Abstract
Activity and reactivity of the default mode network in the brain was studied using functional magnetic resonance imaging (fMRI) in 28 nondemented individuals with mild cognitive impairment (MCI), 18 patients with mild Alzheimer's disease (AD), and 41 healthy elderly controls (HC). The default mode network was interrogated by means of decreases in brain activity, termed deactivations, during a visual encoding task and during a nonspatial working memory task. Deactivation was found in the default mode network involving the anterior frontal, precuneus, and posterior cingulate cortex. MCI patients showed less deactivation than HC, but more than AD. The most pronounced differences between MCI, HC, and AD occurred in the very early phase of deactivation, reflecting the reactivity and adaptation of the network. The default mode network response in the anterior frontal cortex significantly distinguished MCI from both HC (in the medial frontal) and AD (in the anterior cingulate cortex). The response in the precuneus could only distinguish between patients and HC, not between MCI and AD. These findings may be consistent with the notion that MCI is a transitional state between healthy aging and dementia and with the proposed early changes in MCI in the posterior cingulate cortex and precuneus. These findings suggest that altered activity in the default mode network may act as an early marker for AD pathology. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: mild cognitive impairment, Alzheimer's disease, dementia, fMRI
INTRODUCTION
Functional neuroimaging studies typically assess task‐dependent increases in brain activity by subtracting a reference state from an activated state. There is increasing interest in decreases in brain activity occurring during performance of a task, termed “deactivations” [Gusnard and Raichle, 2001; Gusnard et al., 2001; Greicius et al., 2003; Mazoyer et al., 2001; McKiernan et al., 2003; Shulman et al., 1997; Raichle et al., 2001]. Such deactivations occur over a wide range of tasks in the medial frontal, medial parietal, and posterior cingulate cortex. These regions have a high resting state metabolism and are part of a so‐called “default mode network” [Greicius et al., 2003; Raichle et al., 2001]. It has been suggested that this network is engaged in attending to environmental stimuli [Gusnard et al., 2001; Raichle et al., 2001], reviewing past knowledge, and planning of future behaviors [Binder et al., 1999]. Deactivations are likely to represent reallocation of processing resources to areas involved in active task performance and may be due in part to suspension of spontaneous semantic processes that occur during resting state [McKiernan et al., 2003]. Hence, the amount of deactivation during a task in the default mode network is dependent on the amount of attention the task requires and on the amount of activity present during resting state.
Functional imaging with functional MRI (fMRI), blood oxygen level‐dependent (BOLD), is being used to study the pathophysiology of Alzheimer's disease (AD) [Gron et al., 2002; Grossman et al., 2003; Kato et al., 2001; Rombouts et al., 2000; Small et al., 1999; Sperling et al., 2003; Saykin et al., 1999]. Studies of resting state metabolism using PET and SPECT in AD suggest that abnormalities in posterior cingulate cortex and precuneus can be an early marker in AD [Bradley et al., 2002; de Leon et al., 2001; Matsuda, 2001; Mega et al., 1999]. Given the possible relation between resting state metabolism and functional deactivation in fMRI, deactivations could be considered as an alternative early marker in AD. The default mode network shows decreased activity in AD [Greicius et al., 2004]. Further, in one study involving AD patients, parietal and posterior cingulate cortices showed no deactivation as observed in controls, but rather sustained activation [Lustig et al., 2003]. This suggests that activity in (parts of) the default mode network is afflicted in AD. More challenging is the detection of very early changes before clinical dementia is overt.
Mild cognitive impairment (MCI) is of special interest in this respect, since MCI is thought to represent a functional continuum between healthy aging and the earliest signs of dementia [Petersen et al., 2001]. Roughly half of MCI patients will convert to AD in 3–5 years [Petersen et al., 2001]. A change in activation patterns in MCI patients may therefore be indicative of very early AD. Structural MRI in MCI shows atrophy in the medial temporal lobe [Du et al., 2001; Jack et al., 1999; Visser et al., 1999], cingulate cortex [Chetelat et al., 2002], and also more widespread pathology [Van Der Flier et al., 2002]. Functional imaging in MCI with FDG PET shows decreased resting state metabolism associated with cognitive decline in hippocampal regions and posterior cingulate cortex [Chetelat et al., 2003; De Santi et al., 2001]. Using fMRI the task‐related increase in BOLD signal is reduced in the medial temporal lobe with a memory test, but not in the sensorimotor cortex with a motor test [Machulda et al., 2003]. Others used fMRI to show a positive correlation between extent of (para)hippocampal activation with memory performance in MCI patients [Dickerson et al., 2004]. Paradoxically, the same study showed that greater clinical impairment is associated with recruitment of a larger region of the right parahippocampal gyrus [Dickerson et al., 2004].
As an alternative to these existing neuroimaging data in MCI, we interrogated the default mode network by means of deactivations in MCI during two different active fMRI tasks. One task assessed episodic memory, the other working memory with increasing difficulty levels. The focus of our study was how the magnitude and the temporal profile of signal response in regions of deactivations change in MCI and AD compared to controls. Given other fMRI studies of the default mode network in AD [Greicius et al., 2004; Lustig et al., 2003], we hypothesized that (1) deactivation in this network is decreased in AD, and (2) this diminished deactivation is, to a lesser extent, already present in MCI.
SUBJECTS AND METHODS
Subject Recruitment
Patients were recruited at the Alzheimer Center of the VU University Medical Center, Amsterdam, the Netherlands. MCI patients were diagnosed using criteria for amnestic MCI [Petersen et al., 2001], with Mini‐Mental State Examination (MMSE) scores >25 [Folstein et al., 1975], and clinical dementia rating (CDR) scale scores of 0.5 [Morris, 1993]. AD patients were diagnosed using NINCDS‐ADRDA criteria [McKhann et al., 1984] and were mildly afflicted, with MMSE scores >18 and CDR <2. Twenty‐eight MCI, 18 AD, and 41 healthy controls were included (see Table I for subjects' characteristics). The experiment was approved by the Medical Ethics Committee of the VU University Medical Center Amsterdam. All subjects provided informed consent; if necessary, patients under supervision of a lawful caregiver. Subjects were excluded if they had any significant medical, neurological, or psychiatric illness, or if they were taking medication or other substances known to influence cerebral function. In this study only patients were included whose diagnosis had remained unaltered during a 6‐month follow‐up.
Table I.
Subject characteristics
| Controls | MCI | AD | |
|---|---|---|---|
| N | 41 | 28 | 18 |
| MMSE score (mean ± SD) | 29.0 ± 0.9 | 26.9 ± 1.2 | 22.5 ± 2.2 |
| Age (mean ± SD) | 63.1 ± 5.2 | 74.0 ± 7.5 | 74.1 ± 8.0 |
| Age range (yr) | (50–75) | (54–84) | (55–83) |
| Sex (M/F) | 28/13 | 8/20 | 11/7 |
| Education | 2.1 ± 0.7 | 2.2 ± 0.6 | 1.7 ± 0.6 |
| Left/right handed | 2/39 | 3/25 | 1/17 |
Education level was determined on a discrete scale with 3 levels: low = 1, middle = 2, high = 3.
MMSE, Mini‐Mental State Examination.
MR Acquisition
Imaging was carried out on a 1.5 T Sonata MR scanner (Siemens, Erlangen, Germany) using a standard circularly polarized head coil with foam padding to restrict head motion. For fMRI, an echo planar imaging sequence was used (echo time 60 ms, flip angle 90°, matrix 64 × 64, field of view 192 × 192mm), to obtain 21 transverse slices (5 mm thickness, 1 mm interslice gap). Task stimuli were projected on a screen at the head end of the scanner table via an LCD projector located outside the scanner room and viewed through a mirror on the head coil. In each hand subject's held a response‐box to react by pressing a button using their index‐fingers. A T1‐weighted structural MRI‐scan was also acquired (MPRAGE; inversion time: 300 ms, TR = 15 ms; TE = 7 ms; flip angle = 8°; 160 coronal slices, 1 × 1 × 1.5 mm voxels).
Memory Tasks
Two paradigms were used to examine two different memory types: a parametric n‐letter back task for working memory (WM) [Braver et al., 1997] and a face‐encoding task for episodic memory [Small et al., 1999]. The tasks were practiced extensively: the first practice was 1 day before scanning, the second just before scanning, the third within the scanner bore. During the first 10.5 s of each memory‐task, subjects saw a circle indicating time left before the onset of the first condition.
Working Memory Task
In this task three conditions alternated: in each one, 20 letters were presented sequentially (1 s each followed by a 1‐s delay). Each condition was preceded by a written instruction shown for 10 s. Subjects were asked to press a single button using their right index finger when a target appeared. In the “X” condition, the target was the letter X. In the “simple WM” condition subjects had to respond to two identical letters sequentially. In the third, they needed to respond when two identical letters were separated by another letter (increased WM load). Each condition was presented three times in a pseudo‐randomized fashion and responses were recorded. WM conditions were modeled as blocks of activation (see below). The contrasts of interest were “X > simple WM,” “X > increased WM load,” and “simple WM > increased WM load.” Total task duration was 7 min, 40 s. Task performance scores were calculated by subtracting the ratio of false alarms from the ratio of correct hits.
Face Encoding
Two conditions alternated in a block design: face encoding and fixation. In a 42‐s encoding block, six unfamiliar faces were presented sequentially (6 s each, followed by a 1‐s delay). Subjects were instructed to remember each face and to classify gender by pressing one of two buttons (left: male, right: female; instructions below each face). Male and female faces were balanced across blocks. The task started with a 21‐s presentation of a fixation cross. Then four encoding block alternated with four fixation blocks of 44 s each. Responses were recorded and reaction times and gender accuracy‐scores determined. Faces were modeled as events (see below). The contrast of interest was “fixation > encoding.” Total task duration was 6 min, 12 s. Performance accuracy was assessed immediately after encoding using a recognition task. Subjects saw 24 faces sequentially in random order, of which 12 were shown during encoding and 12 were new (presentation time 5 s, followed by a fixation cross for 3 s). Subjects were instructed to indicate whether the faces were familiar or unfamiliar by pressing one of two buttons (left: familiar, right: unfamiliar; instructions appearing below the face). Recognition scores were rated between −1 (all wrong) and 1 (no errors) by subtracting the number of incorrect responses from correct responses, divided by the total number of responses. Hence, the chance level score was 0.
MR Data Analysis
fMRI analysis was carried out using FEAT (fMRI Expert Analysis Tool) v. 5.1, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). Prestatistical processing consisted of motion correction [Jenkinson et al., 2002], nonbrain removal [Smith, 2002], spatial smoothing using a Gaussian kernel of FWHM (full width at half maximum) 6 mm, mean‐based intensity normalization of all volumes by the same factor, and highpass temporal filtering (Gaussian‐weighted LSF straight line fitting, with sigma = 127.5 s for the WM data and sigma = 60.0 s for the encoding data). Time‐series statistical analysis was carried out with local autocorrelation correction [Woolrich et al., 2001]. A boxcar convolved with a “double” gamma hemodynamic response function (HRF) and its temporal derivative was used to model the data, giving images of “contrasts of parameter estimates” and corresponding variance images for each contrast of interest. This double gamma HRF consists of a mixture of a positive and small negative gamma function that together represent a realistic HRF, including the “undershoot.” The contrasts (see above) were calculated for the double gamma HRF; images of parameter estimates of temporal derivatives were calculated for each condition. Contrast images of the temporal derivative explain variance in the data reflecting either rapid or slow responses not accounted for by the double gamma HRF. fMRI images were registered to the individual's structural scan, which was registered to standard space images [Jenkinson and Smith, 2001; Jenkinson et al., 2002]. These transformations were applied to images of contrasts of interest and variances to put them in standard space.
Higher‐level (group level) analysis was carried out using mixed effects analysis [Woolrich et al., 2004]. The general linear model included the three groups (MCI, AD, and controls) and age and gender as covariates across groups in order to account for these covariates. We tested for group averages and differences between groups for each of the contrasts of interest. Z (Gaussianized T/F) statistic images were thresholded using clusters of pixels with Z > 3.1 and a corrected cluster significance threshold of P = 0.05 [Forman et al., 1995; Friston et al., 1994; Worsley et al., 1992]. For the differences between groups, the analysis was limited to regions of deactivation only, allowing less stringent cluster corrections. The average best‐fitted HRF could be reconstructed by adding the parameter estimates multiplied with their corresponding regressor (double gamma HRF and its temporal derivative).
Analysis of Behavioral Data
For each group separate performance scores were calculated. A univariate analysis of variance (ANOVA) was performed using SPSS 9.0 (Chicago, IL), with performance score as dependent variables, “group” as fixed factor, and “age” and “gender” as covariates. If a significant effect of a covariate was found the model was adjusted to contain the relevant covariate as a fixed factor in a subsequent analysis of the group effect.
RESULTS
Face Encoding
AD accuracy scores of gender discrimination differed significantly from controls (P = 0.031) and MCI patients (P = 0.032) (see Table II for estimated marginal means). AD patients had significantly slower reaction times than controls (P = 0.022). Other pairwise comparisons were not significant. Age did not significantly affect accuracy scores (P = 0.088) or latency scores (P = 0.13). No significant effect was found of gender on either accuracy (P = 0.29) or latency scores (P = 0.22). Accuracy scores during face recognition in each patient group differed significantly from the other two groups (P < 0.001). Reaction times were only different between controls and patients (P < 0.001), not between MCI and AD (P = 0.44).
Table II.
Estimated modified population marginal means of accuracy and latency scores of encoding task and working memory task
| Controls | MCI | AD | |
|---|---|---|---|
| Encoding | |||
| GD ACC (SE) | 0.96 (0.02) | 0.96 (0.02) | 0.87 (0.03) |
| GD RT (s) | 1.13 (0.08) | 1.39 (0.08) | 1.50 (0.10) |
| Recognition | |||
| ACC | 0.71 (0.05) | 0.36 (0.05) | 0.15 (0.06) |
| RT | 1.72 (0.12) | 2.41 (0.11) | 2.23 (0.14) |
| WM | |||
| X ACC | 1.00 (0.03) | 0.93 (0.04) | 0.97 (0.04) |
| X RT | 0.45 (0.02) | 0.52 (0.02) | 0.56 (0.02) |
| 1B ACC | 1.00 (0.03) | 0.94 (0.04) | 0.91 (0.04) |
| 1B RT | 0.50 (0.02) | 0.57 (0.02) | 0.62 (0.02) |
| 2B ACC | 0.88 (0.03) | 0.81 (0.04) | 0.55 (0.04) |
| 2B RT | 0.62 (0.02) | 0.69 (0.02) | 0.73 (0.02) |
GD, gender discrimination; ACC, accuracy; SE, standard error; RT, reaction time; WM, working memory; X, ‘X’ condition; 1B, simple WM load; 2B, increased WM load.
fMRI data
Deactivation during face encoding as compared to fixation occurred in controls in orbital gyrus, gyrus rectus, medial frontal gyrus, anterior cingulate cortex, posterior cingulate cortex, and in the precuneus. MCI patients showed smaller regions of deactivation in medial frontal cortex and precuneus. AD patients only showed one area of deactivation in the medial frontal cortex (Fig. 1).
Figure 1.
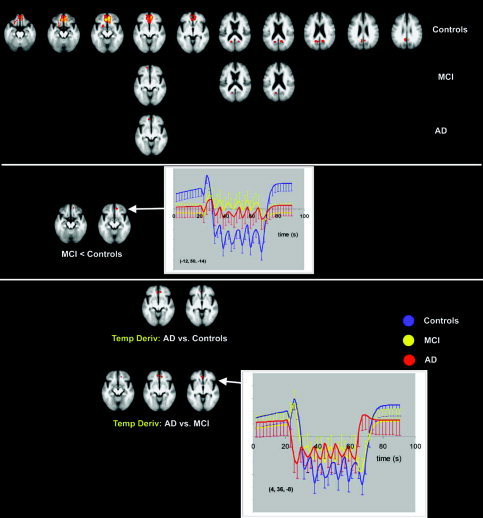
Transverse sections with Z‐statistics of average deactivation in controls, MCI, and AD (top) during the face‐encoding task (P < 0.05, corrected). Z‐scores are color‐coded from 3.1 (red) to 6.2 (yellow). The graphs show the estimated responses with standard deviations. The underlying structural image is the average image of all controls and patients; left in image is left in the brain. MCI show less deactivation in the medial frontal gyrus than controls (middle). AD show a change in the early phase of deactivation (temporal derivative) compared to both MCI and controls (bottom) in the anterior cingulate gyrus.
MCI patients showed significantly less deactivation than controls in the medial frontal gyrus (Fig. 1, Table III). Other pairwise comparisons were not significantly different for the “standard” HRF fit.
Table III.
Talairach coordinates of local maxima showing a significant difference between patients and controls both for the steady state amplitude and temporal derivative
| Task | x, y, z | Z‐score | Region |
|---|---|---|---|
| Encoding task | |||
| Steady state amplitude | |||
| MCI < Controls | −12, 50, −14 | 4.02 | Medial frontal gyrus |
| Temporal derivative | |||
| AD < MCI | 4, 36, −6 | 3.90 | Anterior cingulate gyrus |
| −10, 42, −8 | 3.87 | Anterior cingulate gyrus | |
| AD < Controls | 4, 36, −8 | 3.64 | Anterior cingulate gyrus |
| −10, 42, −8 | 3.95 | Anterior cingulate gyrus | |
| N‐BACK task: temporal derivative | |||
| Simple WM (negative derivative in controls) | |||
| AD > C (AD less negative than controls) | 4, −68, 24 | 3.46 | Precuneus |
| MCI > C | 4, −66, 26 | 3.35 | Precuneus |
| Increased WM load (positive derivative in controls) | |||
| C > AD | −22, 16, −16 | 3.55 | Inferior frontal gyrus |
AD patients showed a significant difference in the temporal pattern of deactivation in the frontal regions. Controls and MCI patients had a significant positive temporal derivative in frontal areas (not in posterior regions of deactivation). This reflected an initial increase in activation, followed by a quick reversal of signal at steady state (Fig. 1). AD patients did not show this initial increase and the temporal derivatives were therefore significantly diminished in AD compared to both MCI and controls in the anterior cingulate gyrus (Table III).
Working Memory
Mean accuracy scores for working memory performance were 0.96 (standard error of the mean (SE) 0.02) in controls, 0.89 (0.03) in MCI, and 0.81 (0.02) in AD (for averages grouped by working memory load, see Table II). AD accuracy scores differed significantly from those of controls (P = 0.0003), but not MCI patients (P = 0.071). Controls and MCI patients did not differ significantly in mean accuracy scores (P = 0.18). Differences in accuracy became especially manifest with increasing WM load (P = 0.0002). Mean reaction times of controls differed significantly from those of MCI (P = 0.025) and AD patients (P = 0.0001). Reaction times of MCI and AD patients were not significantly different (P = 0.32). Age did not significantly affect accuracy scores (P = 0.71) or latency scores (P = 0.21). No significant effect was found of gender on either accuracy (P = 0.27) or latency scores (P = 0.34).
fMRI data
In control subjects, deactivation was found in right insula, lingual gyrus, medial frontal gyrus, anterior cingulate gyrus, posterior cingulate cortex, and precuneus for both simple WM and increased WM load (Fig. 2). During increased WM load, deactivation was also found in the superior temporal gyrus and left insula. Deactivation increased significantly during increased WM load compared to simple WM in the medial frontal gyrus, gyrus rectus, anterior cingulate gyrus, and superior temporal gyrus. This increase in deactivation was not found in posterior regions.
Figure 2.
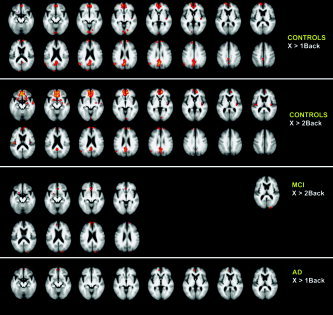
Transverse sections with Z‐statistics of average deactivation in controls, MCI, and AD in the working memory task (P < 0.05, corrected). The underlying structural image is the average image of all controls and patients; left in image is left in the brain. MCI patients only show significant deactivation in the increased WM load condition (2BACK). AD patients only show deactivation in the 1BACK condition. Z‐scores are color‐coded from 3.1 (red) to 8.5 (yellow) for 1BACK, and from 3.1 to 9.9 for 2BACK.
MCI patients did not show a significant deactivation in the simple WM condition, whereas increased WM load deactivated cuneus, anterior cingulate gyrus, and inferior, medial, and superior frontal gyrus. A direct statistical comparison between simple WM and increased WM load in MCI in the same regions showed a significant difference (significantly more deactivation from simple WM to increased WM load). AD patients only showed deactivation in the simple WM load condition in the medial frontal and anterior cingulate gyrus. There was no significant change in deactivation between the two WM conditions in AD.
The apparent differences in deactivation between the groups were not significant for the “standard” HRF. However, the temporal derivatives of the BOLD responses indicated that, in the initial phase of the BOLD signal, differences occurred between patients and controls. Control subjects showed for the simple WM condition a negative temporal derivative in precuneus and posterior cingulate cortex. This showed that these regions had a fast negative response when simple WM started, which was not captured by the default gamma HRF. In the increased WM load condition, controls showed a positive temporal derivative in the anterior cingulate and medial and middle frontal gyrus, meaning that the signal first showed an increase there, and then quickly reversed sign to deactivation. In contrast, these early phases of the BOLD response in the WM task were not significantly different from zero in MCI and AD patients. In the simple WM condition, the precuneus showed significantly less early deactivation in both AD and MCI as compared to controls. During increased WM load, the early increase of inferior frontal activation in controls (followed by a decrease), was significantly less in AD (Fig. 3, Table III). Note that this was only significant in a small region on the edge of the larger deactivated anterior frontal region shown in Figure 2. Other voxels are not shown in the figure since they are below the (corrected) significance threshold.
Figure 3.
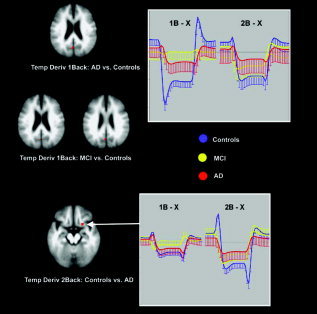
Differences in deactivation during the working‐memory task between patients and controls. The graphs show the estimated responses with standard deviations. Differences in this task are only significant for the very early phase of the deactivation response represented by the parameter estimate of the temporal derivative. 1B = simple working memory; 2B = increased working memory load.
Neither age nor gender was significantly associated with any deactivation in the two tasks. Any subthreshold variance associated with these covariates was removed when comparing the groups due to the multilevel nature of the analysis (see Subjects and Methods).
DISCUSSION
Using two different tasks, we found evidence for deactivation in a common network involving anterior frontal lobe and precuneus and posterior cingulate cortex in healthy elderly. MCI and AD patients showed alterations in this default mode network which was most pronounced in the very early phase of deactivation. In MCI, deactivation was observed in the same network, yet to a lesser extent than in controls. In AD, deactivation was further restricted to anterior frontal regions only. Significant differences occurred in anterior frontal regions between AD and controls, between MCI and controls, and also between AD and MCI. In the precuneus both patient groups were significantly different from controls, but AD deactivation was not significantly different from MCI.
The deactivation is postulated to represent a decrease of activity in the default mode network and depends on the processing demands for the enforced task as well as the degree of network activity during resting state. Healthy elderly controls show deactivation in the gyrus rectus, medial frontal gyrus, anterior cingulate, precuneus, and posterior cingulate cortex in both memory tasks, extended to the superior temporal gyrus, left and right insula, and lingual gyrus in the working memory task only. This is consistent with other studies suggesting that these regions of deactivation are a general phenomenon which may not strongly depend on the type of task a person is involved in (see also Figs. 1 and 2) [Gusnard and Raichle, 2001; Mazoyer et al., 2001; Gusnard et al., 2001; McKiernan et al., 2003; Shulman et al., 1997; Raichle et al., 2001; Greicius et al., 2003]. Most studies of deactivation identify the default mode network using a “rest” or very low level baseline condition [Binder et al., 1999; Shulman et al., 1997; Mazoyer et al., 2001]. The face encoding condition in the current study has such a low‐level baseline condition (fixation on a cross). The working memory task we used has a baseline condition that requires some processing (X). McKiernan et al. [2003] showed that it is not necessary to include a very low‐level baseline to identify the default mode network: with increasing task difficulty, deactivation also increases. Our data also show that either a low‐level baseline as reference or a task requiring some processing (X) both reveal (partly) the same default mode network.
We also found that deactivation in the anterior frontal regions was enhanced by increasing the working memory load, showing that a parametric increase in attentional demand results in a further decrease of activity. Effects of load manipulation on deactivation have not been studied frequently. McKiernan et al. [2003] studied effects of stimulus presentation rate, target discriminability, and short‐term memory load on deactivation in young, healthy controls. Increased short‐term memory load significantly increased deactivation in the anterior cingulate gyrus and superior and middle frontal gyrus. These regions, as well as posterior cingulate gyrus and parietal occipital cortex, showed more deactivation with more difficult target discriminability as well. Comparing our results with that study shows that we find many regions of deactivation located more ventrally to the anterior cingulate region than in the study by McKiernan et al. Increasing working memory load increased deactivation in our study in a region more ventral than the frontal regions found by McKiernan et al. [2003]. One explanation for our more ventrally located regions may have to do with age, which has an effect on deactivation [Lustig et al., 2003]. Clearly, more load manipulation data to study the default mode network are needed to understand load, age, and disease interactions on deactivation.
The altered BOLD signal in the default mode network was not the same in MCI and mild AD as compared to healthy elderly controls. The deactivation in controls was also present in MCI in anterior frontal and posterior cingulate cortex during face encoding and anterior frontal cortex and cuneus during working memory performance. Yet in MCI it was limited to much smaller regions in the default mode network. Deactivation appeared to be further diminished in AD, where it was limited to small anterior frontal regions only, with no deactivation in (pre)cuneus or posterior cingulate cortex. Proper statistical testing showed that the deactivation amplitude was significantly different in the anterior frontal region in MCI compared to controls during face encoding, signifying less deactivation in MCI. Although MCI and AD showed the same behavior in that region, with hardly any signal change, while controls showed very significant deactivation, only the MCI‐controls difference was significant (Fig. 1). Both increased intersubject variance in the AD group and the inclusion of fewer AD patients than MCI patients may have caused this (Fig. 1). All other differences between the three populations were caused by an alteration in the early phase of the BOLD response, represented by the parameter estimate of the temporal derivative in the data analysis, as discussed below.
In anterior frontal regions of deactivation healthy subjects first show a signal increase, and then the signal quickly changes sign to show a decrease. This pattern has been observed in medial parietal and posterior cingulate cortex in other studies of the default mode network also [Lustig et al., 2003]. Hence, our results seem to corroborate the findings in that study, although we found the initial increase located in another region of the default mode network. Based on the currently available data, it is impossible to understand what this initial increase signifies and further studies will be necessary to study the phenomenon more closely. In that early phase the BOLD response behaved differently in patients. The anterior frontal lobe is the only region where there is a difference between MCI and AD due to a difference in early phase deactivation (Fig. 1). The particular sensitivity of the early phase of changes in activity in the default mode network to differences between patients and controls is most probably because the early phase is dependent on the fast reactivity and adaptation of the network. Hence, our data suggest that default mode network activity during resting state is decreased in patients, but the most significant difference with controls is that patients show a slower (and different) adaptation of this network. Therefore, the data analysis of deactivations in dementia should be made sensitive to changes in early stages of the HRF model separately when searching for markers in the BOLD signal of early dementia.
It has been suggested that the default mode network is engaged in preparation for future actions [Binder et al., 1999], which might link the slower reaction times to the altered deactivation in the current study. Further, the default mode network may be engaged in retrieval of past knowledge [Binder et al., 1999; Buckner, 2004]. Evidence for this comes from studies of memory retrieval: although most tasks show a decrease in activity in the default mode network, tasks comparing successful memory retrieval to correctly rejecting new items show an increase in activity in (posterior parts of) the network [Buckner, 2004]. Hence, our data may also link the general observation of memory decline to the altered deactivation in the default mode network in patients.
Lustig et al. [2003] compared the BOLD signal in a priori defined regions of interest between young and elderly controls and AD patients. In that study, AD differed from elderly controls only in the medial parietal/posterior cingulate region. This region was deactivated in young controls, showed no significant signal change in healthy elderly, and showed a significant signal increase in AD. The other two regions that were analyzed, medial frontal cortex (BA 10) and right lateral parietal cortex (BA 40), did not show any difference in AD when compared to healthy elderly. We also found altered deactivation in dementia in posterior regions, although we did not replicate the above‐baseline activation in AD. However, in frontal regions we also find an effect, both between controls and patients and between MCI and AD. Differences in data analysis and patient definitions might (partly) explain these differences. In the study by Lustig et al. [2003] the regions were identified a priori based on typical deactivations in young controls. This may result in different regions of analysis between the two studies: we analyzed regions showing deactivations either in elderly controls or patients. Hence, regions with no deactivation in healthy elderly and positive activation in dementia would not have been analyzed in our study. Additionally, the two studies used different definitions of AD. Lustig et al. [2003] included many patients in the AD group who would meet the MCI definition used in the current study. Given the difference we see in frontal regions between MCI and AD, sensitivity might be decreased when MCI and AD patients are grouped together. Furthermore, we found that almost all differences in the anterior frontal cortex between groups originated from differences in the temporal derivatives, and not in the parameter estimates of the standard HRF.
The pattern we found may be consistent with the notion that MCI is a transitional state between healthy aging and dementia. Already in an early stage of dementia (the MCI stage), altered brain deactivation is detected in the default mode network. This is further affected in AD. A growing number of imaging studies in AD also show that the same regions show pathologic changes. In vivo images of amyloid suggest marked depositions in these areas in AD [Klunk et al., 2004]. Even in the very earliest stages of AD there is atrophy visible in the precuneus and posterior cingulate gyrus, but also in the anterior frontal lobe [Scahill et al., 2002]. Furthermore, the BOLD signal is altered in (some of) these regions in AD as well [Lustig et al., 2003], with evidence for different resting connectivity in AD between orbitofrontal cortex/ventral anterior cingulate cortex, posterior cingulate cortex, and hippocampus [Greicius et al., 2004].
In conclusion, we have found altered deactivation in AD and MCI using two different tasks reflecting decreased resting state activity and adaptation in the default mode network. This alteration was most significant in the very early phase of a task. A change in deactivation could already be observed in MCI, suggesting that reduced deactivation may act as an early marker for AD pathology consistent with decreased resting metabolism.
REFERENCES
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11: 80–95. [DOI] [PubMed] [Google Scholar]
- Bradley KM, O'Sullivan VT, Soper ND, Nagy Z, King EM, Smith AD, Shepstone BJ (2002): Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer's disease. Brain 125: 1772–1781. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC (1997): A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2004): Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron 44: 195–208. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC (2002): Mapping gray matter loss with voxel‐based morphometry in mild cognitive impairment. Neuroreport 13: 1939–1943. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, Sayette V, Viader F, Berkouk K, Landeau B, Lalevee C, Le DF, Dupuy B, Hannequin D, Baron JC, Eustache F (2003): Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126: 1955–1967. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J (2001): Prediction of cognitive decline in normal elderly subjects with 2‐[F‐18]fluoro‐2‐deoxy‐D‐glucose/positron‐emission tomography (FDG/PET). Proc Natl Acad Sci U S A 98: 10966–10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J (2001): Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging 22: 529–539. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA (2004): Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol 56: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW (2001): Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry 71: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans A (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 210–220. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101: 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gron G, Bittner D, Schmitz B, Wunderlich AP, Riepe MW (2002): Subjective memory complaints: objective neural markers in patients with Alzheimer's disease and major depressive disorder. Ann Neurol 51: 491–498. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Detre J, Alsop D, Gee J (2003): Neural basis for semantic memory difficulty in Alzheimer's disease: an fMRI study. Brain 126: 292–311. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E (1999): Prediction of AD with MRI‐based hippocampal volume in mild cognitive impairment. Neurology 52: 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu HY (2001): Dissociation of regional activation in mild AD during visual encoding — a functional MRI study. Neurology 57: 812–816. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004): Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound‐B. Ann Neurol 55: 306–319. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL (2003): Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A 100: 14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, Petersen RC, Boeve BF, Knopman D, Tang‐Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR Jr (2003): Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology 61: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H (2001): Cerebral blood flow and metabolic abnormalities in Alzheimer's disease. Ann Nucl Med 15: 85–92. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Mega MS, Chu T, Mazziotta JC, Trivedi KH, Thompson PM, Shah A, Cole G, Frautschy SA, Toga AW (1999): Mapping biochemistry to metabolism: FDG‐PET and amyloid burden in Alzheimer's disease. Neuroreport 10: 2911–2917. [DOI] [PubMed] [Google Scholar]
- Morris JC (1993): The clinical dementia rating (CDR): current version and scoring rules. Neurology 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985–1992. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Barkhof F, Veltman DJ, Machielsen WCM, Witter MP, Bierlaagh MA, Lazeron RHC, Valk J, Scheltens P (2000): Functional MR imaging in Alzheimer's disease during memory encoding. AJNR Am J Neuroradiol 21: 1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Flashman LA, Frutiger SA, Johnson SC, Mamourian AC, Moritz CH, O'Jile JR, Riordan HJ, Santulli RB, Smith CA, Weaver JB (1999): Neuroanatomic substrates of semantic memory impairment in Alzheimer's disease: patterns of functional MRI activation. J Int Neuropsychol Soc 5: 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC (2002): Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid‐registered serial MRI. Proc Natl Acad Sci U S A 99: 4703–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y (1999): Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol 45: 466–472. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS (2003): fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Flier WM, Van Den Heuvel DM, Weverling‐Rijnsburger AW, Bollen EL, Westendorp RG, Van Buchem MA, Middelkoop HA (2002): Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann Neurol 52: 62–67. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C (1999): Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol 246: 477–485. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM (2004): Multilevel linear modelling for fMRI group analysis using Bayesian inference. Neuroimage 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]


