Abstract
Grating orientation discrimination is employed widely to test tactile spatial acuity. We used functional magnetic resonance imaging (fMRI) to investigate the neural circuitry underlying performance of this task. Two studies were carried out. In the first study, an extensive set of parietal and frontal cortical areas was activated during covert task performance, relative to a rest baseline. The active regions included the postcentral sulcus bilaterally and foci in the left parietal operculum, left anterior intraparietal sulcus, and bilateral premotor and prefrontal cortex. The second study examined selective recruitment of cortical areas during discrimination of grating orientation (a task with a macrospatial component) compared to discrimination of grating spacing (a purely microspatial task). The foci activated on this contrast were in the left anterior intraparietal sulcus, right postcentral sulcus and gyrus, left parieto‐occipital cortex, bilateral frontal eye fields, and bilateral ventral premotor cortex. These findings not only confirm and extend previous studies of the neural processing underlying grating orientation discrimination, but also demonstrate that a distributed network of putatively multisensory areas is involved. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: somatosensory, finger, touch, macrospatial, microspatial, multisensory
INTRODUCTION
Johnson and Phillips [1981] introduced the use of grating orientation discrimination to measure tactile spatial acuity. They applied gratings consisting of alternating ridges and grooves to the immobilized human fingerpad and investigated the subjects' ability to discriminate whether the gratings were oriented along or across the long axis of the finger. They found that this ability increases with groove width and derived a threshold, expressed as the minimal groove width required for reliable orientation discrimination. In a corresponding neurophysiologic study of peripheral afferents in monkeys, the same researchers showed that the spatial resolution underlying performance in this task depends on slowly adapting (Merkel) afferents [Phillips and Johnson, 1981]. Subsequently, a version of this task was developed and validated as a dependable test of tactile function [van Boven and Johnson, 1994a]. This test has been used by several groups to study not only variations in tactile spatial acuity between different locations [Sathian and Zangaladze, 1996; van Boven and Johnson, 1994b] but also alterations in populations such as blind Braille readers [Goldreich and Kanics, 2003; Grant et al., 2000; van Boven et al., 2000], dyslexic individuals [Grant et al., 1999], finger amputees [Vega‐Bermudez and Johnson, 2002], and patients with hand dystonia [Bara‐Jimenez et al., 2000].
Given the widespread use of grating orientation discrimination to test tactile ability, it is of considerable interest to understand the central neural processing that takes place during performance of this task. An earlier study [Sathian et al., 1997] from our laboratory used positron emission tomography (PET) to address whether the task recruits visual cortical areas, because we had observed that tactile discrimination of grating orientation is accompanied by subjective reports of visual imagery. This study used discrimination of grating groove width as a control task. This control (spacing) task is not associated with subjective reports of visual imagery. Relative to the spacing task, the orientation task evoked activity in an extrastriate region of visual cortex contralateral to the stimulated hand, in parietooccipital cortex (POC). This POC region is also active during visual discrimination of grating orientation [Sergent et al., 1992]. We then showed that transcranial magnetic stimulation (TMS) over the POC area interferes with tactile performance of this task [Zangaladze et al., 1999], thus establishing the functional relevance of POC activity. Although both tasks require microspatial resolution, on the order of about 1 mm, the tasks differ in the nature of the critical parameter. In the spacing task, the critical parameter is microspatial, because the task demands comparison of groove widths. In the orientation task, the critical parameter is on a larger spatial scale and can be considered macrospatial. In addition to our studies using gratings [Sathian et al., 1997; Zangaladze et al., 1999], there is substantial other evidence that macrospatial and microspatial stimulus parameters are processed differently [Klatzky et al., 1987; Randolph and Semmes, 1974; Roland et al., 1998; Stoesz et al., 2003].
The studies in the present report used functional magnetic resonance imaging (fMRI) to investigate, with greater sensitivity and spatial resolution, the neural processing involved in tactile discrimination of grating orientation. In an initial exploratory study, we examined the activations during task performance relative to a rest baseline, using a scanner with a field strength of 1.5 T (Study 1). We then conducted a study at 3 T to show task‐specific areas, relative to the spacing control (Study 2). Both studies employed random‐effects analyses to allow generalization of the results to the population, whereas our previous PET study used a fixed‐effects analysis. Preliminary reports of these studies have been presented previously [Mariola et al., 2003; Zhang et al., 2002, 2003].
SUBJECTS AND METHODS
Subjects
Forty‐two neurologically normal subjects took part in the studies described here, after giving informed consent. All procedures were approved by the Institutional Review Board of Emory University. Subjects with callused fingerpads or a history of injury to the hands or their innervation were excluded, as were those with a history of dyslexia, which is associated with tactile impairments [Grant et al., 1999; Sathian et al., 2003]. Twenty subjects (7 males, 13 females; mean age, 36.3 years; age range, 20–58 years) took part in Study 1. Twenty‐two subjects (12 males, 10 females; mean age, 20.9 years; age range, 18–25 years) took part in Study 2.
Tactile Stimulation
The stimuli (Fig. 1) were taken from a set of commercially available, dome‐shaped plastic gratings, each with equal groove and ridge width (JVP Domes; Stoelting, Wood Dale, IL). Gratings were applied manually to the distal part of the fingerpad, normal to it, with a static indentation that was maintained for about 1 s. The right hand was stimulated in all cases. The stimulated finger was immobilized in a plastic mold using double‐sided adhesive tape. For the orientation task, used in both studies, gratings were applied with the ridges oriented either along or across the long axis of the finger (Fig. 1), and subjects verbally reported the orientation of the grating as “along” or “across”. In the spacing task, used in Study 2, one of two gratings differing in groove width was applied in each trial, oriented along the long axis of the finger (Fig. 1), and subjects judged whether the grating had wider or narrower grooves (verbal response “big” or “small”). For both tasks, pseudorandom sequences of the two alternatives were used. Study 1 used the grating with the largest groove width in the set, 3 mm, applied to the pad of either the thumb or little finger. In Study 2, gratings were applied to the pad of the index finger. Preliminary psychophysical testing was carried out to determine which gratings would be used during scanning for the orientation and spacing tasks. The criterion was accuracy of 90% or better. The preliminary testing was carried out outside the scanner with the subject blindfolded and seated. For the orientation task, gratings were presented in blocks of 20 trials. The same grating was used within each block. Testing began with the largest groove width (3 mm) and continued until performance fell below criterion. Preceding each block, five practice trials with feedback were presented using the grating for that block; no feedback was given on test trials. Based on this testing, the gratings chosen for use during scanning had the following groove widths: 3 mm (n = 7); 2 mm (n = 9); 1.5 mm (n = 4); 1.2 mm (n = 1); and 1 mm (n = 1). A similar procedure was followed for the spacing task, except that each block used a pair of gratings that differed in groove width. The pairs employed during subsequent scanning had groove widths of: 3 mm, 1.5 mm (n = 4); 3 mm, 1.2 mm (n = 2); 2 mm, 1.2 mm (n = 7); and 2 mm, 1 mm (n = 9). During scanning, the subject lay supine in the scanner with the right arm outstretched and eyes closed.
Figure 1.
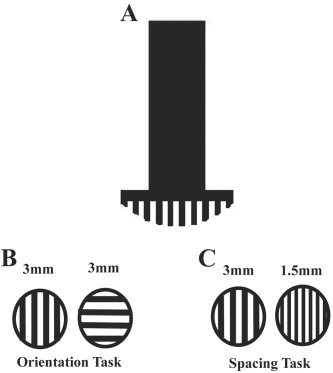
Diagram illustrating stimulus in cross‐section (A), the grating orientation discrimination task (B), and the grating spacing discrimination task (C). Example values of groove width (in mm) are shown in B and C.
MR Scanning
Study 1
A 1.5‐T Philips Intera scanner was used. The subject's head was positioned in a head coil and immobilized using a restraining strap and a suction‐connected bead bag. The subject's arm and hand were immobilized using a mold and foam padding. Anatomic images were acquired using a T1‐weighted spin‐echo sequence with the following parameters: repetition rate (TR) 500 ms; echo time (TE) 25 ms; flip angle (θ) 90 degrees; matrix 256 × 256; and in‐plane resolution 0.9 × 0.9 mm. Blood oxygenation level‐dependent (BOLD) images were acquired using single‐shot gradient‐recalled echo‐planar imaging (EPI); parameters were: TR/TE/θ = 3 s/40 ms/90 degrees; matrix 64 × 64; and in‐plane resolution 3.7 × 3.7 mm. For both anatomic and functional images, 25 slices of 5‐mm thickness were acquired without intervening gaps. A block design was used in which three stimulation blocks alternated with four rest blocks; block duration was 33 s. Gratings were applied to the right thumb and little finger in separate runs. Approximately five stimuli were applied in each orientation within each block. Subjects were instructed to covertly discriminate between the two orientations.
Study 2
This study used a 3‐T Siemens Trio whole‐body scanner and a standard head coil. BOLD images were acquired using an EPI sequence comprising 30 horizontal slices of 4 mm thickness (TR/TE/θ = 3 s/31 ms/90 degrees, matrix 64 × 64, and in‐plane resolution 3.4 × 3.4 mm). High‐resolution anatomic images were acquired using a 3D magnetization‐prepared rapid gradient‐echo imaging (MPRAGE) sequence [Mugler and Brookman, 1990] consisting of 176 sagittal slices of 1‐mm thickness (TR/TE/θ = 2.6 s/3.9 ms/8 degrees, matrix 208 × 256, and in‐plane resolution 1 × 1 mm). Foam padding under the body and beside the right arm was used to minimize arm movement and transfer of vibration from the gradient coil and to ensure the subject's comfort. Head restraint straps and foam pads were utilized to minimize head movement. Ear plugs muffled scanner noise; additional attenuation of extraneous sounds was provided by headphones that also served to convey verbal cues (see below). Once subjects were lying comfortably in the scanner, they were given instructions and a few examples for each task.
A block design paradigm was used in which blocks with and without tactile stimulation alternated. Block duration was 30 s. During the stimulation blocks, an experimenter manually applied the stimuli to the right index fingerpad for 1 s per trial, with a 2‐s interstimulus interval. There were thus 10 trials within each block. In the interstimulus intervals, the subject pressed one of two keys on a fiberoptic response box, using the second or third digit of the left hand, to indicate which stimulus had just been presented. During the blocks without stimulation (blanks), there was no tactile stimulus but subjects continued to press the response keys in alternation. Immediately preceding each block, subjects received an instructional verbal cue (“along–across” for orientation, “big–small” for spacing, and “keep tapping” for blanks) delivered through headphones. Each type of stimulation block was repeated three times (giving a total of 30 trials per condition) with the two discrimination tasks interleaved in a randomly ordered sequence (Fig. 2). The sequence and timing of stimuli were guided by preprogrammed instructions displayed to the experimenter on a computer screen using the Presentation software package (Neurobehavioral Systems, Inc., Albany, CA), which was also used to record responses.
Figure 2.
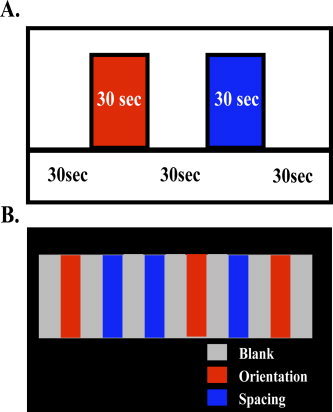
Block design protocol of Study 2. A: Nature and timing of each block type. B: Pseudorandom sequence of blocks and repetitions. OR: orientation; SP: spacing.
Image Processing and Analysis
Study 1
The software used was SPM99 (Wellcome Department of Cognitive Neurology, London, UK). EPI images from each scan were realigned to the first image of the series with motion correction, then coregistered to T1‐weighted anatomic images and normalized into Talairach space. A Gaussian kernel with full‐width half‐maximum (FWHM) of 8 mm was applied before group analysis. Statistical parametric (t) maps were generated by analyzing the time‐course of the MRI signal at each voxel, using a random‐effects general linear model with correction for multiple comparisons at the voxel level.
Study 2
This study used BrainVoyager 2000 v. 4.91 (Brain Innovation, Maastrict, The Netherlands). Each subject's EPI images were realigned to the middle image of the series using a rigid‐body transformation procedure. Functional images were preprocessed utilizing trilinear interpolation for motion correction, sinc interpolation for slice scan time correction, and high‐pass temporal filtering at 1 Hz to remove slow drifts in the data. Anatomic 3‐D images were processed, coregistered with the functional data, and transformed into Talairach [Talairach and Tournoux, 1988] space. For group analysis, the transformed data was spatially smoothed with an isotropic Gaussian kernel (FWHM = 8 mm) and z‐normalized. Statistical analysis used a random‐effects general linear model. Correction for multiple comparisons was accomplished by a cluster analysis method that takes into account both the extent and the intensity of activation [Xiong et al., 1995]. The critical t‐value varies inversely with the extent of contiguously activated voxels.
For both studies, activations were localized with respect to 3‐D cortical anatomy with the help of an MRI atlas [Duvernoy, 1999], and Talairach coordinates were used to compare the locations of activations with those identified in prior studies.
RESULTS
Study 1
As Table I shows, the grating orientation discrimination task activated a widespread set of brain regions when the right thumb was stimulated, relative to the rest baseline used in this study. All the activations detailed survived correction for multiple comparisons in the entire gray matter. There was a large activation in the left postcentral sulcus (PCS) extending posteriorly into the anterior intraparietal sulcus (aIPS), and a separate focus of activation in the left parietal operculum. A right PCS focus was also active. Multiple frontal cortical foci were active, including foci in dorsal and ventral premotor cortex (PMd and PMv, respectively) on the left, the pre‐supplementary motor area (pre‐SMA) and frontal operculum bilaterally, and the right inferior frontal gyrus (IFG). In addition, there were active foci in the right superior temporal gyrus (STG) and the right cerebellar hemisphere. Activations when the right little finger was stimulated were generally similar, but smaller and less intense and were not considered further.
Table I.
Activations in Study 1 for grating orientation discrimination relative to rest baseline
| Site | x | y | z | tmax |
|---|---|---|---|---|
| L PCS | −51 | −39 | 45 | 9.9 |
| L parietal operculum | −60 | −21 | 12 | 6.5 |
| R PCS | 48 | −39 | 48 | 6.5 |
| L PMd | −36 | −6 | 63 | 7.0 |
| L PMv | −54 | 0 | 42 | 6.0 |
| Bilateral pre‐SMA | 3 | 9 | 57 | 6.3 |
| L frontal operculum | −60 | 9 | 9 | 8.6 |
| R frontal operculum | 60 | 12 | 6 | 6.4 |
| R IFG | 60 | 15 | 24 | 7.1 |
| R STG | 48 | 18 | −9 | 6.9 |
| R cerebellum | 21 | −60 | −27 | 6.7 |
For extensive activations, the locus of the peak is identified (x, y, z Talairach coordinates of peak).
PCS, postcentral sulcus; PMd, dorsal premotor cortex; PMv, ventral premotor cortex; pre‐SMA, pre‐supplementary motor area; IFG, inferior frontal gyrus; STG, superior temporal gyrus.
Study 2
Accuracy during scanning was comparable in the orientation and spacing tasks. Mean accuracy (± standard error of the mean [SEM]) was 84 ± 2% in the orientation task and 86 ± 2% in the spacing task. There was no significant difference in accuracy between tasks (paired t test, t = −0.62; P = 0.54).
Relative to the blank (no stimulation) condition, each task elicited activity in a set of regions that was broadly similar to that identified in Study 1. To isolate task‐specific processing, we carried out a contrast between the orientation and spacing conditions. Activations on this contrast are detailed in Table II. Because most activations in Study 1 were cortical and we were interested primarily in cortical activations, these activations were corrected for multiple comparisons within the cortical volume. Foci more active during the orientation than spacing condition were present in the frontal eye fields (FEFs) bilaterally, in the right PCS extending anteriorly into the postcentral gyrus, and the left aIPS (Fig. 3). Less intense activations were also present in the PMv bilaterally and the left POC. Only one focus was more active for spacing than for orientation; it was located in the right angular gyrus (AG). Note that stimuli were applied to the right index finger in this study.
Table II.
Activations in Study 2 on contrast between grating orientation discrimination and grating spacing discrimination
| Site | x | y | z | tmax |
|---|---|---|---|---|
| Orientation > spacing | ||||
| L FEF | −22 | −14 | 52 | 5.4 |
| R FEF | 26 | −12 | 50 | 4.1 |
| L PMv | −39 | −3 | 35 | 3.7 |
| R PMv | 42 | −3 | 32 | 3.3 |
| R PCS | 35 | −32 | 40 | 4.8 |
| L alPS | −29 | −43 | 46 | 4.1 |
| L POC | −15 | −70 | 31 | 2.9 |
| Spacing > orientation | ||||
| R AG | 40 | −62 | 42 | 3.4 |
For extensive activations, the locus of the peak is identified (x, y, z Talairach coordinates of peak).
FEF, frontal eye field; PMv, ventral premotor cortex; PCS, postcentral sulcus; aIPS, anterior intraparietal sulcus; POC, parietooccipital cortex; AG, angular gyrus.
Figure 3.
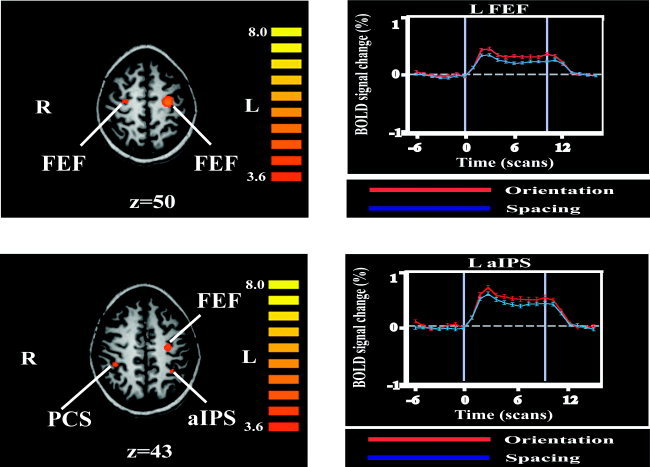
Left: Activations for orientation compared to spacing task (see text for details and abbreviations), displayed on axial MR slices taken from one subject. Display threshold: t > 3.6; Talairach z‐value below each image; color t‐scale at right of each image. Ventral premotor cortex (PMv) and parietooccipital (POC) activations are not shown. Right: Time course of BOLD % signal change (mean ± SEM) at representative activation peaks. Each scan in a time series lasted for 3 s. Abbreviations as in text.
DISCUSSION
Study 1 showed that grating orientation discrimination activated multiple parietal and frontal cortical areas bilaterally, although stimuli were only applied to the right hand. Some of these areas, as well as some other areas, were shown in Study 2 to be more active during the orientation than during the spacing task. Again, foci were activated in both hemispheres despite stimulation being limited to the right hand. Because there were methodologic differences between the two studies, we will restrict ourselves to general comparisons between the studies.
Somatosensory Cortical Activity
Not surprisingly, primary somatosensory cortex (S1) in the PCS was activated bilaterally during grating orientation discrimination relative to rest. This region corresponds to Brodmann's area (BA) 2, based on a study correlating cortical anatomy and cytoarchitectonics [Grefkes et al., 2001]. Bilateral activation of the PCS region has been reported previously during tactile tasks involving moving gratings [Burton et al., 1997] and is consistent with the bilateral somatosensory responsiveness [Iwamura et al., 1994] and callosal connectivity [Killackey et al., 1983] of BA2 in macaques. There was also an active focus in the left parietal operculum. There seem to be two somatosensory fields in this cortical region in humans [Disbrow et al., 2001; Ledberg et al., 1995]. These fields are thought to correspond to the second somatosensory cortex (S2) and the parietal ventral area (PV) of monkeys [Disbrow et al., 2001]. Interestingly, the right PCS site, but not the left, was selective for orientation compared to spacing. This orientation‐selective right PCS activation extended anteriorly into the postcentral gyrus, so that it probably involved BA1 in addition to BA2.
Posterior Parietal and Parieto‐occipital Cortical Activity
The left PCS activation during grating orientation discrimination, compared to that during rest, extended posteriorly into the aIPS. The aIPS part was orientation selective. The aIPS is known to be recruited during haptic shape perception [Binkofski et al., 1999; Bodegård et al., 2001; Stoeckel et al., 2003]. Other studies have shown that this region is multisensory, because various foci in this neighborhood are active during both haptic and visual shape perception [Grefkes et al., 2002; Zhang et al., 2004], visuo‐haptic matching of objects [Grefkes et al., 2002], attending contralaterally in both vision and touch [Macaluso et al., 2002], mentally rotating visual stimuli [Cohen et al., 1996], mentally rotating tactile stimuli [Prather et al., 2004], and motion processing of visual, auditory, or tactile stimuli [Bremmer et al., 2001].
Another orientation‐selective focus was found in the left POC. This focus was activated on the orientation versus spacing contrast in our original PET study [Sathian et al., 1997] and was functionally implicated in the orientation but not spacing task using TMS [Zangaladze et al., 1999]. The POC may be the human homolog of an area in the parietooccipital fissure of macaques that has been termed V6 or PO, and contains many orientation‐selective neurons [Galletti et al., 1991]. This focus is known to be active during visual discrimination of grating orientation [Sergent et al., 1992] and during spatial mental imagery [Mellet et al., 1996], and thus is also multisensory. We have argued previously that these findings are consistent with the idea that POC involvement in this task reflects visual imagery of the tactile stimulus [Sathian and Zangaladze, 2001]. The aIPS may serve a distinct role, that of processing tactile stimulus orientation. Further work is necessary to examine this possible distinction in the function of the aIPS and POC.
The only focus more active during the spacing than orientation task, in the right AG, was also identified on the same contrast in our previous PET study [Sathian et al., 1997], suggesting that this area might be involved specifically in the spacing discrimination task. Another study found a focus close to this one to be active during haptic discrimination of object shape [Stoeckel et al., 2003]. The precise nature of processing mediated by this area remains unclear at present.
Frontal Cortical Activity
A number of frontal cortical areas were more active in the grating orientation task than during rest. These included prefrontal areas with a right‐sided predominance, as found by others [Hagen et al., 2002], and premotor areas bilaterally. Activity in premotor areas was found in both studies, even though Study 1 required no overt motor responses, and in Study 2 identical responses were made in the experimental (orientation) and control (spacing) task. Premotor areas that were active relative to the rest baseline included both medial (pre‐SMA) and lateral areas (PMd and PMv). This fits with previous studies that have found activation of frontal regions involved in motor control, including primary motor cortex [Francis et al., 2000; Moore et al., 2000], PMd [Lloyd et al., 2003], and the SMA [Bushara et al., 2001], during tactile stimulation in the absence of a perceptual task. The exact reason for involvement of these areas is not understood, but may relate to processes linking sensory input and motor output, as suggested by neurophysiologic observations in macaques pointing to a role for neurons in the SMA and pre‐SMA in translating sensory inputs into decisions and ultimately motor output [Romo and Salinas, 2003].
Among premotor areas, the FEF and PMv bilaterally displayed orientation selectivity in the present study. The FEF is close to but separable from PMd [Astafiev et al., 2003]. Subjects' eyes were closed in both studies, so that activity in the FEF and other premotor areas is unlikely to be due to differences between conditions in the number of eye movements. Because the tasks were balanced for difficulty, as evidenced by similar accuracy during scanning, FEF activation is also unlikely to be due to attentional differences. There was also higher activity in the pre‐SMA for orientation compared to that for spacing, but this did not survive the conservative correction applied for multiple comparisons. Broadly speaking, it thus seems that premotor cortical areas are preferentially involved in processing grating orientation compared to spacing. This selectivity was unexpected and the underlying reasons remain obscure. It is of interest that there is evidence for multisensory neuronal responses in both FEF [Russo and Bruce, 1989] and PMv [Graziano et al., 1997] of macaques. Perhaps these multisensory premotor areas are more effectively recruited by macrospatial than microspatial tasks. This requires further study.
CONCLUSIONS
In summary, the studies reported in the present work confirm and extend our previous observations on the neural circuitry involved in tactile discrimination of grating orientation. We conclude that an extensive set of frontal and parietal areas is active during this task, with many of these areas showing selectivity for orientation compared to spacing. These areas include the left aIPS, right PCS, left POC, bilateral FEF, and bilateral PMv. All these areas, with the exception of the right PCS, have been shown to have multisensory properties. Future work should focus on identifying how each of these areas contributes to task performance, and the nature of multisensory processing in each area.
REFERENCES
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M (2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara‐Jimenez W, Shelton P, Hallett M (2000): Spatial discrimination is abnormal in focal hand dystonia. Neurology 55: 1869–1873. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ (1999): A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Bodegård A, Geyer S, Grefkes C, Zilles K, Roland PE (2001): Hierarchical processing of tactile shape in the human brain. Neuron 31: 317–328. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR (2001): Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29: 287–296. [DOI] [PubMed] [Google Scholar]
- Burton H, MacLeod AM, Videen T, Raichle ME (1997): Multiple foci in parietal and frontal cortex activated by rubbing embossed grating patterns across fingerpads: a positron emission tomography study in humans. Cereb Cortex 7: 3–17. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Wheat JM, Khan A, Mock BJ, Turski PA, Sorenson J, Brooks BR (2001): Multiple tactile maps in the human cerebellum. Neuroreport 12: 2483–2486. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Bookheimer SY, Rosen BR, Belliveau JW (1996): Changes in the cortical activity during mental rotation, a mapping study using functional MRI. Brain 119: 89–100. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Poeppel D, Krubitzer L (2001): Evidence for interhemispheric processing of inputs from the hands in human S2 and PV. J Neurophysiol 85: 2236–2244. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1999): The human brain. Surface, blood supply and three‐dimensional sectional anatomy. New York: Springer‐Verlag; 491 p. [Google Scholar]
- Francis ST, Kelly EF, Bowtell R, Dunseath WJR, Folger SE, McGlone F (2000): fMRI of the responses to vibratory stimulation of digit tips. Neuroimage 11: 188–202. [DOI] [PubMed] [Google Scholar]
- Galletti C, Battaglini PP, Fattori P (1991): Functional properties of neurons in the anterior bank of the parieto‐occipital sulcus of the macaque monkey. Eur J Neurosci 3: 452–461. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM (2003): Tactile acuity is enhanced in blindness. J Neurosci 23: 3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AC, Thiagarajah MC, Sathian K (2000): Tactile perception in blind Braille readers: a psychophysical study of acuity and hyperacuity using gratings and dot patterns. Percept Psychophys 62: 301–312. [DOI] [PubMed] [Google Scholar]
- Grant AC, Zangaladze A, Thiagarajah MC, Sathian K (1999): Tactile perception in developmental dyslexia: a psychophysical study using gratings. Neuropsychologia 37: 1201–1211. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Hu XT, Gross CG (1997): Visuospatial properties of ventral premotor cortex. J Neurophysiol 77: 2268–2292. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001): Human somatosensory area 2: observer‐independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14: 617–631. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Weiss PH, Zilles K, Fink GR (2002): Crossmodal processing of object features in human anterior intraparietal cortex: an fMRI study implies equivalencies between humans and monkeys. Neuron 35: 173–184. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Zald DH, Thornton TA, Pardo JV (2002): Somatosensory processing in the human inferior prefrontal cortex. J Neurophysiol 88: 1400–1406. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Iriki A, Tanaka M (1994): Bilateral hand representation in the postcentral somatosensory cortex. Nature 369: 554–556. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Phillips JR (1981): Tactile spatial resolution. I. Two‐point discrimination, gap detection, grating resolution and letter recognition. J Neurophysiol 46: 1177–1191. [DOI] [PubMed] [Google Scholar]
- Killackey HP, Gould HJ, Cusick CG, Pons TP, Kaas JH (1983): The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of New and Old World monkeys. J Comp Neurol 219: 384–419. [DOI] [PubMed] [Google Scholar]
- Klatzky RL, Lederman SJ, Reed C (1987): There's more to touch than meets the eye: the salience of object attributes for haptics with and without vision. J Exp Psychol Gen 116: 356–369. [Google Scholar]
- Ledberg A, O'Sullivan BT, Kinomura S, Roland PE (1995): Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci 7: 1934–1941. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Shore DI, Spence C, Calvert GA (2003): Multisensory representation of limb position in human premotor cortex. Nat Neurosci 6: 17–18. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J (2002): Directing attention to locations and to sensory modalities: multiple levels of selective processing revealed with PET. Cereb Cortex 12: 357–368. [DOI] [PubMed] [Google Scholar]
- Mariola EL, Zhang M, Stilla R, Weisser V, Sathian K (2003): Neural networks active during tactile discrimination of grating orientation. Soc Neurosci Abstr 379.5. [Google Scholar]
- Mellet E, Tzourio N, Crivello F, Joliot M, Denis M, Mazoyer B (1996): Functional anatomy of spatial mental imagery generated from verbal instructions. J Neurosci 16: 6504–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM (2000): Segregation of somatosensory activation in the human Rolandic cortex using fMRI. J Neurophysiol 84: 558–569. [DOI] [PubMed] [Google Scholar]
- Mugler JP, Brookman JR (1990): Three dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MPRAGE). Magn Reson Med 15: 152–157. [DOI] [PubMed] [Google Scholar]
- Phillips JR, Johnson KO (1981): Tactile spatial resolution: II. Neural representation of bars, edges and gratings in monkey primary afferents. J Neurophysiol 46: 1192–1203. [DOI] [PubMed] [Google Scholar]
- Prather SC, Votaw JR, Sathian K (2004): Task‐specific recruitment of dorsal and ventral visual areas during tactile perception. Neuropsychologia 42: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Randolph M, Semmes J (1974): Behavioral consequences of selective subtotal ablations in the postcentral gyrus of Macaca mulatta . Brain Res 70: 55–70. [DOI] [PubMed] [Google Scholar]
- Roland PE, O'Sullivan B, Kawashima R (1998): Shape and roughness activate different somatosensory areas in the human brain. Proc Natl Acad Sci USA 95: 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Salinas E (2003): Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci 4: 203–218. [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ (1989): Auditory receptive fields of neurons in frontal cortex of rhesus monkey shift with direction of gaze. Soc Neurosci Abstr 15: 1204. [Google Scholar]
- Sathian K, Cascio C, Rice D, Morris M, Dancer C, McGlone F (2003): Is tactile temporal processing impaired in developmental dyslexia? Cogn Neurosci Abstr 192. [Google Scholar]
- Sathian K, Zangaladze A (1996): Tactile spatial acuity at the human fingertip and lip: bilateral symmetry and inter‐digit variability. Neurology 46: 1464–1466. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A (2001): Feeling with the mind's eye: the role of visual imagery in tactile perception. Optom Vis Sci 78: 276–281. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, Hoffman JM, Grafton ST (1997): Feeling with the mind's eye. Neuroreport 8: 3877–3881. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, MacDonald B (1992): Functional neuroanatomy of face and object processing. A positron emission tomography study. Brain 115: 15–36. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Weder B, Binkofski F, Buccino G, Shah NJ, Seitz RJ (2003): A fronto‐parietal circuit for tactile object discrimination: an event‐related fMRI study. Neuroimage 19: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Stoesz M, Zhang M, Weisser VD, Prather SC, Mao H, Sathian K (2003): Neural networks active during tactile form perception: common and differential activity during macrospatial and microspatial tasks. Int J Psychophysiol 50: 41–49. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the brain. New York: Thieme Medical Publishers. [Google Scholar]
- van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual‐Leone A (2000): Tactile spatial resolution in blind Braille readers. Neurology 54: 2230–2236. [DOI] [PubMed] [Google Scholar]
- van Boven RW, Johnson KO (1994a): A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain 117: 149–167. [DOI] [PubMed] [Google Scholar]
- van Boven RW, Johnson KO (1994b): The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue and finger. Neurology 44: 2361–2366. [DOI] [PubMed] [Google Scholar]
- Vega‐Bermudez F, Johnson KO (2002): Spatial acuity after digit amputation. Brain 125: 1256–1264. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J‐H, Lancaster JL, Fox PT (1995): Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp 3: 287–301. [Google Scholar]
- Zangaladze A, Epstein CM, Grafton ST, Sathian K (1999): Involvement of visual cortex in tactile discrimination of orientation. Nature 401: 587–590. [DOI] [PubMed] [Google Scholar]
- Zhang M, Stoesz M, Mao H, Sathian K (2002): Activation of somatosensory, visual and motor areas during tactile discrimination of grating orientation: an fMRI study [abstract]. In: Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, Sendai, Japan.
- Zhang M, Weisser VD, Stilla R, Prather SC, Sathian K (2004). Multisensory cortical processing of object shape and its relation to mental imagery. Cogn Affect Behav Neurosci 4: 251–259. [DOI] [PubMed] [Google Scholar]


