Abstract
Limited processing resources are allocated preferentially to events that are relevant for behavior. Research using the novelty “oddball” paradigm suggests that a widespread network of limbic, paralimbic, and association areas supports the goal‐directed processing of task‐relevant target events. In that paradigm, greater activity in diverse brain areas is elicited by rare task‐relevant events that require a subsequent motor response than by rare task‐irrelevant novel events that require no response. Both stimulus infrequency (unexpectedness) and novelty, however, may contribute to the pattern of activity observed using that paradigm. The goal of the present study was to examine the supramodal neural activity elicited by regularly occurring, equiprobable, and non‐novel stimuli that differed in the subsequent behavior they prescribed. We employed event‐related functional magnetic resonance imaging (fMRI) during auditory and visual versions of a Go/NoGo task. Participants made a motor response to the designated “Go” (target) stimulus, and no motor response to the equiprobable “NoGo” (nontarget) stimulus. We hypothesized that task‐relevant Go events would elicit relatively greater hemodynamic activity than would NoGo events throughout a network of limbic, paralimbic, and association areas. Indeed, Go events elicited greater activity than did NoGo events in the amygdala‐hippocampus, paralimbic cortex at the anterior superior temporal sulcus, insula, posterior orbitofrontal cortex, and anterior and posterior cingulate cortex, as well as in heteromodal association areas located at the temporoparietal junction, anterior intraparietal sulcus and precuneus, and premotor cortex. Paralimbic cortex offers an important site for the convergence of motivational/goal‐directed influences from limbic cortex with stimulus processing and response selection mediated within the frontoparietal areas. Hum Brain Mapp 24: 35–49, 2005. © 2004 Wiley‐Liss, Inc.
Keywords: event‐related functional magnetic resonance imaging (fMRI), Go/NoGo, oddball target detection, conjunction analysis, auditory and visual sensory modalities
INTRODUCTION
Effective behavior is predicated in large part on the preferential allocation of limited processing resources to events that are relevant to the organism's goals. Dysfunction in the neural substrate supporting goal‐directed processing may precipitate symptoms such as apathy, which are observed in various psychiatric and neurologic illnesses, including schizophrenia and Alzheimer's disease [Brown and Pluck, 2000]. Within the laboratory setting, goal‐directed processes may be examined readily using paradigms that incorporate the processing of a target stimulus that signals the need to engage in a prescribed motor response. Using event‐related functional magnetic resonance imaging (fMRI), the present study sought to elucidate the supramodal network of brain areas that supports goal‐directed stimulus‐response processing by examining the neural activity elicited during the processing of simple auditory and visual target stimuli.
Previous research using the “oddball” target detection paradigm suggests that multiple brain areas may support goal‐directed processing. In the oddball paradigm, participants are required typically to detect and respond behaviorally to an infrequent target event that occurs against a background of frequent nontarget (standard) events for which no behavioral response is required. A variant of this task additionally incorporates infrequent novel or distracter stimuli that also require no motor response. Based on intracranial recordings made during auditory and visual oddball detection, Halgren et al. [ 1998] reported a supramodal network of brain areas that seemed to be specialized for processing of infrequent target stimuli that specified a behavioral response. This network incorporated medial temporal (hippocampal and perirhinal) cortex, cortex at the superior temporal sulcus, ventrolateral/orbitofrontal cortex, and superior posterior parietal cortex (i.e., cortex at the intraparietal sulcus). Halgren et al. [ 1998] identified additional brain areas that were active during the processing of the task‐relevant target events, but not exclusively, including inferior parietal cortex (at the temporoparietal junction), cingulate, and dorsolateral prefrontal cortex. These areas were active during processing of rare, task‐relevant target stimuli that necessitated a motor response as well as that of rare, task‐irrelevant novel/distracter stimuli for which no behavioral response was required. This finding led Halgren et al. [ 1998] to propose that these areas are specialized for orientation of attention to salient stimuli, regardless of whether or not they are overtly attended.
Using event‐related fMRI during auditory and visual oddball detection, Kiehl et al. [ 2001a, b] confirmed that an infrequent target stimulus elicits relatively greater activation than does frequently occurring nontarget stimuli in a widespread network of brain areas. These regions incorporate limbic cortex in amygdala‐parahippocampal gyrus, paralimbic cortex (i.e., anterior and posterior cingulate gyri, and cortex in the frontal operculum encompassing the anterior superior temporal sulcus [STS], insula, and inferior frontal/orbitofrontal gyrus), as well as bilateral cortex at the temporoparietal junction, intraparietal sulcus (extending medially into precuneus), superior frontal (premotor) cortex, and sensorimotor, subcortical (putamen and thalamus), and cerebellar regions [see also Ardekani et al., 2002; Braver et al. 2001; Clark et al., 2000; Linden et al., 1999; McCarthy et al., 1997; Menon et al., 1997; Stevens et al., 2000].
These results suggest that processing of a stimulus designated for a behavioral response elicits activity within a network of brain areas. However, the ability to infer the brain regions that support goal‐directed processing from the comparison of the activity elicited by the target and nontarget events in the oddball paradigm is confounded by the attentional capture (i.e., orienting) evoked by the relative infrequency of the target event. Using event‐related potentials (ERPs), several investigators [Duncan‐Johnson and Donchin, 1977; Katayama and Polich, 1996; Polich et al., 1996] have demonstrated that the amplitude of the P300 potential elicited by target events increases as target infrequency increases (i.e., target probability decreases). Changes in target probability have also been associated with changes in the hemodynamic response elicited during fMRI of the oddball paradigm [Casey et al., 2001; Horovitz et al., 2002].
Kiehl et al. [ 2001a, b] directly compared the activity evoked by equally infrequent target and novel stimuli that occurred against a background of frequent, regularly repeated nontarget stimuli. Many areas activated by target stimuli were also activated by novel events for which no motor response was required. However, the activation elicited by task‐relevant target events was significantly greater than that elicited by novel events in each area described above for target relative to nontarget processing. Even when comparing events of equivalent infrequency, there thus seems to be preferential recruitment of a widespread network of areas for stimuli relevant to the participant's behavioral goal. However, the contribution of differential familiarity of target and novel stimuli to the observed pattern of activation remains unknown. Although the network seemed to be activated for processing of both auditory [Kiehl et al., 2001b] and visual [Kiehl et al., 2001a] stimuli, assessment of hemodynamic response elicited in multiple stimulus modalities within the same participants is required to confirm whether the network of brain areas recruited during goal‐directed target processing is indeed supramodal (i.e., modality nonspecific).
To distinguish the brain response associated with goal‐directed target processing from that associated with the attentional capture elicited by stimulus infrequency or novelty, one might examine the brain response elicited during the performance of a task in which a target stimulus that requires a behavioural response is equiprobable with a non‐novel stimulus that requires no motor response. An example of this method is the Go/NoGo paradigm. In this paradigm, the salience of the target (Go) and nontarget (NoGo) stimulus may be made equivalent in all respects but the critical aspect, namely, the behavioral goal that is ascribed solely to the target stimulus.
The Go/NoGo paradigm has been used previously to elucidate the brain areas that support the response inhibition processes involved in withholding a motor response to the NoGo event. To optimize examination of response inhibition function, typically a Go/NoGo variant is used that induces a prepotent response tendency by including of few NoGo trials (generally between 6–25%) among a preponderance of Go events [e.g., de Zubicaray et al., 2000; Durston et al., 2002; Ford et al., 2004; Garavan et al., 2002; Kiehl et al., 2000; Mathalon et al., 2003; Mostofsky et al., 2003]. The brain areas supporting response inhibition are then inferred from the brain activity elicited by the NoGo event relative to a Go event baseline or control task. These studies, which have been conducted in the visual modality only, generally concur in reporting activation elicited during response inhibition on NoGo trials in the anterior cingulate cortex, dorsolateral prefrontal cortex, premotor cortex, and posterior parietal cortex, particularly in the right hemisphere. However, NoGo stimulus infrequency in these task variants increases the salience of the Go stimulus, and confounds examination of the NoGo stimulus relative to goal‐directed processing of the task‐relevant Go stimulus. The infrequency of NoGo stimuli enhances an expectation that the next stimulus will be a Go stimulus, thereby promoting a more rapid, and less considered, decision in favor of responding to Go stimuli. Other studies, from our laboratory [Liddle et al., 2001] and others [Konishi et al., 1998; Watanabe et al., 2002], have studied response inhibition using equiprobable visual Go/NoGo tasks during event‐related fMRI. These studies, however, established a prepotent motor response by introducing a cue stimulus (or series of cue stimuli) to signal the impending presentation of the next test event (irrespective of event type), thereby facilitating rapid Go responses and creating a bias toward responding. In such cases, the salience of the NoGo stimulus (and withholding an inappropriate motor response to that stimulus) is again increased relative to the Go stimulus. Similarly, interpretation of the findings of Braver et al. [ 2001], who also employed a task in which Go and NoGo trials were equally probable, is complicated by the inclusion of multiple Go stimulus variants (i.e., any letter that was not an X) versus only a single NoGo (i.e., letter X) stimulus. The differential frequency and familiarity of the Go and NoGo stimuli may have created a difference in salience between the event types that confounds an examination of goal‐directed processing differences.
We employed event‐related fMRI to examine the neural response elicited commonly across auditory and visual modalities during processing of and motor response to a Go stimulus relative to an equiprobable NoGo stimulus requiring no motor response. To remove the attentional effect of event‐infrequency and stimulus novelty, we repeatedly presented only two stimuli, one of which was designated the Go event and the other the NoGo event. Based on the oddball detection studies of Kiehl et al. [ 2001a, b], we hypothesized that goal‐directed processing of the task‐relevant Go stimulus would elicit greater activity than would the NoGo stimulus in a supramodal network incorporating limbic, paralimbic, and neocortical association areas. The target stimuli‐elicited activity in the oddball detection research by Halgren et al. [ 1998] and Kiehl et al. [ 2001a, b] suggests that this network may encompass the amygdala/hippocampus, paralimbic cortex at the frontal operculum and in the anterior and posterior cingulate gyri, as well as association cortex at the temporoparietal junction, the intraparietal sulcus and precuneus, and superior frontal (premotor) cortex. The Go/NoGo variant employed was not designed to elicit marked response inhibition processes on NoGo events. Should participants have acquired a prepotent tendency for motor responding, however, previous response inhibition research suggests that NoGo events might elicit relatively greater activity than would Go events in the anterior cingulate, dorsolateral prefrontal, premotor, and parietal cortices, particularly in the right hemisphere.
SUBJECTS AND METHODS
Participants
Ten right‐handed volunteers (mean age ± SD, 24.4 ± 5.1 years; 5 females) with normal visual and auditory acuity participated in the study [handedness assessed using the questionnaire of Annett, 1970]. Participants were medication‐free and without history of neurologic or psychiatric illness. All procedures complied with University and Hospital ethical requirements, and participants provided written informed consent before scanning.
Task
Visual and auditory stimuli were presented to the participant by a computer‐controlled presentation system (online at http://nilab.psychiatry.ubc.ca/vapp). Visual stimuli were displayed on a rear projection screen mounted at the entrance to the magnet bore. Participants viewed the screen from approximately 2 m away via a mirror system attached to the head coil. The scanning room and magnet bore were darkened to permit easy visualization of the stimuli. During both visual and auditory scanning runs, a white 62 × 32 cm rectangular box was presented continuously on the screen to ensure a restricted fixation space during all tasks.
Only two stimuli (occurrence probability = 0.5) were used in each sensory modality so that Go and NoGo events were equally salient in frequency and familiarity. Designated Go and NoGo stimuli were also counterbalanced across participants to ensure that particular stimulus characteristics would not contribute to salience differences between the Go and NoGo event types. In this way, the Go and NoGo events differed only in terms of the behavioral goal associated with the stimuli.
In the visual task, the Go and NoGo trials were counterbalanced such that five participants were instructed to respond to the stimulus letter X and to make no motor response to the letter K. The other five subjects responded to the letter K and made no motor response to the letter X. The letters were presented in random order, in white font against a black background, for a period of 200 ms and subtended a visual angle of approximately 6 degrees. Two visual stimulus runs, each containing 35 Go and 35 NoGo trials, were presented to the participant. An ISI of 7‐s ensured that the stimuli had an equal probability of occurring 0, 1, or 2 s after commencement of the 3‐s image volume acquisition period (i.e., TR). The hemodynamic response to each stimulus type was thus sampled effectively at 1‐s intervals [Josephs et al., 1997]. A constant ISI of 7 s was also chosen to minimize any orienting response due to unpredictability of stimulus occurrence.
Auditory stimuli were delivered to the participant via insert earphones fitted into 30‐dB sound‐attenuating MRI‐compatible headphones. Five participants were instructed to respond to a 1,000‐Hz tone and make no response to a 1,500‐Hz tone, whereas the other five subjects responded to the 1,500‐Hz tone and made no response to the 1,000‐Hz tone. As in the visual paradigm, stimulus duration was 200 ms with an ISI of 7 s. All stimuli were presented at approximately 80 dB, and each participant indicated their ability to hear and discriminate stimuli from background noise created by the scanner. Two auditory stimulus runs, each containing 35 Go and 35 NoGo stimuli, were collected in each participant. The presentation order of the visual and auditory runs was counterbalanced so that half of the participants completed the two auditory runs before the visual runs.
Across both stimulus modalities, participants were informed of the importance of fast and accurate performance. Participants responded with a right index‐finger button press to the Go stimuli using a commercially available MRI‐compatible fiberoptic response device (Lightwave Medical, Vancouver, BC). Reaction times to Go events were computed for trials in which the participants responded within 1,500 ms of stimulus onset. Failure to respond to a Go event within 1,500 ms of stimulus onset constituted an error of omission. Errors of commission were defined as responses that occurred within 1,500 ms of a NoGo stimulus onset. To ensure comprehension of the task instructions, participants carried out a practice block of 10 trials before scanning.
Post‐hoc examination of randomly ordered stimulus presentation demonstrated that almost 60% of trials involved a switch from one stimulus event type to the other on subsequent trials, with only 41% of stimuli following a stimulus of the same type. If participants had become aware of the slight bias toward switching after each trial, the decision to respond to a stimulus or not may have become slightly easier and thus have evoked a relatively reduced hemodynamic stimulus response. Although this would make it harder to detect the brain response to stimuli relative to baseline, it should not have differentially affected the hemodynamic response elicited by Go and NoGo events.
Imaging Parameters
Functional images were acquired in transaxial planes parallel to the anterior‐posterior commissure line on a standard clinical GE 1.5‐T system fitted with a Horizon Echo‐speed upgrade. A custom head holder was used to prevent movement. Conventional spin‐echo T1‐weighted sagittal localizing images were acquired to view positioning of the participant's head in the scanner and to prescribe the functional image volumes. Blood oxygen level‐dependent (BOLD) contrast images were collected with a gradient‐echo sequence (TR/TE 3,000/40 ms, flip angle 90 degrees, 24 × 24 cm field of view, 64 × 64 matrix, 62.5‐kHz bandwidth, 3.75 mm × 3.75 mm in plane resolution, 5 mm thickness, 29 slices) that effectively covered the entire brain (145 mm axial extent). In each run, 167 brain volumes were acquired. Four images collected before presentation of stimuli were discarded from subsequent analyses to remove effects of the T1 stabilization process.
Image Processing
Functional images were analyzed using the Statistical Parametric Mapping 99 software (SPM99; Wellcome Department of Cognitive Neurology, London, UK; online at http://www.fil.ion.ucl.ac.uk/spm). Images were reconstructed offline. Each scanning series was realigned independently and motion‐corrected using the procedure described by Friston et al. [ 1996]. Corrections for translations and rotations did not exceed 2.5 mm and 3 degrees, respectively, for any participant. A mean functional image was constructed independently for each scanning series (i.e., “session”) in each participant, and used to derive parameters for spatial normalization into the modified Talairach [Talairach and Tournoux, 1988] stereotaxic space implemented in SPM99. Both affine and nonlinear components were used in the normalization [Friston et al., 1995a]. The normalization parameters for each mean image were then applied to the corresponding functional images for each session, and the images resampled into isotropic 4‐mm voxels. The normalized images were subsequently smoothed with an 8‐mm full‐width at half‐maximum (FWHM) Gaussian kernel to optimize the signal‐to‐noise ratio and to compensate for intersubject anatomic variation. High‐frequency noise associated with scanner artefacts was removed using a 0.16‐Hz low‐pass fifth‐order IIR Butterworth filter applied to the fMRI time series at each voxel. A high‐pass filter was applied to remove noise associated with low‐frequency confounds (e.g., respiratory artefact).
Image Analysis
Statistical analysis was carried out using the general linear model approach implemented in SPM99 to estimate and statistically test the effect of the Go and NoGo events on the hemodynamic response within each voxel. Event‐related responses to Go and NoGo stimuli were modelled using a synthetic hemodynamic response function (HRF) comprised of two gamma functions and their temporal derivatives [Friston et al., 1998; Josephs et al., 1997]. The first gamma function modelled the hemodynamic response using a peak latency of 6 s, and the second gamma function modelled the small “overshoot” of the hemodynamic response on recovery. The temporal derivatives of the gamma functions were included to compensate for slight variation in the peak latency of the onset of the hemodynamic response. Errors of commission and omission were modelled separately from correct responses to Go events and correct nonresponses to NoGo events. The confounding effects of fluctuations in global signal intensity between image volumes were removed using an adjusted proportional scaling routine [Desjardins et al., 2001]. Although all coordinates in the present study are reported and displayed in the modified Talairach stereotaxic space implemented in SPM99, a transformation algorithm was applied to these coordinates to localize activations within standard Talairach space [Talairach and Tournoux, 1988; see http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html for the transformation algorithm].
Conjunction analyses across sensory modalities
Contrasts were specified to estimate and test for differences in amplitude of the fitted hemodynamic response between Go and NoGo events, and between each of these event types and baseline (“resting”) activation, using t‐tests at each voxel. Conjunction analyses [Friston et al., 1999a; Price et al., 1997a, b] were carried out to identify brain regions that were activated commonly across auditory and visual modalities. The results from the conjunction analyses form the basis of this study because they enable elucidation of supramodal activity (i.e., activity common across auditory and visual modalities) using a small sample of participants [Friston et al., 1999b]. As t‐tests were conducted in each voxel across the entire brain, a correction for multiple comparisons based on the theory of Gaussian fields was applied (as implemented in SPM99) [Friston et al., 1995b] and all reported voxels satisfy a corrected significance criterion of P ≤ 0.05.
Random‐effects (Between‐subject) analyses within sensory modalities
To verify results obtained in the conjunction analyses with analyses that take into account both inter‐ and intrasubject variability, direct comparisons of amplitudes of fitted hemodynamic response to Go and NoGo events were conducted separately within each sensory modality using second‐level random‐effects analyses [Friston et al., 1999a]. For the auditory and visual modality separately, images of parameter estimates contrasting the amplitude of the fitted response in each voxel for Go relative to NoGo trials, and NoGo relative to Go trials, were computed for each participant. These contrast images were then entered into separate second‐level, one‐sample t‐tests (9 df). The resultant SPM(t) in each sensory modality was thresholded at a t‐value of 2.82 (corresponding to a significance threshold of P ≤ 0.01 uncorrected for multiple comparisons), and significant clusters of activation were determined at a cluster significance of P ≤ 0.05 corrected for multiple comparisons [Friston et al., 1994].
Similar thresholding was applied in further random‐effects analyses that tested for modality‐specific (i.e., non‐supramodal) activations in the direct comparison of Go and NoGo events. Contrast images comparing Go and NoGo event responses for each subject, separately for each modality, were entered into a second‐level, paired t‐test (9 df) to elucidate any brain areas in which the Go relative to NoGo comparison was significantly greater in one sensory modality than in the other.
RESULTS
Behavioral Data
The mean (±SD) reaction time and percentage of correct hits for Go events was 425 ± 87 ms and 99.0%, respectively, in the auditory modality, and 402 ± 46 ms and 99.9%, respectively, in the visual modality. Mean reaction time did not differ significantly across modalities (t[9] = 0.973, P > 0.35). Of 10 participants, 3 made a total of seven errors of omission in the auditory task, and 1 participant made one error of omission in the visual task. The mean (±SD) percentage of false alarms to NoGo events (i.e., errors of commission) was 2.1 ± 1.18% and 5.7 ± 2.75% in the auditory and visual modalities, respectively. Although errors of omission did not differ significantly across modalities (t[9] = 1.765, P > 0.11), participants made significantly more errors of commission during the visual task than during the auditory task (t[9] = −2.785, P = 0.02).
Imaging Data
Conjunction analyses across sensory modalities
Go versus NoGo event comparisons.
Table I and Figure 1 (red colormap) show results of the conjunction analysis conducted across auditory and visual sensory modalities. The results indicate brain areas in which a significantly greater hemodynamic response was elicited by Go events than by NoGo events. Go events elicited greater activity than did NoGo events in widespread neocortex, including in the limbic cortex, bilateral paralimbic cortex in the frontal operculum, caudal anterior cingulate, mid‐cingulate and posterior cingulate gyri, in association cortex at the temporoparietal junction, the anterior intraparietal sulcus extending medially and posteriorly into the precuneus, as well as in bilateral frontal cortex. Additional cortical and subcortical (e.g., basal ganglia and thalamus) regions of activation are reported in Table I.
Table I.
Supramodal brain regions in which a greater hemodynamic response was elicited during the processing Go events than during NoGo events, based on a conjunction analysis of the auditory and visual sensory modalities
| Functional anatomic area (BA) | Talairach coordinates | t | P corr | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Limbic‐paralimbic cortex | |||||
| L amygdala | −28 | 4 | −20 | 5.27 | 0.000 |
| R hippocampus | 32 | −16 | −16 | 3.83 | 0.001 |
| L anterior superior temporal sulcus (38/21/22) | −60 | 4 | −4 | 6.54 | 0.000 |
| R anterior superior temporal sulcus (38/21/22) | 52 | 16 | −8 | 6.32 | 0.000 |
| L orbitofrontal cortex (47) | −20 | 16 | −24 | 4.34 | 0.000 |
| R orbitofrontal cortex (47) | 20 | 16 | −16 | 3.84 | 0.001 |
| L anterior insula (13) | −44 | 12 | −4 | 3.45 | 0.008 |
| R anterior insula (13) | 44 | 16 | −8 | 3.94 | 0.000 |
| Caudal anterior cingulate cortex (24/32) | 0 | 8 | 40 | 8.81 | 0.000 |
| Mid‐cingulate cortex (24) | −4 | −8 | 48 | 9.41 | 0.000 |
| Posterior cingulate cortex (29/30/23) | 0 | −44 | 20 | 3.76 | 0.001 |
| Temporoparietal junction | |||||
| L superior temporal gyrus (22) | −64 | −36 | 20 | 7.10 | 0.000 |
| R superior temporal gyrus (22) | 56 | −40 | 20 | 4.65 | 0.000 |
| L inferior parietal lobule (40/39) | −60 | −40 | 20 | 4.97 | 0.000 |
| R inferior parietal lobule (40/39) | 56 | −40 | 24 | 4.68 | 0.000 |
| Intraparietal sulcus | |||||
| L superior parietal lobule (7) | −16 | −56 | 64 | 6.03 | 0.000 |
| R superior parietal lobule (7) | 32 | −56 | 56 | 3.27 | 0.024 |
| L inferior parietal lobule (40) | −52 | −36 | 48 | 9.71 | 0.000 |
| R inferior parietal lobule (40) | 44 | −40 | 48 | 3.15 | 0.050 |
| L precuneus (7) | −12 | −52 | 68 | 5.59 | 0.000 |
| R precuneus (7) | 8 | −52 | 68 | 5.95 | 0.000 |
| Dorsal and ventral frontal cortex | |||||
| L middle frontal gyrus (9/8) | −40 | 32 | 40 | 3.36 | 0.014 |
| R middle‐superior frontal gyri (10/46) | 40 | 40 | 24 | 3.33 | 0.017 |
| L precentral gyrus (6/4) | −64 | 0 | 24 | 4.46 | 0.000 |
| R precentral gyrus (6/4) | 32 | −16 | 64 | 6.34 | 0.000 |
| Other neocortex | |||||
| R superior frontal gyrus (11) | 24 | 56 | −12 | 3.18 | 0.042 |
| Medial frontal gyrus (6) | −4 | −20 | 52 | 10.83 | 0.000 |
| L postcentral gyrus (3/2/1/5) | −32 | −32 | 68 | 14.63 | 0.000 |
| R postcentral gyrus (3/2/1/5) | 24 | −32 | 68 | 6.01 | 0.000 |
| L superior‐transverse temporal gyrus (42/41/22) | −56 | −28 | 16 | 12.64 | 0.000 |
| R superior‐transverse temporal gyrus (42/41/22) | 60 | −20 | 8 | 5.07 | 0.000 |
| L middle‐inferior temporal/middle occipital gyri (39/19/37) | −48 | −72 | 8 | 5.63 | 0.000 |
| R middle‐inferior temporal/middle occipital gyri (39/19/37) | 56 | −64 | −4 | 5.65 | 0.000 |
| L lingual gyrus/cuneus (18/17) | −16 | −84 | 4 | 4.66 | 0.000 |
| R lingual gyrus/cuneus (18/17) | 4 | −88 | 8 | 4.05 | 0.000 |
| Subcortical structures | |||||
| L thalamus | −20 | −16 | 8 | 5.22 | 0.000 |
| R thalamus | 12 | −4 | 12 | 4.87 | 0.000 |
| R caudate | 12 | 20 | −4 | 3.29 | 0.021 |
| L lentiform (putamen/lateral globus pallidus) | −28 | 0 | 4 | 5.41 | 0.000 |
| R lentiform (putamen/lateral globus pallidus) | 24 | 4 | 0 | 4.48 | 0.000 |
| Midbrain | −4 | −28 | −8 | 4.20 | 0.000 |
| Pons | −8 | −28 | −28 | 3.21 | 0.036 |
| L cerebellum | −24 | −72 | −28 | 5.20 | 0.000 |
| R cerebellum | 12 | −60 | −20 | 10.15 | 0.000 |
Talairach coordinates of each voxel, the t score, and the probability of achieving that t score when a correction for multiple comparisons conducted throughout the whole brain (P corr) is applied, are reported.
Any P given as 0.000 signifies that the value was ≤0.0005.
BA, Brodmann area; L, left; R, right.
Figure 1.
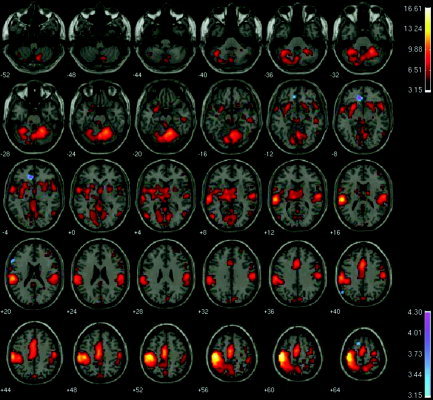
Illustration of the brain regions in which a significantly different amplitude of the hemodynamic response was elicited by Go and NoGo events, as revealed by a conjunction analysis of the auditory and visual sensory modalities. Red colormap: Significantly greater activity elicited during Go than during NoGo events. Blue colormap: The converse condition, in which significantly greater activity was elicited during NoGo than during Go events. Data are presented in the modified Talairach space used in SPM99, and rendered onto transaxial slices of a standard reference brain according to neurologic convention (i.e., the left hemisphere is illustrated on the left). Transaxial slices are presented in 4‐mm increments starting from z = −52 below to z = 64 mm above the AC‐PC plane. The image is thresholded at a significance level of P ≤ 0.05 corrected for multiple comparisons conducted throughout the whole brain. The range of t‐score values in each comparison are defined in the colored bars located at right.
Figure 1 also illustrates the results of the conjunction analysis that tested for regions in which NoGo events elicited a greater hemodynamic response than did Go events (blue colormap). This analysis revealed activation in the rostral extreme of the anterior cingulate cortex (Brodmann area [BA] 32; x, y, z coordinate of the voxel of peak activation = −8, 36, −4, t[9] = 4.30, P < 0.000002 corrected, 12 voxels) and in the left lateral inferior frontal gyrus (BA 45; x, y, z = −56, 24, 20, t[9] = 3.27, P < 0.005 corrected, 2 voxels). Two further single voxels of activation survived correction for multiple comparisons across the whole brain and were located in the superior frontal cortex (BA 6; x, y, z = −56, −64, 40, t[9] = 3.21, P < 0.01 corrected) and in the posterior aspect of the inferior parietal lobule (BA 39; x, y, z = −12, 12, 64, t = 3.11, P < 0.05 corrected).
Go and NoGo events relative to baseline.
Conjunction analyses conducted across the auditory and visual modalities for Go events relative to a baseline of rest and for NoGo events relative to a baseline of rest revealed several brain regions that were activated significantly in both conditions and that did not show a differential effect for Go and NoGo events (Fig. 2, Table II). These areas included superior aspects of the rostral anterior cingulate cortex, sections of insular cortex bilaterally, and ventrolateral and dorsolateral prefrontal‐premotor cortex, particularly in the right hemisphere.
Figure 2.
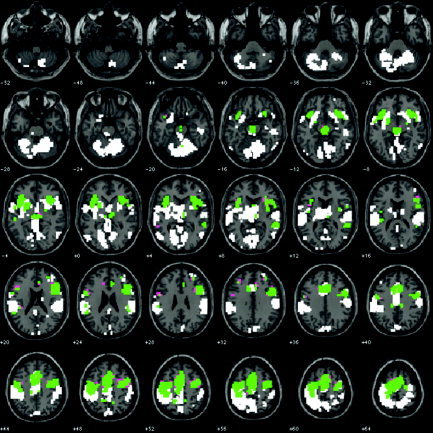
Illustration of brain regions significantly but not differentially activated by Go and NoGo events (each relative to baseline), as shown by a conjunction analysis of the auditory and visual sensory modalities (in green). White areas: Areas in which significantly greater activity was elicited during Go events than during NoGo events (see also Fig. 1). Residual areas in which significant activity was elicited during NoGo events only are indicated in pink. Data are presented in the format used in Figure 1. The image is thresholded at a significance level of P ≤ 0.05 corrected for multiple comparisons conducted throughout the whole brain.
Table II.
Brain regions activated during Go and NoGo events relative to a rest baseline but not differentially active during Go and NoGo events, based on a conjunction analysis of the auditory and visual sensory modalities
| Functional anatomic area (BA) | Go events vs. baseline | NoGo events vs. baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | P corr | x | y | z | t | P corr | |
| L rostral anterior cingulate gyrus (24/32) | −12 | 28 | 24 | 3.34 | 0.015 | −12 | 28 | 24 | 3.17 | 0.044 |
| R rostral anterior cingulate gyrus (24/32) | 8 | 28 | 24 | 4.88 | 0.000 | 8 | 28 | 24 | 4.66 | 0.000 |
| L mid insula | −40 | 0 | 4 | 5.77 | 0.000 | −40 | 4 | 4 | 3.57 | 0.003 |
| R mid insula | 36 | 0 | 8 | 6.74 | 0.000 | 36 | 0 | 8 | 5.30 | 0.000 |
| L inferior‐middle frontal gyrus (44/45/9) | −48 | 16 | 20 | 4.22 | 0.000 | −44 | 16 | 20 | 4.54 | 0.000 |
| R inferior‐middle frontal gyrus (44/45/9) | 48 | 20 | 20 | 7.97 | 0.000 | 48 | 20 | 20 | 7.74 | 0.000 |
| L precentral‐middle frontal gyrus (6) | −52 | −4 | 40 | 5.66 | 0.000 | −44 | −8 | 52 | 7.51 | 0.000 |
| R precentral‐middle frontal gyrus (6) | 44 | −4 | 52 | 7.68 | 0.000 | 44 | −4 | 52 | 7.96 | 0.000 |
Table shows Talairach coordinates of each voxel (x, y, z), t score, and probability of achieving that t score when a correction for multiple comparisons conducted throughout the whole brain (P corr) is applied.
Any P given as 0.000 signifies that the value was ≤ 0.0005.
BA, Brodmann area; L, left; R, right.
Random‐effects analyses conducted within the sensory modalities
Go versus NoGo event comparisons.
The random‐effects analyses conducted separately for auditory and visual sensory modalities closely replicated the pattern of activity revealed in the conjunction analyses across modalities (Table III and IV, Fig. 3 and 4, respectively). In the auditory modality, two clusters of activation survived correction for multiple comparisons conducted across the whole brain, whereas five clusters survived correction in the visual modality. In both modalities, these clusters incorporated bilateral activation in limbic cortex, in paralimbic cortex at the frontal operculum and in the caudal anterior cingulate and posterior cingulate gyri, and in posterior association cortex at the temporoparietal junction and intraparietal sulcus extending medially into the precuneus.
Table III.
Selected local maxima contained within the two significant clusters of activation reported for the auditory sensory modality in which Go events elicited a greater hemodynamic response did than NoGo events
| Functional anatomic area (BA) | Talairach coordinates | t | P corr | P uncorr | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Limbic‐paralimbic cortex | ||||||
| L amygdalaa | −20 | 4 | −20 | 4.76 | 1.000 | 0.001 |
| R amygdalaa | 28 | 0 | −16 | 2.85 | 1.000 | 0.009 |
| L hippocampusa | −24 | −16 | −16 | 3.41 | 1.000 | 0.004 |
| R hippocampusa | 32 | −16 | −16 | 3.97 | 1.000 | 0.002 |
| R hippocampusa | 36 | −20 | −12 | 5.92 | 0.998 | 0.000 |
| L anterior superior temporal sulcus (38/21/22)a | −56 | −4 | 0 | 5.80 | 0.999 | 0.000 |
| R anterior superior temporal sulcus (38/21/22)a | 60 | 4 | −4 | 6.76 | 0.966 | 0.000 |
| L orbitofrontal cortex (47)a | −20 | 20 | −16 | 4.03 | 1.000 | 0.001 |
| R orbitofrontal cortex (47)a | 16 | 20 | −20 | 3.20 | 1.000 | 0.005 |
| L anterior insula (13)a | −40 | 0 | 0 | 4.19 | 1.000 | 0.001 |
| R anterior insula (13)a | 42 | 8 | −4 | 3.46 | 1.000 | 0.004 |
| Caudal anterior cingulate cortex (24/32)a | 4 | 16 | 40 | 6.35 | 0.990 | 0.000 |
| Mid‐cingulate cortex (24)a | 0 | 0 | 44 | 4.52 | 1.000 | 0.001 |
| Posterior cingulate cortex (31)a | −12 | −24 | 44 | 6.14 | 0.995 | 0.000 |
| Temporoparietal junction | ||||||
| L superior temporal gyrus (22)a | −64 | −36 | 8 | 3.87 | 1.000 | 0.002 |
| R superior temporal gyrus (22)a | 64 | −28 | 12 | 2.88 | 1.000 | 0.009 |
| L inferior parietal lobule (40/39)a | −64 | −32 | 28 | 10.65 | 0.025 | 0.000 |
| R inferior parietal lobule (40/39)a | 64 | −28 | 24 | 4.50 | 1.000 | 0.001 |
| Intraparietal sulcus | ||||||
| L superior parietal lobule (7)a | −28 | −52 | 64 | 7.39 | 0.500 | 0.000 |
| R superior parietal lobule (7)a | 24 | −52 | 68 | 4.05 | 1.000 | 0.001 |
| L inferior parietal lobule (40)a | −44 | −40 | 52 | 10.00 | 0.043 | 0.000 |
| R inferior parietal lobule (40)a | 52 | −36 | 44 | 5.30 | 1.000 | 0.000 |
| L precuneus (7)a | −12 | −52 | 56 | 9.18 | 0.088 | 0.000 |
| R precuneus (7)a | 12 | −64 | 64 | 3.91 | 1.000 | 0.002 |
| Dorsal and ventral frontal cortex | ||||||
| L precentral gyrus (6)a | −60 | 0 | 32 | 3.61 | 1.000 | 0.003 |
| R precentral gyrus (4)a | 60 | −20 | 44 | 5.74 | 0.999 | 0.000 |
| R middle‐inferior frontal gyrus (9)a | 52 | 8 | 40 | 4.73 | 1.000 | 0.001 |
| R inferior frontal gyrus (44/45)a | 52 | 12 | 32 | 3.04 | 1.000 | 0.007 |
| R middle frontal/precentral gyri (9/6)a | 52 | 12 | 38 | 4.28 | 1.000 | 0.001 |
| Other neocortex | ||||||
| Medial frontal gyrus (6)a | −4 | 0 | 52 | 11.55 | 0.013 | 0.000 |
| R postcentral gyrus (40)a | 60 | −20 | 20 | 12.05 | 0.009 | 0.000 |
| R postcentral gyrus (3)a | 20 | −40 | 60 | 6.06 | 0.996 | 0.000 |
| L superior‐transverse temporal gyrus (42/41/22)a | −60 | −24 | 12 | 5.07 | 1.000 | 0.000 |
| R superior‐transverse temporal gyrus (42/41/22)a | 60 | −28 | 16 | 5.20 | 1.000 | 0.000 |
| L middle temporal gyrus (39/37)b | −48 | −72 | 8 | 8.83 | 0.120 | 0.000 |
| L cuneus/lingual gyrus (23/17/18)a | −16 | −72 | 8 | 5.95 | 0.998 | 0.000 |
| R cuneus/lingual‐fusiform gyri (23/18)a | 4 | −72 | 8 | 4.17 | 1.000 | 0.001 |
| Subcortical structures | ||||||
| L thalamusa | −20 | −20 | 0 | 7.19 | 0.622 | 0.000 |
| R thalamusa | 12 | −16 | 4 | 5.16 | 1.000 | 0.000 |
| R caudatea | 8 | 8 | 0 | 2.84 | 1.000 | 0.009 |
| L lentiform (putamen/lateral globus pallidus)a | −28 | −12 | −4 | 8.93 | 0.110 | 0.000 |
| R lentiform (putamen/lateral globus pallidus)a | 16 | 4 | 4 | 6.12 | 0.995 | 0.000 |
| Midbraina | 0 | −28 | −12 | 4.59 | 1.000 | 0.001 |
| Ponsa | −8 | −28 | −28 | 3.02 | 1.000 | 0.007 |
| L cerebellum (pyramis)a | −8 | −80 | −36 | 6.39 | 0.988 | 0.000 |
| L cerebellum (uvula)a | −28 | −68 | −32 | 5.35 | 1.000 | 0.000 |
| R cerebellum (culmen)a | 16 | −56 | −24 | 9.64 | 0.059 | 0.000 |
| R cerebelluma | 28 | −52 | −36 | 4.66 | 1.000 | 0.001 |
Table shows Talairach coordinates, t score, and the probability of achieving that t score, both when correcting for multiple comparisons conducted throughout the whole brain (P corr) and when no correction is applied (P uncorr), for each local maximum.
Cluster of 3,274 voxels, P < 0.0005, corrected.
Cluster of 47 voxels, P < 0.041 corrected.
Any P given as 0.000 signifies that the value was ≤0.0005.
BA, Brodmann area; L, left; R, right.
Table IV.
Selected local maxima contained within the five significant clusters of activation reported for the visual sensory modality in which Go events elicited a greater hemodynamic response than did NoGo events
| Functional anatomic area (BA) | Talairach coordinates | t | P corr | P uncorr | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Limbic‐paralimbic cortex | ||||||
| L amygdalaa | −28 | 0 | −16 | 5.38 | 1.000 | 0.000 |
| R amygdalaa | 20 | 4 | −20 | 3.94 | 1.000 | 0.002 |
| L hippocampusa | −36 | −20 | −16 | 3.97 | 1.000 | 0.002 |
| R hippocampusd | 36 | −16 | −16 | 2.88 | 1.000 | 0.009 |
| L anterior superior temporal sulcus (38/21/22)a | −56 | 4 | 0 | 6.29 | 0.987 | 0.000 |
| R anterior superior temporal sulcus (38/21/22)c | 56 | 12 | −8 | 7.36 | 0.518 | 0.000 |
| L orbitofrontal cortex (47)a | −24 | 12 | −20 | 5.42 | 1.000 | 0.000 |
| R orbitofrontal cortex (47)c | 48 | 20 | −4 | 6.27 | 0.988 | 0.000 |
| R orbitofrontal cortex (47)a | 24 | 8 | −16 | 5.71 | 0.999 | 0.000 |
| L anterior insula (13)a | −32 | 8 | −4 | 5.22 | 1.000 | 0.000 |
| R anterior insula (13)c | 42 | 12 | −4 | 4.47 | 1.000 | 0.001 |
| Rostral anterior cingulate cortex (24)a | 0 | 32 | 28 | 3.28 | 1.000 | 0.005 |
| Caudal anterior cingulate cortex (24/32)a | 4 | 16 | 40 | 5.28 | 1.000 | 0.000 |
| Mid‐cingulate cortex (24)a | 8 | −20 | 44 | 7.38 | 0.508 | 0.000 |
| Posterior cingulate cortex (31)a | −12 | −28 | 44 | 7.77 | 0.338 | 0.000 |
| Posterior cingulate cortex (30)a | 8 | −56 | 4 | 3.23 | 1.000 | 0.005 |
| Temporoparietal junction | ||||||
| L superior temporal gyrus (22)a | −64 | −36 | 12 | 7.07 | 0.709 | 0.000 |
| R superior temporal gyrus (22)b | 56 | −36 | 8 | 5.58 | 0.999 | 0.000 |
| L inferior parietal lobule (40/39)a | −60 | −32 | 24 | 9.57 | 0.062 | 0.000 |
| R inferior parietal lobule (40/39)b | 56 | −44 | 24 | 6.74 | 0.958 | 0.000 |
| Intraparietal sulcus | ||||||
| L superior parietal lobule (7)a | −16 | −56 | 64 | 5.55 | 1.000 | 0.000 |
| R superior parietal lobule (7)a | 20 | −52 | 64 | 3.57 | 1.000 | 0.003 |
| L inferior parietal lobule (40)a | −52 | −40 | 56 | 7.55 | 0.426 | 0.000 |
| L precuneus (7)a | 8 | −56 | 64 | 3.04 | 1.000 | 0.007 |
| R precuneus (7)a | 4 | −60 | 68 | 10.11 | 0.039 | 0.000 |
| Dorsal and ventral frontal cortex | ||||||
| L precentral gyrus (4/6)a | −60 | −16 | 44 | 5.89 | 0.998 | 0.000 |
| R inferior frontal gyrus (45)c | 56 | 24 | 4 | 3.30 | 1.000 | 0.005 |
| R inferior frontal/precentral gyrus (44/6)b | 60 | 4 | 20 | 5.82 | 0.998 | 0.000 |
| R precentral gyrus (4)b | 60 | −20 | 40 | 4.19 | 1.000 | 0.001 |
| Other neocortex | ||||||
| Medial frontal gyrus (6)a | −4 | −20 | 52 | 6.75 | 0.957 | 0.000 |
| L postcentral gyrus (2)a | −44 | −24 | 52 | 10.44 | 0.030 | 0.000 |
| L postcentral gyrus (40)a | −56 | −24 | 16 | 5.09 | 1.000 | 0.000 |
| R postcentral gyrus (2/3)b | 60 | −20 | 24 | 8.56 | 0.155 | 0.000 |
| R postcentral gyrus (1/2)b | 56 | −28 | 48 | 3.85 | 1.000 | 0.002 |
| L superior‐transverse temporal gyrus (42/41/22)a | −56 | −28 | 16 | 5.55 | 1.000 | 0.000 |
| R superior‐transverse temporal gyrus (42/41/22)b | 56 | −24 | 12 | 6.94 | 0.937 | 0.000 |
| R middle‐inferior temporal gyri (21)d | 64 | −28 | −16 | 3.15 | 1.000 | 0.006 |
| L inferior‐middle occipital gyri (19/18)a | −44 | −76 | −12 | 3.28 | 1.000 | 0.005 |
| R inferior‐middle occipital/lingual gyri (18)a | 40 | −84 | −12 | 3.88 | 1.000 | 0.002 |
| L cuneus (19)e | 0 | −84 | 32 | 4.09 | 1.000 | 0.001 |
| L cuneus/lingual gyrus (17/18/19)a | −8 | −88 | 4 | 3.61 | 1.000 | 0.003 |
| R cuneus/lingual gyrus (19)a | 24 | −76 | −8 | 5.49 | 1.000 | 0.000 |
| Subcortical structures | ||||||
| L thalamusa | −8 | −12 | 12 | 7.14 | 0.657 | 0.000 |
| R thalamusa | 16 | −8 | 12 | 9.05 | 0.513 | 0.000 |
| L caudatea | −4 | 12 | 0 | 2.86 | 1.000 | 0.009 |
| R caudatea | 12 | 24 | 0 | 5.85 | 0.998 | 0.000 |
| L lentiform (putamen/lateral globus pallidus)a | −28 | 8 | 0 | 6.35 | 0.985 | 0.000 |
| R lentiform (putamen/lateral globus pallidus)a | 24 | 8 | 0 | 3.92 | 1.000 | 0.002 |
| Midbraina | 8 | −32 | −20 | 5.22 | 1.000 | 0.000 |
| Ponsa | 0 | −12 | −32 | 3.46 | 1.000 | 0.004 |
| L cerebellum (cerebellar tonsil)a | −40 | −52 | −40 | 9.40 | 0.072 | 0.000 |
| R cerebellum (culmen)a | 20 | −52 | −24 | 11.23 | 0.016 | 0.000 |
Table shows Talairach coordinates, t score, and the probability of achieving that t score, both when correcting for multiple comparisons conducted throughout the whole brain (P corr) and when no correction is applied (P uncorr), for each local maximum.
Cluster of 2,932 voxels, P < 0.0005, corrected.
Cluster of 312 voxels, P < 0.0005, corrected.
Cluster of 76 voxels, P < 0.004 corrected.
Cluster of 53 voxels; P < 0.032 corrected.
Cluster of 51 voxels, P < 0.038 corrected.
Any P given as 0.000 signifies that the value was ≤0.0005.
BA, Brodmann area; L, left; R, right.
Figure 3.
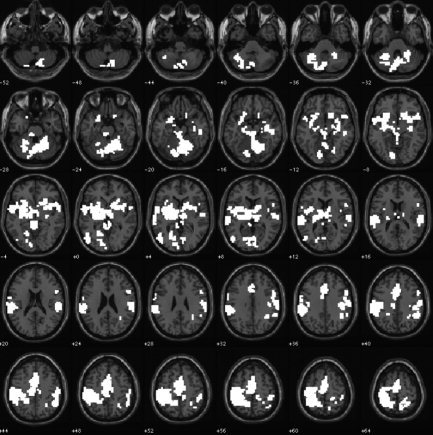
Illustration of the two significant clusters of activation in which Go events elicited a greater hemodynamic response than did NoGo events in the auditory sensory modality. Data are presented in the format used in Figure 1. The image is thresholded at a height of t(9) = 2.82, corresponding to a significance level of P ≤ 0.01 uncorrected for multiple comparisons conducted throughout the whole brain. Clusters are significant at P ≤ 0.05 corrected for multiple comparisons.
Figure 4.
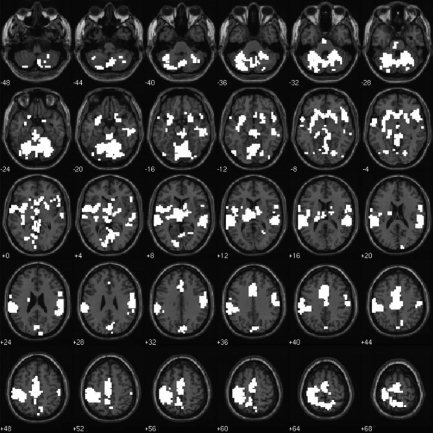
Illustration of the five significant clusters of activation in which Go events elicited a greater hemodynamic response than did NoGo events in the visual sensory modality. Data are presented in the format used in Figure 1. The image is thresholded at a height of t(9) = 2.82, corresponding to a significance level of P ≤ 0.01 uncorrected for multiple comparisons conducted throughout the whole brain. Clusters are significant at P ≤ 0.05 corrected for multiple comparisons.
No significant clusters of activation were reported in the random‐effects analyses that tested for regions in which greater activity was elicited during NoGo events than during Go events. In the auditory modality, a small, nonsignificant cluster of four voxels was observed in rostral anterior cingulate cortex (BA 32; x, y, z coordinate of the voxel of peak activation within the cluster = −8, 36, −8, t = 3.67, P = 0.003 uncorrected). In the visual modality, the corresponding nonsignificant cluster in the rostral anterior cingulate cortex comprised only two voxels (x, y, z coordinate of the voxel of peak activation = −12, 36, −8, t = 3.90, P = 0.002 uncorrected).
Modality of presentation comparisons for go and NoGo events.
The random‐effects analyses that directly compared sensory modalities on the Go relative to NoGo event contrast failed to reveal any brain areas in which the auditory modality was more active than was the visual modality. A cluster of 37 voxels located in the medial frontal cortex (BA 10, x, y, z coordinate of the voxel of peak activation = −8, 52, 20, t[9] = 6.53, P = 0.005 uncorrected) was significantly more active in the visual modality than in the auditory modality. Behavioral data indicated a slightly higher number of false alarms committed to NoGo events in the visual modality than in the auditory modality. These results suggest that discriminating Go and NoGo events in the visual modality may have been slightly more difficult than that in the auditory task. The paucity of differences between modalities, however, is consistent with the concept of a supramodal network for processing and responding to task‐relevant stimuli processing and response.
DISCUSSION
The results demonstrate that a widespread, supramodal network of brain areas is recruited preferentially during processing of a target stimulus that prescribes a subsequent motor response relative to processing of an equally probable and familiar stimulus that does not require a motor response. This network incorporates limbic cortex (amygdala‐hippocampus), paralimbic cortex at the anterior STS, insula, posterior orbitofrontal/inferior frontal gyrus, caudal anterior cingulate, mid‐cingulate, and posterior cingulate gyri, and association cortex at the temporoparietal junction, anterior intraparietal sulcus extending medially and posteriorly into the precuneus, and premotor cortex. These results closely replicate findings from previous fMRI studies of auditory and visual oddball target detection [Kiehl et al., 2001a, b], which demonstrated greater activation in this extended network for infrequent task‐relevant (target) events than for infrequent task‐irrelevant (novel) events. The current findings, however, indicate that this supramodal network of areas is engaged by goal‐directed attention to behaviorally salient stimuli irrespective of stimulus infrequency and novelty.
Although goal‐directed attention may be construed as an emergent property of coordinated activity throughout the network, human and animal research has begun to delineate specialized contributions from the diverse brain regions that make up the network. Cortex at the intraparietal sulcus seems dedicated to identifying characteristics of salient events (especially location) and in specifying cognitive plans/intentions that target these events for behavior [Andersen and Buneo, 2002; Gottlieb, 2002]. Downar et al. [ 2000, 2001, 2002] demonstrated supramodal activation of cortex at the temporoparietal junction during passive or active detection of stimuli that were salient due to their novelty, infrequency, or their task relevance. Together, premotor areas in superior frontal cortex and anterior cingulate play a prominent role in selection, sequencing, and execution of behavior [Mesulam et al., 2001; Posner and Petersen, 1990; Posner and Rothbart, 1998], and in resolving conflict between response alternatives [Carter et al., 1998].
Based on a review of animal and human research, Corbetta and Shulman [ 2002] recently proposed two partially segregated frontoparietal systems that control goal‐directed and stimulus‐driven processing. They describe a dorsal frontoparietal network that embraces the superior frontal cortex and intraparietal sulcus and mediates cognitive selection of stimuli and responses. They also posit a predominantly right‐lateralized ventral frontoparietal network involving the temporoparietal junction and inferior‐middle frontal gyrus. They suggest that this network is modulated by detection of salient events, particularly unattended, infrequent, and novel events, to reorient limited processing resources to these events (i.e., to interrupt or reset ongoing goal‐directed activity in dorsal frontoparietal areas). The results of our study imply that even when stimulus‐response associations are simple and well‐learned, the different response demands associated with otherwise equally probable and familiar stimuli modulate activity bilaterally in both frontoparietal networks identified by Corbetta and Shulman [ 2002].
Mesulam [ 1998] proposed that goal‐directed processing recruits a widely distributed neural network in which motivational and volitional influences from limbic cortex are channeled via paralimbic cortex to neocortical areas involved in perceptual elaboration and behavioral planning. In the present study, coactivation of widespread limbic cortex (amygdala–hippocampus) and paralimbic cortex (i.e., at the anterior STS, insula, orbitofrontal and cingulate cortex) in the Go/NoGo event comparison is compatible with the idea that a motivational component contributes to brain activation differences between the Go and NoGo events in the present task. Until now, the cingulate gyrus has been considered the main site through which motivational, effortful, and emotional influences are brought to bear on selecting targets of attention [Kim et al., 1999; Mesulam, 1981, 1999; Paus, 2001; Winterer et al., 2002]. Indirect limbic inputs to the caudal anterior cingulate areas responsible for cognition and motor control are received via direct amygdala projections to the rostral anterior cingulate cortex [Morecraft and Van Hoesen, 1998]. Connectivity between limbic cortex and cingulate gyrus, and output from cingulate motor cortex to primary and supplementary motor cortex, putamen, and spinal cord, suggests that cingulate cortex is situated ideally to mediate limbic influences on voluntary motor behavior [Paus, 2001]. Several studies have described activity in anterior cingulate cortex that is specific to conflict between response options rather than conflict at the level of stimulus identification [Milham et al., 2001; van Veen et al, 2001]. By contrast, posterior cingulate cortex may mediate cue‐induced anticipatory attention toward events of intrinsic or experientially acquired salience [Hopfinger et al., 2000; Kim et al., 1999; Mesulam et al., 2001], and thus provides an early site for motivational biasing of exogenous stimulus processing.
The present results imply that activity in paralimbic cortex around the frontal operculum also contributes to goal‐directed processing of task‐relevant stimuli. Paralimbic cortex in the anterior STS (along with insular and orbitofrontal cortex) provides an additional interface between limbic cortex in the medial temporal lobes and frontoparietal association cortices [Mesulam, 1998; Mesulam and Mufson, 1982a, b]. The anterior STS receives projections from both the dorsal “where” visual pathway in posterior parietal cortex and the ventral “what” visual pathway in inferior temporal cortex [Baizer et al., 1991]. Likewise, auditory “where” and “what” processing streams converge within rostral superior temporal cortex [Karnath, 2001; Rauschecker and Tian, 2000]. The anterior STS also receives input from other paralimbic regions (cingulate, insula, and orbitofrontal cortex) and subcortical structures (claustrum, thalamus, and brainstem) [Markowitsch et al., 1985; Moran et al., 1987]. It is thus situated ideally to link the multimodal perception of stimuli occurring in posterior neocortex and frontal executive processes that select, initiate, and monitor behavior with motivational/goal‐directed influences from limbic centres.
In the present study, greater bilateral activation was elicited by Go stimuli than by NoGo stimuli in additional brain areas known to directly mediate motor behavior, including primary motor cortex (M1), basal ganglia, and thalamus. Using the current paradigm, the contribution of goal‐directed attention to activation in these areas cannot be distinguished from activation associated with motor responding. Likewise, activation in supplementary and cingulate motor areas and in lateral premotor areas may reflect in part the direct connectivity of these regions with M1 and spinal motorneurons innervating hand muscles [Dum and Strick, 2002]. The role of these regions in goal‐directed processing (i.e., beyond generation of the motor response per se) may be ascertained more successfully using paradigms that require only covert responses to target stimuli (e.g., counting target stimuli).
By requiring solely a right‐handed button‐press response, the current task is also not suited to investigating laterality of brain activity during goal‐directed processing. Many studies have reported right hemisphere dominance in certain attentional processes (e.g., vigilance) [Pardo et al., 1991], observable particularly in lateral prefrontal and superior parietal cortex [see also Corbetta and Shulman, 2002; Mesulam, 1999; Posner and Petersen, 1990]. Our data showed that Go and NoGo events both elicited predominantly right‐sided activation of lateral prefrontal cortex. In many brain regions, however, activation elicited by the Go stimuli in this study was bilateral, and in some instances, there was a tendency toward greater activation in the left hemisphere than in the right. As would be expected from the fact that the participants responded with their right index finger, Go stimuli elicited greater activation of primary sensorimotor cortex, supplementary motor cortex and thalamus on the left than on the right (although in the cerebellum, greater activity was observed on the right). Go stimuli, however, also elicited activation of temporoparietal junction and mid‐cingulate that seemed more marked on the left than on the right. Braver et al. [ 2001] observed greater activation in left mid‐cingulate and temporoparietal cortex during right‐handed responses to target stimuli than during right‐handed responses in a response choice task. They concluded that the observed left hemisphere bias during target processing was unlikely to be due to the handedness of the response. Future research that separates detection of Go stimuli from an immediate behavioral response (e.g., by delayed cue signaling for the commission of a motor response) may provide insight into laterality of goal‐directed processing and information about the time‐course over which limbic and paralimbic influences are brought to bear on frontoparietal association areas during goal‐directed processing.
In addition to those areas differentially activated by Go and NoGo events, other frontal regions were activated similarly by both event types, including sites in ventral and dorsal frontal cortex, rostral anterior cingulate cortex, and regions of insular cortex. In contrast to studies employing Go/NoGo variants that established a prepotent response tendency that was inhibited subsequently on NoGo trials, the present study identified few voxels in which NoGo stimuli elicited greater activation than did the Go stimuli. These few voxels were located most consistently in the rostral extreme of anterior cingulate cortex. Although this activation might reflect a residual amount of response inhibition on NoGo trials, previous observations of anterior cingulate activation during the correct inhibition of NoGo stimuli responses have typically located the peak of activity in more caudal portions of anterior cingulate gyrus [e.g., Braver et al., 2001; Durston et al., 2002; Kiehl et al., 2000; Menon et al., 2001]. The relatively greater activation in the rostral extreme of anterior cingulate cortex for NoGo compared to Go events in this study might rather be explained by previous reports that activation in this area is inversely proportional to the degree to which an individual is engaged in carrying out a difficult task [Paus et al., 1998; Simpson et al., 2001]. The observation of greater activation in more caudal anterior cingulate areas and lesser activation in the rostral extreme of cingulate during Go trials (and vice versa for NoGo trials) is consistent with the requirement for greater processing resources in Go compared to NoGo trials. Overall, activation elicited by NoGo events (relative to resting baseline) was restricted largely to frontal cortex. In parietal cortex, NoGo stimuli elicited little activation, whereas Go stimuli elicited extensive activation. These observations are consistent with evidence that the P300 event‐related potential elicited by task‐irrelevant novel/distracter stimuli is maximal at frontocentral sites and attenuated over posterior cortex, whereas that elicited by target stimuli is maximal over parietal cortex [Friedman et al, 2001; Katayama and Polich, 1998].
We have described a widespread, supramodal network of brain areas that is preferentially recruited during processing of and motor response to a task‐relevant target (Go) stimulus relative to processing of an equally probable and familiar stimulus for which no motor response is required. We suggest that activation within this network reflects in part a motivational/goal‐directed component that elicits preferential processing of task‐relevant events. To elucidate further this mechanism, future research should manipulate explicitly the motivational value of Go and NoGo stimuli, for example, by assigning a differential monetary reward to correctly engaging in a motor response and withholding an inappropriate motor response.
Acknowledgements
We thank L. Vandenbeld, C. Ruff, and A. Vouloumanos for assistance with data collection; and MR technicians T. Shaw, S. Renneberg, J. McCord, and Drs. A. MacKay, K. Whittall, and B. Forster for fMRI scanning assistance and advice. We also thank Dr. T. Woodward for commenting on an earlier version of the article. This work was supported in part by grants from the Dr. Norma Calder Foundation for Schizophrenia Research and the Medical Research Council of Canada. K.R.L. was supported by the Gertrude Langridge Graduate Scholarship in Medical Sciences and a University of British Columbia Graduate Fellowship.
REFERENCES
- Andersen RA, Buneo CA (2002): Intentional maps in posterior parietal cortex. Annu Rev Neurosci 25: 189–220. [DOI] [PubMed] [Google Scholar]
- Annett M (1970): A classification of hand preference by association analysis. Br J Psychol 61: 303–321. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Choi SJ, Hossein‐Zadeh GA, Porjesz B, Tanabe JL, Lim KO, Bilder R, Helpern JA, Begleiter H (2002): Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res Cogn Brain Res 14: 347–356. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Ungerleider LG, Desimone R (1991): Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J Neurosci 11: 168–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A (2001): Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Brown RG, Pluck G (2000): Negative symptoms: the “pathology” of motivation and goal‐directed behaviour. Trends Neurosci 23: 412–417. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC (2001): Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp 13: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L (2000): Responses to rare visual target and distractor stimuli using event‐related fMRI. J Neurophysiol 83: 3133–3139. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Andrew C, Zelaya FO, Williams SC, Dumanoir C (2000): Motor response suppression and the prepotent tendency to respond: a parametric fMRI study. Neuropsychologia 38: 1280–1291. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF (2001): Removal of confounding effects of global signal in functional MRI analyses. Neuroimage 13: 751–758. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2001): The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event‐related fMRI study. Neuroimage 14: 1256–1267. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2002): A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. J Neurophysiol 87: 615–620. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL (2002): Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682. [DOI] [PubMed] [Google Scholar]
- Duncan‐Johnson CC, Donchin E (1977): On quantifying surprise: the variation of event‐related potentials with subjective probability. Psychophysiology 14: 456–467. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ (2002): The effect of preceding context on inhibition: an event‐related fMRI study. Neuroimage 16: 449–453. [DOI] [PubMed] [Google Scholar]
- Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH (2004): Acquiring and inhibiting prepotent responses in schizophrenia: event‐related brain potentials and functional magnetic resonance imaging. Arch Gen Psychiatry 61: 119–129. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H (2001): The novelty P3: an event‐related brain potential (ERP) sign of the brain's evaluation of novelty. Neurosci Biobehav Rev 25: 355–373. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS (1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999a): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ (1999b): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA (2002): Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage 17: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gottlieb J (2002): Parietal mechanisms of target representation. Curr Opin Neurobiol 12: 134–140. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P (1998): Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 106: 156–164. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3: 284–291. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Skudlarski P, Gore JC (2002): Correlations and dissociations between bold signal and P300 amplitude in an auditory oddball task: a parametric approach to combining fMRI and ERP. Magn Reson Imaging 20: 319–325. [DOI] [PubMed] [Google Scholar]
- Josephs O, Turner R, Friston KJ (1997): Event‐related fMRI. Hum Brain Mapp 5: 1–7. [DOI] [PubMed] [Google Scholar]
- Karnath HO (2001): New insights into the functions of the superior temporal cortex. Nat Rev Neurosci 2: 568–576. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J (1996): P300, probability, and the three‐tone paradigm. Electroencephalogr Clin Neurophysiol 100: 555–562. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J (1998): Stimulus context determines P3a and P3b. Psychophysiology 35: 23–33. [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB (2000): Error processing and the rostral anterior cingulate: an event‐related fMRI study. Psychophysiology 37: 216–223. [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF (2001a): An event‐related fMRI study of visual and auditory oddball tasks. J Psychophysiol 15: 221–240. [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF (2001b): Neural sources involved in auditory target detection and novelty processing: an event‐related fMRI study. Psychophysiology 38: 133–142. [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM (1999): The large‐scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage 9: 269–277. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y (1998): No‐go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci 10: 1209–1213. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM (2001): Event‐related fMRI study of response inhibition. Hum Brain Mapp 12: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T (1999): The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cereb Cortex 9: 815–823. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B (1985): Cortical and subcortical afferent connections of the primate's temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. J Comp Neurol 242: 425–458. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. (2003): Anatomy of an error: ERP and fMRI. Biol Psychol 64: 119–141. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman‐Rakic P (1997): Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 77: 1630–1634. [DOI] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A (1997): Combined event‐related fMRI and EEG evidence for temporal‐parietal cortex activation during target detection. Neuroreport 8: 3029–3037. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10: 309–325. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121: 1013–1052. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (1999): Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci 354: 1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982a): Insula of the old world monkey. I. Architectonics in the insulo‐orbito‐temporal component of the paralimbic brain. J Comp Neurol 212: 1–22. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ (1982b): Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212: 38–52. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR (2001): Heterogeneity of cingulate contributions to spatial attention. Neuroimage 13: 1065–1072. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen, NJ , Wszalek T, Kramer AF (2001): The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM (1987): Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol 256: 88–103. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW (1998): Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull 45: 209–232. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ (2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17: 419–430. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C (1998): Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulate cortex: a review of 107 PET activation studies. Neuroreport 9: 37–47. [DOI] [PubMed] [Google Scholar]
- Polich J, Ellerson PC, Cohen J (1996): P300, stimulus intensity, modality, and probability. Int J Psychophysiol 23: 55–62. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK (1998): Attention, self‐regulation and consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997a): Cognitive conjunction: a new approach to brain activation experiments. Neuroimage 5: 261–270. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Friston KJ (1997b): Subtractions, conjunctions, and interactions in experimental design of activation studies. Hum Brain Mapp 5: 264–272. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B (2000): Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA 97: 11800–11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR Jr, Snyder AZ, Gusnard DA, Raichle ME (2001): Emotion‐induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci USA 98: 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, Gore JC (2000): Event‐related fMRI of auditory and visual oddball tasks. Magn Reson Imaging 18: 495–502. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. 3‐dimensional proportional system: an approach to cerebral imaging. Stuttgart, Germany: Thieme. [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS (2001): Anterior cingulate cortex, conflict monitoring and levels of processing. Neuroimage 14: 1302–1308. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R (2002): The human prefrontal and parietal association cortices are involved in no‐go performances: An event‐related fMRI study. Neuroimage 17: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Winterer G, Adams C, Jones D, Knutson B (2002): Volition to action‐an event‐related fMRI study. Neuroimage 17: 851–858. [PubMed] [Google Scholar]


