Abstract
We used functional magnetic resonance imaging (fMRI) to study the role of the striatum in inhibitory motor control. Subjects had to refrain from responding to designated items (STOP trials) within a similar series of motor stimuli. Striatal activation was increased significantly compared to that when responding to all targets within a series of motor stimuli, indicating that the striatum is more active when inhibitory motor control over responses is required. The likelihood of a STOP trial was varied parametrically by varying the number of GO trials before a STOP trial. We could thus measure the effect of expecting a STOP trial on the fMRI response in the striatum. We show for the first time in humans that the striatum becomes more active when the likelihood of inhibiting a planned motor response increases. Our findings suggest that the striatum is critically involved in inhibitory motor control, most likely by controlling the execution of planned motor responses. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: basal ganglia, inhibition, functional magnetic resonance imaging, fMRI, motor behavior, striatum
INTRODUCTION
Many everyday life movements, such as grasping an apple, are carried out automatically. When that apple lies in a basket among other apples, conscious control is needed to select one. Such control is thought to arise from cortical areas, especially the frontal cortex [Fuster, 1997]; however, execution of this control is likely mediated by subcortical areas [Alexander et al., 1986; Alexander and Crutcher, 1990]. Of these subcortical areas, the striatum is considered a critical nexus by directly regulating the chain of neuronal responses leading to motor acts [Aron et al., 2003; Band and van Boxtel, 1999; Kaji, 2001; Mink, 1996]. The striatum has been linked to initiating movements in monkeys [Lebedev and Nelson, 1999], but also with suppression of movements during anti‐saccades [Raemaekers et al., 2002] and primed responses in humans [Aron et al., 2003], suggesting that striatal cell ensembles are involved in both initiation and inhibition of motor responses. Clinical evidence for a role of the striatum in motor control comes from neurologic illnesses associated with dysfunctional motor control, such as Parkinson's, Huntington's, and Tourette's diseases, which are all linked to impaired functioning of the striatum [Dubois et al., 1995; Saint‐Cyr et al., 1995].
Recent primate studies suggest that the striatum does more than simply mediate movement inhibition and execution. For example, a subset of striatal neurons exhibit a change in firing pattern when an external stimulus is presented repeatedly [Sardo et al., 2000]. Moreover, some striatal interneurons exhibit activity before motor acts, i.e., in anticipation of stimuli [Apicella et al., 1991; Jaeger et al., 1993; Kermadi and Boussaoud, 1995; Mink, 1996], with the level of anticipatory activity depending upon the likelihood that a response is required [Blazquez et al., 2002]. The striatum may also be involved in the preparation of motor acts based on learned contextual information [Apicella, 2002; Graybiel et al., 1994]. We focus on the role of the striatum during motor function in humans using functional magnetic resonance imaging (fMRI). Based on recent primate studies, we hypothesize that the striatum regulates execution of a planned motor response. We use a modified stop‐signal task [Logan and Cowan, 1984] (Fig. 1). In this task, subjects have to refrain from responding to designated items (STOP trials) within a series of stimuli (GO trials). Within such a series (GO/STOP), the expectancy of a STOP trial is varied parametrically by pseudorandomly presenting STOP trials in such a way that at least two but no more than six GO trials separate two STOP trials. In that way, the striatal response to varying levels of anticipation of having to inhibit a planned response can be assessed. Baseline motor‐related activation is measured during separate blocks consisting of only GO trials (GO ONLY). In these latter blocks, GO trials are presented at a fixed pace to induce a high level of automaticity in responding. To allow statistical comparison of correct and incorrect responses on STOP trials, task difficulty is tuned individually so that the number of correct and incorrect responses is equal for all subjects.
Figure 1.
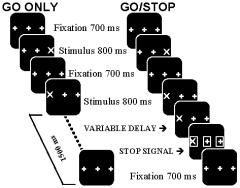
Schematic display of the task. Sequence of events for the GO ONLY task, consisting of only GO trials, and for the GO/STOP task, which consisted of 80% GO trials and 20% STOP trials. A screen with the word PRACTICE or TASK was presented to indicate the beginning of a GO ONLY or GO/STOP block, respectively. Fixation was followed by a stimulus presented on the location of either the right or left plus sign, requiring a right or left button‐press response, respectively. In STOP trials, three open squares surrounding the stimulus and two plus signs were presented after a variable delay after stimulus presentation. Subjects were instructed to respond to the GO trials but to withhold their response to STOP trials. The likelihood that a STOP signal would occur was parametrically modified by pseudorandomly varying the number of GO trials between two consecutive STOP trials from two to six.
If the striatum is actively involved in regulating the execution of planned movements, the striatal response to GO trials would be enhanced when the task calls for inhibition of motor responses as opposed to when it does not (Hypothesis 1). This is assessed by comparing brain responses to GO trials during the GO ONLY task, where STOP‐trials are not expected, to GO responses during the GO/STOP task with a 20% chance of a STOP stimulus. Furthermore, enhancement of the striatal response should be proportional to the probability that the motor response has to be inhibited (Hypothesis 2). This is tested by comparing brain responses to GO stimuli with a low STOP likelihood to responses to GO stimuli with a high STOP likelihood. Finally, failure to enhance the striatal response should result in failure to inhibit that motor response (Hypothesis 3). This is assessed by comparing the brain response to STOP trials when the response is correctly inhibited to the response to STOP trials when the response is executed (i.e., when an error is made).
SUBJECTS AND METHODS
Subjects
In total, 20 right‐handed subjects (10 males, 10 females; mean age, 20 ± 3.7 years) participated in this study. Subjects were excluded if they or their first‐degree relatives had a history of psychiatric or neurologic disorder. Other exclusion criteria included a history of head trauma or substance abuse, and pregnancy in women. The study was approved by the ethical committee of the UMC Utrecht in accordance with the Declaration of Helsinki.
Motor Inhibition Task
The motor inhibition task was based on the stop‐signal paradigm [Logan and Cowan, 1984]. Stimuli were presented in five blocks of 160 trials, alternated with rest periods of 30 s. Each block consisted of a series of GO and STOP trials (GO/STOP), which was preceded and followed by a series of only GO trials (GO ONLY). Before the beginning of the experiment, two practice blocks of 50 trials each were presented so that subjects could familiarize themselves with the stimuli and the magnetic resonance imaging (MRI)‐compatible response device. Stimuli were projected onto a screen placed across the bore of the MR magnet at 1.8 m from the subject's eyes. Subjects were lying in a supine position and observed the screen through a mirror placed at 45 degrees above their eyes. Three plus signs (each of diameter 2.2 degrees) formed the background that was displayed continuously throughout the task (Fig. 1). The two peripheral plus signs were spaced 3 degrees from the center of the display. Each trial consisted of the replacement of either the left or the right plus sign by an X, leaving the other two plus signs in place. Upon stimulus presentation, a response had to be made by pressing the corresponding left or right button of the response box as fast as possible using the right thumb. The stimulus was presented for 800 ms, in which time a response had to be made if the trial was a GO trial. The display was then cleared, leaving the background in place for 700 ms. Stimulus location was determined pseudorandomly to ensure that the same side of the display was not used more than four times successively. Each GO/STOP block consisted of 96 GO trials (80%) and 24 STOP trials (20%). GO trials were identical to those in the GO ONLY blocks. STOP trials (see following paragraph) were presented pseudorandomly between the GO trials, so that at least two but no more than six GO trials separated subsequent STOP trials. The beginning of each block was communicated to the subject by presentation of the word PRACTICE on the screen before GO ONLY blocks, and TASK before a GO/STOP block, so that subjects were informed about the upcoming task (i.e., whether or not to expect STOP trials).
STOP trials differed from GO trials in that shortly after the GO stimulus a STOP signal appeared, which instructed the subject not to respond to that GO stimulus. The STOP signal consisted of squares presented around the stimulus and the two remaining plus signs (Fig. 1). These squares were 2.25 degrees in size. STOP signals were presented at three different delay times, which were determined individually and adjusted during the experiment. Initially, the middle STOP signal was presented 150 ms before the time of the mean reaction time (RT) of the correct training trials, which was calculated for each subject individually (Fig. 2; adapted from Logan and Cowan [1984]). Timing of the other two STOP signal delays were one standard deviation (SD) shorter or longer than that of the middle one. These times corresponded roughly to points in the RT distribution of the training trials at the 25th, 50th, and 75th percentile, minus the time needed for processing of the STOP signal. Presenting a STOP signal at these points in the distribution should therefore result in an accuracy level of 75, 50, and 25%, respectively. These percentages were used as targets to which actual performance was compared during the experiment. While the task was running, delay times were adjusted real‐time as follows. For each of the three STOP conditions, performance of four consecutive STOP trials was recorded and analyzed. If actual performance was lower than the target percentage was, delay time for that specific STOP signal delay condition was decreased by 10 ms, and vice versa for higher performance. If actual performance was the same as the target percentage, no change was made. Within a GO/STOP block, every STOP condition occurred eight times. Actual delay time was therefore adjusted twice each block for each subject individually.
Figure 2.
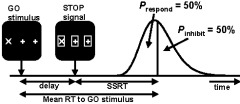
Schematic representation of the STOP signal mechanism, adapted from Logan and Cowan [1984]. The distribution depicts the individual reaction time (RT) distribution for the GO trials obtained during the training session. The STOP signal is presented after a variable delay after stimulus presentation. The stop‐signal reaction time (SSRT) reflects the speed at which the STOP signal is processed. For the middle STOP condition, the delay is chosen so that in 50% of the trials a response is given before the STOP signal is processed fully (Prespond), so that an accuracy level of 50% is expected for this particular delay time (i.e., Pinhibit is 50%).
Image Acquisition
Brain imaging data were collected on a 1.5‐T Philips ACS‐NT MRI scanner (Philips Medical Systems, Best, The Netherlands) with fast gradients (PT6000). The head was held in place with a strap and padding. Structural and functional images were acquired in transverse orientation from the same section of the brain. For functional scans, a navigated 3D‐PRESTO (i.e., principles of echo‐shifting with a train of observations) pulse sequence [Ramsey et al., 1998] was used with the following parameters: echo/repetition time [TE/TR] 35/24 ms; flip angle 10 degrees; matrix 48 × 64 × 24; field of view (FOV) 192 × 256 × 96 mm; voxel size 4 mm isotropic; scan duration 1,500 ms/24‐slice volume. Immediately after functional scans, an additional PRESTO scan of the same volume of brain tissue was acquired with a high (30 degrees) flip angle (FA30) for the image coregistration routine. In total, 920 functional images were acquired for each subject.
Data Analysis
For data analysis of fMRI scans, in‐house software and software developed by the Montreal Neurological Institute (MNI; Canada) was used. All functional scans were registered to the FA30 volume [Ramsey et al., 1998] using a rigid‐body affine transformation. The structural image was then registered to the FA30 using a least‐squares difference routine [Thevenaz et al., 1998], so that functional and structural images were spatially aligned. The first analysis was carried out to address Hypotheses 1, 2, and 3, regarding the difference in striatal activation during GO ONLY, GO/STOP, and correct versus incorrect STOP trials, respectively. In this analysis, for each individual subject regressor‐coefficients for each voxel were obtained from a general linear model using a factor matrix that modeled hemodynamic responses during GO ONLY, GO from GO/STOP, correct STOP trials, and incorrect STOP trials [Friston et al., 1995].
The second hypothesis regarding the effects of increasing STOP probability was addressed using three separate analyses. In the first, the GO trials within the GO/STOP task were divided in two factors: factor GO1,2 representing low STOP probability, being the first two GO trials after a STOP trial; and factor GO3–6, representing higher STOP probability, consisting of the third, fourth, fifth, and sixth GO trial after a STOP trial. This procedure allowed contrasting brain activation during low versus high STOP likelihood. The second analysis consisted of a parametric analysis of brain activation depending on the hypothesized amount of inhibitory motor control. Whereas during GO ONLY some control is needed as compared to rest, the amount of control is thought to increase as STOP probability increases. The parametric regressor coding the amount of control therefore ranged from 1 to 7 in steps of 1, with 1 being GO ONLY and 7 being the sixth GO trial in which STOP probability was highest. This procedure would yield only those brain areas in which the activation level increases in a linear fashion similar to the parametric regressor. Finally, a third analysis was carried out to map the effect of STOP signal occurrence in the GO trials from the GO/STOP blocks. The first two GO trials were combined, as STOP likelihood was 0% in both trials. In the following GO trials, STOP likelihood increased from 20% (third GO trial; the STOP trial could occur in the third, fourth, fifth, sixth, or seventh GO trial) to 50% (sixth GO trial; the STOP trial could occur in this trial or else it would occur in the next as there is a maximum sequence of six consecutive GO trials). In contrast to the second analysis, this analysis makes no assumptions on how brain activation is related to increasing amount of motor control. Each of these GO conditions was modeled using a separate regressor. In total, six regressors and an intercept were included in this regression analysis.
Regressor‐coefficient volumes per subject were spatially registered to a T1‐weighted MNI standard brain to enable group‐wise comparisons, using transformation parameters of the MNI registered structural volume. A 3‐D Gaussian filter (8 mm full‐width at half‐maximum [FWHM]) was then applied these statistical volumes. Group activation maps were generated for each factor using the pooled standard deviation approach [Worsley, 1994]. Group results were tested for significance (P < 0.05) with Bonferroni correction for the number of voxels (approximately 16,000, resulting in a critical z‐value of 4.5 for each voxel). For assessment of significant effects, a threshold of z = 4.5 was this applied to all image analysis results.
RESULTS
Performance Data
Performance data are presented in Table I. Responses were significantly faster for GO ONLY trials compared to those for GO trials from the GO/STOP task (t[19] = 4.60; P < 0.001), confirming that responses to the latter GO trials required more control. RT on these GO trials increased linearly as a function of STOP expectancy (R 2 = 0.83; P < 0.05; see Fig. 3), in that higher STOP likelihood was associated with increased RT. Mean STOP signal RTs (SSRTs) were calculated individually by subtracting the mean delay time for each of the three STOP conditions from the 25th, 50th, and 75th percentile RT on GO trials, respectively (see Fig. 2) [Logan and Irwin, 2000]. These results are within the normal range of 200–230 ms [Band and van Boxtel, 1999; Logan et al., 1997]. Mean accuracy on STOP trials did not differ significantly from the mean target percentage of 50% (46%; t[9] = 1.25; P = 0.227), confirming that performance calibration was effective.II
Table I.
Overview of the behavioral data
| Condition | Reaction time (ms) | Accuracy (%) |
|---|---|---|
| GO | ||
| Practice | 460 ± 10.4 | 98 ± 0.003 |
| Go from GO/STOP | 531 ± 16.2 | 97 ± 0.01 |
| Go from GO ONLY | 479 ± 11.2 | 99 ± 0.002 |
| STOPa | ||
| STOP1 | 238 ± 9.4 | 69 ± 0.04 |
| STOP2 | 228 ± 8.8 | 44 ± 0.04 |
| STOP3 | 218 ± 10.6 | 25 ± 0.02 |
| Overall mean | 228 ± 5.6 | 46 ± 0.03 |
Values are given as means ± SEM.
For STOP condition, RT is stop‐signal reaction time (SSRT).
Figure 3.
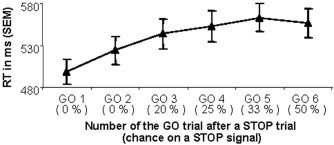
Effect of increasing STOP likelihood on GO reaction time (RT; in ms ± SEM). Between two consecutive STOP trials, at least two but no more than six GO trials could be presented. The chance of a STOP signal occurring in a specific GO trial (in brackets) therefore increases as more GO trials follow a STOP trial.
Table II.
Coordinates and Z‐value of peak voxel within group activation regions
| Condition | Region | Hem | BA | Peak voxel location | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| GO/STOP and GO ONLY | Striatum | R | — | −22 | 5 | −4 | 6.9 |
| L | — | 22 | 5 | −4 | 5.0 | ||
| M1 | L | 3, 4 | 42 | −23 | 56 | 22.7 | |
| SMA‐ACC | R, L | 6, 24 | 2 | −3 | 56 | 10.5 | |
| GO/STOP > GO ONLY | Striatum | R | — | −18 | 10 | 0 | 7.6 |
| L | — | 18 | 10 | −4 | 5.1 | ||
| SMA‐ACC | R, L | 6 | −6 | 1 | 60 | 5.5 | |
| Insula | R | 13 | −42 | 18 | −4 | 6.3 | |
| Parametric analysis | Striatum | R | — | −18 | 10 | 0 | 8.6 |
| L | — | 18 | 10 | −4 | 6.3 | ||
| SMA‐ACC | R, L | 6, 24 | −6 | 10 | 48 | 6.7 | |
| Insula | R | 13 | −42 | 18 | −4 | 8.3 | |
| Correct > incorrect STOP | Striatum | R | — | −22 | 10 | −4 | 6.4 |
| L | — | 22 | 10 | −4 | 5.8 | ||
BA, Brodmann area; R, right; L, left; Hem, hemisphere; M1, primary motor cortex; SMA, supplementary motor cortex; ACC, anterior cingulate cortex.
Imaging Data
Hypothesis 1: GO ONLY versus GO/STOP
In the baseline (GO ONLY) task, subjects responded to the targets in the absence of STOP trials. Areas activated during the GO ONLY task were consistent with those commonly associated with right‐handed motor processing, being predominantly left hemispheric and including precentral gyrus, postcentral gyrus, parietal lobe, and supplementary motor area (SMA). The latter activation region extended into the anterior cingulate gyrus. In Figure 4A, activation associated with all GO trials is presented. Responding to GO trials during GO/STOP, which required more motor control than did GO ONLY trials, was associated with additional brain activation bilateral in the striatum, the right insula, and the left SMA extending to the anterior cingulate (Fig. 4B).
Figure 4.
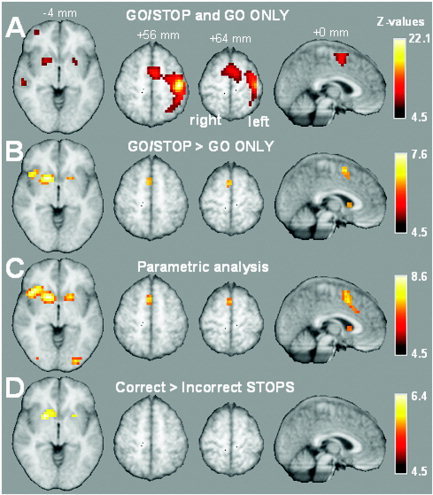
Overview of brain activation overlaid on selected slices of the mean anatomic group image thresholded at P < 0.05 corrected for multiple comparisons (i.e., z = 4.5). A: Combined brain activation during all GO trials from both GO ONLY and GO/STOP. B: Contrast in brain activation between GO trials from GO ONLY and GO/STOP task (GO trials from GO/STOP > GO ONLY). C: Brain activation correlated with the amount of control required on GO trials. D: Contrast in brain activation between correct and incorrect STOP trials (correct STOP > incorrect STOP).
Hypothesis 2: Effects of increasing STOP likelihood
To investigate the effect of increased control over motor responses, activation during GO trials within the GO/STOP task with no STOP likelihood was contrasted with activation during GO trials with an increased STOP likelihood. Between two subsequent STOP trials, there were at least two but no more than six GO trials. The STOP expectancy during the first two GO trials (GO1,2) after a STOP trial therefore was low (in fact, this should be zero, but as subjects were not informed about this rule they might have had some expectancy). STOP trials could occur during the third until the sixth GO trial (GO3–6). These two conditions were modeled in two factors, representing low and high STOP probability, respectively. A region‐of‐interest (ROI) strategy was adopted to compare activity for low versus high STOP likelihood within GO/STOP. The ROIs were defined as those voxels that were significantly more active during GO trials in GO/STOP tasks than during the GO ONLY task (Fig. 4B). Four regions were found and were selected for further analyses. Only activation in striatum (right, t[19] = 2.6, P < 0.05; left, t[19] = 2.7, P < 0.05), but not in the SMA‐anterior cingulate (t[19] = 1.8; P = 0.088) nor in the right insula (t[19] = 0.4, P = 0.97) was significantly higher when the likelihood of a STOP trial was higher (see Fig. 5).
Figure 5.
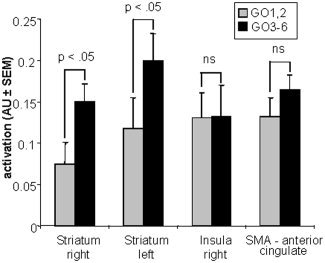
Mean activation level in the regions of interest selected from the GO from GO/STOP vs. GO ONLY contrast (Fig. 4B), being left and right striatum, right insula and the anterior cingulate (±SEM) during the first two GO trials after a STOP trial (GO1,2; 240 events, gray bars) and third until the sixth GO trial after a stop (GO3–6; 240 events, black bars).
Correlation of STOP likelihood with brain activation
A second, parametric analysis was carried out in which all GO trials were coded corresponding to the hypothesized degree of motor control needed, ranging from 1 (GO ONLY) to 6 (the sixth GO trial after a STOP trial, corresponding to that GO trial with the highest STOP probability; Fig. 6). Results are presented in Figure 4C, and show that activation within the striatum bilaterally, the right insula, and the SMA‐anterior cingulate increased in a linear fashion with the degree of motor control. In contrast, the motor cortex is not correlated with this factor (i.e., no significant effect at P < 0.05, corrected for multiple comparisons). Even upon lowering the threshold to z = 3.09 (P < 0.001, uncorrected for multiple comparisons), no effect in the motor cortex was observed. We take this as indicating that the observed correlation between the degree of motor control and striatal activation is not caused merely by more or less overall motor activity. The finding that the insula shows a significant effect despite absence of a significant difference between low and high STOP probability within the GO/STOP task (Fig. 5) is due mainly to the difference in activation between GO ONLY and GO/STOP (see also Fig. 4B).
Figure 6.
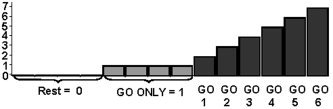
Schematic representation of the parametric factor coding the hypothesized amount of control required on GO trials, ranging from 1 (GO ONLY; no STOP likelihood) to 7 (GO 6; highest STOP likelihood).
Increasing brain activation with increasing inhibitory motor control
To illustrate in more detail the activation levels (i.e., magnitude of the blood oxygenation level‐dependent [BOLD] response) within the ROIs (see Fig. 4B), a third analysis was carried out in which the GO trials from the GO/STOP blocks were modeled. In Figure 7, the activation levels within these regions are mapped against the likelihood of a STOP signal occurring. Testing these activation levels against the hypothesized linear increase in brain activation as STOP likelihood increased (ranging from 0% in the first two GO trials to 50% in the sixth GO trial; see Subjects and Methods section) resulted in a significant linear effect in both left and right striatum (F[1,13] = 21.99, P < 0.001 and F[1,13] = 12.98, P < 0.01, respectively), but not in the SMA–anterior cingulate (F[1,13] = 1.83, not significant), nor in the right insula (F < 1). These results, although obtained with a different regression matrix, are consistent with those of the first analysis.
Figure 7.
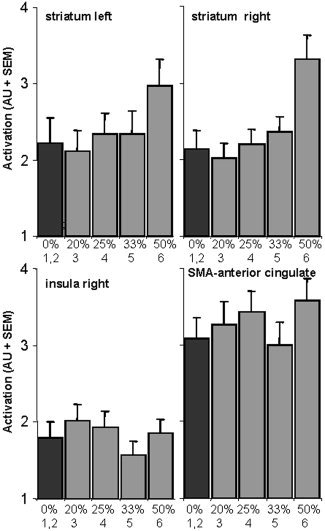
Mean maximum brain activation (AU ± SEM) contrasted against the chance of having to inhibit the response (in %) in the regions of interest selected from the GO from GO/STOP vs. GO ONLY contrast (Fig. 4B). Black bars denote baseline (first two GO trials after a STOP trial; 0% chance of a STOP signal). Gray bars denote the third (3) until the sixth (6) GO trial after a STOP trial. Note that the distance between points on the x‐axis is not constant.
Hypothesis 3: Inhibition of a motor response
By contrasting correctly performed STOP trials with incorrectly performed STOP trials (i.e., trials where subjects failed to inhibit the response), activation associated with successful control over the motor response could be detected. Contrasting activation during successful and unsuccessful STOP trials revealed activation bilaterally, although it was predominantly in the right striatum. This indicates that successful inhibition of a motor response depends upon increased activation in the striatum (see Fig. 4D).
DISCUSSION
In this study, subjects had to withhold their response to designated stimuli within a series of motor stimuli. We have shown for the first time in humans that activity in the striatum in response to these motor stimuli increased as the likelihood of having to stop this motor response increased. In addition, RT to these motor stimuli also increased when it became more likely that the response had to be inhibited. It therefore seems that the striatum plays an important role in inhibitory motor control, possibly by holding back prepared motor responses. This notion is supported further by the finding that when striatal activity was low, subjects failed to inhibit the motor response.
The striatum is considered a critical relay station in the cortico‐striato‐cortical motor loop involved in controlling ongoing movements both in humans and in primates [Alexander et al., 1986; Alexander and Crutcher, 1990; Band and van Boxtel, 1999; Graybiel et al., 1994; Kaji, 2001; Kimura, 1992; Nambu et al., 2002]. Frontal and parietal cortical areas as well as the thalamus project to the striatum, thereby controlling striatal activity [Rolls, 1994]. In turn, striatal neurons project to the globus pallidus and substantia nigra, which are the main output structures of the basal ganglia [Alexander et al., 1986; Alexander and Crutcher, 1990; Jueptner and Weiller, 1998]. The globus pallidus projects to the thalamus, and thus affects the information relay within cortico‐striato‐cortical loops. These projections are inhibitory (GABAergic) and can be either facilitated or inhibited, so that ongoing movements can be executed or inhibited when required by the context [Alexander et al., 1986; Alexander and Crutcher, 1990; Kaji, 2001].
In primates, it has been shown that when responses can be given in a temporally predictable manner (i.e., no anticipation of inhibition), activity of striatal interneurons is low [Blazquez et al., 2000]. These interneurons are thought to regulate the activation level within the striatum by regulating the firing of medium spiny projection neurons [Graybiel et al., 1994; Mink, 1996], which make up the majority of striatal neurons. Consistent with these findings is the reduced striatal activity during a series of only GO trials (GO ONLY task) compared to that with GO trials intermixed with STOP trials (GO/STOP task). Whereas in the GO ONLY task a motor response is given to every stimulus, this response predictability is reduced during the GO/STOP task. STOP signals could occur pseudorandomly within the series of GO trials. Furthermore, the STOP signal could be presented at three varying delays after the stimulus. This procedure ensured that the instance of a STOP signal was difficult to predict. Because the presentation of the STOP signal was delayed after the GO stimulus, it was not immediately clear to the subject whether the response has to be executed or not. As the behavioral data indicate, RTs increased as a function of STOP signal likelihood, suggesting that subjects used the context (i.e., awareness of the number of preceding consecutive GO trials) to assess the likelihood of STOP signal incidence for each GO trial. As the GO trials were presented at a fixed pace during both the GO ONLY and GO/STOP task, responses to the GO cues were presumably prepared automatically [Osman et al., 2003] due to a strong stimulus–response coupling. The reduced predictability of response execution in the GO/STOP task due to STOP signals does not diminish this response preparation per se, but rather leads to a delay in the execution of the prepared response until it becomes clear whether or not it has to be inhibited. Our data show that within the striatum, activation was increased as a result of this reduced predictability of response execution. This finding is consistent with recent data showing that striatal activation is increased when stimuli requiring a motor response are presented in an unpredictable compared to a predictable order [Dreher and Grafman, 2002]. Furthermore, Jueptner et al. [1997] found that the anterior part of the striatum is activated in nonroutine motor operations, but not when motor behavior is carried out automatically with a minimum of attention to the performance of the task. Indeed, attention to action is likely to affect neuronal activity in the basal ganglia because patients with damage to these structures, such as that in Parkinson's disease, show attentional deficits [Owen et al., 1992]. It thus seems that the striatum becomes more activated when subjects respond in a more controlled manner, i.e., when subjects pay more attention to their actions to prevent them from being executed automatically. The more likely it becomes that a prepared response will have to be inhibited due to a STOP signal, the more subjects attend to their actions by increasing inhibitory motor control over the preparation and execution of their response.
Stimulus‐induced striatal activity is thought to be modulated within the striatum by a small set of tonically active neurons (TANs) [Apicella, 2002; Apicella et al., 1991; Graybiel et al., 1994]). These TANs, presumed cholinergic interneurons, are believed to be important in regulating activity of the medium spiny projection neurons within the striatum [Graybiel et al., 1994; Mink, 1996]. Such an intrastriatal regulatory mechanism may explain the present findings in that with increasing likelihood of having to inhibit a response, the striatum becomes less prone to letting a response be executed automatically, potentially by controlling the regulatory activity of these TANs. In turn, these TANs may act to hyperpolarize the medium spiny neurons, thereby preventing fast movement initiation. As a result, responding to a target may hence require additional activation within the striatum to overcome this global inhibition. We hypothesize that as it becomes more likely that a planned response to a stimulus will not have to be executed (i.e., chance of a STOP signal increases), intrastriatal inhibition increases to prevent a spontaneous motor response. The level of neuronal activation to overcome this inhibition to generate a response thus becomes higher. Taken together with the fact that frontal regions regulate striatal activity [Alexander et al., 1986; Alexander and Crutcher, 1990; Hauber, 1998; Rolls, 1994], it is likely that the striatum exerts its inhibitory actions under the control of regions involved in cognitive processing.
Due to a relative large voxel size (4 mm isotropic) and spatial filter (Gaussian kernel with 8 mm FWHM), the exact location of activation within the striatum was difficult to determine. As the striatum consists of multiple brain regions (putamen, caudate head, caudate body, and nucleus accumbens), it would be interesting to determine in more detail the exact location of activation within these regions. Using smaller voxel sizes would allow for a more detailed localization, and thus could add to the understanding of what parts of the striatum are involved in inhibitory motor control.
During GO ONLY simple baseline motor processing, in which responses had to be given to regular occurring cues, activation was found in the SMA extending to the anterior cingulate cortex, primary motor cortex, and superior parietal cortex. In addition to the striatum, the insula and the SMA‐anterior cingulate showed increased activation when more control over response execution was required. Anatomic studies have shown that the insula has extensive connections with the motor and somatosensory cortices and is involved in motor programming, execution, and control [Augustine, 1996; Mesulam, 1998]. The SMA is linked anatomically with the basal ganglia by the corticobasal ganglia motor loop and is therefore thought to be involved in motor planning and preparation [for review see Tanji, 1994].
The current results show that the striatum, as well as the right insula and the SMA–anterior cingulate, is critically involved in inhibitory motor control. When subjects anticipate that they probably do not need to execute the prepared response, the striatum acts to keep this response on hold to prevent immediate execution. More specifically, our data show for the first time in humans that striatal activation rises when the likelihood of a response cancellation increases, suggesting a direct link between inhibitory motor control and striatal activation levels. In sum, a successful response strategy depends critically upon control over the striatum.
REFERENCES
- Alexander GE, Crutcher MD (1990): Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Apicella P (2002): Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. Eur J Neurosci 16: 2017–2026. [DOI] [PubMed] [Google Scholar]
- Apicella P, Scarnati E, Schultz W (1991): Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res 84: 672–675. [DOI] [PubMed] [Google Scholar]
- Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, Sahakian BJ, Robbins TW (2003): Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease. Brain 126: 713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Band GP, van Boxtel GJ (1999): Inhibitory motor control in stop paradigms: review and reinterpretation of neural mechanisms. Acta Psychol (Amst) 101: 179–211. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Fujii N, Kojima J, Graybiel AM (2002): A network representation of response probability in the striatum. Neuron 33: 973–982. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J (2002): The roles of the cerebellum and basal ganglia in timing and error prediction. Eur J Neurosci 16: 1609–1619. [DOI] [PubMed] [Google Scholar]
- Dubois B, Defontaines B, Deweer B, Malapani C, Pillon B (1995): Cognitive and behavioral changes in patients with focal lesions of the basal ganglia. Adv Neurol 65: 29–41. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS (1995): Characterizing evoked hemodynamics with fMRI. Neuroimage 2: 157–165. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1997): The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Philadelphia: Lippincott Williams and Wilkins; 333 p. [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M (1994): The basal ganglia and adaptive motor control. Science 265: 1826–1831. [DOI] [PubMed] [Google Scholar]
- Hauber W (1998): Involvement of basal ganglia transmitter systems in movement initiation. Prog Neurobiol 56: 507–540. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Gilman S, Aldridge JW (1993): Primate basal ganglia activity in a precued reaching task: preparation for movement. Exp Brain Res 95: 51–64. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997): Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 77: 1313–1324. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998): A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121: 1437–1449. [DOI] [PubMed] [Google Scholar]
- Kaji R (2001): Basal ganglia as a sensory gating devise for motor control. J Med Invest 48: 142–146. [PubMed] [Google Scholar]
- Kermadi I, Boussaoud D (1995): Role of the primate striatum in attention and sensorimotor processes: comparison with premotor cortex. Neuroreport 6: 1177–1181. [DOI] [PubMed] [Google Scholar]
- Kimura M (1992): Behavioral modulation of sensory responses of primate putamen neurons. Brain Res 578: 204–214. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Nelson RJ (1999): Rhythmically firing neostriatal neurons in monkey: activity patterns during reaction‐time hand movements. J Neurophysiol 82: 1832–1842. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB (1984): On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91: 295–327. [DOI] [PubMed] [Google Scholar]
- Logan GD, Irwin DE (2000): Don't look! Don't touch! Inhibitory control of eye and hand movements. Psychon Bull Rev 7: 107–112. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R (1997): Impulsivity and inhibitory control. Psychol Sci 8: 60–64. [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Mink JW (1996): The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Nambu A, Kaneda K, Tokuno H, Takada M (2002): Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol 88: 1830–1842. [DOI] [PubMed] [Google Scholar]
- Osman A, Moore CM, Ulrich R (2003): Temporal organization of covert motor processes during response selection and preparation. Biol Psychol 64: 47–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW (1992): Fronto‐striatal cognitive deficits at different stages of Parkinson's disease. Brain 115: 1727–1751. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF (2002): Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3‐dimensional event‐related functional magnetic resonance imaging. Arch Gen Psychiatry 59: 313–320. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, van den Brink JS, van Muiswinkel AM, Folkers PJ, Moonen CT, Jansma JM, Kahn RS (1998): Phase navigator correction in 3D fMRI improves detection of brain activation: quantitative assessment with a graded motor activation procedure. Neuroimage 8: 240–248. [DOI] [PubMed] [Google Scholar]
- Rolls ET (1994): Neurophysiology and cognitive functions of the striatum. Rev Neurol (Paris) 150: 648–660. [PubMed] [Google Scholar]
- Saint‐Cyr JA, Taylor AE, Nicholson K (1995): Behavior and the basal ganglia. Adv Neurol 65: 1–28. [PubMed] [Google Scholar]
- Sardo P, Ravel S, Legallet E, Apicella P (2000): Influence of the predicted time of stimuli eliciting movements on responses of tonically active neurons in the monkey striatum. Eur J Neurosci 12: 1801–1816. [DOI] [PubMed] [Google Scholar]
- Tanji J (1994): The supplementary motor area in the cerebral cortex. Neurosci Res 19: 251–268. [DOI] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M (1998): A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7: 27–41. [DOI] [PubMed] [Google Scholar]
- Worsley KJ (1994): Local maxima and the expected Euler characteristic of excursion sets of Chi square, F and t fields. Adv Appl Probability 26: 13–42. [Google Scholar]


