context, Fuster [2001] and Koechlin et al. [2003] also suggest that the middle prefrontal cortex has a general role in monitoring or temporal organization of cognitive processes necessary to carry out the ongoing task. Consequently, the prefrontal cortex would be involved in the temporal organization necessary to selection and initiation of the function to be carried out in accordance with the task rules and goals, whereas parietal areas would be involved, as discussed previously, in the enactment of attentional sets responsible for the reactivation and suppression of working memory contents necessary to carry out the executive task [Kübler et al., 2003]. This is generally consistent with Miyake et al.'s [2000] proposition that commonalities between their nine executive tasks could be due to the selection and control of goal and context information concerning the current task.
Finally, the question remains of why the parietal areas are more active than prefrontal areas are during the performance of executive tasks. One reason could be that prefrontal areas underlie processes that are more strategic and that are not used in the same way (or to the same extent) by all subjects, unlike parietal areas, which are involved in more “basic” attentional processes. Indeed, there are often several ways of performing an executive task [Rabbit, 1997] and we cannot assert that all subjects used exactly the same strategy in each task (i.e., giving more or less priority to speed of processing or accuracy of response). Moreover, the comparison of studies exploring a similar executive task demonstrated a significant degree of heterogeneity in prefrontal areas, as evidenced with different groups of subjects [for a review, see Collette and Van der Linden, 2002]. If there is a slight variability between subjects in the exact anterior cerebral areas required by the executive tasks, this will thus be reflected by a lower activation threshold at the random effect level, exploring the consistency of activation between subjects.
Limitations on the conjunction activation studies
There remain some limits on the use of tasks associated with cognitive factors identified based on variable latent analyses to explore the neural substrates of executive functioning. Indeed, the use of such a procedure depends on strong theoretical hypotheses about the structure of the factors whose neural substrates are explored. At present, theoretical models of executive functioning are not specified well enough at a cognitive level to allow easily the conceptualization of such working hypotheses. Moreover, the model obtained with this method remains constrained by the choice of tasks and the possibility cannot be ruled out that a change in the task battery would modify the structure of the factors obtained. Finally, it is not certain that there is a strict correspondence between the factors identified at a cognitive level and changes in rCBF. For example, the running of a specific executive process could be expressed by the synchronization of cerebral activation between several cerebral areas and not by an increase in cerebral activity in a specific brain region.
To obtain a better representation of the cerebral areas underlying executive functioning, it is thus important to explore the relationships between the data obtained using various experimental designs. Although conjunction analyses highlight common cerebral areas involved in the accomplishment of several different tasks, it seems essential to construct a more specific experimental design using subtraction and interaction analyses or exploring effective and functional connectivity, to understand better the functional role of these regions [for a detailed discussion of the advantages and limitations of these statistical analyses, see Friston and Price, 2001; Price et al., 1997].
CONCLUSIONS
The aims of this study were to identify cerebral areas that were shared in common or specific to the executive processes of updating, shifting, and inhibition. We capitalized on a cognitive study carried out by Miyake et al. [2000] that demonstrated that these executive processes are clearly separable but share some commonalities, indicating both unity and diversity of executive functioning at a cognitive level. In agreement with their hypothesis, we have shown that each executive process is associated with specific cerebral areas and more importantly, that lateral and superior parietal areas, as well as lateral prefrontal gyri, are commonly activated by all three executive processes. The results obtained in this study are very close to those obtained by Wager and Smith [2003] using a metaanalysis procedure. The data from both studies support the conceptualization of executive functioning in terms of interrelationships between anterior and posterior cerebral areas and stress the critical role of parietal areas in executive functioning.
Acknowledgements
F. Collette and S. Laureys are Research Associates at the National Fund for Scientific Research (FNRS) of Belgium.
REFERENCES
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA (1999): A comment on the functional localization of the phonological storage subsystem of working memory. Brain Cogn 4: 27–38. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, Friston KJ, Paulesu E, Frackowiak RSJ, Dolan RJ (1993): Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia 31: 907–922. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D (2003): Praat‐A system for doing phonetics by computer [Computer Software]. The Netherlands: Institute of Phonetic Sciences, University of Amsterdam. [Google Scholar]
- Burgess PW, Shallice T (1996a): Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia 34: 263–273. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T (1996b): Bizarre responses, rule detection and frontal lobe lesions. Cortex 32: 241–259. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998): The counting Stroop: an interference task specialized for functional neuroimaging. Validation study with functional MRI. Hum Brain Mapp 6: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Sriram N, Siong Soon C, Ming Lee K (2000): Dorsolateral prefrontal cortex and the implicit association of concepts and attributes. Neuroreport 11: 135–140. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrielli JD (2000): The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28: 168–186. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE (1997): Temporal dynamics of brain activation during a working memory task. Nature 386: 604–607. [DOI] [PubMed] [Google Scholar]
- Collette F, Salmon E, Van der Linden M, Chicherio C, Belleville S, Degueldre C, Delfiore G, Franck G (1999): Regional brain activity during tasks devoted to the central executive of working memory. Brain Res Cogn Brain Res 7: 411–417. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Delfiore G, Degueldre C, Luxen A, Salmon E (2001): The functional anatomy of inhibition processes investigated with the Hayling task. Neuroimage 14: 258–267. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M (2002): Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev 26: 105–125. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE (1995): Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 270: 802–805. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Natl Rev Neurosci 31: 201–215. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD (1998): Differential activation of right superior parietal cortex and intraparietal sulcus by spatial and nonspatial attention. Neuroimage 8: 176–187. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA., Alsop CD, Shin RK, Atlas S, Grossman M (1995): The neural basis of the central executive of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Grossman M (1996): The physiological basis of executive function and working memory. Neuroscientist 2: 345–352. [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J (1999a): Maintenance versus manipulation of information held in working memory: an event‐related fMRI study. Brain Cogn 41: 66–86. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE (1999b): The neural substrates and temporal dynamics of interference effects in working memory as revealed by event‐related functional MRI. Proc Natl Acad Sci USA 96: 7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: an event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483. [DOI] [PubMed] [Google Scholar]
- Evans JC, Lauber EJ, Meyer DE, Rubinstein J, Gmeindl L, Junck L, Koeppe RA. (1996): Brain areas in the executive control of task switching as revealed by PET. Soc Neurosci Abstr 22: 7. [Google Scholar]
- Frackowiak R, Friston K, Frith C, Dolan R, Mazziotta JC (1997): Human brain function. London: Academic Press; 528 p. [Google Scholar]
- Friedman NP, Miyake A (2004): The relations among inhibition and interference control functions: a latent‐variable analysis. J Exp Psychol Gen 133: 101–135. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ (2001): Generative models, brain function and neuroimaging. Scand J Psychol 42: 167–177. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ (1996): The trouble with cognitive substraction. Neuroimage 4: 97–104. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1993): Frontal lobes. Curr Op Neurobiol 3: 160–165. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2001): The prefrontal cortex—an update: Time is of the essence. Neuron 30: 319–333. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross T, Li, SJ , Stein EA (2000): A parametric manipulation of central executive functioning. Cereb Cortex 10: 585–892. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT (2002): Lateral prefrontal damage affects processing selection but not attention switching. Brain Res Cogn Brain Res 13: 267–279. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Rosinsky N, Ring H, Casey BJ, Trimble MR, Horwitz B, Herscovitch P, Post RM (1994): Regional brain activity when selecting a response despite interference: an H2O15 PET study of the Stroop and emotional Stroop. Hum Brain Mapp 1: 194–209. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic P (1995): Architecture of the prefrontal cortex and the central executive In: Grafman J, Holyoak KJ, Boller F, editors. Structure and functions of the human prefrontal cortex (Annals of the New York Academy of Sciences, Vol 769). NY: New York Academy of Science; p 71–84. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic P (1996): The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci 351: 1445–1453. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR (2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain 125: 1024–1038. [DOI] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Sharma T (2000): Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage 12: 495–503. [DOI] [PubMed] [Google Scholar]
- Imaruoka T, Yanagida T, Miyauchi S (2003): Attentional set for external information activates the right intraparietal area. Brain Res Cogn Brain Res 16: 199–209. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G, Fuller R, Frith CD (2000): The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage 12: 713–725. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter‐Lorenz PA (1998): Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95: 8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998): Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 2: 148–156. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M (2000): Modulation of task‐related neural activity in task‐switching: an fMRI study. Brain Res Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F (2003): The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–1185. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y (1999): Common inhibitory mechanism in human inferior prefrontal cortex revealed by event‐related functional MRI. Brain 122: 981–991. [DOI] [PubMed] [Google Scholar]
- Kübler A, Murphy K, Kaufman J, Stein EA, Garavan H (2003): Co‐ordination within and between verbal and visuospatial working memory: network modulation and anterior frontal recruitment. Neuroimage 20: 1298–1308. [DOI] [PubMed] [Google Scholar]
- Larrue V, Celsis P, Bès A, Marc‐Vergnes JP (1994): The functional anatomy of attention in humans: cerebral blood flow changes induced by reading, naming and the Stroop effect. J Cereb Blood Flow Metab 14: 958–962. [DOI] [PubMed] [Google Scholar]
- Lauber EJ, Meyer DE, Evans JC, Koeppe RA (1997): Converging evidence from EEG and PET that prefrontal and superior parietal regions are involved in the executive control of task switching. Soc Neurosci Abstr 23: 1120. [Google Scholar]
- Le TH, Pardo JV, Hu X (1998): 4‐T fMRI study of nonspatial shifting of selective attention: cerebellar and parietal contribution. J Neurophysiol 79: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Logan GD (1994): On the ability to inhibit thought and action: a user's guide to the stop‐signal paradigm In: Dagenbach D, Carr TH, editors. Inhibition processes in attention, memory and language. San Diego, CA: Academic Press; p 189–239. [Google Scholar]
- MacLeod CM, Dodd MD, Sheard ED, Wilson DE, Bibi U (2003): In opposition to inhibition. The Psychology of Learning and Motivation 43: 163–214. [Google Scholar]
- Milner B (1963): Effects of different lesions on card sorting. Arch Neurol 9: 90–100. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A (2000): The unity and diversity of executive functions and their contribution to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Morris N, Jones DM (1990): Memory updating in working memory: the role of the central executive. Brit J Psychol 81: 111–121. [Google Scholar]
- Morris RG (1994a): Recent developments in the neuropsychology of dementia. Int Rev Psychiatry 6: 85–107. [Google Scholar]
- Morris RG (1994b): Working memory in Alzheimer‐type dementia. Neuropsychology 8: 544–554. [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J (1996): Cerebral activation during performance of a card sorting test. Brain 119: 1667–1675. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H (2001): Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 11: 85–92. [DOI] [PubMed] [Google Scholar]
- Navon D (1977): Forest before trees: the precedence of global features in visual perception. Cogn Psychol 9: 353–383. [Google Scholar]
- Nelson JK, Reuter‐Lorenz PA, Sylvester CY, Jonides J, Smith EE (2003): Dissociable neural mechanisms underlying response‐based and familiarity‐based conflict in working memory. Proc Natl Acad Sci USA 100: 11171–11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2000): On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull 126: 220–246. [DOI] [PubMed] [Google Scholar]
- Owen AM (2000): The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp Brain Res 133: 33–43. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990): Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia 28: 1021–1034. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M (1996): Evidence for a two‐stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 6: 31–38. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990): The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87: 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M (1999): Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci USA 96: 12959–12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Friston KJ (1997): Subtractions, conjunctions and interactions in experimental design of activation studies. Hum Brain Mapp 5: 264–272. [DOI] [PubMed] [Google Scholar]
- Rabbit P. (1997): Methodologies and models in the study of executive In: Rabbit P, editor. Methodology of frontal and executive function. Hove: Psychology Press; p 1–38. [Google Scholar]
- Rees G, Frackowiak R, Frith C (1997): Two modulatory effects of attention that mediate object categorization in human cortex. Science 275: 835–838. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hager LD, Heron C (1994): Prefrontal cognitive processes: Working memory and inhibition in the anti‐saccade task. J Exp Psychol Gen 123: 374–393. [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW (2000): Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 12: 142–162. [DOI] [PubMed] [Google Scholar]
- Salmon E, Van der Linden M, Collette F, Delfiore G, Maquet P, Degueldre C, Luxen A, Franck G (1996): Regional brain activity during working memory tasks. Brain 119: 1617–1625. [DOI] [PubMed] [Google Scholar]
- Shallice T (1982): Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 298: 199–209. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Anderson JR, Rottenberg DA. (1999): Are brain functions really additive? Neuroimage 6: 490–496. [DOI] [PubMed] [Google Scholar]
- Smith EE, Marshuetz C, Geva A (2002): Working memory: findings from neuroimaging and patient studies In: Grafman J, editor. Handbook of neuropsychology (2nd ed.). Amsterdam: Elsevier; p. 55–72. [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000): The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR (1935): Studies of interference in serial verbal reactions. J Exp Psychol 6: 643–661. [Google Scholar]
- Talairach J, Tournoux P. 1988. Co‐planar stereotaxic atlas of the human brain: 3‐dimensional proportional system: an approach to cerebral imaging. Stuttgart: Thieme; 122 p. [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA (1997): Isolation of specific interference processing in the Stroop task: PET activation studies. Neuroimage 6: 81–92. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Brédart S, Beerten A (1994): Age‐related differences in updating working memory. Brit J Psychol 85: 145–152. [DOI] [PubMed] [Google Scholar]
- Van der Linden M, Collette F, Salmon E, Delfiore G, Degueldre C, Luxen A, Franck G (1999): The neural correlates of updating of information in verbal working memory. Memory 7: 549–560. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: a meta‐analysis. Neuroimage 22: 1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: a meta‐analysis. Cogn Affect Behav Neurosci 3: 255–274. [DOI] [PubMed] [Google Scholar]
- Weinberger DR (1993): A connectionist approach to the prefrontal cortex. J Neuropsychiatr Clin Neurosci 5: 241–253. [DOI] [PubMed] [Google Scholar]
- Wilkinson DT, Halligan PW, Marshall JC, Büchel C, Dolan RJ (2001): Switching between the forest and the trees: brain systems involved in local/global changed‐level judgments. Neuroimage 13: 56–67. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N (1999): The generality of parietal involvement in visual attention. Neuron 23: 747–764. [DOI] [PubMed] [Google Scholar]


