Abstract
Recent neuroimaging studies have lead to the proposal that rest is characterized by an organized, baseline level of activity, a default mode of brain function that is suspended during specific goal‐oriented mental activity. Previous studies have shown that the primary function subserved by the default mode is that of an introspectively oriented, self‐referential mode of mental activity. The default mode of brain function hypothesis is readdressed from the perspective of the presence of low‐frequency blood oxygenation level‐dependent (BOLD) functional magnetic resonance imaging (fMRI) signal changes (0.012–0.1 Hz) in the resting brain. The results show that the brain during rest is not tonically active in a single mode of brain function. Rather, the findings presented here suggest that the brain recurrently toggles between an introspectively oriented mode (default mode) and a state‐of‐mind that tentatively might be interpreted as an extrospectively oriented mode that involves a readiness and alertness to changes in the external and internal environment. Hum. Brain Mapping, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: human brain, spontaneous mental activity, functional magnetic resonance imaging, blood oxygenation level‐dependent contrast, resting state
INTRODUCTION
Recently, there has been increased interest in the use of neuroimaging techniques to investigate what happens in the brain when it is not engaged in a specific goal‐oriented task. In other words, we are interested in the neuronal processes that subserve what would be referred to commonly as being at rest or, simply put, when we “do nothing.” The characterization of a resting state in the human brain is of paramount interest to the field of neuroimaging, because it pertains to identification of a baseline or control state against which other conditions of interests can be compared [Stark and Squire, 2001]. The idea that there might be a specific network of brain regions active when we are at rest came originally from the observations of deactivation in the brain that were consistent across many goal‐directed tasks [Shulman et al., 1997; for early accounts of deactivations during an attention‐demanding task see Baker et al., 1996; Ghatan et al., 1995]. These observations together with independent positron emission tomography (PET) measurements of cerebral blood flow (CBF) and oxygen consumption led Raichle et al. [Gusnard and Raichle, 2001; Raichle et al., 2001] to propose the theory of a “default mode” of brain function. This theory attributes the observation of signal decreases during various cognitive tasks in PET studies to an organized mode of brain function that is present as a baseline or default state and attenuated during specific goal‐directed tasks [Raichle et al., 2001]. Chief components of the default mode are the precuneus/posterior cingulate cortex (PCC) and medial prefrontal cortex (MPFC)/ventral anterior cingulate cortex (vACC). A subsequent study by the same group has shown that the latter is involved preferentially in self‐referential mental activity [Gusnard et al., 2001].
Studies of the resting brain have been carried out in parallel using functional magnetic resonance imaging (fMRI) techniques. In this context, the occurrence of low‐frequency fluctuations in resting‐state fMRI signal intensity time‐series has come to attract the attention of researchers. First observed by Biswal et al. [1995], the term low‐frequency fluctuations, or physiological noise [Krueger and Glover, 2001], refers to the presence of spontaneous oscillations/fluctuations of the blood oxygenation level‐dependent (BOLD) signal during rest. These spontaneous signal changes that are synchronized in time between distant brain regions are thought to arise mainly from fluctuations in metabolic demands in the resting brain and reside in the frequency interval below 0.1 Hz, unrelated to cardiac and respiratory effects. Initial fMRI studies probing for signal changes in the low‐frequency range demonstrated a functional connectivity in M1 across hemispheres during rest [Biswal et al., 1995; Xiong et al., 1999]. Subsequent studies have shown a functional connectivity for other sensory modalities [Cordes et al., 2000] and language areas [Hampson et al., 2002]. Moreover, Laufs et al. [2003b] correlated electroencephalograph (EEG) activity with low‐frequency BOLD signal fluctuations in the resting brain. Similarly, a network analysis of resting‐state activity using fMRI has been reported recently [Greicius et al., 2003].
In the present study, the default mode of brain function hypothesis is readdressed from the perspective of spontaneous low‐frequency fluctuations in BOLD fMRI data. We sought to extend the previous results by Greicius et al. [2003] and Laufs et al. [2003b] by addressing the question of whether there exists a spatial heterogeneity of low‐frequency signal fluctuations in the resting brain. If so, the hypothesis would be that the variance in resting‐state fMRI time‐series attributable to spontaneous low‐frequency fluctuations would be significantly larger in specific brain regions assigned previously to belong to the default mode of brain function. The rationale for this assumption is that subjects that are instructed to rest and refrain from engaging in any structured, goal‐oriented activity will automatically initiate self‐referential mental processes that can be characterized as introspective. These mental processes have been described as task‐unrelated imagery and thought (TUITs) [Giambra, 1995], self‐reflection [Johnson et al., 2002], or streams of consciousness [Andreasen, et al., 1995]. A second objective was to study the interregional connectivity among the brain regions that exhibit low‐frequency BOLD fluctuations during a resting state. The hypothesis was that regions prone to spontaneous low‐frequency fluctuations during rest would also show a synchronicity in time. The aim of the study therefore was to carry out a functional connectivity analysis of the networks of brain regions involved in sustaining a resting state in the brain.
To this end, functional MR images of 15 subjects were acquired while they were at rest and instructed to keep their eyes closed or looking at a fixation cross and to refrain from initiate any goal‐directed, attention‐demanding activity during the scanning sessions. The spatial distribution of low‐frequency BOLD oscillations in the brain during rest was assessed at an individual and multi‐subject level of analysis. The temporal characteristics of brain regions that exhibited a high degree of activity, in the sense that they contained variance attributed to low‐frequency spontaneous fluctuations during rest, was examined using region‐of‐interest (ROI)‐based functional connectivity analysis.
SUBJECTS AND METHODS
Participants
Fifteen healthy subjects (7 females; age range, 20–38 years) with no history of major neurologic and psychiatric disease participated in this study. All MR examinations were carried out according to the ethical guidelines and declarations of the Declaration of Helsinki (1975). Written informed consent was obtained from all participating subjects before the study and the subjects were scanned as a part of a technical development project within the Karolinska University Hospital.
Paradigm Design
Each volunteer underwent resting brain scans during which they kept their eyes closed throughout the entire scanning session or, alternatively, were instructed to keep their eyes open and look at a fixation cross (white cross on a black background) presented in the center of the subjects field‐of‐view. Functional MR image volumes of the two conditions were acquired in separate scanning session. Subjects were asked explicitly to refrain from engaging in any cognitive task or otherwise related effort. Simply put, the subjects were told to do nothing during all functional MRI scans. They were also asked to move as little as possible during scanning and to stay awake at all times. After scanning, all subjects were asked to describe: (1) whether they were able to stay fully awake through all scanning sessions; (2) how well they thought they have achieved the instruction to do nothing; and more specifically, (3) whether they experienced (and how frequently) that their mind was no longer “blank.” One subject reported difficulty in staying fully awake during MR scanning. Data from this subject were discarded and not used for any further analysis. The remaining 14 subjects reported that during scanning they experienced frequent episodes of thoughts that could be described as self‐referential mental processing. Typically, these thoughts dealt with autobiographic reminiscences, mental images, inner speech, or planning for the future. In addition, MR images from another subject were lost due to technical problems in the image acquisition process; therefore, all analysis and results are based on data obtained from 13 subjects.
MR Imaging
MR imaging was carried out using a 1.5‐T General Electric Signa Horizon MR scanner. Structural T1‐weighted images were acquired in a coronal orientation employing a 3‐D‐spoiled gradient recalled (SPGR) sequence (repetition/echo time [TR/TE] = 24/6 ms; flip angle = 35 degrees) with a voxel size of 0.9 × 1.5 × 0.9 mm3. MR images sensitized to changes in BOLD signal levels (TR/TE = 2,000/40 ms; flip angle = 80 degrees) were obtained by a gradient‐echo echo‐planar imaging (EPI) sequence. The slice thickness was set to 5 mm (slice gap = 1 mm) with a matrix size of 64 × 64 and a field‐of‐view (FOV) of 220 × 220 mm2, resulting in a voxel size of 3.44 × 3.44 × 6 mm3. Each brain volume comprised 24 axial sections and each functional run contained 300 image volumes. To assess the possibility of signal contamination arising from aliasing of cardiac pulsations, additional functional EPI image volumes were acquired in a subset of subjects with an increased temporal resolution (TR = 500 ms; flip angle = 30 degrees) at the expense of reduced spatial coverage (seven axial sections, slice thickness = 9 mm, centered at the precuneus/PCC and MPFC/vACC).
Data Analysis
Image preprocessing and statistical inference was carried out using the SPM2 software package (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). For each subject, all EPI images were realigned to the first image in the first series. In addition to realignment, EPI images were also unwarped to correct for susceptibility‐by‐movement interaction [Andersson et al., 2001]. The T1‐weighted high‐resolution image volume was coregistered to the mean EPI image and spatially normalized to the approximate Talairach space [Talairach and Tournoux, 1988] as defined by the Montreal Neurological Institute (MNI) T1‐weighted template in SPM2. Finally, EPI images were spatially smoothed using an isotropic Gaussian filter (12‐mm full‐width half‐maximum [FWHM]).
Low‐frequency spontaneous fluctuations in BOLD signal levels were modeled within the framework of the general linear model as implemented in SPM2 [Friston et al., 1995]. In detail, a discrete cosine basis set containing 120 regressors that together spanned the frequency range of 0–0.1 Hz was used. The discrete cosine basis set constituted an effective model of any signal change (within the given frequency range) as a linear combination of the individual basis functions. The inclusion of this particular frequency interval in the model was motivated by previous studies of low‐frequency functional connectivity [Biswal et al., 1995; Lowe et al., 1998; Peltier et al., 2002]. Statistical parametrical maps for all voxels showing a presence of slow physiologic BOLD signal oscillations in the brain were constructed by computing F‐contrasts comparing the effect of signal fluctuations in the range of 0.012–0.1 Hz to that of the complete frequency range spanned by the cosine basis set. Very slow signal changes in the range of 0–0.012 Hz that are related predominately to nonphysiologic sources, such as MR scanner drift, were thus treated as covariates of no interest by the statistical analysis. All EPI signal intensities time‐courses were globally normalized within SPM2. At an individual level of inference, statistical parametrical maps were created and thresholded at F > 1.69 corresponding to a P value of 0.001, uncorrected for multiple comparisons.
Obviously, the slow fluctuations in the BOLD signal intensity time‐course that occur during rest in a given subject will not be time‐locked to any external stimuli. The signal would therefore be modeled by a subset of regressors that would vary from individual to individual depending on differences in the exact temporal profile of the BOLD signal intensity time‐course. This implies that a statistical inference of the presence of low‐frequency fluctuations effects at a population level based on a conventional random‐effects analysis, e.g., t tests of each parameter estimate in the cosine basis set taken over subjects, is not straightforward. An alternative route to multi‐subject analysis was therefore taken using a masking procedure. A binary mask was created from each individual F‐contrast image by setting each pixel value to one if the corresponding F value exceeded 1.69; otherwise, it was set to zero. A final mask was calculated by multiplying the binary values of all of the individual masks. The result was a mask that showed voxels in the brain for which the corresponding F value exceeded 1.69 in all subjects that participated in the study. Although a less statistically rigorous approach, the masking procedure allowed for investigation of brain areas that exhibited slow oscillations in the BOLD signal during rest that generalized to the subjects that participated in the study.
As a final step, the default brain mode hypothesis was tested using an ROI‐based functional connectivity analysis. The mean signal intensity time course from the voxels inside a spherical ROI (radius = 10 mm) in the PCC/precuneus area (0, −56, 30) was extracted from the resting‐state scan in all subjects. The choice of the precuneus area as a seed point for functional connectivity analysis was motivated by the fact that it was only cluster together with considerably smaller clusters in the MPFC and the left angular gyrus to survive a threshold of the individual F‐contrast images of F > 2.64 (P < 0.5 × 10−3, corrected) in all subjects. The average signal intensity time‐course was inserted as a regressor in a general linear model and statistical parametrical contrast images were calculated at the subject level pertaining to brain regions that either were significantly correlated, positively or negatively, with the PCC/precuneus area. All data entering the ROI‐based functional connectivity analysis was bandpass filtered using a phase‐insensitive filter (passband: 0.012–0.1 Hz). Subsequently, contrast images constituting subject‐specific effects of the parameters obtained at the first level were entered into a second‐level model (one‐sample t test) for each contrast, yielding a random‐effects model [Holmes and Friston, 1998]. The resulting SPM[T] maps of activated voxels were thresholded at P < 0.01, corrected for multiple comparison using the false discovery rate (FDR)‐criterion [Genovese et al., 2002].
RESULTS
Low‐Frequency BOLD Signal Oscillations During Rest
In individual subjects, spontaneous fluctuations in the resting‐state fMRI signal were detected in widespread areas throughout the brain. The signal fluctuations during resting state were found predominately in gray matter (Fig. 1, eyes closed). A recurrent finding was a marked occurrence of low‐frequency fluctuations in the MPFC/vACC and the PCC/precuneus area and, to some lesser extent, in the dorsolateral prefrontal cortex (PFC). In addition, spontaneous signal changes were also present in the parietal and occipital lobes. Results from the multi‐subject analysis are shown in Figure 2 (eyes closed). In accordance with the results from the analysis in individual subjects, maps of low‐frequency fluctuations across subjects showed activity in the MPFC/vACC, precuneus/PCC, and the dorsolateral PFC. Additional clusters of activity were observed in the parietal cortex together with small clusters of activity in the occipital cortex. The corresponding analysis for the eyes‐open resting‐state condition yielded very similar results, in terms of presence of low‐frequency BOLD signal fluctuations at both the individual and the multi‐subject level of analysis (data not shown). In an attempt to gauge the relative strength of low‐frequency fluctuations among different brain regions across subjects, the individual statistical F‐contrast maps (eyes closed) were thresholded at F > 2.64 (P < 0.5 × 10−3, corrected) before applying the masking procedure. Apart from a few very small clusters, this considerably more conservative thresholding level resulted in a large cluster of surviving voxels that were localized to the precuneus/PCC (cluster size = 4.2 mL), as well as a smaller cluster in the MPFC (cluster size = 0.75 mL) and left angular gyrus (cluster size = 0.49 mL; Fig. 3, eyes closed). These findings suggest that resting‐brain BOLD fluctuations are particularly strong in the precuneus/PCC area and the MPFC.
Figure 1.
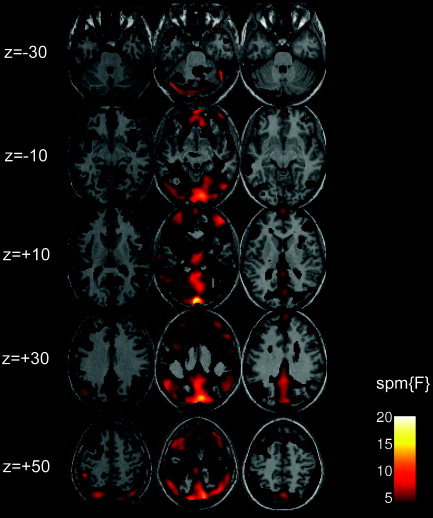
Spontaneous low‐frequency BOLD signal fluctuations during rest (eyes closed) in individual subjects. Images of F‐contrast statistical parametrical maps thresholded at F > 1.69 (P < 0.001, uncorrected) showing low‐frequency BOLD signal fluctuations in three representative subjects (left, middle, and right column, respectively). Spontaneous BOLD signal changes during rest are present in widespread areas in the brain that are localized predominately to gray matter. Of note is the particular strong presence in the MPFC, precuneus and the PCC in all three subjects.
Figure 2.
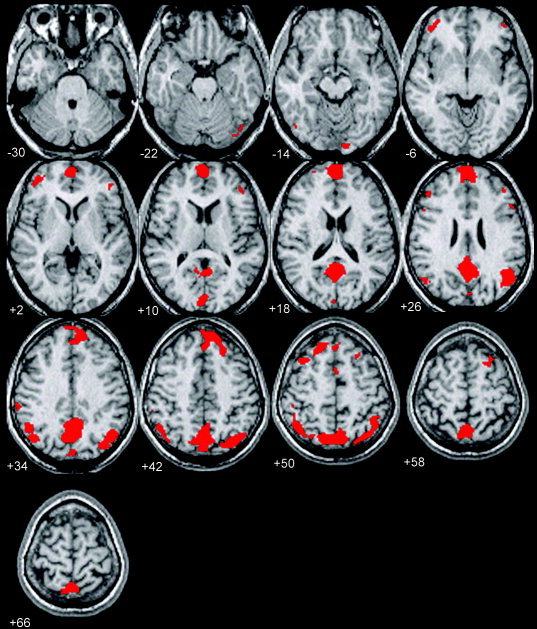
Multi‐subject analysis of BOLD signal fluctuations during rest with eyes closed. A binary mask for each subject was created by thresholding the individual F‐contrast images at F > 1.69 (P < 0.001 uncorrected). A final binary mask image showing voxels in the brain exhibiting low‐frequency fluctuations in all subjects at the specified statistical threshold was constructed by multiplying together all the individual binary mask images. Voxels that met these criteria are depicted in red and superimposed on a T1‐weighted high‐resolution MR image of one subject in the study. Spontaneous, low frequency BOLD signal changes at a multi‐subject level are observed in the prefrontal cortex with clusters centered foremost in the medial PFC and bilaterally in the dorsolateral cortices. Extensive areas of activity are present in the parietal cortex bilaterally and the precuneus and PCC.
Figure 3.
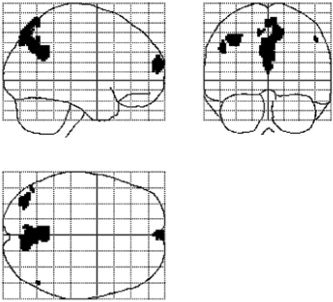
Multi‐subject analysis of BOLD signal fluctuations during rest (eyes closed). Maximum intensity projection (MIP) images showing voxels that exhibited low‐frequency fluctuations in all subjects at the statistical threshold of F > 2.64 (P < 0.5 × 10−3, corrected). Low‐frequency BOLD fluctuations are observed in the MPFC, precuneus/PCC, and the left angular gyrus.
Functional Connectivity During Rest
The temporal synchronicity among active brain regions during rest was assessed using correlation analysis. The strong occurrence of low‐frequency fluctuations in the precuneus/PCC region across all subjects warranted its use as a seed point (spherical ROI centered at 0, −52, 30; 10‐mm radius) for the functional connectivity analysis. Brain regions that correlated positively with the precuneus/PCC during rest are shown in a red‐yellow color‐scale in Figure 4 (eyes closed) and Figure 5 (eyes open). In both conditions, a strong positive correlation with the precuneus was observed in the MPFC (Brodmann areas [BA] 8/9/10), including the vACC (BA24/32) and extending dorsolaterally along BA8 and 9. Moreover, in both conditions a positive correlation was found bilaterally in the angular gyrus (BA39) and anterolateral temporal cortex (BA21). In the eyes‐closed condition, the bilateral parahippocampal gyrus (BA36), inferior frontal cortex (BA47), and the temporal pole (BA38), together with the thalamus, pons, and the right cerebellum, correlated positively with the precuneus/PCC during rest (Table I, eyes closed; Table III, eyes open). For the eyes‐open condition, the involvement of the parahippocampal gyrus and inferior frontal cortex was restricted to the left hemisphere, whereas the activity in the temporal pole was limited to the right hemisphere. A bilateral activation pattern was found for these regions when thresholding the statistical parametrical maps at P < 0.05, corrected. Similarly, pons and thalamus correlated positively with the precuneus/PCC during the eyes‐open condition, although at a lower statistical threshold (P < 0.05, corrected).
Figure 4.
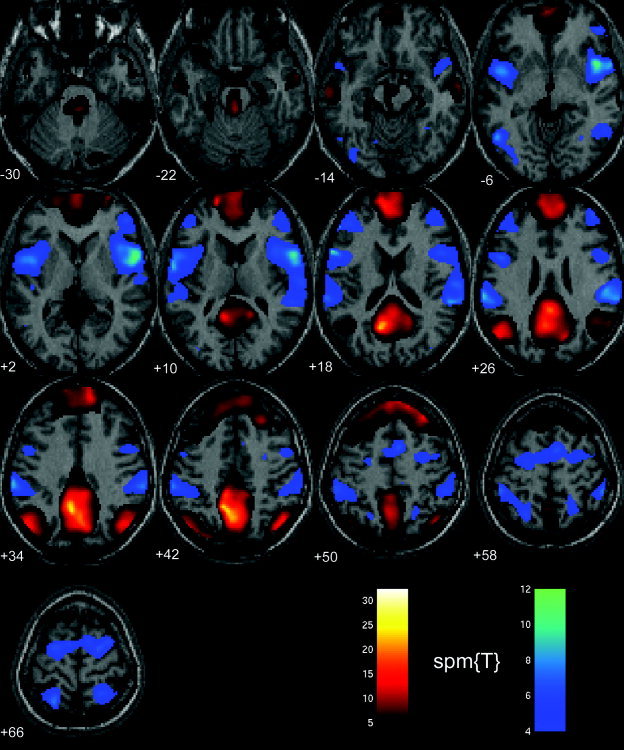
Functional connectivity analysis of low‐frequency BOLD signal changes during rest with eyes closed. The figure shows two statistical parametrical maps SPM[T] thresholded at P < 0.01, (corrected for multiple comparisons) superimposed on a single high‐resolution T1‐weighted image from one subject in the study. Areas color‐coded in a red‐yellow color scale correlate positively with the precuneus/PCC region and included medial and dorsolateral parts of prefrontal cortex, angular gyrus, anterolateral section of the temporal lobe, parahippocampal gyrus, thalamus, and pons. Brain regions that correlate negatively with the precuneus/PCC region are color‐coded in blue‐magenta color scale and included premotor cortex bilaterally, dorsolateral prefrontal cortex, supplementary motor cortex, inferior parietal lobe, occipital cortex, and the insula bilaterally.
Figure 5.
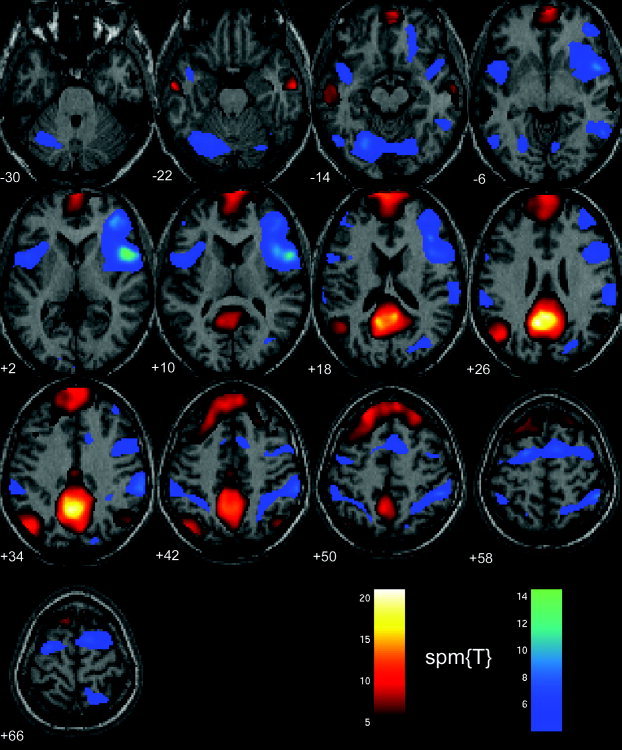
Functional connectivity during rest (eyes open). Brain regions that in the eyes‐open condition correlated positively (red‐yellow color‐coded) and negatively (blue‐magenta color‐coded) with the precuneus/PCC area are matched very closely to the corresponding brain regions involved for the eyes‐closed condition (see Fig. 4 for comparison). Minor differences between the two conditions exist. For example, the positive correlation in the parahippocampal gyrus and the inferior frontal cortex was only found in the left hemisphere in the eyes‐open condition. Moreover, a negative correlation with the precuneus was found in the lateral cerebellum for the eyes‐open condition but not in the eyes‐closed condition.
Table I.
Brain regions that correlated positively with the precuneus/PCC (ROI centered at [0, −52, −30]) during resting state and with eyes closed
| Region | x | y | z | BA | Peak Z score |
|---|---|---|---|---|---|
| Prefrontal cortex | |||||
| L MPFC | −14 | 66 | 0 | 10 | 7.19 |
| R MPFC | 26 | 60 | 4 | 10 | 7.94 |
| L MPFC | −12 | 62 | 30 | 9 | 8.50 |
| R MPFC | 12 | 54 | 30 | 9 | 16.25 |
| L DLPFC | −12 | 44 | 44 | 8 | 8.50 |
| R DLPFC | 30 | 34 | 48 | 8 | 13.29 |
| L MPFC/vACC | −10 | 48 | 0 | 24/32 | 6.89 |
| L inferior PFC | −26 | 20 | −20 | 47 | 5.18 |
| R inferior PFC | 36 | 26 | −22 | 47 | 5.11 |
| Parietal cortex | |||||
| L angular gyrus | −50 | −72 | 30 | 39 | 19.22 |
| R angular gyrus | 46 | −70 | 42 | 39 | 14.72 |
| Temporal cortex | |||||
| L anterolateral | −62 | −16 | −18 | 21 | 8.44 |
| R anterolateral | 60 | −2 | −26 | 21 | 7.44 |
| L temporal pole | −46 | 6 | −38 | 38 | 4.67 |
| R temporal pole | 52 | 8 | −36 | 38 | 4.72 |
| Subcortical | |||||
| L parahippocampal gyrus | −28 | −30 | −22 | 36 | 4.18 |
| R parahippocampal gyrus | 30 | −22 | −26 | 36 | 5.04 |
| L thalamus | −8 | −30 | 2 | — | 4.09 |
| Pons | 2 | −34 | −26 | — | 12.26 |
| L cerebellum | −34 | −82 | −34 | — | 4.70 |
| R cerebellum | 22 | −84 | −34 | — | 6.52 |
PCC, posterior cingulate cortex; ROI, region of interest; BA, Brodmann area; L, left; R, right; DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; vACC, ventral anterior cingulate cortex.
Table III.
Brain regions that correlated positively with the precuneus/PCC (ROI centered at [0, −52, −30]) during resting state and eyes open
| Region | x | y | z | BA | Peak Z score |
|---|---|---|---|---|---|
| Prefrontal cortex | |||||
| L MPFC | −8 | 70 | 20 | 10 | 12.89 |
| R MPFC | 10 | 72 | 14 | 10 | 10.83 |
| L MPFC | 6 | 60 | 36 | 9 | 10.58 |
| R MPFC | −4 | 48 | 36 | 9 | 9.70 |
| L DLPFC | −22 | 38 | 50 | 8 | 11.15 |
| R DLPFC | 26 | 36 | 50 | 8 | 9.15 |
| L MPFC/vACC | −4 | 48 | 2 | 24/32 | 4.49 |
| L inferior PFC | −48 | 30 | −12 | 47 | 5.55 |
| Parietal cortex | |||||
| L angular gyrus | −46 | −72 | 34 | 39 | 11.36 |
| R angular gyrus | 58 | −62 | 28 | 39 | 6.86 |
| Temporal cortex | |||||
| L anterolateral | −60 | −10 | −22 | 21 | 11.04 |
| R anterolateral | 62 | −10 | −22 | 21 | 9.99 |
| R temporal pole | 52 | 8 | −36 | 38 | 4.77 |
| Subcortical | |||||
| L parahippocampal gyrus | −26 | −20 | −20 | 36 | 4.73 |
| R cerebellum | 26 | −82 | −36 | — | 5.13 |
| R cerebellum (vermis) | 6 | −62 | −50 | — | 5.21 |
PCC, posterior cingulate cortex; ROI, region of interest; BA, Brodmann area; L, left; R, right; DLPFC, dorsolateral prefrontal cortex; MPFC, medial prefrontal cortex; vACC, ventral anterior cingulate cortex.
Several brain regions were found to correlate negatively with the precuneus. These regions are shown in a blue‐magenta color scale in Figure 4 (eyes closed) and Figure 5 (eyes open). Regions that correlated negatively with the precuneus/PCC in both conditions comprised the dorsolateral PFC (BA46) and the premotor (PM) cortex (BA6) including the inferior frontal gyrus (BA44), all bilaterally. In addition, the bilateral insula (BA13/14) correlated negatively with the precuneus/PCC. In the parietal lobe, negatively correlated regions included the supramarginal gyrus (SII, BA40) and the posterior parietal cortex (PPC, BA5). Finally, a negative correlation was found in the extrastriate cortex (BA39/19) in the occipital lobe that in the right hemisphere included the fusiform gyrus. A slightly larger area of negative correlation in extrastriate visual areas, including the bilateral fusiform gyrus and the cerebellum, could be observed in the eyes‐open condition. A full description of all regions that correlated negatively with the precuneus during rest is given in Table II (eyes closed) and Table IV (eyes open).
Table II.
Brain regions that correlated negatively with the precuneus/PCC (ROI centered at [0, −52, −30]) during resting state with eyes closed
| Region | x | y | z | BA | Peak Z score |
|---|---|---|---|---|---|
| Prefrontal cortex | |||||
| R DLPFC | 48 | 42 | −2 | 46 | 7.09 |
| L DLPFC | −48 | 42 | 20 | 46 | 6.35 |
| R dorsal PM | 30 | 0 | 64 | 6 | 6.45 |
| R dorsal PM | 30 | −6 | 46 | 6 | 5.57 |
| L dorsal PM | −20 | −6 | 72 | 6 | 7.56 |
| SMA | 6 | 4 | 54 | 6 | 7.02 |
| R ventral PM | 58 | 8 | 6 | 44 | 10.94 |
| L ventral PM | −62 | −2 | 10 | 44 | 8.88 |
| Parietal cortex | |||||
| R IPL (SII) | 64 | −34 | 26 | 40 | 8.04 |
| L IPL (SII) | −66 | −32 | 26 | 40 | 9.18 |
| L inferior | −56 | −38 | 58 | 40 | 7.09 |
| R inferior | 52 | −38 | 58 | 40 | 7.46 |
| R PPC | 30 | −54 | 70 | 5 | 8.36 |
| L PPC | −24 | −58 | 68 | 5 | 7.59 |
| Temporal cortex | |||||
| L medial | −52 | −62 | −4 | 37 | 8.36 |
| R medial | 54 | −56 | −6 | 37 | 5.69 |
| Occipital cortex | |||||
| R fusiform gyrus | 34 | −58 | −10 | 37 | 4.85 |
| L inferior | −38 | −78 | −10 | 19 | 5.38 |
| Subcortical | |||||
| L insula | −46 | 2 | −2 | 13/14 | 8.20 |
| R insula | 48 | 10 | −4 | 13/14 | 10.58 |
PCC, posterior cingulate cortex; ROI, region of interest; BA, Brodmann area; L, left; R, right; DLPFC, dorsolateral prefrontal cortex; PM, premotor cortex; SMA, supplementary motor area; IPL, inferior parietal lobe; PPC, posterior parietal cortex.
Table IV.
Brain regions that correlated negatively with the precuneus/PCC (ROI centered at [0, −52, −30]) during resting state with eyes open
| Region | x | y | z | BA | Peak Z score |
|---|---|---|---|---|---|
| Prefrontal cortex | |||||
| R DLPFC | 46 | 42 | 6 | 46 | 8.86 |
| L DLPFC | −50 | 40 | 14 | 46 | 4.31 |
| R dorsal PM | 10 | 4 | 60 | 6 | 7.55 |
| L dorsal PM | −20 | 0 | 58 | 6 | 8.21 |
| R dorsal PM | 50 | 6 | 32 | 6/44 | 6.11 |
| SMA | 2 | 2 | 64 | 6 | 8.17 |
| R ventral PM | 56 | 10 | 6 | 44 | 14.52 |
| L ventral PM | −48 | 8 | 4 | 44 | 6.96 |
| Parietal cortex | |||||
| L IPL (SII) | 68 | −26 | 20 | 40 | 7.19 |
| R IPL (SII) | −66 | −36 | 24 | 40 | 6.25 |
| R inferior | 50 | −42 | 56 | 40 | 9.55 |
| L inferior | −50 | −46 | 56 | 40 | 5.13 |
| R PPC | 24 | −56 | 70 | 5 | 5.10 |
| Temporal cortex | |||||
| L medial | −54 | −64 | −14 | 37 | 7.49 |
| R inferior | 56 | −56 | −8 | 37 | 8.65 |
| Occipital cortex | |||||
| L fusiform gyrus | −24 | −66 | −12 | 37/19 | 8.51 |
| R fusiform gyrus | 18 | −72 | −12 | 19 | 5.18 |
| Subcortical | |||||
| L insula | −48 | 8 | 4 | 13/14 | 6.96 |
| R insula | 56 | 10 | 6 | 13/14 | 14.52 |
| L cerebellum | −28 | −44 | −46 | — | 5.06 |
| R cerebellum | 30 | −48 | −48 | — | 4.99 |
PCC, posterior cingulate cortex; ROI, region of interest; BA, Brodmann area; L, left; R, right; DLPFC, dorsolateral prefrontal cortex; PM, premotor cortex; SMA, supplementary motor area; IPL, inferior parietal lobe; PPC, posterior parietal cortex.
The temporal profiles of the two networks of brain regions that showed a functional connectivity during rest suggest that a signal decrease in the negatively correlated brain regions occurs whenever there is a signal increase in the positively correlated brain regions. In the reverse situation, with a signal decrease in positively correlated regions, one should accordingly expect to observe a corresponding signal increase in the negatively correlated regions. BOLD signal intensity time‐courses for a subsample of regions belonging to either of the two networks are shown in Figure 6 during a time excerpt of one 10‐min resting‐state scanning session (eyes closed) in one representative subject. Whereas the upper panel shows the signal intensity time‐course for brain regions that correlated positively with the precuneus/PCC, the lower panel shows the corresponding signal intensity time‐courses for the negatively correlated brain regions. Indeed, a signal increase in the brain regions shown the upper panel is accompanied by a signal decrease for the brain regions presented in the lower panel. Similarly, in the reverse situation with a signal decrease in the regions depicted in the upper panel, a corresponding signal increase is found for the regions shown in the lower panel. Taken together, these findings suggest that from the perspective of low‐frequency BOLD fMRI signal changes, there exist at least two networks of brain regions that are active during rest. The term networks is used here solely to denote the existence of two ensembles of activated clusters. No claims regarding functional correspondence among individual clusters of activation are therefore made beyond the fact that they either correlated negatively or positively with the PCC/precuneus during rest.
Figure 6.
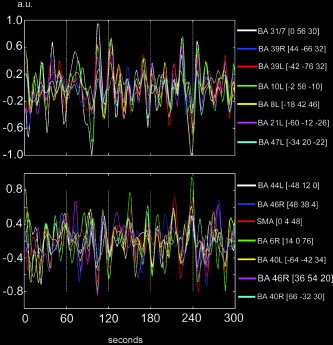
BOLD signal intensity time‐courses during rest (eyes closed) for a selected set of region‐of‐interests in one representative subject. Each signal intensity time course represents the mean time course from all voxels inside a spherical (radius = 10 mm) ROI positioned in proximity to the local maxima of activated clusters given by the connectivity analysis shown in Figure 4. Top: Signal intensity time course for a selected set of regions that correlated positively with the precuneus/PCC. Bottom: Temporal profile for a set of regions that correlated negatively with the precuneus/PCC. All signal intensity time courses were bandpass filtered.
In this context, a possible confounding factor when analyzing signal changes in this low‐frequency range is the possibility that the signal is compromised by cardiac‐related noise. This could possibly occur because sampling frequency used here (0.5 Hz) does not critically sample conceivable signal contributions from cardiac‐related signal fluctuations [Lund, 2001]. According to the Nyqvist theorem, if the critical sampling frequency condition is not met, cardiac‐related noise may be aliased into the frequency spectrum of interest. To rule out the possibility that the results were affected by contamination from cardiac noise, additional resting‐brain scanning sessions were acquired at a sampling frequency of 2 Hz that encompassed a reduced spatial resolution and coverage but nevertheless sampled the precuneus/PCC and the MPFC/vACC. Virtually identical results in individual subjects in terms of maps of low‐frequency fluctuations were obtained in the scanning sessions with the higher sampling frequency compared to the lower sampling frequency scans.
DISCUSSION
The temporal and spatial characteristics of low‐frequency fluctuations in subjects that were instructed to rest and refrain from any cognitive effort were investigated in the present study. An explicit modeling of the possible contents of spontaneous low‐frequency fluctuations in resting‐state fMRI signal time‐series revealed widespread clusters of activity in the brain with a predominance to gray matter.
Spontaneous Low‐Frequency BOLD Signal Oscillations During Rest
Importantly, a strong presence of fluctuations was observed in the precuneus/PCC and MPFC area in all subjects. These results are in agreement with the notion that occurrence of low‐frequency fluctuations are not distributed evenly throughout the brain. Rather, the findings show an increased level of spontaneous fluctuation in brain regions that have been shown to decrease their activity during goal‐directed, attention‐demanding tasks [Shulman et al., 1997]. Several other PET and fMRI studies that have shown that the precuneus/PCC and the MPFC/vACC consistently decrease their activity in variety of cognitive tasks all have in common the fact that they direct the subjects' attention toward externally generated events and suppress attention toward internally generated processes [Binder et al., 1999; McKiernan et al., 2003; Lawrence et al., 2003]. Moreover, the MPFC/vACC and the precuneus/PCC have also been implicated in internally generated processes such as self‐reflection/self‐awareness [Johnson et al., 2002] or self‐referential judgments of emotionally laden pictures [Gusnard and Raichle, 2001]. The observed heterogeneity of spontaneous low‐frequency fluctuations, with emphasis on the MPFC/vACC and the precuneus/PCC, thus provides further experimental support to the idea originating from the default mode hypothesis, namely that these regions are involved crucially in self‐referential mental processing during resting‐state baseline conditions.
Functional Connectivity During Rest
The precuneus/PCC was taken as a starting‐point for the functional connectivity analysis (0, −52, 30). During rest and independent of resting‐state condition, the precuneus/PCC correlated positively with the MPFC, bilateral angular gyrus, bilateral anterolateral temporal cortex, bilateral parahippocampal gyrus, thalamus, and pons. In addition to signal decreases during various goal‐directed tasks and signal increases for self‐reflective thought, the MPFC, as well as the angular gyrus (BA39) and the anterolateral temporal cortex/temporal pole (BA21/38), has been suggested to play a vital role for tasks that involve “mentalizing.” The concept of mentalizing is associated with the ability to represent not only the mental states such as thoughts, feelings, and beliefs about oneself but also representing the mental states of other people [Frith and Frith, 2003]. Furthermore, the MPFC, together with the angular gyrus, anterolateral temporal cortex/temporal pole, thalamus, and medial temporal lobe, has been attributed to a network of brain regions that sustain a memory‐retrieval system for autobiographic events [Maguire and Mummery, 1999]. A recently published review of the neuroimaging literature on activity in the PCC suggested that it is activated consistently for processing emotional states and episodic memory retrieval processes and might have a integrative role between emotion and episodic memory retrieval [Maddock, 1999]. Taken together, the precuneus/PCC network of active brain regions during rest described here is in overall agreement with the network that supposedly supports a default mode of brain function [Raichle et al., 2001]. Further, the data reported here supports the previously proposed idea that this network is most likely invoked to support introspectively oriented mental activity [Gusnard and Raichle, 2001]. The finding of a network showing a functional connectivity during rest is in good agreement with a recent functional connectivity study of the resting brain [Greicius et al., 2003], although those results deviate from the present data because in their study, activity in the angular and parahippocampal gyri is lateralized to the left hemisphere. A possible explanation for this discrepancy might stem from the fact that the scanning sessions used by Greicius et al. [2003] were less than half as long as the scanning duration used here (4 vs. 10 min), which results in a reduced sensitivity compared to that in the present study.
In addition to the analysis of brain regions that correlated positively with the PCC/precuneus, an examination of negatively (or inversely) correlated brain regions was carried out. This analysis revealed a network of regions that showed a negative correlation with the precuneus/PCC during rest. The network comprised the dorsolateral PFC (BA46), dorsal premotor cortex (BA6), including the supplementary motor area (SMA) and the inferior frontal gyrus/ventral premotor cortex (BA4), all bilaterally. In addition, negatively correlated brain regions included the bilateral supramarginal gyrus (SII, BA40), the PPC (BA5), insula (BA13/14), and the extrastriate cortex (BA39/19). The premotor cortex (PM) has been attributed to preparation and organization of movement [Wise, 1985]. More recent neuroimaging findings have elaborated on these general ideas to include the concept that the PM maintains a short‐term representation of structured dynamics based on which either sensory prediction or action planning can be carried out, i.e., the PM is an action‐related forward model of what the brain expects to experience in the short term [Schubotz and von Cramon, 2003]. Similarly, it has been suggested that the PM, together with the SMA, takes part in imagined movement or neuronal simulation of action [Jeannerod, 2001]. The understanding of the PM and SMA as being critical components of the neuronal network that supports preparation and readiness for voluntary movement is in agreement with recent EEG [Ball et al., 1999], magnetoencephalography (MEG) [Erdler et al., 2000], and fMRI [Cunnington et al., 2003] studies of neuronal correlates of the Breitschaftspotential [Kornhuber and Deecke, 1965].
It has been proposed that the dorsolateral PFC (BA46) is associated with willed action, i.e., responses that are generated internally [Frith et al., 1991]. Other studies have implicated the dorsolateral PFC in tasks that involves working memory. Recent studies have formulated the function of the dorsolateral PFC to be “attention to the selection of responses” [Lau et al., 2004]. In this analysis, coactivation was observed bilaterally in the PPC, an area that previous studies have described to constitute an “association area” assigned to associate different sensory modalities. Within this conceptual framework, the role of the PPC has been extended to include sensorimotor integration, perception and interpretation of spatial relationships, and accurate body maps [Andersen and Buneo, 2003]. The putative second somatosensory area (SII) located in BA40 was activated bilaterally in this study. Previous studies have indicated that the SII area adjacent to the lateral sulcus contains a complete representation of the body, although with considerably larger receptive fields than that in the SI. The SII has therefore been attributed to the sensorimotor integration of sensory input across large body parts. Moreover, human and nonhuman studies suggest that the SII region is critical for coordination tasks involving somatic and motor activation of multiple body parts [Disbrow et al., 2000]. The insula has been implicated largely in interoceptive perception in terms of monitoring of feelings such as pain, temperature, itch, vasomotor activity, visceral sensations, hunger, and thirst. In a recent review, the anterior insula was labeled as the “interoceptive cortex” and argued to be a part of a system that constitutes a representation of “the material me” [Craig, 2002].
From a collective network‐oriented perspective, the findings presented here speak in favor of an interpretation that suggests that these brain regions might support a state‐of‐mind that is extrospectively oriented. An extrospectively oriented state‐of‐mind, whenever it is active, implies that the subjects' attention at that specific moment is oriented toward potential changes that are not necessarily limited to changes in the extra‐personal space, but also toward changes in the inner milieu, e.g., an augmented sensation of pain or thirst. Tentatively, the findings presented here suggest that the primary function of this network, whenever engaged, is to enter a mode of preparedness and alertness for possible changes, in terms of bodily states and external events that are of immediate relevance to the individual.
Interestingly, the heterogeneity in terms of the presence of low‐frequency fluctuations in the brain and networks of functional connectivity active during rest are very similar during the eyes‐closed compared to the eyes‐open condition. This similarity is in line with previous resting‐state results obtained by metaanalysis of PET data collected during eyes‐closed conditions [Mazoyer et al., 2001] and passive fixation [Shulman et al., 1997; for review see Wicker et al., 2003]. Both PET studies reported an increased activity during rest in the MPFC, angular gyrus, precuneus/PCC, and vACC. As pointed out by Shulman et al. [1997] and Mazoyer et al. [2001], self‐reflective thought as well as monitoring of internal and external environment and emotional states could be at work in passive viewing conditions as well as during the eyes‐closed condition. Moreover, the similarity in terms of the observed oxygen extraction factor in both conditions has been discussed previously by Gusnard and Raichle [2001].
Two Default Modes of Brain Function?
The results from the experiments described herein corroborate previous studies of the resting brain with regard to the idea that during rest, we activate brain regions that most likely are engaged for self‐reflective and self‐referential mental activity [Greicius et al., 2003; Gusnard and Raichle, 2001]. The results from the functional connectivity analysis presented in Figures 4, 5, 6 suggest that during rest the brain is not tonically active in a single, self‐reflective, default mode of brain function. Based on these findings, it is speculated that that the hyperfrontal activity during rest that supposedly characterizes inner thought, self‐reflective thinking in terms of planning for the future, or simulation of behavior [Ingvar, 1979], is interrupted on a routine basis. During these interruptions, it is hypothesized that the resting state is shifted temporarily from introspective processes into a state‐of‐mind that perhaps can be characterized best as extrospective, in terms of increased attention and readiness, and an engagement of networks that support sensorimotor planning for future routes of action in response to potential changes in the inner and outer environment. It seems that recurrent interruptions from the otherwise continuous streams of consciousness during rest have a survival value to the individual in that it may provide a neuronal mechanism for a survey of environmental changes that may pose a potential threat or danger to the individual.
The ideas outlined here are in agreement with recent studies that have employed simultaneous fMRI and EEG recordings of the brain during rest [Laufs et al., 2003a, b]. A strong positive correlation was detected by Laufs et al. [2003b] between the 17–23 Hz (β‐2) frequency range of EEG activity and the fMRI BOLD signal in the precuneus/PCC, bilateral angular gyrus, and MPFC. This observation links the activity in the β‐2 frequency range with the network of brain regions that underlies the default mode of brain function. In addition, the same study showed a negative correlation during rest between fMRI BOLD signal changes and EEG activity in the α frequency range (8–12 Hz) in a frontoparietal network of brain regions. Moreover, this frontoparietal network showed large similarities with the extrospectively oriented network of brain regions in the present study. As pointed out by Laufs et al. [2003b], the interpretation of a frontoparietal network that is invoked to set the brain in a orienting mode is in good agreement with the classic interpretation that the α blockade is an orienting reaction rather than a sensory process.
It is interesting to view the current results in the perspective of the neuronal networks governing control of attention. According to the work by Corbetta and Shulman [2002], bottom‐up or stimulus‐driven control of attention is driven largely by a ventral frontoparietal network that includes the intraparietal lobule/superior temporal gyrus (BA40), SMA, and the inferior frontal gyrus/middle frontal gyrus (BA6/44). Goal‐directed or top‐down control is driven by a dorsal frontoparietal network that encompasses the intraparietal sulcus, superior parietal lobe, and the human analogue of the frontal eye fields (BA40/5/7). The areas implicated in these two systems of attention largely lie within the network of cortical regions that herein was found active in the extrospective mode. The fact that both top‐down and bottom‐up control of attention are active simultaneously during periods of extrospection is in line with the notion that orienting in humans is controlled by the interaction of the two attention systems [Corbetta and Shulman, 2002]. This might be interpreted to mean that top‐down and bottom‐up processes of attentional control need to interact dynamically to survey and react to behaviorally relevant stimuli in the external environment. These attentional mechanisms, at least in part, seem to be multimodal and thus be active independently of sensory modality [Downar et al., 2000].
There are methodologic limitations of the current study that should be considered when interpreting the results. First, it is well known that the most ventral parts of the forebrain, including the orbitofrontal cortex and the anteroventral part of the medial temporal lobe, suffers from MR signal losses due to macroscopic susceptibility artifacts caused by tissue–air interfaces [Ojemann et al., 1997]. An absence of activity in these areas therefore should be interpreted with caution. Second, we know from the questionnaires that all subjects experienced frequent episodes of self‐referential mental processing such as autobiographic reminiscences during scanning. The exact temporal profile of these thoughts in every individual scan cannot be determined in the present experimental design, although they are accurately modeled using the discrete cosine basis function set. It should also be kept in mind that the changes in neuronal activity responsible for the temporal characteristics of the toggle between introspection and extrospection are filtered by the hemodynamic response function.
In summary, the data presented here suggest that there exists a close relationship between the spatial distribution of spontaneous low‐frequency fluctuations of BOLD fMRI signals and the brain regions suggested to be active in the resting brain. It is proposed that the default mode of brain function as described by Raichle et al. [2001] could be augmented with a second mode of brain function that tentatively reflects the existence of an extrospectively oriented attention network in the brain that is engaged occasionally in the resting brain. The results presented herein extend previous studies on the resting‐state condition in that an instruction to “to do nothing” might entail a recurring switch between an introspective versus an extrospectively oriented state‐of‐mind.
NOTE ADDED IN PROOF
The usage of global normalization of EPI data sets has been debated [see Aguirre et al., Neuroimage 1998;8:302–306]. As a check of consistency, our data were re‐analyzed without global normalization. This resulted in very minor changes for the introspectively oriented network, whereas an overall reduction of the corresponding t‐scores were obtained for the extroceptive network. However, essentially all regions attributed to the extroceptive network were retained at P < 0.07 (corrected).
Acknowledgements
I thank K.M. Petersson, J. Andersson, and M. Ingvar for helpful discussions, and K. Gehlsdorf for linguistic support.
REFERENCES
- Aguirre GK, Zarahn E, D'Esposito M (1998): The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage 8: 302–306. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA (2003): Sensorimotor integration in posterior parietal cortex. Adv Neurology 93: 159–177. [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K (2001): Modeling geometric deformations in EPI time series. Neuroimage 13: 903–915. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD (1995): Remembering the past: two facets of episodic memory explored with position emission tomography. Am J Psychiatry 152: 1576–1585. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AD, Frith CD, Dolan RJ, Frackowiak RSJ, Robbins TW (1996): Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia 6: 515–526. [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Luecking CH, Kristeva‐Feige R (1999): The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. Neuroimage 10: 682–694. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–214. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski A, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MR imaging. ANJR Am J Neuroradiol 21: 1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E (2003): The preparation and readiness for voluntary movement: a high‐field event‐related fMRI study of the Bereitschafts‐BOLD response. Neuroimage 20: 404–412. [DOI] [PubMed] [Google Scholar]
- Craig BD (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–665. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Krubitzer L (2000): Somatotopic organization of cortical fields in the lateral sulcus of homo sapiens: evidence for SII and PV. J Comp Neurol 418: 1–21. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283. [DOI] [PubMed] [Google Scholar]
- Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windishberger C, Lindinger G, Deecke L (2000): Supplementary motor areas activation preceding voluntary movement is detectable with a whole‐scalp magnetoencephalographic system. Neuroimage 11: 697–707. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KP, Poline JB, Frith CD, Frackowiak RS (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS (1991): Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD (2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Wirsen‐Meurling A, Wredling R, Eriksson L, Stone‐Elander S, Levander S, Ingvar M (1995): Brain activation induced by the perceptual maze test: a PET study of cognitive performance. Neuroimage 2: 112–124. [DOI] [PubMed] [Google Scholar]
- Giambra LM (1995): A laboratory method for investigating influences on switching attention to task‐unrelated imagery and thought. Conscious Cogn 4: 1–21. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: a network analysis of the default mode of brain function. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC (2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalisability, random effects and population inference. Neuroimage 7(Suppl): 754. [Google Scholar]
- Ingvar DH (1979): “Hyperfrontal” distribution of cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol Scand 60: 12–25. [DOI] [PubMed] [Google Scholar]
- Jeannerod M (2001): Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14(Suppl): 103–109. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP (2002): Neural correlates of self‐reflection. Brain 125: 1808–1814. [DOI] [PubMed] [Google Scholar]
- Kornhuber HH, Deecke L (1965): Hirnpotentialänderungen bei Willkuerbewegungen und passiven Bewegungen den Menschen: Bereitschaftspotential und reafferente Potentiale. Pflugers Arch Gesamte Physiol Menschen Tiere 284: 1–17. [PubMed] [Google Scholar]
- Krueger G, Glover GH (2001): Physiological noise in oxygenation‐sensitive magnetic resonance imaging. Magn Reson Med 46: 631–637. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N., Passingham RE (2004): Willed action and attention to the selection of responses. Neuroimage 21: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek‐Haddadi A, Preibisch C, Krakow K (2003)a: EEG‐correlated fMRI of human alpha activity. Neuroimage 19: 1463–1476. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek‐Haddadi A, Kleinschmidt A (2003)b: Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci USA 100: 11053–11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multi‐slice echoplanar imaging using resting‐state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]
- Lund TE (2001): fcMRI—mapping functional connectivity or correlation cardiac‐induced noise? Magn Reson Med 46: 628. [DOI] [PubMed] [Google Scholar]
- Maddock RJ (1999): The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 22: 310–316. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ (1999): Differential modulation of a common retrieval network revealed by positron emission tomography. Hippocampus 9: 54–61. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287–298. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE (1997): Anatomic localization and quantitative analysis of gradient refocused echo‐planar fMRI susceptibility artifacts. Neuroimage 6: 156–167. [DOI] [PubMed] [Google Scholar]
- Peltier SJ, Noll DC (2002): T2* dependence of low frequency functional connectivity. Neuroimage 16: 985–992. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz R, von Cramon D (2003): Functional–anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage 20: 5120–5131. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9: 648–663. [DOI] [PubMed] [Google Scholar]
- Stark C, Squire L (2001): When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98: 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 122 p. [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT (1999): Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp 8: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P (2003): A relation between rest and the self in the brain? Brain Res Brain Res Rev 43: 224–230. [DOI] [PubMed] [Google Scholar]
- Wise SP (1985): The primate premotor cortex: past, present and preparatory. Annu Rev Neurosci 8: 1–19. [DOI] [PubMed] [Google Scholar]


