Abstract
Afferent signals from the body play an important role for emotional and motivational aspects of behavior. Nevertheless, little is known about the cortical and subcortical structures involved in interoceptive processes. Recently, a functional MRI study demonstrated that insula, somatomotor, and cingulated cortices are activated when subjects focus attention on their heartbeats. Aside from the use of imaging data, cardiac awareness has frequently been studied by using the heartbeat‐evoked potential (HEP), a brain wave that appears contingent on the heartbeat. The present study aimed at localizing sources of the HEP. Multichannel EEG was recorded in 44 subjects while they performed a heartbeat perception task. This task was used to quantify interoceptive awareness and to subdivide the subjects into good and poor heartbeat perceivers. Analyses showed highest HEP amplitudes over frontal and frontocentral electrode locations in the time range of later than 200 ms after R‐wave onset. By means of a BESA dipole‐source‐analysis, four sources of the HEP were identified which were located in the anterior cingulate, the right insula, the prefrontal cortex, and the left secondary somatosensory cortex. Good heartbeat perceivers showed both significantly higher HEP amplitudes and higher dipole strength than poor heartbeat perceivers in all four cortical sources. We conclude that the identified structures are involved in the processing of cardiac signals, whereby anterior cingulate and right insula seem to serve as interoceptive centers for cardioception. Hum Brain Mapping, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: evoked potentials, electroencephalography, principal component analysis, BESA, heartbeat perception, visceral sensation, insula, anterior cingulate, prefrontal cortex, S2
INTRODUCTION
Afferent signals from the inner organs play a significant role in the control of a homeostatic milieu. This information about events in the organism initiates regulatory mechanisms like the reflex control of breathing or blood pressure, which serve adaptation to actual body needs. Beyond such purely physiological processes, afferent signals are also important for the long‐term behavioral regulation of the organism [e.g., Vaitl,1996; Zagon,2001; Craig,2002].
Little is known about the mechanisms of the interaction between bodily signals from the periphery and the so‐called higher mental functions. Processing of visceral information is supposed to be closely related to emotional and motivational aspects of behavior. In many theories of emotions the perception of signals arising from the body plays an important role: This theoretical approach started with James' [1884] view of emotions as a result of the perception of bodily changes subsequent to an emotional stimulus. Recently, Damasio [1999] specified neural networks underlying emotion, feeling, and the consciousness of feelings, pointing out that the body is the main stage for emotions, either directly or via its representations in somatosensory areas of the brain. Within these theoretical considerations, the perception of bodily processes—so‐called interoception—and the cortical and subcortical structures involved in this process are of essential importance for a general understanding of emotions and feelings.
Concerning interoception, the most extensively studied interoceptive process is heartbeat perception, which has undergone investigations concerned with many of its different aspects. One common observation is the existence of substantial, interindividual differences in heartbeat perception. The ability to perceive cardiac activity may depend on such factors as gender, percentage of body fat, and physical fitness [Jones,1994; Cameron,2001]. Significant differences in heartbeat perception ability were also observed in different clinical samples: Mussgay et al. [1999] demonstrated a tendency towards lower perception scores in patients with depressive, somatoform, and personality disorders, whereas Ehlers et al. [1992,2000] reported more accurate heartbeat perception in panic patients. Patients suffering from arrhythmias and benign palpitations [Ehlers et al.,2000], and patients with diabetic neuropathy [Leopold and Schandry,2001] showed a decreased heartbeat perception ability compared to healthy controls. Thus, differences in heartbeat perception are of clinical relevance and may be used for describing and classifying patients suffering from various somatoform and psychological problems.
Apart from the investigation of influences of cardiac perception on behavior, a second line of research is concerned with the cortical and subcortical processes relating to cardiac awareness. Knowledge of the cortical projection and functional organization of cardiac sensation, compared to that involving somatosensation, is sparse. It has to be assumed that signals from the cardiac mechanoreceptors (baroreceptors) enter the brain primarily via the vagus nerve. The majority (80–85%) of vagal fibers are afferent, projecting viscerotopically to the nucleus of the solitary tract (NTS) of the brain stem [Jaenig,1995,1996]. Axons from cardiovascular structures (carotid body and aortic arch baroreceptors) terminate within the dorsomedial portion of the NTS. Most of the fibers from the NTS project to the parabrachial nucleus, which provides projections to multiple higher centers such as the hypothalamus, thalamus, and cerebral cortex. Here, the insular cortex plays a major role as a cortical projection area of viscerosensory input [Saper,2002; Cechetto et al.,1990; Bennaroch,1993; Augustine,1996; Craig,2002]. Correspondingly, the most probable cardioafferent pathway giving rise to conscious visceral perception appears to be the NTS‐parabrachial‐thalamus‐insula. Recently, Critchley et al. [2004] performed a functional MRI study investigating the neural systems supporting the perception cardiac activity. They demonstrated that insula, somatomotor, and cingulated cortices are activated when subjects focus attention on their heartbeats. Except for the mentioned fMRI study, the cortical processing of signals from the cardiovascular system has frequently been studied using the heartbeat‐evoked potential (HEP), a brain wave that appears contingent on the heartbeat [Schandry et al.,1986; Jones et al.,1986; Dirlich et al.,1997,1998; Montoya et al.,1993; Riordan et al.,1990; Schandry and Montoya,1996; Leopold and Schandry,2001; Pollatos and Schandry,2004]. Concerning the scalp distribution of the HEP, congruent results exist, showing highest HEP activity primarily at frontocentral electrodes [Pollatos and Schandry,2004; Montoya et al.,1993; Schandry and Montoya,1996; Leopold and Schandry,2001]. This frontocentral activation pattern could reflect sources in the insular, the anterior cingulate, or the somatosensory cortices [Pollatos and Schandry,2004], an assumption further confirmed by studies of the esophageal‐evoked potentials [Aziz et al.,1995,2000; Franssen et al.,1996; Furlong et al.,1998; Schnitzler et al.,1999]. In accordance with this, an fMRI study showed analogous activation patterns during stimulation of the distal esophagus in the somatosensory cortices, right insula, anterior cingulated gyrus, and the dorsolateral prefrontal gyrus [Aziz et al.,2000].
Although the cortical processing of signals from the cardiovascular system has been studied using HEP, no data exists concerning possible sources of the HEP. It is of theoretical and clinical relevance to identify anatomical regions dealing with interoceptive information. These regions may be involved in emotion and feelings and could be of importance for understanding the mechanisms underlying panic and anxiety in clinical samples. The present study aimed at localizing sources of the HEP using a multichannel EEG. Since the HEP can be used as an indicator of cardiac awareness, we were interested in interactions between the activity of HEP and its sources with the accuracy of heartbeat perception.
SUBJECTS AND METHODS
Subjects
The sample consisted of 44 students (16 male, 28 female) from the University of Munich. Subjects received € 20 (about $20) for their participation. Their age ranged from 18 to 36 years (mean 25.5 years, standard deviation [SD] 4.5 years). Using a heartbeat perception task, about 100 subjects were screened and, according to the ability to perceive one's heartbeats accurately, a total of 22 subjects (eight male, 14 female) with a high degree of interoceptive awareness were identified and contrasted to 22 subjects with poor interoceptive awareness who had been matched with regard to age and gender.
Experimental Procedure
Upon arrival, subjects were given written information about the experiment, their informed consent was obtained, and they filled in a general questionnaire concerning age, educational level, etc. Subsequently, electrodes were attached. The positions of scalp electrodes and of surface landmarks (nasion, right/left preauricular points) were digitized using the Zebris Digitizer System. The heartbeat perception tasks were then performed consisting of 11 heartbeat‐counting phases (intervals lasting for 150, 120, 130, 100 150, 100, 140, and 110 cardiac cycles as identified by EKG). During these intervals, participants were asked to count their own heartbeats silently. The beginning and end of the counting phases were signaled by a start and stop tone. During heartbeat counting, subjects should not take their pulse or attempt to use other manipulations facilitating the counting of heartbeats. In case the perception of heart activity was poor or lacking, participants should try to count in the presumed rhythm of their heartbeats. After the stop signal, subjects verbally reported the number of heartbeats counted. Subjects were neither informed about the length of the counting phases nor about their performance. After every two intervals a short break was inserted. The heartbeat perception tasks lasted for a total of 30 min.
Psychophysiological Recording
During the heartbeat counting phases, EEG activity was recorded over both hemispheres from 62 leads with DC amplifiers (bandpass: 0.01–100 Hz; SYNAMPS, NeuroScan, El Paso, TX) and digitized at a sampling rate of 1,000 Hz. Electrode positions (see Fig. 1) were determined with an electrode cap without electrodes (Easy cap, Falk Minow Services, Germany). The electrode positions and certain landmarks were digitized using the Zebris Digitizing system. All activity was referenced to the tip of the nose and grounded with an electrode on the left cheek. Offline, EEG was re‐referenced to linked mastoids. For the BESA analysis, data assessed with the original reference was used.
Figure 1.
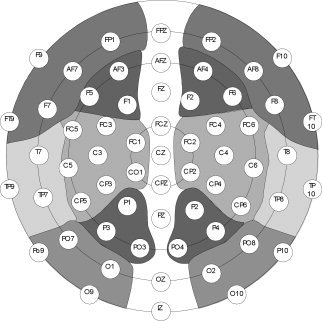
Pooling of the 12 electrode pools (right and left anterior–superior, anterior–inferior, medial–superior, medial–inferior, posterior–superior, posterior–inferior).
An electrooculogram (EOG) was recorded with two electrodes placed lateral to the outer canthus of each eye (EOGH) and a bipolar montage placed above and below the left eye (EOGV). Nonpolarizable Ag/AgCl electrodes were used. Electrode resistance was maintained below 5 k°.
ECG Recording Paradigms
ECG electrodes were placed at the left chest and the right clavicula. Nonpolarizable Ag/AgCl electrodes were used. ECG activity was recorded analogous to the EEG using a DC amplifier (bandpass: 0.01–100 Hz; SYNAMPS, NeuroScan) and digitized at a sampling rate of 1,000 Hz. The raw ECG was stored in an analogous manner to that of the EEG. Additionally, ECG R‐waves were detected online and were stored on a separate trigger channel to be used for later offline EEG averaging.
Data Analysis
Analysis of heartbeat perception.
A heartbeat perception score was calculated according to the following equation:
High scores (maximum 1) indicate an absolutely accurate heartbeat perception ability. A cut‐off score of 0.85 was used to categorize subjects either as good or poor heartbeat perceivers. This is in accordance with the cut‐off selected by Pollatos and Schandry [2004], Montoya et al. [1993], and Weitkunat and Schandry [1995].
Analysis of ERP data.
The stored EEG was examined for EOG, muscle, and other sources of electrophysiological artifacts. The analysis software (Brain Vision, Japan) performed EOG correction for blinks based on the blink correction method suggested by Gratton et al. [1983]. Epochs were rejected from the analysis if the scalp EEG exceeded ±80 μV in any channel. Trials contaminated by artifacts were eliminated prior to averaging: these accounted for approximately 8% of the trials. EEG epochs were computed extending from 200 ms prior to and up to 824 ms after the R‐wave. Data were corrected for cardiac field artifacts by using the Hjorth source derivation method [Hjorth,1975]. This method yields an estimation of the source activity as it appears at the scalp surface for each individual electrode. It is realized in the equidistant electrode montage basically as an analogous superposition of five bipolar derivations, forming a star‐like configuration around each electrode. The weighted activity of the surrounding electrodes is subtracted from each electrode; thus, cardiac field effects should be removed to a great extent in the remaining EEG activity. Montoya et al. [1993] described this method as being sufficient to substantially reduce the cardiac field.
For the purpose of statistical analysis, the mean amplitudes of the HEP were averaged for 12 regions, designated by hemisphere (right/left), horizontal plane (anterior, medial, posterior), and vertical plane (inferior, superior; see Fig. 1). In accordance with former results indicating the highest HEP amplitudes in the latency range of 250–450 ms [Pollatos and Schandry,2004; Leopold and Schandry,2001; Schandry et al.,1986], analyses were performed on the mean voltage within this time window.
Main effects and interactions, as well as differences between groups were investigated by submitting the data to ANOVAs with two levels of hemisphere (right/left), six levels of region (antero‐inferior, antero‐superior, medial‐inferior, medial‐superior, postero‐inferior, postero‐superior), and two levels of heartbeat perception (good/poor HP). Where appropriate, degrees of freedom were adjusted using the Greenhouse‐Geiser method. In the following, uncorrected F‐values are reported together with the Greenhouse‐Geiser epsilon values and corrected probability levels.
Source analysis.
The localization of the dipoles generating the HEP activity was modeled using the BESA software package [Scherg, 1992]. For a detailed description see, for example, Scherg [1990, 1992] and Berg and Scherg [1994]. Basically, BESA is a program that allows spatiotemporal modeling of multiple simultaneous sources over defined intervals. The first step in spatiotemporal dipole modeling is to select the interval for dipole localization. For this reason, a PCA for the whole curve complex was conducted to identify time intervals of interest and of the main waveforms, and to establish their contribution to the total variance. Then, a three‐step strategy for localizing the generators of the HEP was applied independently to the waveforms of each subject.
First, to determine how many sources should be used to fit the data, a second PCA was carried out in the selected time interval using the grand average of all subjects.
Second, a source analysis was performed on the grand average beginning with the amount of dipoles suggested by the results of the PCA (two dipoles for each principal component). The locations and orientations of the dipoles were calculated by an iterative, least‐squares method. The goodness of fit (GOF) was expressed as a percentage of the total variance.
Third, the model developed on the grand average of all subjects was applied to the individual data. The dipoles that presumably reflected cardiac field artifacts were modeled individually, i.e., they could vary in location and orientation. The remaining dipoles, presumably representing brain activity, were fixed in location and orientation. Their course of activation, their peak latencies, and their dipole strength, as well as the GOF, were assessed for each subject.
Main effects and interactions as well as differences between groups were investigated by submitting the data to ANOVAs with the factors Dipole, Location, and Heartbeat Perception (good/poor heartbeat perceivers). Where appropriate, degrees of freedom were adjusted using the Greenhouse‐Geiser method. In the following, uncorrected F‐values are reported together with the Greenhouse‐Geiser epsilon values and corrected probability levels.
The relationship between heartbeat perception and the HEP dipole strength was examined with bivariate, nonparametric correlation analyses (Spearman‐Rho) between the heartbeat perception score and the peak dipole strength in the latency range of 250–450 ms.
RESULTS
Heartbeat Perception
The mean heartbeat perception score was 0.78 (SD 0.19). In the group of good heartbeat perceivers, the mean heartbeat perception score was 0.92 (SD 0.04; minimum 0.87, maximum 0.98). In the group of poor heartbeat perceivers, the mean heartbeat perception score was 0.64 (SD 0.16; minimum 0.14, maximum 0.82).
HEP Morphology and Scalp Distribution
Grand averages of all electrode positions for good and poor heartbeat perceivers are depicted in Figure 2. HEP polarity varied across electrode locations. Cardiac field influences are primarily visible at the outermost positions. In the latency range from 250–450 ms, which has repeatedly been shown [Pollatos and Schandry,2004; Leopold and Schandry,2001] to be closely related to heart activity, good heartbeat perceivers showed an enhanced mean activity (0.69 μV across all electrodes) as compared to poor heartbeat perceivers (0.39 μV). These differences were most pronounced over anterior and medial electrode positions, showing a stronger positivity for good heartbeat perceivers at anterior superior (0.47 μV vs. 0.14 μV), medial superior (0.67 μV vs. 0.52 μV), medial inferior (0.97 vs. 0.57 μV), and posterior superior regions (0.98 μV vs. 0.73 μV).
Figure 2.
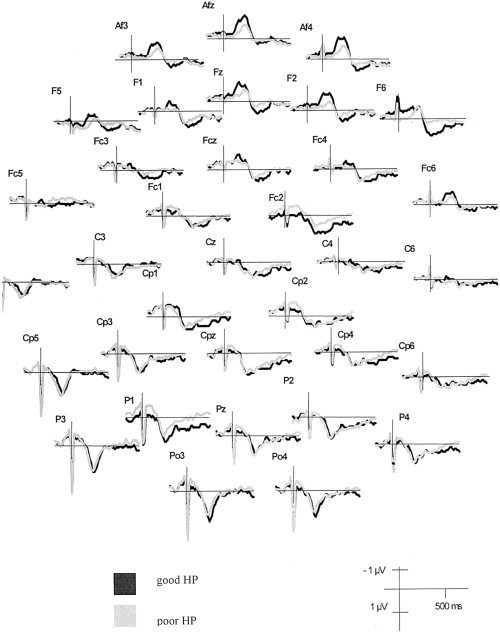
HEP (uncorrected) contrasting good and poor heartbeat perceivers at 32 electrode positions.
After the Hjorth correction method, HEP appeared as a relatively broad waveform at most electrodes in the latency range of 250–600 ms. The polarity of this waveform varies across electrode locations. However, in the relevant latency range from 250–450 ms, the picture becomes more unequivocal. The scalp distributions of the group means for the HEP amplitude of good vs. poor heartbeat perceivers are depicted in Figure 3 for the latency range of 250–450 ms. ANOVA performed on the corrected data assessed a significant main effect of Heartbeat Perception (F(1,42) = 4.74, η2 = 0.10, ε = 0.57), indicating an enhanced mean activity when comparing good heartbeat perceivers to poor heartbeat perceivers (cf. Fig. 3).
Figure 3.
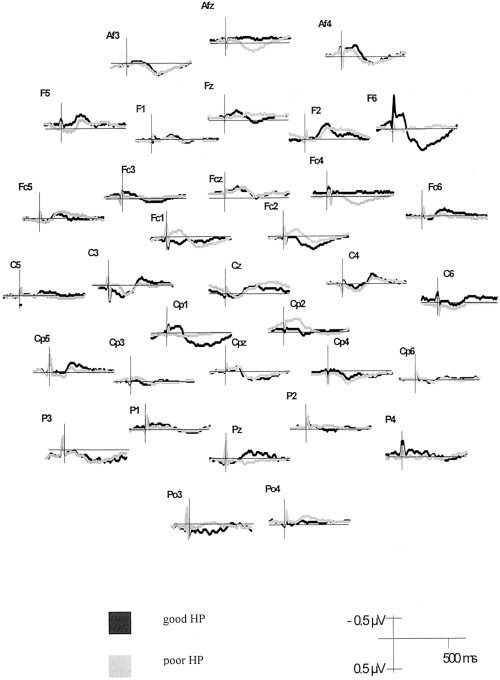
HEP (Hjorth‐corrected) contrasting good and poor heartbeat perceivers at 32 electrode positions.
A main effect of Region (F(5,210) = 8.42, η2 = 0.17, ε = 1.00) showed that anterior superior, medial, and posterior superior sites contributed most to the positive potential in this latency range.
EKG Analysis
In the latency range of 250–450 ms (F = 2.48; P = n.s.), EKG mean scores did not differ significantly between good and poor heartbeat perceivers.
Spatiotemporal Source Analysis
The PCA of the grand average revealed four principal components (see Fig. 4: criterion: explained variance >1%). The first component accounted for 87.8% of the total variance and showed a waveform very similar to the ECG, so that it was assumed to represent the cardiac field artifacts. The following three components explained variances of 7.3%, 3.0%, and 1.4%, and differed more clearly from the ECG activation pattern: They presumably reflected the processing of cardiac information. To select a relevant time interval, it was essential to consider the peak latencies of the three components not reflecting cardiac artifacts. The second PCA component with an explained variance of 7.3% revealed an activation peak at about 350 ms and the third component at 260 ms, whereas no clear activation peak could be identified for the fourth PCA component. We therefore chose a time interval of 250–450 ms to catch both the main activity of the second and third PCA component, and to fit in a time interval with a high goodness of fit. The selected time interval was also in accordance with the HEP data analysis and with former studies.
Figure 4.
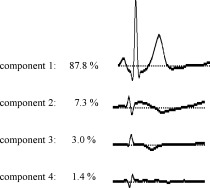
Principal component analysis of the HEP (n = 44).
The subsequent PCA for the time interval of 250–450 ms showed three components: Again, a first component reflecting the ECG artifact (explained variance 93.0%) and a further two components (explained variances 5.3% and 1.2%). Based on the PCA, a model with six dipoles was developed and fitted in the time interval of 250–450 ms using the iterative, least‐square algorithm implemented in the BESA software. This “cardiac model” explained 96% of the total variance in the whole HEP interval and 97% in the time interval of 250–450 ms; the location of the six dipoles is depicted in Figure 5. Table I shows the Talairach coordinates and the orientation and anatomical correlation of the six dipoles modeling the HEP.
Figure 5.
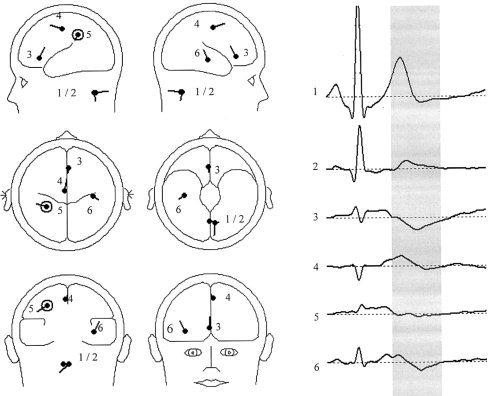
Model of cardiac signal transformation with six dipoles (n = 44).
Table I.
HEP dipoles with coordinates, orientation, anatomical correlate, peak latency and peak dipole strength based on the grand average (n = 44)
| Dipole | Talairach coordinates (x, y, z) | Dipole orientation (x, y, z) | Anatomical correlate (BA) | Peak latency (ms) | Peak dipole strength (nAm) |
|---|---|---|---|---|---|
| 1 | 0.8, −59.7, −61.7 | −0.7, −0.1, −0.7 | Extracortical | 258 | 23.66 |
| 2 | −6.7, −63, −62.1 | 0.1, −0.1, −0.2 | Extracortical | 253 | 11.70 |
| 3 | 2.2, 28.8, 7.4 | 0, −0.6, 0.8 | Anterior cingulate (BA 24) | 284 | 10.50 |
| 4 | 4, −16.5, 52.0 | −0.2, 0.9, −0.4 | Medical frontal gyrus (BA 6) | 270 | 8.83 |
| 5 | −30.9, −42.5, 38.5 | −0.8, 0.4, −0.5 | Inferior parietal gyrus (BA 7/40) | 354 | 2.84 |
| 6 | 39.4, −19.9, −3.1 | 0.4, −0.4, 0.8 | Insula (BA 13) | 374 | 5.63 |
Sources 1 to 2 were located beyond cortical and subcortical structures. They were characterized by an almost identical activation curve with an almost coincidental peak latency of 253 and 258 ms (based on the grand average of 44 subjects), so we assumed them to represent the cardiac field artifact. The remaining four sources (dipoles 3–6) were located in the anterior cingulate (dipole 3), the medial frontal gyrus (dipole 4), the insula (dipole 5), and the associative parietal cortex (dipole 6).
The model obtained with six dipoles was transferred to the individual datasets. The two artifact sources were individually fitted for the time interval of 250–450 ms, whereas the four cortical dipoles were fixed in location and orientation. The dipole peak strength, the peak latencies, and the goodness of fit were assessed for the four cortical dipoles in each subject and submitted to repeated ANOVA measures with the factor's dipole location (dipoles 3–6) and heartbeat perception group (good/poor heartbeat perceivers). Table II shows the averages obtained, separated for both heartbeat perception groups.
Table II.
The four cortical dipoles with coordinates, orientation, anatomical correlate, peak latency and peak dipole based on the averages of good and poor heartbeat perceivers (n = 44)
| Peak dipole strength (nAM) | Latency (ms) | |||||
|---|---|---|---|---|---|---|
| Good HP | Poor HP | P | Good HP | Poor HP | P | |
| Anterior cingulate | 19.99 (9.79) | 10.56 (5.54) | <0.01 | 285 (27.84) | 295 (30.66) | n.s. |
| Medial frontal cortex | 13.86 (7.55) | 9.82 (3.55) | <0.05 | 270 (20.87) | 269 (17.26) | n.s. |
| Somatosensory cortex | 8.39 (4.25) | 5.93 (3.39) | <0.05 | 359 (30.80) | 362 (27.93) | n.s. |
| Insula | 11.93 (7.22) | 6.26 (3.50) | <0.01 | 390 (35.21) | 397 (25.05) | n.s. |
Values are expressed as mean (SD), unless otherwise indicated.
n.s., not significant.
For dipole strength, a significant main effect of Heartbeat Perception was observed (F(1,42) = 17.75, P < 0.01, η2 = 0.30, ε = 0.98), indicating a higher activation in good heartbeat perceivers (13.54 nAm) compared to poor heartbeat perceivers (8.15 nAm).
The factor of Dipole Location was also significant (F(3,126) = 22.49, P < 0.01, η2 = 0.35, ε = 1.00), with the highest dipole strength being observed for the anterior cingulate (15.28 nAm), followed by the medial frontal gyrus (11.84 nAm) and the insula (9.10 nAm). The lowest activation score was found for the dipole in the somatosensory cortex (7.16 nAm; post‐hoc tests, P < 0.05).
A significant interaction effect between Heartbeat Perception and Dipole Location (F(3,126) = 4.06, P < 0.01, η2 = 0.09, ε = 0.76) was observed. Post‐hoc ANOVAs showed that good heartbeat perceivers yielded significantly higher dipole strengths for all four cortical dipoles, with most pronounced differences for the dipoles lying in the anterior cingulate and the insula (P < 0.05).
Concerning the peak latencies, only the factor of Dipole Location was significant (F(3,126) = 202.23, P < 0.001, η2 = 0.83, ε = 1.00). Post‐hoc tests showed that the latencies of the sources in the medial frontal gyrus and the anterior cingulate were significantly shorter than those of the somatosensory cortex and the insula (P < 0.05).
The mean GOF was 94.31% (SD 3.09) in the sample, with a mean of 94.88% (SD 2.43) in the group of good heartbeat perceivers and a mean of 93.74% (SD 3.60) for poor heartbeat perceivers. This difference was not significant (F(1,42) = 1.53, P = n.s.).
Correlation Analysis
Nonparametric Spearman Rho correlation analyses were calculated between the heartbeat perception score and the dipole strength of the four cortical dipoles. All observed correlations were positive: The correlation coefficients for the anterior cingulate (r = 0.34) and the insula (r = 0.33) reached significance (P < 0.05).
DISCUSSION
HEP Amplitude
The heartbeat‐evoked potential was observed as a broad waveform, with a polarity depending on the latency and the position of the electrodes. The observed distribution of the HEP is in accordance with former results [Pollatos and Schandry,2004; Montoya et al.,1993; Riordan et al.,1990; Schandry et al.,1986; Schandry and Montoya,1996], showing highest HEP amplitudes over frontal and frontocentral electrode locations in the time range later than 200 ms after R‐wave onset.
Source Analysis
We identified sources of the HEP located in the anterior cingulate, the right insula, the prefrontal cortex, and the left secondary somatosensory cortex through the use of dipole‐source‐analyses. These regions are highly congruent with functional imaging data by Critchley et al. [2004] obtained during subjects focusing their attention on their heartbeats: They reported activation clusters in the anterior cingulate, the insula, the inferior parietal gyrus, the somatomotor cortex, and the inferior frontal gyrus. The observed sources of the HEP are also congruent with sources obtained by distal esophagus stimulation located in somatosensory cortices, the insula, the anterior cingulated gyrus, and the dorsolateral prefrontal gyrus [Aziz et al.,2000; Franssen et al.,1996]. Although the observed dipoles are located with high congruence to these stated research data, one must keep in mind that BESA does not offer the optimal tools for modeling the heart. The incomplete modeling of the heart will lead to some misallocation of the remaining cortical dipoles; thus, the exact coordinates of the dipoles must be interpreted with caution. Future research aiming at a more complete modeling of the heart would be of great interest because this would provide a higher precision when performing the subsequent source localization. The thus obtained dipoles and their coordinates could be located with a higher precision and then compared to the activation coordinates assessed by fMRI. Nevertheless, the present study points out the general importance of the somatosensory cortices, the insula, the anterior cingulated gyrus, and the dorsolateral prefrontal gyrus for HEP.
In the present study, the highest dipole strength occurred at the anterior cingulate, followed by the medial frontal gyrus, the insula, and the somatosensory cortex. Concerning the observed anatomical structures identified as generators of HEP, the dipole in the anterior cingulate, the prefrontal cortex, and the insula is in accordance with the results of former studies [Critchley et al.,2000,2001; Williamson et al.,1999,2002; King et al.,1999] demonstrating activation by cardiovascular arousal.
We found the highest activation for the dipole in the anterior cingulate that could reflect activity in that part of area 24 subserving visceromotor control [Vogt et al.,1992]. It is a fact of interest that the anterior cinguli (ACC) plays an important role in the regulation of cognitive, emotional, and visceral processes [Cameron,2001,2002; Devinsky et al.,1995; Bennaroch,1993; Allman et al.,2001]. The anterior cingulate is thus a candidate for a possible link between visceroception and emotion processing.
Interoceptive awareness also correlated with dipole strength in the right insula. The insula integrates numerous visceral and somatosensory afferent inputs and is another essential structure for the processing of cardiovascular signals [Bennaroch,1993; Cameron,2001,2002; Cechetto et al.,1990, Augustine,1996]. The role of insula as a “primary viscerosensory cortex” [Bennaroch,1993] is supported by studies measuring electrical activity evoked by esophageal balloon distention of visceral regions in humans and observed electrical potential generators in the insular cortex [Augustine,1996; Aziz et al.,1995; Furlong et al.,1998, Franssen et al.,1996; Hecht et al.,1999]. The right insula is especially activated by cardiovascular arousal [Critchley et al.,2000,2001; Williamson et al.,1999,2002; King et al.,1999] and plays a major role for conscious perception of visceral signals in the theoretical framework of Craig [2002].
Concerning the dipole located in the prefrontal cortex, empirical data exists showing interconnections with all sensorimotor systems and also a strong involvement in the processing of cardiovascular signals [Cameron,2002; Critchley et al.,2000,2001; Williamson et al.,1999,2002; King et al.,1999]. Besides, it plays a major role in the synthesis of diverse information needed for complex behavior [Miller and Cohen,2001]. In the present studies, the prefrontal dipole shows the second highest dipole strength, which could reflect the importance of higher processing centers in the analyzed time range.
An HEP dipole source was also located in the somatosensory cortex. This source may reflect early stages of information processing, as this area is considered to be significant for providing visceral pain and temperature sensations [Hendry, 1999; Craig,2002]. The dipole observed in the secondary somatosensory cortex could represent early sensory information processing related to the processing of cardiac signals. This assumption is confirmed by studies using esophageal stimulations: Schnitzler et al. [1999] could show that nonpainful, electrical stimulation of visceral afferents in the distal esophagus activated the S2, while the somatosensory stimulation was associated with the activation of S1 and S2 as well. Following this result, the authors postulated that S1 is primarily involved in discriminative aspects of somatosensory information processing, while visceral afferents from the distal esophagus are mainly projected to S2, the activity of which represents the process of visceral sensation. Similar results were reported by other authors [Aziz et al.,1995,2000; Furlong et al.,1998], who also identified sources located in somatosensory areas of the cortex as demonstrating a reaction to esophageal stimulation.
Concerning the peak latencies, the frontal sources in the anterior cingulate and in the medial frontal gyrus were activated earlier than both insula and somatosensory cortices. This observation is in congruence with data by Franssen et al. [1996] using esophageally evoked potentials, which described a shorter latency for the source in the anterior cingulate (about 280 ms) as compared to bilateral sources in the insula (about 320 ms). Concerning the HEP and taking into account that heartbeats cause a stimulation curve rather than a clear, sharply distinguishable stimulus onset, one would expect that early components of the evoked potential (e.g., N100) are less prominent than later ones. Besides, the chosen evaluation time range of 250–450 ms probably captures later occurring HEP components, and thus modeled source activation does not reflect primary sensory activation, but activity components occurring later in the evoked response. For this reason, activity in the frontal dipoles (peaking at about 280 ms) could precede that observed in the somatosensory and insula dipoles (peaking at about 350 ms).
HEP Amplitude, Source Modeling, and Interoceptive Awareness
Good heartbeat perceivers revealed significantly higher HEP amplitudes in the observed latency range, with the most pronounced differences over frontal and central electrode locations. This result confirms former EEG studies using fewer than 64‐channels [for example, Pollatos and Schandry,2004] and points out the importance of frontal sources attributing to the HEP. When we modeled the HEP, the dipole strength in all four cortical sources was significantly higher for the group of good heartbeat perceivers. This was most pronounced for the dipoles in the anterior cingulate and the right insula, whose dipole strength also correlated positively with the heartbeat perception score. This result confirms the data of Critchley et al. [2004], who demonstrated activation in the anterior cingulate and the insula when subjects focused on their heartbeats. Another congruent observation is that Critchley et al. [2004] reported a significantly positive correlation between interoceptive ability and the BOLD response in the right insula. Comparing the activation in the right insula found by Critchley et al. [2004] with the exact coordinates of the observed dipole in the right insula, in the present study a more posterior part of the insula was activated. As the source localization method in general does not provide the anatomical precision reached by fMRI, and keeping in mind that due to the incomplete modeling of the heart some misallocation of the remaining dipoles is to be expected, the different methods used in both studies probably account for the observed deviance in the activation of the right insula. Thus, the right insula and anterior cingulate seem to be related to the ability of cardiac perception to the highest degree. As cited above, the work of Craig [2002] suggests that activity of the right insula reflects the conscious perception of visceral signals. We propose that in addition to the right insula, parts of the anterior cingulate are also important modules in the network subserving interoception and cardiac awareness.
REFERENCES
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P (2001): The anterior cingulated cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci 935: 107–117. [PubMed] [Google Scholar]
- Augustine JR (1996): Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev 22: 229–244. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Furlong PL, Barlow J, Hobson A, Alani S, Bancewicz J, Ribbands M, Harding GF, Thompson DG (1995): Topographic mapping of cortical potentials evoked by distension of the human proximal and distal esophagus. Electroencephalograph Clin Neurophysiol 96: 219–228. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Thompson DG, Hamdy S, Sarkar S, Brammer MJ, Bullmore ET, Hobson A, Tracey I, Gregory L, Simmons A, Williams SCR (2000): Cortical processing of human somatic and visceral sensation. J Neurosc 20: 2657–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennaroch EE (1993): The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68: 998–1001. [DOI] [PubMed] [Google Scholar]
- Berg P, Scherg M (1994): A multiple source approach to the correction of eye artefacts. Electroencephalograph Clin Neurophysiol 90: 229–241. [DOI] [PubMed] [Google Scholar]
- Cameron OG (2001): Interoception: the inside story — a model for psychosomatic processes. Psychosom Med 63: 697–710. [DOI] [PubMed] [Google Scholar]
- Cameron OG (2002): Visceral sensory neuroscience. Oxford: Oxford University Press. [Google Scholar]
- Cechetto DF, Saper, CB , Yasui Y (1990): Cardiovascular area of the insular cortex and its efferent projections in the rat. Neurosci Res 15: 23. [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000): Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ (2001): Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain 124: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1999): The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Dirlich G, Vogl L, Plaschke M, Strian F (1997): Cardiac field effects on the EEG. Electroencephalograph Clin Neurophysiol 102: 307–315. [DOI] [PubMed] [Google Scholar]
- Dirlich G, Dietl T, Vogl L, Strian F (1998): Topography and morphology of heart action‐related EEG potentials. Electroencephalograph Clin Neurophysiol 108: 299–305. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Margraf J, Roth WT (1992): Imipramine and alprazolam effects on stress test reactivity in panic disorder. Biol Psychiatry 31: 35–51. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Mayou R, Sprigings DC, Birkhead J (2000): Psychological and perceptual factors associated with arrhythmias and benign palpitations. Psychosom Med 62: 693–702. [DOI] [PubMed] [Google Scholar]
- Franssen H, Weusten B, Wineneke GH, Smout A (1996): Source modelling of esophageal evoked potentials. Electroencephalograph Clin Neurophysiol 100: 85–95. [DOI] [PubMed] [Google Scholar]
- Furlong PL, Aziz Q, Singh KD, Thompson DG, Hobson A, Harding GFA (1998): Cortical localisation of magnetic fields evoked by esophageal distension. Electroencephalograph Clin Neurophysiol 108: 234–243. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E (1983): A new method for off‐line removal of ocular artefact. Electroencephalogr Clin Neurophysiol 55: 468–484. [DOI] [PubMed] [Google Scholar]
- Hecht M, Kober H, Claus D, Hilz M, Vieth J, Neundörfer B (1999): The electrical and magnetical cerebral responses evoked by electrical stimulation of the esophagus and the location of their cerebral sources. Clin Neurophysiol 110: 1435–1444. [DOI] [PubMed] [Google Scholar]
- Heuser‐Link M, Dirlich G, Berg P, Vogl L, Scherg M (1992): Eyeblinks evoke potentials in the occipital brain region. Neurosci Lett 143: 31–34. [DOI] [PubMed] [Google Scholar]
- Hjorth B (1975): An on‐line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalogr Clin Neurophysiol 39: 526–530. [DOI] [PubMed] [Google Scholar]
- Jaenig W (1995): Visceral afferent neurons: neuroanatomy and functions, organ regulations and sensations In: Vaitl D, Schandry R, editors. From the heart to the brain. Frankfurt am Main: Peter Lang; p 5–34. [Google Scholar]
- Jänig W (1996): Neurobiology of visceral afferent neurons: neuroanatomy functions, organ regulations and sensations. Biol Psychol 42: 29–51. [DOI] [PubMed] [Google Scholar]
- James W (1884): What is an emotion? Mind 9: 188–205. [Google Scholar]
- Jones GE (1994): Perception of visceral sensations: a review of recent findings, methodologies, and future directions In: Ackles P, Jennings JR, Coles MGH, editors. Advances in psychophysiology, vol. 5 London: Jessica Kingsley Publishers; p 55–192. [Google Scholar]
- Jones GE, Leonberger TF, Rouse CH, Caldwell JA, Jones KR (1986): Preliminary data exploring the presence of an evoked potential associated with cardiac visceral activity. Psychophysiology 23: 445 (Abstract). [Google Scholar]
- King AB, Menon RS, Hachinski V, Cechetto DF (1999): Human forebrain activation by visceral stimuli. J Comp Neurol 413: 572–582. [PubMed] [Google Scholar]
- Leopold C, Schandry R (2001): The heartbeat‐evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clin Neurophysiol 112: 674–682. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Montoya P, Schandry R, Müller A (1993): Heartbeat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr Clin Neurophysiol 88: 163–172. [DOI] [PubMed] [Google Scholar]
- Mussgay L, Klinkenberg N, Rueddel H (1999): Heart beat perception in patients with depressive, somatoform, and personality disorders. J Psychophysiol 13: 27–36. [Google Scholar]
- Pollatos O, Schandry R (2004): Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat‐evoked brain potential. Psychophysiology 41: 476–482. [DOI] [PubMed] [Google Scholar]
- Riordan H, Squires NK, Brenner J (1990): Cardio‐cortical potentials: electrophysical evidence for visceral perception. Psychophysiology 27: 559. [Google Scholar]
- Saper CB (2002): The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25: 433–469. [DOI] [PubMed] [Google Scholar]
- Schandry R, Montoya P (1996): Event‐related brain potentials and the processing of cardiac activity. Biol Psychol 42: 75–85. [DOI] [PubMed] [Google Scholar]
- Schandry R, Sparrer B, Weitkunat R (1986): From the heart to the brain: A study of heartbeat contingent potentials. Int J Neurosci 30: 261–275. [DOI] [PubMed] [Google Scholar]
- Scherg M, Simpson GV, Ritter W, Vaughan HG (1990): Localization and temporal dynamics of human ERP sources. Electroencephalogr Clin Neurophysiol 75: 141.1689637 [Google Scholar]
- Schnitzler A, Ploner M, Schmitz F, Freund HJ (1999): Parallel activation if primary and secondary somatosensory cortices in human pain processing. J Neurophysiol 81: 3100–3104. [DOI] [PubMed] [Google Scholar]
- Vaitl D (1996): Interoception. Biol Psychol 42: 1–27. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR (1992): Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 2: 435–443. [DOI] [PubMed] [Google Scholar]
- Weitkunat R, Schandry R (1995): Cortical evoked potentials and heartbeat perception In: Vaitl D, Schandry R, editors. From the heart to the brain. Frankfurt am Main: Peter Lang; p 105–120. [Google Scholar]
- Williamson JW, McColl R, Mathews D, Ginsburg M, Mitchell JH (1999): Activation of the insular cortex is affected by the intensity of exercise. J Appl Physiol 87: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Williamson JW, McColl R, Mathews D, Mitchell JH, Raven PB, Morgan WP (2002): Brain activation by central command during actual and imagined handgrip under hypnosis. J Appl Physiol 92: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Zagon A (2001): Does the vagus nerve mediate the sixth sense? Trends Neurosci 24: 671–673. [DOI] [PubMed] [Google Scholar]


