Abstract
Human behavior reflects a continual negotiation of automatic and directed actions. The oculomotor network is a well‐characterized neural system in which to study this balance of behavioral control. For instance, saccades made toward and away from a flashed visual stimulus (prosaccades and antisaccades, respectively) are known to engage different cognitive processes. Brain regions important for such controlled execution include the presupplementary motor area (pre‐SMA), frontal eye fields (FEF), and intraparietal sulcus (IPS). Recent work has emphasized various elements of this network but has not explored the functional interactions among regions. We used event‐related fMRI to image human brain activity during performance of an interleaved pro/antisaccade task. Since traditional univariate statistics cannot address issues of functional connectivity, a multivariate technique is necessary. Coherence between fMRI time series of the pre‐SMA with the FEF and IPS was used to measure functional interactions. The FEF, but not IPS, showed significant differential coherence between pro‐ and antisaccade trials with pre‐SMA. These results suggest that the pre‐SMA coordinates with FEF to maintain a controlled, preparatory set for task‐appropriate oculomotor execution. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: coherence, neural networks, oculomotor control, preparatory set, neuroimaging
INTRODUCTION
Behavioral control is an effortful process whereby we act contrary to an overlearned or reflex‐like tendency. Our daily activities are a constant negotiation of automatic and supervised forces, for instance, in the oculomotor control of saccades [Carpenter,2000; Pierrot‐Deseilligny et al.,1995; Tehovnik et al.,2000]. We navigate our visual world through a series of saccades: some made automatically toward abrupt visual events, some exploratory, and some directed to avoid salient cues (e.g., antisaccades). Antisaccades are especially illustrative, as they place control and reflex in direct opposition and have been shown to engage different cognitive processes than prosaccades [Connolly et al.,2000,2002; Curtis and D'Esposito,2003; Everling and Munoz,2000; Guitton et al.,1985; Hallett,1978; Hanes et al.,1998; Kimmig et al.,2001; Merriam et al.,2001; Munoz and Everling,2004; Rivaud et al.,1994; Schlag‐Rey et al.,1997].
The functional anatomy of the cortical oculomotor network is dominated by the frontal eye fields (FEF) and the intraparietal sulcus (IPS) [Andersen and Buneo,2002; Bruce et al.,1985; Luna et al.,1998; Paus,1996; Pierrot‐Deseilligny et al.,1995; Snyder et al.,2000], both of which have been implicated in preparatory aspects of eye movements. Oculomotor control is thought to originate in frontal cortical regions such as the presupplementary motor area (pre‐SMA) and supplementary eye fields (SEF) [Coe et al.,2002; Curtis et al.,2005; Curtis and D'Esposito,2003; Deiber et al.,1999; Hikosaka et al.,1996; Luna et al.,1998; Picard and Strick,2001; Schlag and Schlag‐Rey,1987], and the dorsolateral prefrontal cortex (DLPFC) [Funahashi et al.,1993; Hasegawa et al.,2000]. Recent human imaging work has begun to characterize the relative functional roles that these regions play. For instance, Curtis and D'Esposito [2003] compared activity during a delay interval before pro‐ and antisaccades and determined that pre‐SMA, as opposed to SEF or DLPFC, is a key source of oculomotor control. The pre‐SMA was the only region in their analysis that showed greater prestimulus preparatory activity seconds prior to antisaccades compared to prosaccades. Moreover, this preparatory activity was critically associated with saccade suppression; it predicted whether or not unwanted saccades made later in the trial were successfully inhibited. The FEF and IPS, as well as SEF, showed significant task differences, but these differences were most prominent at the time the pro‐ or antisaccade was generated. Connolly et al. [2002] also compared pro‐ and antisaccades, and determined that FEF but not IPS was critically involved in maintaining a preparatory set for eye movements. Only in FEF did activity indicate both the instructional cue (pro‐ or antisaccade) and the duration of delay preceding the appearance of a target. These imaging results, in addition to those from other labs [Cornelissen et al.,2002; DeSouza et al.,2003] and from single‐cell electrophysiology [Gottlieb and Goldberg,1999], support the idea that parietal and frontal elements of the oculomotor network emphasize sensory and executive/motor‐planning aspects of a task, respectively.
The experimental designs of Curtis and D'Esposito [2003] and of Connolly et al. [2002] are complementary, highlighting different functional aspects of the oculomotor network. Connolly et al. parametrically varied the delay between instructional cue and target appearance but did not monitor the success of inhibitory control during antisaccades. Their design was consequently effective at elucidating preparatory set but less so at evaluating control processes per se. Their analysis, moreover, was limited to only two regions of interest, FEF and IPS. The design in Curtis and D'Esposito, alternatively, was aimed more toward control processes. In‐scanner eyetracking of a challenging antisaccade task yielded trialwise behavioral results and a significant proportion of control‐failures. Although they had no parametric control for preparatory activity, this design allowed them to uniquely identify pre‐SMA as a key source of top‐down control.
Considering the two studies together, one might speculate that pre‐SMA exerts its control upon FEF, and not IPS, to maintain the more difficult preparatory set for antisaccades. We would therefore expect increased functional connectivity between pre‐SMA and FEF for antisaccades vs. prosaccades, and no change in the connection between pre‐SMA and IPS. There is, however, no way to address such a hypothesis using a univariate analysis. Since univariate statistics only test activity for each brain region in isolation, they cannot provide information about neural network interactions. Not only are univariate mean activity differences unreliable indicators for changes in functional connectivity [Sun et al.,2004], but in Curtis and D'Esposito [2003] FEF and IPS show the same univariate profile across tasks. No univariate analysis, in practice or in principle, can possibly distinguish the functional interactions among such regions. In this study, we analyze event‐related fMRI time series with a multivariate statistic called coherence that allows us to contrast directly the functional interactions for automatic (prosaccade) vs. controlled (antisaccade) eye movements. Only with such a network method can we establish how pre‐SMA cooperates with other regions to exert successful oculomotor control.
MATERIALS AND METHODS
Experimental Methods
This report uses data first published in Curtis and D'Esposito [2003], in which a full description of the methods appears. Here, we summarize the main points. Eleven healthy participants (five females; ages 21–33) gave informed consent according to procedures approved by the University of California. Each subject performed 80 antisaccade, 40 fixation, and 32 prosaccade trials in a nonoverlapping, randomly interleaved order. Each trial began with a central fixation dot (1,000 ms) that briefly changed colors (1,000 ms) instructing the participant to make a prosaccade, antisaccade, or fixation at the end of a delay. The instruction was followed by a 6,000 ms fixation (delay) and then a 200 ms gap, after which the peripheral saccade stimulus appeared in one of eight radial positions (6° from center). A 12,000 ms intertrial interval (ITI) followed the saccade stimulus, for a total length of 22,000 ms per trial. Eye‐movement data, acquired with an infrared videographic camera (Model 504LRO; Applied Sciences Laboratories, http://www.a-s-l.com), were used to determine correct and incorrect trials. Only correct antisaccade and prosaccade trials were used in this analysis.
Functional images were acquired during eight runs lasting 418 s each, resulting in a total of 1,672 volumes covering the dorsal cortex. T2*‐weighted echo planar images (EPI) sensitive to blood oxygenation level‐dependent (BOLD) contrasts were acquired at 4 T with an MR scanner (Varian INOVA; http://www.varianinc.com) and a TEM send‐and‐receive RF head coil (http://www.highfieldcoils.com) using a 2‐shot gradient‐echo EPI sequence (22.4 cm2 field of view with a 64 × 64 matrix size resulting in an in‐plane resolution of 3.5 × 3.5 mm for each of 18 5‐mm axial slices with 0.5 mm interslice gap; repetition time = 1 s per half of k‐space (2 s total), echo time = 28 ms, flip angle = 20°). High‐resolution MP‐Flash 3D T1‐weighted scans were acquired for anatomical localization.
Preprocessing
Functional images acquired from the scanner were reconstructed using an algorithm that linearly interpolates consecutive one‐half k‐space shots of equal ordinal rank to double the nominal sampling rate. Image volumes were corrected for slice‐timing skew using temporal sinc‐interpolation, and corrected for movement using a rigid‐body transformation to spatially align all volumes. Time series were high‐pass‐filtered to remove low‐frequency trends and other noise. All voxels outside of the brain were discarded by masking to remove extraneous signals.
Univariate Analysis
For all participants, a hemodynamic response function (HRF) was empirically derived using an event‐related HRF estimation task, where subjects made 20 saccades toward a flickering‐checkerboard (20 Hz) stimulus briefly presented (200 ms) every 16–20 s to the left or right hemifield. The mean trial‐averaged response of significant voxels within an anatomically defined mask of the bilateral FEF was used as the subject's HRF [Aguirre et al.,1998]. For the purposes of determining an HRF, the FEF was defined by the region extending laterally along the precentral sulcus of the dorsolateral frontal cortex, beginning at the junction with the superior frontal gyrus.
To model task‐related activity we convolved the subject's HRF with independent variables representing each epoch of every trial (instructional cue, preparatory delay, and saccade response). These covariates were entered into the modified general linear model (GLM) for analysis using VoxBo software (http://www.voxbo.org). Parameter estimates reflecting the percent signal change relative to baseline, or the intercept term in the GLM, were estimated for each covariate. Statistical parametric maps (t‐statistics) of contrasts were generated for the group after individual subject data were resampled to 2 mm isotropic voxels, smoothed by an 8 mm full‐width at half‐maximum (FWHM) Gaussian kernel, and spatially normalized into the standard Montreal Neurological Institute (MNI) atlas space using routines from SPM99 (http://www.fil.ion.ucl.ac.uk/spm).
Coherence Analysis
To investigate interregional interactions we performed a seed‐coherence analysis, calculating the coherence between a reference region and two other regions of interest (ROIs) [Sun et al.,2004]. Coherence measures how well one signal can be represented by a linear transformation of another. It is thus an indication of the functional connectivity between brain areas.
Definition of coherence.
The coherence between time series x and y is defined by:
where f xy (λ) is the cross‐spectrum of x and y at frequency λ, and f xx (λ) is the power spectrum of x [Brillinger,2001; Muller et al.,2001]. It is a normalized measure from 0 to 1, where 0 indicates an absence of any linear relation, and 1 indicates that the signals are perfectly related by a linear magnitude and phase transform. One advantage of coherence is its invariance to HRF differences between regions, due to the roughly linear transformation from neural activity to the BOLD response [Dale and Buckner,1997]. Unlike correlation, the coherence of brain regions with very different HRFs will be high as long as they have similar underlying neural activity, even if the HRFs have a relative phase lag (Fig. 1).
Figure 1.
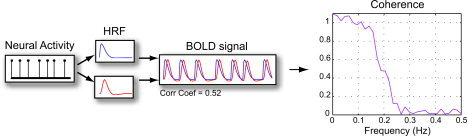
Coherence is invariant to timing and shape differences in the hemodynamic response function (HRF). Time series generated by convolving the same impulse function with two different HRFs have a high average coherence across the hemodynamic frequency range (0–0.15 Hz), reflecting a highly linear relation between the time series. The correlation coefficient of these time series reflects a difference in the shape of the HRF, and is therefore considerably lower (adapted from Sun et al. [2004], with permission). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The coherence analysis we used is summarized by the following steps and described in further detail below.
-
1
Identify seed voxels within an anatomically defined mask.
-
2
Generate condition‐specific coherence maps for the seed voxels.
-
3
Compare coherence across conditions within an ROI.
Identifying seed voxels.
We defined an anatomical mask of the pre‐SMA for each subject to be on the dorsomedial wall, rostral to the vertical plane of the anterior commissure (VAC), and above the cingulate sulcus [Picard and Strick,2001; Rizzolatti and Luppino,2001]. Within each masked region we identified the voxels with the most task‐related activity by choosing those with a significant F‐value for all pro‐ and antisaccade task periods (P < 0.05, corrected for multiple comparisons) (Fig. 2). Most subjects showed 12–20 significant voxels, but several subjects had many more. In those individuals we chose the most significant 20 voxels to maintain a similar seed size across the group. A single, averaged time series was derived from each seed region.
Figure 2.
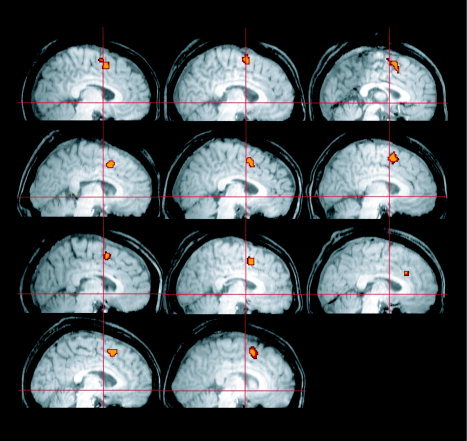
Seed regions for coherence analysis. The locations of the pre‐SMA seed are shown for all eleven subjects, overlaid on a parasagittal section of each subject's brain. Crosshairs indicate the location of the anterior commissure in the y and z dimensions. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Generating time series and coherence maps.
Time series for every brain voxel were separated into nonoverlapping, condition‐specific segments (i.e., prosaccade or antisaccade). Each 22‐s segment was mean‐centered and tapered with a 4‐point split‐cosine bell function to minimize spectral leakage due to segmented edge effects [Bloomfield,1976]. Because the distribution of the coherence estimate is sensitive to the total number of segments in the time series [Brillinger,2001], we removed alternating correct antisaccade trials to yield an equal number of trials per condition. Condition‐specific segments were then concatenated to form a continuous time series. We estimated the band‐averaged (0–0.15 Hz) condition‐specific coherence of the seed region with all other voxels using Welch's averaged periodogram method in Matlab (http://www.mathworks.com) [Oppenheim and Schafer,1989]. A band‐averaged coherence of 1 would indicate that the time series are perfectly related by a linear transform across all frequencies in the band, and a coherence of 0 would indicate the total absence of such a relationship. Condition‐specific coherence maps were generated for each seed region using this coherence measure.
Contrasting coherence across conditions.
To facilitate interpretation across studies, we selected ROIs in the right FEF and right IPS based on Connolly et al. [2002]. We concentrated on the right hemisphere because previous studies have shown that both inhibitory control and saccadic/manual exploration is right‐lateralized [Garavan et al.,1999; Gitelman et al.,1996,2002; Mesulam,1999]. Using the Talairach Daemon (http://ric.uthscsa.edu/projects/talairachdaemon.html) we identified the gray‐matter Talairach (TAL) coordinates for the rFEF ([25 −8 45] TAL) and rIPS ([28 −47 43] TAL) closest to the peak coordinates reported in Connolly's localizer saccade task (rFEF: [21 −10 45] TAL, rIPS: [28 −46 42] TAL). After transforming the coordinates into MNI space (http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html), we then selected the 8‐mm sphere surrounding these coordinates as the ROI. To investigate the functional interactions with the seed region across conditions, we subtracted the Gaussian‐normalized coherence maps for the prosaccade condition from the antisaccade condition. The normalization transformation, accomplished using the arc‐hyperbolic tangent function, allows us to apply a parametric random‐effects group analysis on the difference maps [Rosenberg et al.,1989]. A t‐test was performed across subjects, within each ROI, with significance set at P < 0.05.
RESULTS
Coherence Maps Reveal Functional Networks
We identified the pre‐SMA seed or reference region for the coherence maps as the most significant task‐related voxels within a broadly defined anatomical ROI. The peak voxels were determined with an F‐test across pro‐ and antisaccades (P < 0.05, corrected for multiple comparisons). This ensured that the seed was highly relevant while avoiding any bias in its association with either task. Using the average signal from the peak voxels provided a lower‐noise estimate for our reference time series. In 10 of 11 subjects the seed was located immediately anterior to the vertical plane through the anterior commissure, dorsal to the cingulate sulcus [Picard and Strick,2001; Rizzolatti and Luppino,2001] (Fig. 2). Coherence maps were calculated for each condition, pro‐ and antisaccade. A representative subject's coherence map during the antisaccade task appears in Figure 3. The general characteristics of the maps were similar across both tasks for all subjects. The most coherent voxels cluster around the seed itself, highly localized to the medial wall. Other peaks in the functional network associated with the seed include the bilateral FEF, intraparietal sulci, SEF, and several regions in the dorsolateral prefrontal cortex.
Figure 3.
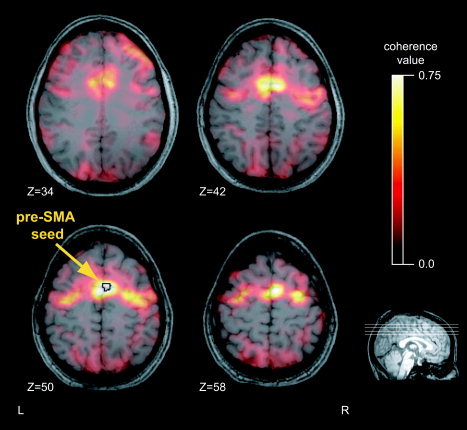
Coherence maps exhibit specific peaks. A typical condition‐specific coherence map is shown for a single subject's pre‐SMA seed during antisaccades. Lighter colors indicate greater coherence. Location of the seed region is indicated with an arrow and a black outline. Z indicates the vertical coordinates of the anatomical slices (MNI).
Anti‐Pro Coherence Contrast Reveals Differential Interactions With the Oculomotor System
The coherence contrast map quantifies how functional interactions with the seed region differ between anti‐ and prosaccades. The group contrast map appears in Figure 4 overlaid on a representative single subject's brain anatomy. Our hypothesis addressed the very regions that Connolly et al. [2002] tested for involvement in preparatory set; therefore, our regions of interest in right FEF and IPS were identified using coordinates from their report [see Methods in Connolly et al.,2002]. The two regions are illustrated in Figure 4 by circles of lighter shading, and the coherence contrast is superimposed. A strong positive peak occurs in FEF (P < 0.05, t = 2.67 [28 −8 49] MNI), indicating that FEF has significantly greater coherence with pre‐SMA during antisaccades vs. during prosaccades. No such differential coherence is evident in right IPS, demonstrating that there is no significant change in the functional interaction between pre‐SMA and IPS between pro‐ and antisaccades.
Figure 4.
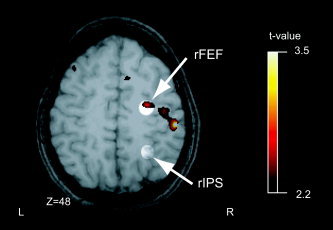
Coherence contrast maps with the pre‐SMA seed. The antisaccade‐prosaccade group difference map for the coherence analysis is shown. The regions defined by the rFEF and rIPS ROIs are highlighted. Within the rFEF ROI, the significant voxels indicate increased coherence between the rFEF and the pre‐SMA during the antisaccade task as compared to the prosaccade task. No significant voxels were detected in the ROI for the rIPS. Z indicates the vertical coordinates of the anatomical slice (MNI).
In addition to our hypothesized effects, there were several other qualitatively notable aspects of the coherence contrast maps. In a post‐hoc test, no significant differential coherence occurred in the corresponding FEF and IPS regions of the left hemisphere, or in SEF. Prominent peaks were observed, however, in the premotor‐motor strip caudolateral to the right FEF locus and in bilateral DLPFC (Fig. 4).
DISCUSSION
Functional Connectivity in Oculomotor Control
FEF and IPS are highly connected structures important for oculomotor tasks [Schall et al.,1995; Stanton et al.,1995] but their roles are distinguished by their interactions with pre‐SMA, an area sensitive to successful top‐down control. The increased coherence between pre‐SMA and FEF during antisaccades shows that controlled movements engage this network more than reflex‐like movements. Univariate analyses are principally incapable of supporting such a distinction, as empirically demonstrated by the common involvement of FEF and IPS in pro‐ vs. antisaccade tasks [Curtis and D'Esposito,2003]. No analysis of mean activity changes in FEF and IPS could possibly distinguish their differential connectivity with pre‐SMA. Our results therefore illustrate the essential nature of multivariate approaches when testing hypotheses of functional connectivity.
The reports by Connolly et al. [2002] and Curtis and D'Esposito [2003] motivated this study and consequently provide the richest context for interpreting our results. In this light, our findings support the view that pre‐SMA exerts control by coordinating with FEF, where the more difficult preparatory set during antisaccades is maintained. Specifically, this analysis supports the hypothesis of Curtis and D'Esposito, who proposed that pre‐SMA activity reflects a highly flexible, abstract, and directionally undetermined eye‐movement goal that biases the activity in other oculomotor centers such as the FEF. They suggested that this bias reduces the likelihood that a reflex‐like saccade to the exogenous cue will be generated, either through the excitation of fixation‐related neurons in the FEF, or through the inhibition of movement‐related neurons [Everling and Munoz,2000].
IPS, another candidate region for oculomotor planning, failed to show significant interactions with pre‐SMA. While the IPS is undoubtedly important for oculomotor tasks, it may be more involved with maintaining visuospatial representations and executing sensorimotor transformations rather than issuing preparatory instructions for the saccade itself [Curtis et al.,2004,2005; Gottlieb and Goldberg,1999; Ro et al.,2001]. Our findings are therefore consistent with the idea that frontal oculomotor areas are necessary for executive or motor planning aspects of a task, and parietal oculomotor areas are more involved with visuospatial or sensorimotor aspects.
No current technique using BOLD time series can unequivocally specify direction of influence. Nevertheless, a wealth of anatomical and functional evidence in humans and nonhuman primates suggests that the dominant influence is from pre‐SMA to FEF rather than vice versa [Coe et al.,2002]. While FEF is most consistently involved in the execution of eye movements [Tehovnik et al.,2000], pre‐SMA may serve a role more similar to heteromodal association areas such as prefrontal cortex. For example, pre‐SMA has greater anatomical interconnectivity with prefrontal cortex than neighboring premotor regions, such as SMA proper [Bates and Goldman‐Rakic,1993; Lu et al.,1994; Luppino et al.,1993]. Moreover, functional imaging work clearly implicates pre‐SMA in cognitive control processes that must precede saccade execution activity in the oculomotor system. These include response inhibition during go/no‐go and flanker tasks [Garavan et al.,1999; Hazeltine et al.,2000; Humberstone et al.,1997; Kiehl et al.,2000; Menon et al.,2001; Rubia et al.,2001; Ullsperger and von Cramon,2001] and the updating or switching of essential visual–motor associations [Grosbras et al.,2001; Heide et al.,2001; Kawashima et al.,1998; Rushworth et al.,2002; Sakai et al.,1999; Shima et al.,1996].
While we are confident that the dominant direction of influence is from pre‐SMA to FEF, coherence analysis does not presume monosynaptic connectivity. There are no known direct connections from pre‐SMA to FEF, at least in the monkey [Bates and Goldman‐Rakic,1993; Huerta and Kaas,1990; Luppino et al.,1993; Schall et al.,1993], but the influence of pre‐SMA may be transmitted via an intermediary. Since this study was a strict test of an a priori hypothesis, we did not explore all brain regions functionally connected to pre‐SMA [Johansen‐Berg et al.,2004]. Based on anatomy, however, a possible intermediary would be SEF, which has strong reciprocal connections with both pre‐SMA and FEF. Although activity in SEF, unlike pre‐SMA, failed to show significant differences in the prestimulus, preparatory delay period, its activity did discriminate between antisaccades and prosaccades at the time of the stimulus/response [Curtis and D'Esposito,2003]. Moreover, SEF showed relatively high coherence with pre‐SMA in both saccade conditions, suggesting an integrated role in this task. A similar role could be played by DLPFC [DeSouza et al.,2003], which in addition to its strong anatomical connections with pre‐SMA and FEF, also showed indications of differential coherence with pre‐SMA between anti‐ and prosaccades. Alternatively, DLPFC may influence both pre‐SMA and FEF to enable or potentiate successful top‐down control and maintenance of preparatory set. Whatever the case, it is important to recognize that a seemingly simple task, such as making an eye‐movement toward or away from a single spot of light, depends on a large and widespread network of brain regions. Univariate analyses of fMRI time series do not characterize the interactions between the nodes of these networks.
Multivariate Methods Are Essential to Complement Univariate Information
Univariate measures could provide reliable (and necessarily indirect) information about functional neural networks if only one network were ever engaged at a time; if that network were fully, or exhaustively, connected; and if all regions of the network showed significant mean activity changes across tasks. Brain dynamics, though, are far more complex than this. Coherence contrast maps show patterns of interaction that depend on the seed region and could never be achieved through any combination of univariate contrasts. This report demonstrates one way in which the complementary information of univariate and multivariate analyses may be unified to yield an understanding inaccessible through either alone. Namely, a specific functional hypothesis was proposed based on the task‐related variance of individual regions. Then, the hypothesis was addressed in a targeted way using a robust measure of functional interactions among the areas. For certain limited questions, of course, univariate analyses will suffice. However, given our current appreciation for the interactive nature of brain processes, solely univariate results may be more suitably considered the starting point rather than the endpoint of an investigation.
Coherence has been applied successfully across brain regions in electroencephalography, magnetoencephalography, and single‐cell electrophysiology [Friston,1997; Miller and Schreiner,2000; Rosenberg et al.,1989; Shaw,1984] and within visual and motor cortices using fMRI [Marchini and Ripley,2000; Muller et al.,2001; Sun et al.,2004]. Although correlation has also proved valuable [Biswal et al.,1995; Cordes et al.,2000], coherence has several distinct advantages over its time‐domain counterpart, making it particularly appropriate for BOLD signals [Muller et al.,2001; Sun et al.,2004]. One exciting advantage is that it is independent of an estimate of the HRF, and therefore avoids the associated biases in analysis. Coherence is also an important addition to the existing array of multivariate methods for imaging data [Buchel et al.,1999; Horwitz,1991; McIntosh et al.,1996; McIntosh and Gonzalez‐Lima,1994; Moeller and Strother,1991]. Since each method has unique strengths, the more we command the more likely we will have the appropriate tool for a given study. For instance, coherence as applied here is less model‐dependent than structural equation modeling, which requires the user to incorporate all relevant connections of a network in order to parse the covariances among regions. On the other hand, coherence allows more explicit hypothesis testing than a method such as partial‐least squares [McIntosh et al.,1996], which instead of evaluating a user‐defined task contrast, finds contrasts that explain the most variance regardless of their theoretical applicability. Since our objective was to compare task‐related, model‐independent functional connectivity that was independent of interregional hemodynamic differences, coherence was the appropriate tool.
We show that when top‐down control over gaze is necessary, interactions between key nodes of an oculomotor network change to facilitate optimal behavior. Prefrontal regions, such as pre‐SMA, and premotor regions, such as FEF, show greater functional connectivity during antisaccades than prosaccades. In addition to addressing a pivotal question raised by the recent literature, we illustrate how hypotheses about functional connectivity that develop from univariate analyses can be addressed specifically and quantitatively with a method such as coherence.
Acknowledgements
We thank Ben Inglis and John Jinks‐Ollinger for technical assistance.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): The variability of human, BOLD hemodynamic responses. Neuroimage 8: 360–369. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA (2002): Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220. [DOI] [PubMed] [Google Scholar]
- Bates JF, Goldman‐Rakic PS (1993): Prefrontal connections of medial motor areas in the rhesus monkey. J Comp Neurol 336: 211–228. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Bloomfield P (1976): Fourier analysis of time series: an introduction. New York: John Wiley & Sons. [Google Scholar]
- Brillinger DR (2001): Time series data analysis and theory. Philadelphia: SIAM; p 255–257. [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB (1985): Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734. [DOI] [PubMed] [Google Scholar]
- Buchel C, Coull JT, Friston KJ (1999): The predictive value of changes in effective connectivity for human learning. Science 283: 1538–1541. [DOI] [PubMed] [Google Scholar]
- Carpenter RH (2000): The neural control of looking. Curr Biol 10: R291–293. [DOI] [PubMed] [Google Scholar]
- Coe B, Tomihara K, Matsuzawa M, Hikosaka O (2002): Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision‐making task. J Neurosci 22: 5081–5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Desouza JF, Menon RS, Vilis T (2000): A comparison of frontoparietal fMRI activation during anti‐saccades and anti‐pointing. J Neurophysiol 84: 1645–1655. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP (2002): Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5: 1345–1352. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MR imaging. AJNR Am J Neuroradiol 21: 1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cornelissen FW, Kimmig H, Schira M, Rutschmann RM, Maguire RP, Broerse A, Den Boer JA, Greenlee MW (2002): Event‐related fMRI responses in the human frontal eye fields in a randomized pro‐ and antisaccade task. Exp Brain Res 145: 270–274. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M (2003): Success and failure suppressing reflexive behavior. J Cogn Neurosci 15: 409–418. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Cole MW, Rao VY, D'Esposito M (2005): Canceling planned action: an fMRI study of countermanding saccades. Cereb Cortex; DOI:10.1093/cercor/bhi011 [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M (2004): Maintenance of spatial and motor codes during oculomotor delayed response tasks. J Neurosci 24: 3944–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL (1997): Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 5: 329–340. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M (1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81: 3065–3077. [DOI] [PubMed] [Google Scholar]
- DeSouza JF, Menon RS, Everling S (2003): Preparatory set associated with pro‐saccades and anti‐saccades in humans investigated with event‐related FMRI. J Neurophysiol 89: 1016–1023. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP (2000): Neuronal correlates for preparatory set associated with pro‐saccades and anti‐saccades in the primate frontal eye field. J Neurosci 20: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ (1997): Another neural code? Neuroimage 5: 213–220. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman‐Rakic PS (1993): Prefrontal neuronal activity in rhesus monkeys performing a delayed anti‐saccade task. Nature 365: 753–756. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: an event‐related functional MRI study. Proc Natl Acad Sci U S A 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Alpert NM, Kosslyn S, Daffner K, Scinto L, Thompson W, Mesulam MM (1996): Functional imaging of human right hemispheric activation for exploratory movements. Ann Neurol 39: 174–179. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, Friston KJ, Mesulam MM (2002): Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage 15: 970–982. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME (1999): Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci 2: 906–912. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Leonards U, Lobel E, Poline JB, LeBihan D, Berthoz A (2001): Human cortical networks for new and familiar sequences of saccades. Cereb Cortex 11: 936–945. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM (1985): Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal‐directed saccades. Exp Brain Res 58: 455–472. [DOI] [PubMed] [Google Scholar]
- Hallett PE (1978): Primary and secondary saccades to goals defined by instructions. Vision Res 18: 1279–1296. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF 2nd, Schall JD (1998): Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol 79: 817–834. [DOI] [PubMed] [Google Scholar]
- Hasegawa RP, Matsumoto M, Mikami A (2000): Search target selection in monkey prefrontal cortex. J Neurophysiol 84: 1692–1696. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack R, Gabrieli JD (2000): Neural activation during response competition. J Cogn Neurosci 12(Suppl 2): 118–129. [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund HJ, Kompf D (2001): Activation of frontoparietal cortices during memorized triple‐step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci 13: 1177–1189. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Putz B (1996): Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 76: 617–621. [DOI] [PubMed] [Google Scholar]
- Horwitz B (1991): Functional interactions in the brain: use of correlations between regional metabolic rates. J Cereb Blood Flow Metab 11: A114–120. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Kaas JH (1990): Supplementary eye field as defined by intracortical microstimulation—connections in macaques. J Comp Neurol 293: 299–330. [DOI] [PubMed] [Google Scholar]
- Humberstone M, Sawle GV, Clare S, Hykin J, Coxon R, Bowtell R, Macdonald IA, Morris PG (1997): Functional magnetic resonance imaging of single motor events reveals human presupplementary motor area. Ann Neurol 42: 632–637. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM (2004): Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci U S A 101: 13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Tanji J, Okada K, Sugiura M, Sato K, Kinomura S, Inoue K, Ogawa A, Fukuda H (1998): Oculomotor sequence learning: a positron emission tomography study. Exp Brain Res 122: 1–8. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: an event‐related fMRI study. Psychophysiology 37: 216–223. [PubMed] [Google Scholar]
- Kimmig H, Greenlee MW, Gondan M, Schira M, Kassubek J, Mergner T (2001): Relationship between saccadic eye movements and cortical activity as measured by fMRI: quantitative and qualitative aspects. Exp Brain Res 141: 184–194. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL (1994): Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol 341: 375–392. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA (1998): Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex 8: 40–47. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G (1993): Corticocortical connections of area F3 (SMA‐proper) and area F6 (pre‐SMA) in the macaque monkey. J Comp Neurol 338: 114–140. [DOI] [PubMed] [Google Scholar]
- Marchini JL, Ripley BD (2000): A new statistical approach to detecting significant activation in functional MRI. Neuroimage 12: 366–380. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez‐Lima F (1994): Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp 2: 2–22. [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL (1996): Spatial pattern analysis of functional brain images using partial least squares. Neuroimage 3: 143–157. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EP, Colby CL, Thulborn KR, Luna B, Olson CR, Sweeney JA (2001): Stimulus‐response incompatibility activates cortex proximate to three eye fields. Neuroimage 13: 794–800. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1999): Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LM, Schreiner CE (2000): Stimulus‐based state control in the thalamocortical system. J Neurosci 20: 7011–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller JR, Strother SC (1991): A regional covariance approach to the analysis of functional patterns in positron emission tomographic data. J Cereb Blood Flow Metab 11: A121–135. [DOI] [PubMed] [Google Scholar]
- Muller K, Lohmann G, Bosch V, von Cramon DY (2001): On multivariate spectral analysis of fMRI time series. Neuroimage 14: 347–356. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S (2004): Look away: the anti‐saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5: 218–228. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW (1989): Discrete‐time signal processing. Englewood Cliffs, NJ: Prentice Hall; p 730–742. [Google Scholar]
- Paus T (1996): Location and function of the human frontal eye‐field: a selective review. Neuropsychologia 34: 475–483. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Pierrot‐Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI (1995): Cortical control of saccades. Ann Neurol 37: 557–567. [DOI] [PubMed] [Google Scholar]
- Rivaud S, Muri RM, Gaymard B, Vermersch AI, Pierrot‐Deseilligny C (1994): Eye movement disorders after frontal eye field lesions in humans. Exp Brain Res 102: 110–120. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001): The cortical motor system. Neuron 31: 889–901. [DOI] [PubMed] [Google Scholar]
- Ro T, Rorden C, Driver J, Rafal R (2001): Ipsilesional biases in saccades but not perception after lesions of the human inferior parietal lobule. J Cogn Neurosci 13: 920–929. [DOI] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM (1989): The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E (2001): Mapping motor inhibition: conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002): Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B (1999): Presupplementary motor area activation during sequence learning reflects visuo‐motor association. J Neurosci 19: RC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Morel A, Kaas JH (1993): Topography of supplementary eye field afferents to frontal eye field in macaque: implications for mapping between saccade coordinate systems. Vis Neurosci 10: 385–393. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J (1995): Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci 15: 4464–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag J, Schlag‐Rey M (1987): Evidence for a supplementary eye field. J Neurophysiol 57: 179–200. [DOI] [PubMed] [Google Scholar]
- Schlag‐Rey M, Amador N, Sanchez H, Schlag J (1997): Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390: 398–401. [DOI] [PubMed] [Google Scholar]
- Shaw JC (1984): Correlation and coherence analysis of the EEG: a selective tutorial review. Int J Psychophysiol 1: 255–266. [DOI] [PubMed] [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J (1996): Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci U S A 93: 8694–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA (2000): Intention‐related activity in the posterior parietal cortex: a review. Vision Res 40: 1433–1441. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME (1995): Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol 353: 291–305. [DOI] [PubMed] [Google Scholar]
- Sun FT, Miller LM, D'Esposito M (2004): Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage 21: 647–658. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH (2000): Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev 32: 413–448. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001): Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]


