Abstract
A number of neuroimaging studies have implicated an involvement of Broca's area, particularly of the pars opercularis of the left inferior frontal gyrus (IFG), in the processing of complex (permuted) sentences. However, functional interpretations of this region's role range from very general (e.g., in terms of working memory) to highly specific (e.g., as supporting particular types of syntactic operations). A dissociation of these competing accounts is often impossible because in most cases, the language internal complexity of permuted sentence structures is accompanied invariably by increasing costs of a more general cognitive nature (e.g., working memory, task difficulty, and acceptability). We used functional magnetic resonance imaging to explore the precise nature of the pars opercularis activation in the processing of permuted sentences by examining the permutation of pronouns in German. Although clearly involving a permutation operation, sentences with an initial object pronoun behave like simple, subject‐initial sentences (e.g., in terms of acceptability) because of a rule stating that pronouns should generally precede non‐pronominal arguments. The results of the experiment show that in contrast to non‐pronominal permutations, sentences with a permuted pronoun do not engender enhanced pars opercularis activation. Our findings therefore speak against both language‐related working memory and transformation‐based accounts of this region's role in sentence comprehension. Rather, we argue that the pars opercularis of the left IFG supports the language‐specific linearization of hierarchical linguistic dependencies. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: language comprehension, word order, inferior frontal gyrus, pars opercularis, linearization, hierarchization
INTRODUCTION
The most fundamental challenge posed by human language arguably lies in determining whether linguistic regularities are somehow “special” or whether they can be derived from the properties of other, independently warranted systems. Although some researchers have, for example, associated linguistic knowledge with constraints on action and perception [Rizzolatti and Luppino, 2001] or statistical distributions of language use [Jurafsky, 1996], others have defended the claim that language cannot be fully accounted for in terms of more general cognitive abilities [e.g., Hauser et al., 2002; Pinker and Jackendoff, 2005]. Within the field of cognitive neuroscience, the debate on the nature of language has focused extensively on the role of Broca's area, i.e. the pars opercularis and triangularis of the left inferior frontal gyrus (IFG). On the one hand, this cortical region has been associated selectively with properties deemed to be particular to language (e.g., transformations [Grodzinsky, 2000] or recursion [Friederici, 2004]). On the other hand, it has also been found to be involved in the processing of nonlinguistic information, such as music, [Koelsch et al., 2002], sequencing [Schubotz and von Cramon, 2002a, b], and action recognition [Hamzei et al., 2003].
The linguistic manipulations employed to ascertain whether Broca's region is selectively sensitive to language‐specific properties typically vary sentence complexity. Complex sentences have been argued to instantiate properties of language that cannot be associated straightforwardly with analogues in other domains such as action and perception. For example, complexity may be increased by the permutation of sentence constituents, as in the sentence Snails, I could never imagine eating. Here, the object snails appears before the subject rather than after the verb, as is typical in English. Indeed, it has been argued that Broca's area responds selectively to such permutations or transformations [Ben‐Shachar et al., 2003, 2004; Grodzinsky, 2000]. The inherent difficulty in using complex sentences to argue for a language‐specific function of Broca's area lies in the fact that by their very nature these sentences occur less frequently [e.g., Kempen and Harbusch, 2004a, b], are judged to be less acceptable [e.g., Bader and Meng, 1999; Gibson, 1998], and give rise to increased processing costs in behavioral psycholinguistic paradigms such as self‐paced reading [Gibson, 1998; King and Just, 1991]. In this way, there are typically inherent differences between complex and simple sentences that cannot be reduced fully to the linguistic manipulation per se.
Indeed, several researchers have argued that the increased processing cost for complex (permuted) sentences is grounded in the higher working memory demands engendered by these structures [Caplan et al., 2000; Fiebach et al., 2005; Kaan and Swaab, 2002; Müller et al., 2003]. From this perspective, the enhanced inferior frontal (Broca's area) activation for permuted (object‐initial) sentences is thought to result from the fact that “patient‐before‐agent sentences impose a larger burden on working memory, because the first noun phrase (corresponding to the eventual patient) cannot be syntactically and thematically integrated until the verb is encountered, and must be retained in working memory until that point” [Kaan and Swaab, 2002: 351]. Although the specific type of working memory thought to be involved in this process is not always defined clearly, the two most explicit claims on the relationship between Broca's region, permuted sentences, and working memory [Caplan et al., 2000; Fiebach et al., 2004] both assume a crucial involvement of syntactic working memory. This type of approach thus accounts for the activation of Broca's area in the processing of complex sentences by appealing to an interaction between language‐internal properties and more general cognitive constraints.
In summary, previous results regarding the role of Broca's region during sentence comprehension have been interpreted both in terms of language‐inherent properties such as transformations or recursion [Friederici, 2004; Norris, 2000] and as a result of more general capacity restrictions. However, a dissociation of these competing accounts is often impossible because in most cases the language‐internal complexity of permuted sentence structures is accompanied invariably by increasing costs of a more general cognitive nature (e.g., working memory, task difficulty, and acceptability).
We capitalize upon the particular properties of German to tease apart some of these competing factors. In contrast to English, for which deviations from a subject‐before‐object order are associated invariably with increased processing costs that are independent of the particular experimental method chosen, German permits “unmarked” permuted orders under particular circumstances. This is illustrated by the following sentences:1
1. Dann hat dem Gärtner der Lehrer den Spaten gegeben.
then has [the gardener]IOBJ [the teacher]SUBJ [the spade]DOBJgiven
“Then the teacher gave the spade to the gardener.”
2. Dann hat ihm der Lehrer den Spaten gegeben.
then has himIOBJ [the teacher]SUBJ [the spade]DOBJgiven
“Then the teacher gave him the spade.”
The indirect object precedes the subject in Sentences 1 and 2. In this way, the linear order of the sentential arguments no longer corresponds to the hierarchy of participant roles specified in the lexical entry of the verb (in this case: Agent [the teacher] > Benefactive/Recipient [the gardener/him] > Patient/Theme [the spade]).
Both sentences are therefore permuted in the sense that they do not allow a direct mapping from the surface ordering of the arguments to the conceptual structure of the verb frame [e.g., Baker, 1988; Perlmutter and Postal, 1984; Wunderlich, 1997]. In this way, the two sentence types both involve a transformation and induce increased working memory costs in the sense that the indirect object must be maintained in memory until it can be integrated. Moreover, the frequency disadvantage for object‐initial structures in comparison to their subject‐initial counterparts is comparable for Sentence 1 and 2 [Schlesewsky et al., 2003].
Despite these commonalities, it is undisputed from both a theoretical and an empirical perspective that Sentence 1 and 2 differ in important respects. In particular, pronouns are subject to a linearization rule that specifies that pronouns should precede non‐pronominal arguments in the medial portion of the German clause (the so‐called “middlefield”2) independently of their grammatical function. A sentence such as Sentence 1 therefore is defined typically as unmarked in the sense that it can be felicitously uttered in the absence of any constraining context [e.g., Siwierska, 1988]. In this respect, sentences such as Sentence 2 behave like subject‐initial sentences and contrast with sentences involving the permutation of non‐pronominal objects (Sentence 1), which require contextual licensing. These considerations, which are standard in the theoretical literature on German [Hoberg, 1981; Lenerz, 1977, 1993; Müller, 1995; Wöllstein‐Leisten et al., 1997], are also supported by a number of empirical findings using a variety of experimental methods. On the one hand, sentences such as Sentence 1 are judged to be less acceptable than are their subject‐initial counterparts [e.g., Pechmann et al., 1996; Röder et al., 2000], engender higher activation in the pars opercularis of the left IFG (i.e., part of Broca's region [Fiebach et al., 2004; Röder et al., 2002]), and elicit a left, frontocentral negativity in terms of event‐related brain potential (ERP) measures at the position of the permuted object [Bornkessel et al., 2002; Rösler et al., 1998; Schlesewsky et al., 2003]. In striking contrast to these findings, the permutation of object pronouns (as in Sentence 2) leads neither to a comparable reduction of sentence acceptability [Bader and Meng, 1999], nor to any ERP effect in comparison to subject‐initial control sentences [Schlesewsky et al., 2003]. Although pronoun permutation shares all of the domain‐general disadvantages for object‐initial structures with the permutation of non‐pronominal arguments, it is thus licensed by a language‐specific grammatical rule and therefore behaves like a subject‐initial structure in terms of linearization properties.
We use the special status of pronouns in German as a diagnostic tool to differentiate between the competing factors that have been implicated in the debate on the precise role of Broca's area during the processing of permuted (complex) sentences. Using functional magnetic resonance imaging (fMRI), we manipulated the factors permutation (permuted vs. non‐permuted) and NP‐type (first noun phrase pronominal vs. first noun phrase non‐pronominal). The critical sentence conditions resulting from this manipulation are shown in Table I.
Table I.
Critical sentence conditions
| Condition | Example |
|---|---|
| N‐SO | Dann | hat | der Lehrer | dem Gärtner | den Spaten | gegeben. |
| then has [the teacher]SUBJ [the gardener]IOBJ [the spade]DOBJ given | |
| “Then the teacher gave the spade to the gardener.” | |
| P‐SO | Dann | hat | er | dem Gärtner | den Spaten | gegeben. |
| then has heSUBJ [the gardener]IOBJ [the spade]DOBJ given | |
| “Then he gave the spade to the gardener.” | |
| N‐OS | Dann | hat | dem Gärtner | der Lehrer | den Spaten | gegeben. |
| then has [the gardener]IOBJ [the teacher]SUBJ [the spade]DOBJ given | |
| “Then the teacher gave the spade to the gardener.” | |
| P‐OS | Dann | hat | ihm | der Lehrer | den Spaten | gegeben. |
| then has himIOBJ [the teacher]SUBJ [the spade]DOBJ given | |
| “Then the teacher gave him the spade.” | |
| COMB | Dann | hat | ihm | den Spaten | der Lehrer | gegeben. |
| then has himIOBJ [the spade]DOBJ [the teacher]SUBJ given | |
| “Then the teacher gave him the spade.” |
Stimulus segmentation is indicated by the vertical bars.
N, non‐pronominal noun phrase; SO, subject‐before‐object (non‐permuted); P, pronoun; OS, object‐before‐subject (permuted); COMB, combined condition, involving the permutation of both a pronoun and a non‐pronominal argument; SUBJ, subject; DOBJ, direct object; IOBJ, indirect object.
Based on the sentence types in Table 1, the following hypotheses can be formulated. Firstly, we expect to replicate previous findings of increased activation in the pars opercularis of the left IFG for the permutation of non‐pronominal objects (N‐OS) in comparison to subject‐initial control sentences (N‐SO) [Fiebach et al., 2004; Röder et al., 2002]. If this activation is engendered by increased syntactic working memory load in the sense discussed above, permuted pronominal sentences (P‐OS) should give rise to a similar activation increase in this region. From the perspective of transformation‐based accounts of the function of the left IFG in language comprehension [Ben‐Shachar et al., 2003, 2004; Grodzinsky, 2000], there are essentially two possibilities. Firstly, if both subject and object pronouns move to a syntactic position reserved for them at the left edge of the middlefield [e.g., Haider and Rosengren, 1998; Müller, 1999], both pronominal conditions (P‐SO/P‐OS) should be expected to show increased activation as compared to the non‐permuted non‐pronominal condition (N‐SO). A second possibility is that, in accordance with the often‐assumed ban on string‐vacuous movement [Chomsky, 1986], only the object‐initial pronominal condition (P‐OS) requires a transformation operation whereas the subject‐initial pronominal condition (P‐SO) does not. An explanation along these lines would predict a similar activation pattern as the working‐memory based account, namely increased activation for the object‐initial (P‐OS) but not for the subject‐initial pronominal condition (P‐SO) in comparison to the non‐pronominal control (N‐SO). Finally, if the IFG activation observed previously reflects the application of language‐specific linearization rules that govern the mapping from hierarchical linguistic structure to sequential language input/output [e.g., Bornkessel et al., 2005],3 the two pronominal conditions (P‐SO/P‐OS) should both be expected to behave similarly to the non‐permuted non‐pronominal condition (N‐SO) in terms of the activation pattern for this region.
To examine more closely the possible differences between the permutation of pronominal and non‐pronominal arguments, we introduced a further condition involving both (COMB). Here, both the pronoun ihm (“himIOBJ”) and the non‐pronominal argument den Spaten (“[the spade]DOBJ”) precede the subject. Based on the results reported by Fiebach et al. [2004], which showed an increase of activation in the left pars opercularis as a function of the number of permutations, we predict that if pronoun permutation (P‐OS) gives rise to increased IFG activation, condition COMB should show higher activation in this region than both conditions N‐OS and P‐OS. By contrast, if there is no such activation for condition P‐OS, condition COMB should behave like condition N‐OS.
SUBJECTS AND METHODS
Participants
Sixteen participants (seven females; mean age, 25.4 years; age range: 21–32 years) took part in the fMRI study. All were monolingual, native speakers of German, had normal or corrected‐to‐normal vision, and were right‐handed as assessed by a German version of the Edinburgh Inventory [Oldfield, 1971]. Informed written consent was obtained from all participants before the scanning session. One further participant was excluded from the final data analysis on account of having consistently failed to respond within the set time limit.
Materials
Participants read 34 sentences in each of the critical conditions in Table I. All sentences comprised a sentence‐initial adverb, followed by a finite auxiliary, three arguments, and a clause‐final participle. The critical sentences were interspersed with a further 34 ungrammatical sentences to balance out the acceptability for the behavioral task (see below). The ungrammatical fillers were of a similar form as the critical sentences but contained an incorrectly positioned participle. As previous studies have shown that sentences involving multiple permutations are judged to be very close to unacceptable on multipoint judgment scales [e.g., Fiebach et al., 2004; Pechmann et al., 1996; Röder et al., 2000], participants were thus confronted with 102 acceptable sentences (conditions N‐SO, P‐SO, and P‐OS), 68 sentences of a markedly degraded acceptability (condition COMB and the filler sentences), and 34 sentences of medium acceptability (condition N‐OS). Finally, 34 null events (empty trials) were introduced to improve statistical evaluation of the data [Miezin et al., 2000], thus resulting in a total number of 238 trials per participant.
Procedure
Participants read the experimental sentences via LCD goggles (Visuastim; Magnetic Resonance Technology, Northridge, CA). To control for reading strategies, sentences were presented in a segmented manner, with a presentation time of 400 ms per segment and an interstimulus interval (ISI) of 100 ms (segmentation indicated in Table I). Each trial began with the presentation of an asterisk (300 ms plus 200‐ms ISI) and ended with a 500‐ms pause, after which a question mark signaled to participants that they should judge the acceptability of the preceding sentence. The participants carried out the judgment task by pressing one of two pushbuttons with their right index and middle fingers and were given maximally 2,500 ms to respond. The assignment of fingers to acceptable and unacceptable was counterbalanced across participants.
Trials were presented with variable onset delays of 0, 400, 800, 1,200, or 1,600 ms, thereby leading to an oversampling of the actual image acquisition time of 2,000 ms by a factor of five [Miezin et al., 2000]. All trials had a length of 8 s, thus resulting in a total measurement time of 32 min, which was separated into two functional runs.
Each participant completed a short practice session before entering the scanner.
fMRI Data Acquisition
The experiment was carried out on a 3T scanner (Medspec 30/100; Bruker, Ettlingen). Twenty axial slices (19.2 cm field of view [FOV], 64 × 64 matrix, 4‐mm thickness, and 1‐mm spacing), parallel to the anterior commissure–posterior commissure (AC–PC) plane and covering the whole brain were acquired using a single‐shot, gradient‐recalled echo planar imaging (EPI) sequence (repetition time [TR] 2,000 ms, echo time [TE] 30 ms, and 90‐degree flip angle). Two functional runs of 476 time points were collected, with each time point sampling over the 20 slices. Before the functional runs, 20 anatomical T1‐weighted MDEFT [Norris, 2000; Ugurbil et al., 1993] images (data matrix 256 × 256, TR 1.3 s, and TE 10 ms) and 20 T1‐weighted EPI images with the same geometrical parameters as the functional data were acquired.
fMRI Data Analysis
The fMRI data were analyzed using the LIPSIA software package [Lohmann et al., 2001], which contains tools for preprocessing, registration, statistical evaluation, and presentation of fMRI data.
Functional data were corrected for motion using a matching metric based on linear correlation. To correct for the temporal offset between the slices acquired in one scan, a cubic‐spline interpolation based on the Nyquist‐Shannon‐Theorem was applied. A temporal high‐pass filter with a cutoff frequency of 1/112 Hz was used for baseline correction of the signal and a spatial Gaussian filter with 5.65 mm full width half‐maximum (FWHM) was applied.
To align the functional data slices onto a 3‐D stereotactic coordinate reference system, a rigid linear registration with six degrees of freedom (three rotational and three translational) was carried out. The rotational and translational parameters were acquired based on the MDEFT and EPI‐T1 slices to achieve an optimal match between these slices and the individual 3‐D reference data set. This 3‐D reference data set was acquired for each subject during a previous scanning session. The MDEFT volume data set with 160 slices and 1‐mm slice thickness was standardized to the Talairach stereotactic space [Talairach and Tournoux, 1988]. The same rotational and translational parameters were normalized, i.e., transformed to a standard size via linear scaling. The resulting parameters were then used to transform the functional slices using trilinear interpolation, so that the resulting functional slices were aligned with the stereotactic coordinate system. This linear normalization process was improved by a subsequent processing step that carries out an additional nonlinear normalization [Thirion, 1998].
The statistical evaluation was based on least‐squares estimation using the general linear model for serially autocorrelated observations [see also Aguirre et al., 1997; Friston et al., 1995; Worsley and Friston, 1995; Zarahn et al., 1997]. The design matrix was generated with a boxcar function convolved with the hemodynamic response function. The model equation, including the observation data, the design matrix, and the error term, was convolved with a Gaussian kernel of dispersion of 4 s FWHM to deal with the temporal autocorrelation [Worsley and Friston, 1995]. Contrast maps were then generated for each subject. As the individual functional datasets were all aligned to the same stereotactic reference space, a group analysis was carried out. The single‐participant contrast images were entered into a second‐level random‐effects analysis for each of the contrasts. The group analysis consisted of a one‐sample t test across the contrast images of all subjects that indicated whether observed differences between conditions were significantly distinct from zero [Holmes and Friston, 1998]. Subsequently, t values were transformed into z scores. To protect against false positive activations, only regions with a z score greater than 3.1 (P < 0.001 uncorrected) and with a volume greater than 216 mm3 (6 measured voxels) were considered [Braver and Bongiolatti, 2002; Forman et al., 1995].
RESULTS
Behavioral Data
The mean acceptability ratings and reaction times collected in the behavioral task are shown in Figure 1 for each of the critical conditions.
Figure 1.

Mean acceptability ratings and reaction times for each of the critical sentence conditions. Error bars indicate the standard error of the mean.
For the statistical analysis of the behavioral data, we first computed one‐way repeated‐measures analyses of variance (ANOVA) involving the factor condition (COND). When the main effect of COND reached significance, we tested for possible differences between the critical conditions and the non‐permuted, non‐pronominal control (N‐SO) by computing planned comparisons between the control condition and each of the other four conditions. Furthermore, to examine possible differences among the permuted conditions, we also compared the combined condition (COMB) with the non‐pronominal permuted condition (N‐OS) and the pronominal permuted condition (P‐OS). The probability levels for planned comparisons were adjusted according to a modified Bonferroni procedure [Keppel, 1991].
With regard to the acceptability ratings, the global analysis showed a main effect of COND (F[4,60] = 88.90; P < 0.001). The subsequent planned comparisons revealed significant differences for the permuted, non‐pronominal condition (N‐OS; F[1,15] = 62.64; P < 0.001) and the combined condition (COMB; F[1,15] = 62.64; P < 0.001) in comparison to the control (N‐SO). The two pronominal conditions (P‐SO and P‐OS), by contrast, did not differ significantly from N‐SO (F < 1). The comparisons among the permuted conditions showed significant differences between COMB and N‐OS (F[1,15] = 43.94; P < 0.001) and COMB and P‐OS (F[1,15] = 133.46; P < 0.001).
For the analysis of the reaction times, the main effect of COND also reached significance (F[4,60] = 13.81; P < 0.001). Here, all conditions differed significantly from the control (P‐SO vs. N‐SO: F[1,15] = 6.44, P < 0.05; N‐OS vs. N‐SO: F[1,15] = 39.69, P < 0.001; P‐OS vs. N‐SO: F[1,15] = 10.40, P < 0.01; COMB vs. N‐SO: F[1,15] = 8.66, P < 0.05). However, there were no significant differences for COMB versus N‐OS (P > 0.26) and COMB versus P‐OS (P > 0.19).
The acceptability rates are in line with the theoretical assumptions concerning the experimental manipulation. Although the permuted non‐pronominal condition (N‐OS) was judged to be significantly less acceptable than the control condition (N‐SO) was, no such acceptability decrease was observable for either of the pronominal conditions (P‐SO/P‐OS). The comparable acceptability for permuted pronominal structures and non‐permuted non‐pronominal structures thus provides converging support for the claim that pronoun permutation is an unmarked operation in German, because it is licensed by an independent rule governing the positioning of pronouns. Finally, the acceptability ratings also showed that the combined condition, which involved two permutation operations, is less acceptable than are the two conditions including single permutations.
As for the differences in reaction times, these are somewhat difficult to interpret because participants were only responding under very moderate time pressure [for example, see Bornkessel et al., 2004]. Nonetheless, a cautious association of the increased reaction times for all noncontrol conditions with higher processing load or decision difficulty is consistent with the assumptions underlying the present experimental manipulation. The acceptability decreases for both the single non‐pronominal permuted condition (N‐OS) and the combined condition (COMB) thus were mirrored in increased reaction times. The reaction time increase for the pronominal permuted condition (P‐OS) may on the one hand reflect the fact that this condition also engendered increased syntactic working memory costs in comparison to the control condition. On the other hand, the reaction time increase for condition P‐OS might stem from more general processes applying to the pronominal sentences, because reaction times were also longer for the non‐permuted condition (P‐SO) in comparison to that for the non‐pronominal control (N‐SO). From this perspective, the general latency increase for the pronoun conditions could reflect the additional difficulties associated with judging as acceptable a sentence with a pronoun that has no antecedent.
fMRI Data
To identify the neural network sensitive to argument permutation, we firstly computed a direct contrast between the permuted and non‐permuted non‐pronominal conditions (N‐OS vs. N‐SO). The activations observable in this contrast are shown in Figure 2 and Table II.
Figure 2.
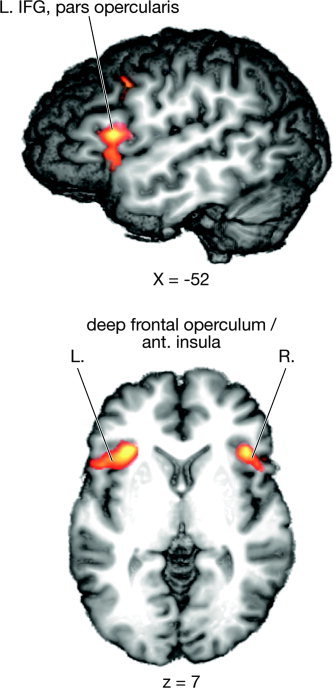
Averaged activation with a z‐value > 3.1 for the contrast between the non‐pronominal permuted condition (N‐OS) and the control condition (N‐SO).
Table II.
Talairach coordinates, maximal Z‐values, and volumes of the activated region for the local maxima in the contrast between permuted non‐pronominal (N‐OS) and non‐permuted non‐pronominal (N‐SO) sentences
| Region | Talairach coordinates | Maximum Z value | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L. deep frontal operculum/anterior insula | −32 | 20 | 3 | 4.75 | 5,297 |
| L. inferior frontal gyrus, pars opercularis | −52 | 14 | 15 | 4.44 | — |
| L. frontomedian cortex (pre‐SMA/BA8) | −2 | 32 | 30 | 4.13 | 4,384 |
| L. inferior frontal junction area | −38 | 8 | 38 | 4.53 | 1,309 |
| R. inferior frontal sulcus | 44 | 26 | 18 | 4.05 | 638 |
| R. deep frontal operculum/anterior insula | 38 | 20 | 6 | 4.31 | 2,070 |
| R. inferior frontal gyrus, pars opercularis | 46 | 11 | 9 | 3.97 | — |
Only activation with a Z value > 3.09 and a volume of at least 216 mm3 (6 measured voxels) were considered. Local maxima were defined as the largest Z‐value exceeding 3.09 within a 10‐mm radius.
L., left; R., right; SMA, supplementary motor area; BA, Brodmann area.
As is apparent from Figure 2 and Table II, the present study replicates previous findings on the permutation of non‐pronominal arguments in German [Fiebach et al., 2004; Röder et al., 2002] in showing increased bilateral pars opercularis activation for permuted structures. In the present study, this activation extended into the deep frontal operculum/anterior insula. Further activations were observed in the frontomedian cortex (pre‐supplementary motor area [SMA]/Brodmann area [BA] 8), the left inferior frontal junction area (IFJ), and the right inferior frontal sulcus (IFS).
To examine the differences between conditions with respect to the hypotheses formulated in the introduction, we extracted the time course of the underlying blood oxygenation level‐dependent (BOLD) signal for the regions shown in Table II. Within these regions, the percent signal change for the voxel with the highest Z value and the 26 adjacent voxels (relative to the mean signal intensity over all time points per voxel) was averaged for each condition and participant, with subsequent averaging over all 16 participants. The time course of the null events was subtracted from the averaged single‐event time courses for the critical sentence conditions [Burock et al., 1998]. Figure 3 visualizes the results of the time course analysis by showing the mean percent signal change in a time window from 8 to 12 s post sentence onset for each of the critical conditions.
Figure 3.
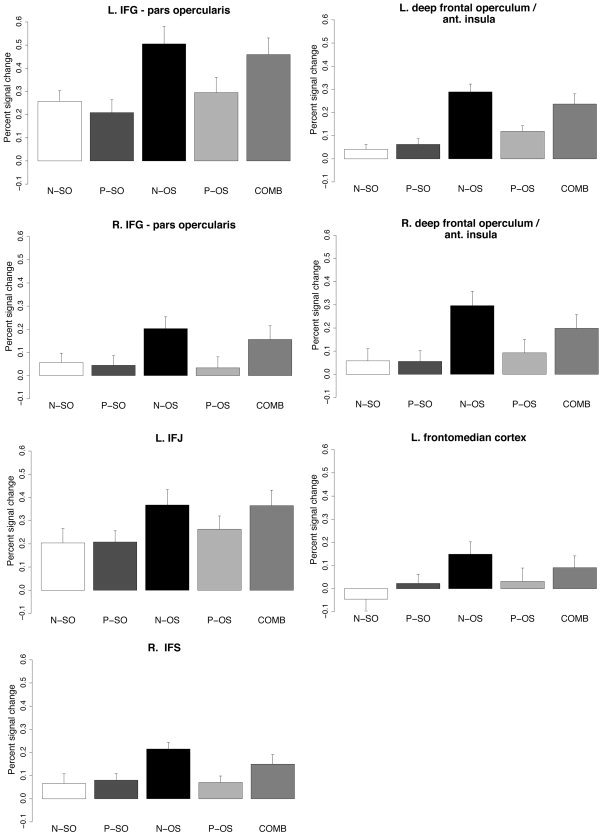
Average percent signal change (8 to 12 s relative to sentence onset) for regions showing a significant effect of permutation for the non‐pronominal conditions (N‐OS vs. N‐SO). Error bars indicate the standard error of the mean.
The averaged time courses were subjected to repeated measures analyses of variance (ANOVAs) involving the factor condition (COND). The results of this analysis are summarized in Table III, as are the planned comparisons between individual conditions for those regions showing a main effect of COND. The significance level of the planned comparisons was adjusted according to a modified Bonferroni procedure [Keppel, 1991].
Table III.
Summary of the global statistical analysis for the averaged percent signal change for the voxel with the maximal activation and the 26 adjacent voxels in each of the regions showing a significant effect of permutation for the non‐pronominal conditions (N‐OS vs. N‐SO)
| Region | COND | P‐SO vs. N‐SO | N‐OS vs. N‐SO | P‐OS vs. N‐SO | COMB vs. N‐SO | COMB vs. P‐OS | COMB vs. N‐OS |
|---|---|---|---|---|---|---|---|
| L. inferior frontal gyrus (pars opercularis) | 9.83c | NS | 19.74c | NS | 9.84a | 6.06a | NS |
| L. deep frontal operculum/anterior insula | 18.64c | NS | 55.09c | M (5.53) | 22.18c | 7.33a | NS |
| R. inferior frontal gyrus (pars opercularis) | 6.91c | NS | 19.64c | NS | 6.23a | M (5.45) | NS |
| R. deep frontal operculum/anterior insula | 10.92c | NS | 27.57c | NS | 9.25a | NS | NS |
| L. frontomedian cortex | 7.08c | NS | 34.81c | NS | 10.29b | NS | NS |
| L. inferior frontal junction area | 12.30c | NS | 33.63c | 5.63a | 16.02b | 8.06a | NS |
| R. inferior frontal sulcus | 9.26c | NS | 26.97c | NS | 9.74a | 8.04a | 8.94a |
Each cell gives the significance level for an effect,, and the F‐value for significant effects. Degrees of freedom were df 1 = 4, df 2 = 60 for the global analysis involving the factor COND and df 1 = 1, df 2 = 15 for the planned comparisons. The probability levels for the planned comparisons are Bonferroni corrected.
P < 0.05;
P < 0.01;
P < 0.001.
P, pronoun; N, non‐pronominal noun phrase; SO, subject‐before‐object (non‐permuted); OS, object‐before‐subject (permuted); COMB, combined condition, involving the permutation of both a pronoun and a non‐pronominal argument; L., left; R., right; NS, not significant; M, marginal (P < 0.07).
The analyses summarized in Table III show that in the pars opercularis of the left IFG, the permuted non‐pronominal (N‐OS) and the combined (COMB) conditions engender increased activation in comparison to the control (N‐SO). By contrast, neither the non‐permuted (P‐SO) nor the permuted pronominal condition (P‐OS) differs significantly from the control condition in this region. Finally, the combined condition (COMB) shows significantly more activation than do N‐SO and P‐OS, but does not differ from N‐OS.
Similar activation patterns were observed in the right hemisphere homologue of the pars opercularis and in the left deep frontal operculum/anterior insula. By contrast, the right deep frontal operculum/anterior insula and left frontomedian cortex failed to show a significant difference between COMB and P‐OS, whereas the right IFS showed a significant difference between COMB and N‐OS and the left IFJ responded more strongly to the P‐OS than to the N‐SO condition.
DISCUSSION
The present study aimed to shed light on the precise role of the pars opercularis of the IFG in the processing of permuted (complex) word orders by examining permuted German sentences that behave like subject‐initial (non‐permuted) sentences. With regard to the permutation of non‐pronominal arguments, this study replicated previous findings of increased bilateral activation in the pars opercularis of the IFG. In contrast to earlier experiments, this activation additionally extended into the deep frontal operculum/anterior insula. Crucially, the permutation of pronominal arguments did not lead to an activation increase in these cortical regions in comparison to the non‐pronominal, subject‐initial control condition. Similarly, the subject‐initial pronominal condition also did not show an activation increase. Finally, the combined condition, which involved the permutation of a pronominal and a non‐pronominal argument, behaved like the single non‐pronominal permutation in terms of pars opercularis activation, engendering increased activation in comparison to both the non‐permuted, non‐pronominal control and the permuted pronominal condition. In the following, we discuss the implications of these findings for the different accounts regarding the function of the pars opercularis and, more generally, of Broca's area during the comprehension of permuted (complex) sentences.
Broca's Region, Language, and Working Memory
As discussed above, in terms of syntactic working memory costs, the permuted pronominal condition should behave similarly to the non‐pronominal permuted condition, because the lower‐ranking argument in the argument hierarchy of the verb must be maintained until the higher‐ranking argument(s) have been processed [Gibson, 1998; Kaan and Swaab, 2002]. If the role of Broca's area (or more precisely, of the pars opercularis of the left IFG) in language processing is crucially tied to working memory resources [e.g., Caplan et al., 2000; Fiebach et al., 2005], the permuted pronominal condition (P‐OS) should show a similar activation increase in comparison to the non‐permuted non‐pronominal condition (N‐SO) as the permuted non‐pronominal condition (N‐OS). However, this was not the case; the permuted pronominal condition did not differ from the non‐permuted non‐pronominal condition in this region. In this way, these findings indicate that working memory is not the decisive factor involved in the increased pars opercularis activation during the processing of complex sentences.4 Rather, the data call for a language‐specific explanation.
Broca's Region, Language, and Transformations
Perhaps the most prominent language‐inherent account of Broca's area activation during the processing of complex (permuted) sentences is the transformation‐based hypothesis put forward by Grodzinsky [2000] and Ben‐Shachar et al. [2003, 2004]. Although this type of account can derive previous findings on argument permutation in German and various other languages, the present findings speak against a transformation‐based explanation of word order‐based activations of Broca's region.
As was laid out in the introduction, transformation‐based accounts can essentially derive two possible predictions with respect to the positioning of pronouns in German. Firstly, it has been assumed that pronouns must generally (i.e., independently of their grammatical function) undergo a dislocation from the position determined by the argument structure of the verb to the left edge of the German middlefield [e.g., Haider and Rosengreen, 2003; Lenerz, 1977; Müller, 1998; see also Schlesewsky et al., 2003]. From this perspective, both of the pronominal conditions (P‐SO/P‐OS) involve a transformation as compared to the non‐pronominal control condition (N‐SO). In terms of a transformation‐based account, both should thus be expected to show increased activation in Broca's area, as argued, for example, by Ben‐Shachar et al. [2004] for both subject‐ and object‐initial wh‐questions in comparison to yes–no questions in Hebrew. With regard to the present study, the time course analysis showed that this hypothesis is not borne out, because neither of the two pronominal conditions engenders increased activation in Broca's area in comparison to the non‐pronominal, non‐permuted control.
A second possibility is that only the object‐initial pronominal condition requires a transformation, whereas the subject‐initial pronominal condition (P‐SO) does not. From the perspective of this analysis and assuming the transformational account, only the object‐initial (P‐OS) condition should be expected to show increased activation in comparison to the non‐pronominal control (N‐SO). Again, the results of the present study are incompatible with such an account, because there is no increased IFG activation for P‐OS in comparison to N‐SO.
One final possibility to salvage the transformation‐based account would be to assume that pronouns are simply “inserted” (or base generated) at the left edge of the middlefield independently of their grammatical function. This possibility not only seems stipulated in view of the absence of independent evidence in its favor, but is also undesirable from a theoretical perspective because it would result in the abandonment of one of the most fundamental assumptions of the form‐to‐meaning mapping that lies at the core of language. It is thus generally assumed that a verb's lexical entry contains a hierarchical representation of its arguments, which essentially corresponds to the relations holding between the arguments' participant roles [e.g., Baker, 1988; Perlmutter, 1978; Van Valin and LaPolla, 1997; Wunderlich, 1997].
In basic, non‐permuted sentences, the syntactic structure directly reflects this lexical argument hierarchy, thus guaranteeing the correspondence between meaning and form. Indeed, the very concept of transformations is based on this assumption because if the form‐to‐meaning mapping could be achieved by other means, there would be no need to reconstruct a surface ordering to an underlying ordering. The present activation pattern thus does not seem to derive from the differential application of transformation operations.
Broca's Region and Sentence Acceptability
One of the critical properties of the permuted pronominal sentences is that their acceptability is in no way degraded in comparison that of non‐permuted sentences (97% as opposed to 41% for the permuted non‐pronominal sentences in the present study). At a first glance, the pattern of pars opercularis activation observed here thus might seem to mirror the surface acceptability of the structures under examination.
Several observations indicate that the pars opercularis activation for permuted sentences does not simply mirror sentence acceptability. Firstly, consider the results of a previous study contrasting grammatical and ungrammatical sentences in German [Fiebach et al., 2004]. This study employed very complex but nonetheless grammatical structures involving the permutation of two non‐pronominal objects. Due to the high complexity of these structures, they were reliably rated as unacceptable by linguistically naive participants [Pechmann et al., 1996; Röder et al., 2000]. Despite the overtly comparable degree of (un)acceptability of the complex and ungrammatical sentences, the two types of structures engendered distinct patterns of activation in inferior frontal cortex: whereas the complex, grammatical condition gave rise to increased activation of the inferior portion of the pars opercularis of the IFG, the ungrammatical condition resulted in a stronger activation of the posterior deep frontal operculum. This dissociation suggests that it is not acceptability per se that covaries with the activation of the pars opercularis.
Upon closer consideration, the findings of the present study also preclude an explanation in terms of acceptability. Consider the behavior of the combined condition (COMB), which involved the permutation of both a pronoun and a non‐pronominal argument. The acceptability of this condition was significantly lower than was that of the condition with a single permuted non‐pronominal argument (N‐OS) 19 vs. 41%. An acceptability‐based account of the pars opercularis activation observed here should therefore also predict increased activation for condition COMB in comparison to condition N‐OS. However, as is apparent from the averaged signal time courses in Figure 3 and the statistical analyses in Table III, there was no difference between these two conditions in the pars opercularis. In this way, the relationship between sentence acceptability and pars opercularis activation is not one‐to‐one and the activation patterns therefore call for a more principled explanation.
Broca's Region and the Linearization of Linguistic Hierarchies
As discussed above, the pattern of pars opercularis activation in the present experiment seems derivable neither in terms of general properties such as working memory requirements or sentence acceptability nor as a function of (language‐inherent) transformation operations. Rather, we propose that the present findings are most naturally accounted for in terms of a model assuming that the pars opercularis of the IFG engages selectively in the linearization of hierarchical linguistic dependencies [see also Bornkessel et al., 2005]. Hierarchical dependencies of various types abound in natural language; for example, objects may be viewed as hierarchically dependent on subjects (at least in European languages) because all syntactic operations that can affect objects can also affect subjects but not vice versa. Similarly, in terms of the conceptual relationship holding between sentential arguments, arguments that are Undergoers of an event are typically thought to be dependent upon arguments that are Actors, because the event that causes the Undergoer to be affected must have been caused by some other participant (the Actor). Due to the sequential nature of language, such dependencies often map onto linearization preferences such that subjects preferentially precede objects and Actors preferentially precede Undergoers, for example. Although these linearization principles often correlate with frequency of occurrence, this need not be the case, thus suggesting that the preferences in question cannot be reduced to structural frequency [e.g., Bornkessel et al., 2002; Schlesewsky et al., 2003].
Despite certain tendencies that are shared across languages, linearization principles are generally language specific. From this perspective, it is thus not surprising that there are sentences in German in which the preference for subjects to precede objects is overridden by a further linearization rule specific to this language, namely that pronouns should precede non‐pronominal arguments in the middlefield. This second principle therefore licenses pronoun‐initial orders even when the pronoun is an object and precedes the (non‐pronominal) subject. Under the assumption that the pars opercularis of the left IFG is sensitive to such linearization principles, the absence of increased activation in the permuted pronoun condition as compared to that in the non‐pronominal control condition is straightforwardly derivable.
A possible theoretical foundation for such a linearization‐based account of pars opercularis function lies in Jackendoff's [2002] tripartite language architecture. This model assumes parallel representations for syntactic, semantic, and phonological information, which then interact with one another at so‐called interface levels. Word order permutations arise when the syntax permits different possible orderings and the optimal linearization is determined at the interfaces (e.g., by semantic information such as animacy or phonological information such as constituent “weight”). From this perspective, the pars opercularis could be viewed as engaging in interface‐level functions, which integrate several different information types to evaluate potential sequential orderings.
The Role of the Deep Frontal Operculum/Anterior Insula
In contrast to previous findings, the activation associated with argument‐order permutations in the present study was not confined to the lateral surface of the pars opercularis, but rather extended into the deep frontal operculum/anterior insula. This observation raises two important questions: (1) whether these adjacent cortical regions perform similar or distinct functions; and (2) why previous studies did not report the deep fronto‐opercular/insular activation.
With regard to possible distinct functions of the pars opercularis and the deep frontal operculum, it has been suggested recently that the former engages in the processing of complex (permuted) sentences whereas the latter is crucially involved in the detection of ungrammaticality [Friederici, 2004]. This hypothesis was based on a number of empirical findings showing activation of the deep frontal operculum rather than of the IFG in response to ungrammatical sentences [Fiebach et al., 2004; Friederici et al., 2003; Kuperberg et al., 2000]. By contrast, the present study failed to reveal systematic differences between the activation pattern of the pars opercularis and that of the deep frontal operculum. Moreover, neither of these regions showed a direct correlation with sentence acceptability.
Alternatively, the activation differences observed in the deep frontal operculum/anterior insula in the present study as opposed to previous findings [Fiebach et al., 2004; Röder et al., 2002] might be attributable to more general processes involved in the evaluation of linguistic structures. In particular, the involvement of anterior insular cortex may be telling in this respect. As part of the paralimbic system, the anterior insula is involved in the mediation of subjective feeling states [Craig, 2002] and reacts to changes in the state of autonomic arousal [e.g., Critchley et al., 2001]. However, a number of studies have also implicated an involvement of the anterior insula in decision making in the presence of uncertainty [e.g., Paulus et al., 2001; Ullsperger and von Cramon, 2001; Volz et al., 2004]. Linking this to the present experimental paradigm, recall that the permuted non‐pronominal stimuli used here are possible in German, but of degraded acceptability. Sentences of this type are thus perceived by speakers as neither perfectly well‐formed nor fully impossible, thereby rendering the degree of uncertainty associated with a two‐way forced choice judgment much higher. Moreover, because constructions of this type are often considered poor style in prescriptive grammars of German, participants were instructed that they should judge the sentences based on their own linguistic intuition and that there are no right and wrong answers. This mode of instruction also differs from those employed in previous studies, in which participants were asked to judge whether sentences were grammatical or ungrammatical. As such, the environment for the present judgment task, and particularly for the conditions involving the permutation of a non‐pronominal object, was one of high uncertainty.
Possibly, then, the deep fronto‐opercular/anterior insular activation observed here may have resulted from the involvement of partly intuitive evaluative decision mechanisms that apply in the absence of any clear rule‐system on which responses might be based. An explanation along these lines accounts for why the activation of the deep frontal operculum was not observed in previous studies that did not employ an explicit judgment task, and why there is no direct correlation between the activation of this region and surface sentence acceptability (i.e., the level of acceptability of a particular sentence structure is in principle independent of the ease or difficulty involved in making this judgment). Nonetheless, the present results indicate that the precise role of the deep frontal operculum/anterior insula in linguistic judgments remains an important topic for future research.
CONCLUSIONS
The present study set out to distinguish between several competing accounts regarding the function of Broca's area, particularly the pars opercularis of the left IFG, during the processing of complex (permuted) sentences. By employing permuted German sentences that behave like simple, subject‐initial sentences, we were able to show that permutation per se does not engender increased activation in this region. The predictions of working memory‐based and transformation‐based accounts of Broca's area function thus are not borne out. Rather, our results suggest that the pars opercularis is selectively sensitive to the language‐specific linearization of hierarchical linguistic dependencies, a proposal that not only accounts for the present findings, but also derives previously reported cross‐linguistic differences in the activation of Broca's region.
Acknowledgements
The research reported here was carried out at the Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig. This work was supported in part by the graduate program “Neuronal representations and action control” (GK 885/1 to T.G.), which is funded by the German Research Foundation (DFG). We thank A. Friederici and F. Rösler for fruitful discussions related to the present research and M. Naumann, A. Wiedemann, and S. Wipper for acquisition of fMRI data.
Footnotes
Abbreviations used in the German sentence examples: SUBJ = subject; DOBJ = direct object; IOBJ = indirect object.
The middlefield is defined as the part of a German clause between a complementizer (e.g., dass, “that”) or a finite verb in second position (cf. example 1) and a clause‐final participle, infinitive, or particle.
In fact, the grammatical rule that pronouns should precede non‐pronominal arguments is only one of a whole number of principles that govern linear order in the German middlefield. Although the most important underlying principle at work in this portion of the clause is the argument hierarchy specified by a verb (see above), further modulating principles include, for example, that animate arguments should precede inanimate arguments and that definite arguments should precede indefinite arguments [cf. Lenerz, 1977]. Essentially, these different factors all encode hierarchical relations between different argument types such that the surface order in the middlefield may be viewed as the output of a mechanism that maps these hierarchical dependencies onto a linear sequence.
Of course, this explanation does not exclude that working memory‐based processes are involved in the comprehension of sentences with permuted pronominal arguments; indeed, we would assume that these processes certainly are required for such sentences to be understood successfully. Nonetheless, different demands on working memory cannot account for the contrast between pronominal and non‐pronominal permutation.
REFERENCES
- Aguirre GK, Zarrahn E, d'Esposito M (1997): Empirical analyses of BOLD fMRI statistics II. Spatially smoothed data collected under null‐hypothesis and experimental conditions. Neuroimage 5: 199–212. [DOI] [PubMed] [Google Scholar]
- Bader M, Meng M (1999): Subject‐object ambiguities in German embedded clauses: an across‐the‐board comparison. J Psycholinguist Res 28: 121–143. [Google Scholar]
- Baker M (1988): Incorporation: a theory of grammatical function changing. Chicago: University of Chicago Press. [Google Scholar]
- Ben‐Shachar M, Hendler T, Kahn I, Ben‐Bashat D, Grodzinsky Y (2003): The neural reality of syntactic transformations: evidence from fMRI. Psychol Sci 14: 433–440. [DOI] [PubMed] [Google Scholar]
- Ben‐Shachar M, Palti D, Grodzinsky Y (2004): Neural correlates of syntactic movement: converging evidence from two fMRI experiments. Neuroimage 21: 1320–1336. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, McElree B, Schlesewsky M, Friederici AD (2004): Multidimensional contribution to garden‐path strength: dissociating phrase structure from case marking. J Mem Lang 51: 495–522. [Google Scholar]
- Bornkessel I, Schlesewsky M, Friederici AD (2002): Grammar overrides frequency: evidence from the online processing of flexible word order. Cognition 85: 21–30. [DOI] [PubMed] [Google Scholar]
- Bornkessel I, Zysset S, von Cramon DY, Friederici AD, Schlesewsky M (2005): Who did what to whom? The neural basis of argument hierarchies during language comprehension. Neuroimage (in press). [DOI] [PubMed] [Google Scholar]
- Braver T, Bongiolatti S (2002): The role of frontopolar cortex in subgoal processing during working memory. Neuroimage 15: 523–536. [DOI] [PubMed] [Google Scholar]
- Burock MA, Bruckner RL, Woldorff MG, Rosen BR, Dale AM (1998): Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A (2000): Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp 9: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N (1986): Barriers. Cambridge, MA: MIT Press; 102 p. [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001): Neural correlates of first and second‐order representation of bodily states. Nat Neurosci 4: 207–212. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Bornkessel I, Friederici AD (2004): Distinct neural correlates of legal and illegal word order variations in German: how can fMRI inform cognitive models of sentence processing In: Carreiras M, Clifton C, Jr, editors. The on‐line study of sentence comprehension. New York: Psychology Press; p 357–370. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD (2005): Revisiting the localization of syntax: syntactic integration vs. syntactic working memory. Hum Brain Mapp 24: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S, Cohen J, Fitzgerald M, Eddy W, Mintun M, Noll D (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2004): Processing local transitions versus long‐distance syntactic hierarchies. Trends Cogn Sci 8: 245–247. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rueschemeyer S‐A, Hahne A, Fiebach C (2003): The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex 13: 1047–3211. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RW (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Gibson E (1998): Linguistic complexity: locality of syntactic dependencies. Cognition 68: 1–76. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y (2000): The neurology of syntax: language use without Broca's area. Behav Brain Sci 23: 1–71. [DOI] [PubMed] [Google Scholar]
- Haider H, Rosengreen I (2003): Scrambling: nontriggered chain formation in OV languages. J Germ Ling 15: 203–267. [Google Scholar]
- Haider H, Rosengren I (1998): Scrambling. Sprache und Pragmatik. Lund: Germanistisches Institut; 49. [Google Scholar]
- Hamzei F, Rijntjes M, Dettmers C, Glauche V, Weiller C, Büchel C (2003): The human action recognition system and its relationship to Broca's area: an fMRI study. Neuroimage 19: 637–644. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT (2002): The faculty of language: what is it, who has it, and how did it evolve? Science 298: 1569–1579. [DOI] [PubMed] [Google Scholar]
- Hoberg U (1981): Die Wortstellung in der geschriebenen deutschen Gegenwartssprache. Heutiges Deutsch I/10. München: Huber. [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalisability, random effects and population inference. Neuroimage 7: 754. [Google Scholar]
- Jackendoff R (2002): Foundations of language. Brain, meaning, grammar, evolution. New York: Oxford University Press; 477 p. [DOI] [PubMed] [Google Scholar]
- Jurafsky D (1996): A probabilistic model of lexical and syntactic access and disambiguation. Cogn Sci 20: 137–194. [Google Scholar]
- Kaan E, Swaab TY (2002): The brain circuitry of syntactic comprehension. Trends Cogn Sci 6: 350–356. [DOI] [PubMed] [Google Scholar]
- Kempen G, Harbusch K (2004a): A corpus study into word order variation in German subordinate clauses: animacy affects linearization independently of grammatical function assignment In: Pechmann T, Habel CH, editors. Multidisciplinary approaches to language production. Berlin: Mouton De Gruyter; p 173–181. [Google Scholar]
- Kempen G, Harbusch K (2004b): How flexible is constituent order in the midfield of German subordinate clauses? A corpus study revealing unexpected rigidity In: Kepser S, Reis M, editors. Pre‐Proceedings of the International Conference on Linguistic Evidence. Tübingen: p 81–85. [Google Scholar]
- Keppel G (1991): Design and analysis: a researcher's handbook. Englewood Cliffs: Prentice Hall; 594 p. [Google Scholar]
- King J, Just M (1991): Individual differences in syntactic processing: the role of working memory. J Mem Lang 30: 580–602. [Google Scholar]
- Koelsch S, Gunter TC, von Cramon DY, Zysset S, Lohmann G, Friederici AD (2002): Bach speaks: a cortical “Language‐Network” serves the processing of music. Neuroimage 17: 956–966. [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe‐Hesketh S, Wright IC, Lythgoe DJ, Williams SC, David AS (2000): Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci 12: 321–341. [DOI] [PubMed] [Google Scholar]
- Lenerz J (1977): Zur Abfolge nominaler Satzglieder im Deutschen. Tübingen: Narr. [Google Scholar]
- Lenerz J (1993): Zu Syntax und Semantik deutscher Personalpronomina In: Reis M, editor. Wortstellung und Informationsstruktur. Tübingen: Niemeyer; p 117–154. [Google Scholar]
- Lohmann G, Müller K, Bosch V, Mentzel H, Hessler S, Chen L, Zysset S, von Cramon DY (2001): LIPSIA—a new software system for the evaluation of functional magnetic resonance images of the human brain. Comput Med Imaging Graph 25: 449–457. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL (2000): Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage 11: 735–759. [DOI] [PubMed] [Google Scholar]
- Müller G (1995): A‐bar syntax. A study in movement types. Berlin: Mouton de Gruyter. [Google Scholar]
- Müller G (1998): German word order and optimality theory. Arbeitspapiere des SFB 340 126: 45. [Google Scholar]
- Müller G (1999): Optimality, markedness, and word order in German. Linguistics 37: 777–818. [Google Scholar]
- Müller RA, Kleinhans N, Courchesne E (2003): Linguistic theory and neuroimaging evidence: an fMRI study of Broca's area in lexical semantics. Neuropsychologia 41: 1199–1207. [DOI] [PubMed] [Google Scholar]
- Norris DG (2000): Reduced power multi‐slice MDEFT imaging. J Magn Reson Imaging 11: 445–451. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL (2001): Prefrontal, parietal, and temporal cortex networks underlie decision‐making in the presence of uncertainty. Neuroimage 13: 91–100. [DOI] [PubMed] [Google Scholar]
- Pechmann T, Uszkoreit H, Engelkamp J, Zerbst D (1996): Wortstellung im deutschen Mittelfeld. Linguistische Theorie und psycholinguistische Evidenz In: Habel CH. Kanngießer S, Rickheit G, editors. Perspektiven der Kognitiven Linguistik: Modelle und Methoden. Wiesbaden: Westdeutscher Verlag; p 257–299. [Google Scholar]
- Perlmutter DM (1978): Impersonal passives and the unaccusativity hypothesis In: Jaeger JJ, editor. Proceedings of the fourths annual meeting of the Berkeley Linguistics Society. Berkeley: Berkeley Linguistics Society; p 157–189. [Google Scholar]
- Perlmutter DM, Postal PM (1984): Studies in relational grammar. Chicago: University of Chicago Press. [Google Scholar]
- Pinker S, Jackendoff R (2005): The faculty of language: what's special about it? Cognition 95: 201–236. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001): The cortical motor system. Neuron 31: 889–901. [DOI] [PubMed] [Google Scholar]
- Röder B, Schicke T, Stock O, Heberer G, Rösler F (2000): Word order effects in German sentences and German pseudo‐word sentences. Sprache Kogn 19: 31–37. [Google Scholar]
- Röder B, Stock O, Neville H, Bien S, Rösler F (2002): Brain activation modulated by the comprehension of normal and pseudo‐word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage 15: 1003–1014. [DOI] [PubMed] [Google Scholar]
- Rösler F, Pechmann T, Streb J, Röder B, Hennighausen E (1998): Parsing of sentences in a language with varying word order: word‐by‐word variations of processing demands are revealed by event‐related brain potentials. J Mem Lang 38: 150–176. [Google Scholar]
- Schlesewsky M, Bornkessel I, Frisch S (2003): The neurophysiological basis of word order variations in German. Brain Lang 86: 116–128. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2002a): A blueprint for target motion: fMRI reveals perceived sequential complexity to modulate premotor cortex. Neuroimage 16: 920–935. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2002b): Predicting perceptual events activates corresponding motor schemes in lateral premotor cortex: an fMRI study. Neuroimage 15: 787–796. [DOI] [PubMed] [Google Scholar]
- Siwierska A (1988): Word order rules. London: Croom Helm; 304 p. [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 122 p. [Google Scholar]
- Thirion JP (1998): Image matching as a diffusion process: an analogy with Maxwell's demons. Med Image Anal 2: 243–260. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Garwood M, Ellermann J, Hendrich K, Hinke R, Hu X, Kim SG, Menon R, Merkle H, Ogawa S, Salmi R (1993): Imaging at high magnetic fields: initial experiences at 4T. Magn Reson Q 9: 259. [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY (2001): A dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- Van Valin RD, LaPolla R (1997): Syntax: structure, meaning and function. Cambridge: Cambridge University Press. [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY (2004): Why am I unsure? Internal and external attributions of uncertainty dissociated by fMRI. Neuroimage 21: 848–857. [DOI] [PubMed] [Google Scholar]
- Wöllstein‐Leisten A, Heilmann A, Stephan P, Vikner S (1997): Deutsche Satzstruktur‐Grundlagender syntaktischen Analyse. Tübingen: Stauffenburg. [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited‐again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Wunderlich D (1997): Cause and the structure of verbs. Ling Inq 28: 27–68. [Google Scholar]
- Zarahn E, Aguirre GK, d'Esposito M (1997): Empirical analyses of BOLD fMRI statistics in spatially unsmoothed data collected under null‐hypothesis conditions. Neuroimage 5: 179–197. [DOI] [PubMed] [Google Scholar]


