Abstract
Emotional behavior is organized along two psychophysiologic dimensions: (1) valence, varying from negative to positive, and (2) arousal, varying from low to high. Behavioral responses along these dimensions are assumed to be mediated by different brain circuits. We recorded startle reflex modulation and skin conductance responses in healthy volunteers during functional magnetic resonance imaging (fMRI) while they viewed a set of emotional pictures and took verbal ratings of the emotional valence and arousal of each picture after scanning. Response‐related multiple correlation analysis revealed differential brain activity in five brain regions. Startle reflex changes, associated with the valence of a stimulus, correlated with activity in the amygdala, while verbal reports of negative emotional valence varied with insular activity. Peripheral physiologic and verbal responses along the arousal dimension varied with thalamic and frontomedial activity. Peripheral physiologic responses along both dimensions correlated with activity in somatosensory association areas in the anterior parietal cortex. In the valence dimension, activity in the left anterior parietal cortex was associated with highly correlating peripheral physiologic and verbal responses, suggesting that verbal reports of emotional valence might depend partly on brain circuits representing peripheral physiologic changes. Our data provide direct evidence for a functional segregation of brain structures underlying peripheral physiologic responses and verbal ratings along the emotional dimensions of valence and arousal. Hum. Brain Mapp. 23:200–209, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: response‐related fMRI, emotional valence, arousal, peripheral physiologic responses, startle reflex, skin conductance response, multiple correlation analysis
INTRODUCTION
A large body of literature describes the neural correlates of human emotion. Many of these studies have been guided by the notion that different emotions are associated with the activation of circumscribed neural networks [e.g., Damasio et al., 2000; Lane et al., 1997b; Paradiso et al., 1997; Phillips et al., 1997; Reiman et al., 1997]. A recent meta‐analysis of 55 neuroimaging studies [Phan et al., 2002], investigating the neural correlates of fear, anger, disgust, sadness, and happiness, could not confirm significant differential activation during different emotions with two exceptions: fear was associated more often with amygdala activity, and sadness with activity in the subcallosal cingulate cortex, than any other emotion. Other stimulus‐related approaches have included the comparison of different induction methods and attentional demands during emotional processing. Visual emotional stimuli lead to activity in the occipital cortex and the amygdala, whereas memories or imagery of emotional events seem associated with activity in the insular and anterior cingulate cortex. Similarly, emotional stimuli lead more often to activity in the insular cortex when additional stimulus processing is required than during passive viewing [Phan et al., 2002].
Few studies have correlated activity in different brain areas with individual emotional responses in different response systems. This might be due partly to the difficulty of measuring emotional responses during functional imaging. However, the interpretation of stimulus‐related functional magnetic resonance imaging (fMRI) data is limited by the simultaneity of the many different components of the response to an emotional stimulus [see Davis et al., 2002]. Multivariate analysis of verbal responses to emotional stimuli has shown that most of the variance in descriptions of emotions can be explained by two factors: (1) valence, varying from negative to positive, and (2) arousal, varying from low to high [Mehrabian and Russel, 1974]. The two‐dimensional organization of the verbal emotional space seems to be mirrored in peripheral physiologic responses. Startle reflex amplitudes, among other behaviors, increase with reported negative valence and decrease with positive valence [for a comprehensive review, see Bradley and Vrana, 1993]. Skin conductance (SCR), an indicator for cholinergically mediated sweat gland activity, increases with reported arousal, independent of emotional valence. Verbal reports and peripheral physiologic responses are significantly and differentially correlated along the two dimensions of valence and arousal [Greenwald et al., 1989; Lang et al., 1993]. It has been proposed that the two‐dimensional organization of behavioral responses reflects the functional organization of brain circuits mediating emotional responses [Lang et al., 1993].
We investigated whether brain activity in regions seen activated frequently in neuroimaging studies would correlate with different components of emotional responses. We recorded verbal reports of experienced emotional valence and arousal and peripheral physiologic responses evoked by a set of emotional pictures during fMRI, and conducted a response‐related multiple correlation analysis to detect brain activity specifically correlated with peripheral physiologic or verbal valence or arousal responses. To avoid a systematic bias introduced by monitoring one's own emotional experience [Lane et al., 1997a] or verbal labeling [Hariri et al., 2000; Lange et al., 2003], subjects were not informed that they would be asked to rate their subjective feelings of emotional valence and arousal as experienced during scanning after scanning.
To increase statistical power, we focused our interest on the two most often activated subcortical and higher cortical structures in a recent comprehensive review of neuroimaging studies on emotional processing (amygdala, thalamus, insula, and medial orbitofrontal cortex) [Phan et al., 2002]. Based on previous studies, we expected amygdalar responses to be correlated specifically with peripheral physiologic responses [Davis, 1992], and insular responses to be associated more closely with verbal responses [Critchley et al., 2002; Phan et al., 2002]. The thalamus and the frontomedial cortex were expected to be closely associated with peripheral physiologic and/or verbal arousal responses [Tranel, 2000]. In addition to these four regions, we included the anterior parietal cortex in our analysis, based on a number of patient studies [Adolphs et al., 2000] that suggest a particular role of the anterior parietal cortex in the mediation between peripheral physiologic and verbal emotional responses.
SUBJECTS AND METHODS
Data Acquisition
Experiments were conducted in a 1.5 Tesla MR Scanner (Magnetom Vision; Siemens, Erlangen, Germany). Sixteen right‐handed volunteers with no report of neurologic or psychiatric disorders (9 women, 7 men; mean age 27.1 years; age range 21–43 years) underwent T2*‐weighted echoplanar imaging (EPI) of the whole brain [Klose et al. 1999] (44 coronal slices, slice thickness 3 mm + 1.5 mm gap, 56 × 64 voxels, in‐plane resolution 3 × 3 mm2, TE 33 ms, effective TR 4 s) while they viewed, through a 45‐degree angled mirror, a set of emotional pictures back‐projected onto a translucent screen. Coronal slice orientation and a short TE were chosen to minimize susceptibility artefacts. The set consisted of 20 human and 20 animal pictures from the International Affective Picture System (IAPS) [Lang et al., 1997]. Pictures depicted one to three humans or animals and varied largely and independently in valence and arousal. Content and visual complexity were balanced across previous valence and arousal ratings (Table I). Pictures, subtending a visual angle of 17 degrees vertically and 10–28 degrees horizontally, were presented for 12 s in pseudorandom order with an intertrial interval of 24, 32, or 40 s and an additional jittering of 0–3 s relative to scan onset. The long stimulus presentation time was chosen to permit the consecutive recording of skin conductance responses (SCR) and startle amplitudes. During the intertrial interval, the screen was dark with a white fixation cross. Scanning and picture presentation was divided into five blocks with eight picture presentations. Each block consisted of 69 fMRI scans, and the first five scans were discarded to suppress T1 saturation effects.
Table I.
Pictorial stimuli from the International Affective Pictures System (IAPS)
| IAPS number | Description | Valence | Arousal |
|---|---|---|---|
| 1710 | Puppies | 8.3 | 5.4 |
| 4220 | Wet female | 8.0 | 7.2 |
| 2550 | Old couple | 7.8 | 4.7 |
| 2360 | Family | 7.7 | 3.7 |
| 1811 | Chimps | 7.6 | 5.1 |
| 1620 | Springbok | 7.4 | 3.5 |
| 2370 | Tuxedos | 7.2 | 2.9 |
| 1604 | Butterfly | 7.1 | 3.3 |
| 4608 | Couple | 7.1 | 6.5 |
| 2352 | Interracial | 6.9 | 5.0 |
| 1650 | Jaguar | 6.7 | 6.2 |
| 1810 | Hippo | 6.5 | 4.5 |
| 1450 | Gannet | 6.4 | 2.8 |
| 1640 | Coyote | 6.2 | 5.2 |
| 1560 | Hawk | 6.0 | 5.5 |
| 1670 | Cow | 5.8 | 3.3 |
| 2220 | Male face | 5.0 | 4.9 |
| 4302 | Female shaving | 5.0 | 5.7 |
| 4561 | Man in water | 5.0 | 4.4 |
| 4770 | Females | 4.9 | 5.9 |
| 2190 | Man | 4.8 | 2.4 |
| 2830 | Lady in red | 4.7 | 3.6 |
| 1112 | Snake | 4.7 | 4.6 |
| 1230 | Spider | 4.6 | 4.0 |
| 1310 | Leopard | 4.6 | 6.0 |
| 1390 | Bees | 4.5 | 5.3 |
| 1931 | Shark | 4.0 | 6.8 |
| 2100 | Angry face | 3.9 | 4.5 |
| 2110 | Angry face | 3.7 | 4.5 |
| 1270 | Roaches | 3.7 | 4.8 |
| 1300 | Pit bull | 3.6 | 6.8 |
| 2590 | Old woman | 3.3 | 3.9 |
| 2490 | Old man | 3.3 | 4.0 |
| 9180 | Seals | 3.0 | 5.0 |
| 9561 | Sick kitty | 2.7 | 4.8 |
| 2900 | Crying boy | 2.5 | 5.1 |
| 9560 | Cormorant in oil | 2.1 | 5.5 |
| 2205 | Hospital | 2.0 | 4.5 |
| 3030 | Mutilation | 1.9 | 6.8 |
| 3170 | Baby tumor | 1.5 | 7.2 |
Valence and arousal ratings are from a large representative reference sample [Lang et al., 1997]. Pictures are ordered by valence ratings.
White noise standard startle probes [Berg and Balaban, 1999], adjusted individually to be unpleasant but not painful, were presented through headphones (HD 570; Sennheiser, Germany, modified after Baumgart et al. [1998] for use with fMRI) during each trial 6–9 s after stimulus onset and 3, 4, 9, or 10 s after stimulus offset. Intervals were chosen such that startle probes always occurred 2 s after scan onset. The eye blink component of the startle response [Anthony, 1985] was recorded at 1,000 Hz using infrared oculography. In this method, infrared light is projected through two optic fibre cables onto the subject's right eye, reflected by sclera and iris, and picked up by two photodiodes at the end of the optic fibres outside the scanner room. A plastic eyepiece is adjusted so that the light is projected onto the eye's horizontal axis. Eyeblink amplitudes are measured as differential voltage between the two photodiodes during lid closure [Anders et al., 2004]. Skin conductance was recorded at 16 Hz with commercial recording equipment (Vitaport II; Becker Meditec, Karlsruhe, Germany) using standard Ag/AgCl electrodes filled with unibase electrolyte affixed to the skin surface underneath the m. abductor hallucis of the left foot halfway between phalanx and calcaneus.
For superposition of functional maps upon brain anatomy, a T1‐weighted structural MRI (MPRAGE, isotropic resolution 1 mm3) was obtained from each subject. After the scanning procedure, subjects reported the valence and arousal of each picture as experienced in the scanner on scales ranging from unpleasant (1) to pleasant (9) and calm (1) to aroused (9) using a paper‐and‐pencil version of the Self‐Assessment Manikin (SAM) [Bradley and Lang, 1994; Lang, 1980]. Before scanning, subjects were instructed to watch the pictures attentively throughout the presentation time but were not informed that they should rate the pictures afterwards. The study was approved by the Ethics Committee of the University of Tübingen Medical School and all subjects gave their written informed consent before participation.
Data Analysis
Peripheral physiologic data and verbal reports
Peripheral physiologic data were processed in a MATLAB environment (MATLAB5; The Mathworks Inc., Natick, MA). Eyeblink data were smoothed (10‐ms Gaussian kernel) and inspected visually for artefacts. Eye blinks that did not show a Gaussian shape time‐course were excluded from amplitude analysis. Data of four subjects and 6% of the eye blinks of the remaining subjects were discarded due to this criterion. Eyeblink amplitude was determined as the maximal differential voltage between 21–150 ms after startle probe onset, relative to the mean of a 20‐ms baseline beginning with startle probe onset. Skin conductance data were smoothed with a 1‐s Gaussian kernel. Amplitude of SCR was determined as the largest change in conductance between 1 and 10 s after picture onset, relative to the preceding smallest value in the interval. SCRs to the first pictures of the first and second blocks were discarded to eliminate orienting responses that occurred at the beginning of the first blocks.
For statistical analysis, eyeblink amplitudes were scaled to session mean, and SCRs were log transformed (log[SCR+1]) [Lang et al., 1993]. All measures were standardized to a mean of zero and standard deviation of unity per subject, and missing values in the eyeblink and skin conductance data were padded with zeros. To compare verbal reports and peripheral physiologic responses, peripheral physiologic response amplitudes were averaged across pictures with the same valence or arousal rating, respectively. Eyeblink amplitude, SCR, reported valence, reported arousal, and two additional regressors representing the interaction between eyeblink amplitude and reported valence and between skin conductance and reported arousal were used for multiple correlation analysis with fMRI data.
Correlations between peripheral physiologic and verbal responses and fMRI data
fMRI data were processed using statistical parametric mapping (SPM99; Wellcome Department of Cognitive Neurology, London, UK). Before statistical analysis, volumes were realigned spatially, slice time corrected, normalized into Montreal Neurological Institute (MNI) space [Collins et al., 1994], and smoothed spatially (15‐mm Gaussian kernel) and temporally (4‐s Gaussian kernel) to remove high‐frequency artefacts and permit application of random field theory for statistical inference [Worsley et al., 1996].
Hemodynamic responses amplitudes were estimated using standard regressors, one per picture, constructed by convolving a boxcar function, representing the stimulus duration, with a synthetic hemodynamic response function using standard SPM99 parameters. Additional regressors representing estimated head movements (translation and rotation with six degrees of freedom [df]) were added as covariates of no interest into these models to account for artefacts due to head movements during scanning [Friston et al., 1996].
Estimated hemodynamic response amplitudes were subjected to within‐subject multiple correlation analyses with peripheral physiologic responses and verbal reports. A model was constructed for each subject that contained six regressors representing peripheral physiologic responses, verbal ratings, and their interaction. Regressors for interaction between peripheral physiologic and verbal responses were constructed as pairwise products of startle amplitudes and valence ratings, and SCRs and arousal ratings, respectively. An example of data used for multiple correlation analysis for one subject is given in Figure 1.
Figure 1.
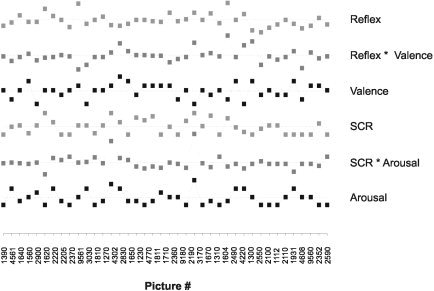
A typical example of behavioral responses during picture processing (from one subject) used for multiple regression analysis with fMRI data. Picture numbers refer to IAPS numbers (Table I). Pictures are in pseudorandom order, from left to right, as during the experiment. Squares represent, from top to bottom, standardized amplitudes of the eyeblink component of the startle reflex, pairwise products of startle reflex amplitude and reported emotional valence used for interaction analysis, reported emotional valence, skin conductance responses, interaction between skin conductance response and reported arousal, and reported arousal. For simple correlation analyses of frontomedial activity, only one of the regressors depicted in the figure was included in each model.
Correlation coefficients were derived with standard SPM99 algorithms as follows. For each regression coefficient, t‐values were calculated and correlation coefficients were derived from t‐values according to:
with r denominating the partial correlation between fMRI and peripheral physiologic responses or verbal reports, and n the number of stimuli. After Fisher transformation, these correlation coefficients were used for random effect statistics. By using Fisher‐transformed correlation coefficients instead of regression coefficients for random effect analysis, we eliminated scaling effects that might have biased regression coefficients while ensuring that the prerequisite of normal distribution for second level parametric testing was met (Fisher z transformation provides a better approximation to the normal distribution than does Student's t transformation) [Rosner, 1994].
Compared to conventional regression analyses, a multiple regression analysis differentiating between response components has relatively little statistical power. To account for this, we confined our analysis to five regions of interest and adopted an approach suggested by Friston et al. [1994] that allows one to lower the height threshold to P < 0.05 while controlling for false positives in terms of spatial extent of the activated region. Significance of response‐correlated brain activity within regions of interest was thus assessed at cluster level based on random field theory (one‐sample t‐tests, height threshold P < 0.05, uncorrected; extent threshold P < 0.05 corrected for each predefined region of interest) [Friston et al., 1994; Worsley et al., 1996]. Regions of interest were marked using an anatomic atlas for automatic labeling of structural brain images normalized into MNI space [Tzourio‐Mzoyer et al., 2002] and included: (1) the amygdala; (2) the thalamus; (3) the anterior parietal cortex (postcentral gyrus, supramarginal gyrus, and the part of the inferior parietal gyrus anterior to the posterior end of the Sylvian fissure); (4) the insular cortex (insular gyrus, the opercular part of the inferior frontal gyrus, and the rolandic operculum); and (5) the frontomedial cortex (rectal gyrus, medial orbital gyrus, and the anterior cingulate gyrus below the horizontal anterior commissure–posterior commissure plane).
RESULTS
Peripheral Physiologic and Verbal Responses
Mean valence and arousal ratings, taken after scanning, were similar to valence and arousal ratings from a large representative reference sample taken in a standard laboratory setting [Lang et al., 1997] with one exception: when pictures were divided into four groups of unpleasant, mildly unpleasant, mildly pleasant, and pleasant pictures, pleasant pictures were rated significantly less positive in our study than in the reference sample (paired t‐test, P < 0.05). Because the range of valence ratings was biased toward negative valence in our study, we report only brain activity that increased with reported negative valence. Reports of negative valence were significantly and positively correlated with startle eyeblink amplitude within the group (r = −0.79, df = 7, two‐tailed P < 0.05). Arousal reports were significantly and positively correlated with skin conductance response (r = 0.75, df = 7, two‐tailed P < 0.05). These correlations are similar to those reported previously for valence ratings and startle eyeblink amplitude and emotional ratings and other peripheral physiologic measures [Lang et al., 1993]. Valence or arousal reports did not correlate significantly with each other or any other peripheral physiologic or interaction measure (Table II).
Table II.
Correlations between verbal reports and mean peripheral physiologic responses
| Valence | Reflex × valence | Reflex | Arousal | SCR × arousal | SCR | |
|---|---|---|---|---|---|---|
| Valence | 1 | 0.44 | −0.79a | −0.01 | 0.34 | −0.14 |
| Arousal | 0.35 | 0.53 | 0.61 | 1 | 0.61 | 0.75a |
P < 0.05, two‐tailed.
SCR, skin conductance response.
Brain Activity
Multiple correlation analysis revealed significant correlations in four of five predefined brain regions (Fig. 2A). Responses along the emotional valence dimension were associated with significant clusters in the amygdalae, the anterior parietal cortex, and the insular cortex. Bilateral amygdalar activity varied significantly with amplitude of startle reflex and the interaction of startle reflex and reported valence, whereby startle amplitude related activity was centered more dorsomedially in the amygdala, and activity associated the interaction of startle and reported valence more ventrolaterally. Startle reflex‐related activity was also found in the right postcentral gyrus. Activity in the left supramarginal gyrus increased with interaction of startle reflex and reported valence, and activity in the left anterior insula increased with reported negative valence. Responses along the arousal dimension were associated with significant clusters in two regions: activity in the right supramarginal gyrus varied with SCR, and thalamic activity varied with the interaction of SCR and reported arousal, and with reported arousal.
Figure 2.
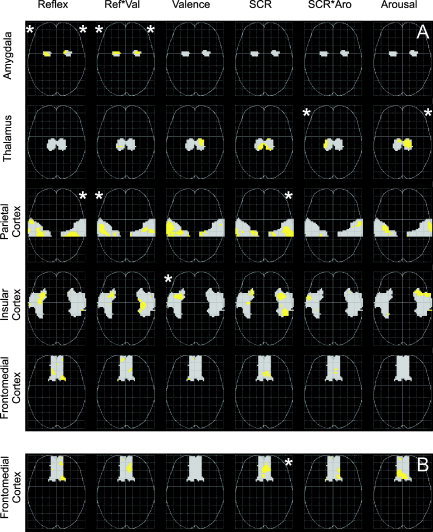
Axial projections of brain activity varying with peripheral physiologic and verbal responses in five predefined regions of interest. Gray areas represent regions of interest. Activated voxels are demarcated in light gray (height threshold P < 0.05, uncorrected). Asterisks denote significant clusters (extent threshold *P < 0.05 corrected for the search volume). A: Partial correlations. B: Correlations with single regressors (only for the frontomedial region, see text). Projections are in neurologic convention (right is right). Reflex, amplitude of the eyeblink component of the startle reflex; Ref*Val, interaction between startle reflex amplitude and valence report; Valence, reported valence; SCR, skin conductance response, SCR*Aro, interaction between skin conductance response and arousal report; Arousal, reported arousal.
Multiple correlation analysis, testing the correlation between a regressor and hemodynamic activity with a model that contains several regressors, demonstrated no significant correlation in the frontomedial cortex. Assuming that the lack of significant correlation in this region might be due to the poor contrast‐to‐noise ratio in frontobasal cortices in fMRI [Ojemann et al., 1997], we used a less stringent simple correlation approach to test this region: only one of the regressors depicted in Figure 1 was included in each within‐subject model, resulting in six separate correlation analyses for each subject. With this approach, we found that activity in the medial orbital gyri varied significantly with SCR (Fig. 2B).
The anatomic localization of significant clusters within each region is depicted in Figure 3. Histograms of correlation coefficients from the highest activated voxel of each cluster show that correlation coefficients representing significant correlations are considerably larger than all other coefficients in the respective voxel, illustrating that not only the extent but also the strength of covariation between peripheral physiologic and verbal responses and brain activity is different in different regions.
Figure 3.
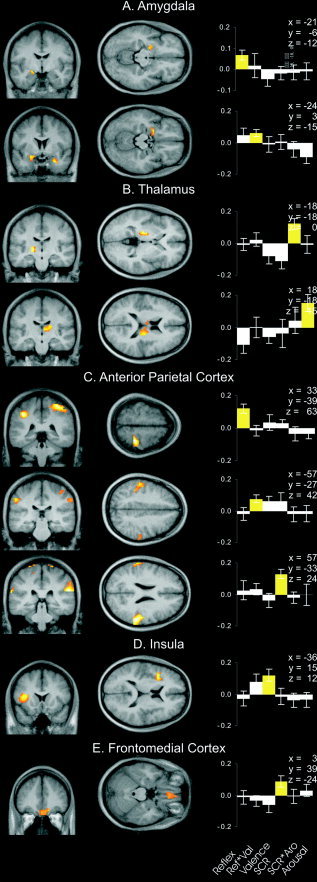
Statistical parametric maps (SPM) of brain activity varying significantly with peripheral physiologic and verbal responses mapped onto an averaged structural MRI derived from all subjects. A: Amygdalar activity varying with startle reflex amplitude (top), and startle reflex and valence report interaction (bottom). B: Thalamic activity varying with the interaction of skin conductance response and reported arousal (top) and with reported arousal (bottom). C: Activity in the right postcentral gyrus varying with startle reflex amplitude (top), activity in the left supramarginal gyrus varying with startle reflex and valence report interaction (middle), and activity in the right supramarginal gyrus varying with skin conductance response (bottom). D: Insular activity varying with reported valence. E: Frontomedial activity varying with skin conductance response. A–D represent results of multiple regression analyses; E represents results of single regression analyses. Coordinates are in MNI space relative to the anterior commissure. Sections are in neurologic convention (right is right). Histograms on right represent mean correlation coefficients and standard errors between brain activity and behavioral responses in the highest activated voxel of each cluster. Mean correlation coefficients and standard errors are retransformed from means and standard errors of Fisher z‐transformed within‐subject correlation coefficients. Yellow bars, correlation coefficients from significant clusters. Reflex, amplitude of the eyeblink component of startle reflex; Ref*Val, interaction between startle reflex amplitude and valence report; Valence, reported valence; SCR, skin conductance response; SCR*Aro, interaction between skin conductance response and arousal report; Arousal, reported arousal.
DISCUSSION
Emotional responses to pictorial stimuli were significantly and positively correlated with activity in the amygdalae, thalamus, anterior parietal cortex, insular cortex, and the frontomedial cortex. Activity in each of these regions was associated with a particular aspect of the emotional response. These correlations cannot be explained simply by picture content because there was a large variability in peripheral physiologic and verbal responses to individual pictures across subjects. Peripheral physiologic, verbal, and hemodynamic response amplitudes were averaged over a relatively long stimulation interval and thus rapid changes of emotional processing during stimulation might have been missed. However, averaged verbal ratings and correlations between verbal ratings and averaged peripheral physiologic recordings are in good agreement with previous studies in which recordings were made in a standard laboratory setting [Greenwald et al., 1989; Lang et al., 1993, 1997]. Specifically, reports of valence correlated with eyeblink amplitude, but not with SCR, and arousal reports correlated with SCR but not with eyeblink amplitude. Responses along the valence dimension were correlated positively with activity in the amygdalae and the insular cortex, and arousal responses were correlated with thalamic and frontomedial activity. Our data therefore support the hypothesis that different aspects of the emotional response are mediated by different brain circuits.
The second important finding of this study is the functional segregation of brain structures underlying peripheral physiologic responses and verbal reports. Startle reflex augmentation was associated with amygdalar activity and SCRs with frontomedial activity, whereas verbal reports of valence and arousal were associated with insular and thalamic activity, respectively. Dissociations between peripheral physiologic and verbal responses in learning and discriminatory tasks have been reported in patients with focal brain lesions. Patients with amygdalar lesions, for example, learn the association between a neutral and an aversive stimulus on the verbal but not the peripheral physiologic level. Patients with hippocampal lesions, in contrast, demonstrate associative learning on the peripheral physiologic level but report not being aware of the association between the two stimuli [Bechara et al., 1995]. Patients with frontomedial lesions can discriminate verbally between familiar and unfamiliar faces but show no discriminatory SCRs, whereas prosopagnosic patients show discriminatory SCRs to faces they cannot discriminate verbally [Tranel et al., 1995]. A recent study [Williams et al., 2001] demonstrated that amygdalar and frontomedial activity is associated with SCRs during the processing of fearful faces, whereas processing of fearful faces in the absence of SCR is associated with hippocampal and lateral frontal activity.
The amygdala is widely recognized to play a crucial role in emotional behavior in both animals and humans. Indeed, the role of the amygdala is so prominent that amygdala activity has been used sometimes as the sole indicator for emotional processing in functional imaging studies [Morris et al., 1998, 2001; Whalen et al., 1998]. Animal and human lesion studies suggest a more refined role of the amygdala in emotional behavior. Amygdalar lesions prevent startle reflex augmentation in the presence of an aversive stimulus in animals [Hitchcock and Davis, 1986] and humans [Angrilli et al., 1996]. The excitatory projections from the central nucleus of the amygdala to the nucleus reticularis pontis caudalis of the startle circuit are well described [Hitchcock and Davis, 1991; Koch and Ebert, 1993]. We found bilateral dorsomedial amygdalar activity associated with startle reflex amplitude, and bilateral ventrolateral amygdalar activity associated with the interaction between startle amplitude and reported valence. This differential activation might reflect a different involvement of the central and the lateral nucleus in emotional processing. The dorsomedially located central nucleus is the major output nucleus of the amygdala, while the lateral nucleus receives and integrates information from cortical regions [LeDoux, 2000].
The role of the amygdala in SCRs is not so clear. Although the central nucleus of the amygdala projects to the hypothalamus (one key structure modulating sympathetic activity) [LeDoux et al., 1988], there is evidence from animal and human lesion studies that amygdalar damage interferes with acquisition of SCRs in avoidance learning but not with SCRs per se [Bechara et al., 1995; Phillips and LeDoux, 1992; Tranel and Damasio, 1989]. In contrast to a study by Williams et al. [2001] that compared amygdala activity during face processing with and without electrodermal activity, but did not relate hemodynamic activity and electrodermal activity directly, we did not observe skin conductance‐related activity in the amygdala. Our study and others [Lane et al., 1997c; Reiman et al., 1997] provide evidence that thalamic and frontomedial activity is associated with arousal changes in humans. Consistent with our findings, the generation of SCRs, especially to complex stimuli, has been associated with frontomedial activity [Critchley et al., 2000; Tranel and Damasio, 1994]. Tranel [2000] speculates that autonomic nervous system responses might be modulated directly or indirectly by visual and auditory projections to the thalamus or the frontomedial cortex, bypassing the amygdala. The thalamus has also been implicated in a variety of nonemotional attention and arousal functions [e.g., Portas et al., 1998]. Cortical arousal in emotional and nonemotional settings might be mediated by unspecific thalamic nuclei that project to widespread cortical regions, and thalamic reticular nuclei that control the relay of sensory information [Heilman, 2000].
Large startle amplitudes and SCRs led to increased activity in the right postcentral and supramarginal gyri, regions that have been associated with the representation of cortical maps of an organism's internal state [Damasio et al., 2000] and activation of “as‐if‐body‐loops” underlying recognition of emotional states in others [Adolphs et al., 2000]. Strikingly, pictures that elicited highly correlated peripheral physiologic and verbal responses along the valence dimension were associated with activity in the left supramarginal gyrus. One might speculate that in cases in which valence reports and startle amplitudes correlated, verbal reports of valence were based largely on the subject's perception of internal state, whereas in other cases valence ratings might have been influenced more strongly by conceptual knowledge related to the presented picture. Left supramarginal activity might then reflect awareness of internal states associated with the emotional valence of a stimulus.
The insular cortex has been reported to be activated when cognitive processing of emotional stimuli or recall of autobiographic emotional information is required [Fink et al., 1996; Reiman et al., 1997] but not during passive viewing [Phan et al., 2002] or subliminal stimulus presentation [Critchley et al., 2002]. Damasio et al. [2000] found that autobiographic recall of four different emotions always led to insular activity and concluded that insular activity might constitute part of the neural correlate of experienced feeling. In the present study, verbal reports of emotional valence were taken after scanning and subjects were not instructed to monitor their feelings during scanning. Although verbal reports after scanning might have been dependent on recall of subjective experience of emotion during scanning, which in turn might be associated with insular activity, it seems equally likely that the correlation between insular activity and verbal reports of negative valence reflects activation of cognitive information associated with negative scenes that underlies verbal reports but might not be associated with the actual experience of negative emotion.
In the present study, the most positive stimuli led to only mildly positive emotional valence ratings in the scanning environment; thus no conjecture about differential brain activity associated with the pleasantness of a stimulus can be drawn. It has been proposed both that the unpleasantness and pleasantness of a stimulus represent polar opposites of the psychophysiologic dimension of valence [Lang et al., 1993], and that positive and negative valence are two separate dimensions [Cacioppo and Berntson, 1994] and thus may be mediated by different brain circuits. Candidate structures for the mediation of responses associated with the positive valence of a stimulus are the ventral striatum and orbitofrontal cortices. Imaging studies with a modified set of pictures might clarify this point.
In summary, peripheral physiologic and verbal responses along the emotional dimensions of valence and arousal activated different brain circuits. Our data thus provide direct evidence of the validity of the two‐dimensional view of the organization of emotions first formalized scientifically by the founder of experimental psychology, Wilhelm Wundt [1874]. The study further separates brain regions more closely involved in mediation of peripheral physiologic verbal responses along these axes. Peripheral physiologic responses are represented in somatosensory association areas in the anterior parietal cortex but, presumably depending on changing levels of attention and cognitive processing during stimulus perception, the degree to which peripheral physiologic responses and verbal reports of valence and arousal are correlated varies.
Acknowledgements
This work was supported in part by the Volkswagen Foundation (to S.A.), the Junior Science Program of the Heidelberger Academy of Sciences and Humanities (to S.A.), and the German Research Council (to N.B.). We thank A. Lindner for support with eyeblink recordings, and N. Weiskopf and H. Flor for comments on earlier versions of the article.
REFERENCES
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR (2000): A role for somatosensory cortices in the visual recognition of emotion as revealed by three‐dimensional lesion mapping. J Neurosci 20: 2683–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, Flor H, Birbaumer N, Sartori G, di Paola F (1996): Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain 119: 1991–2000. [DOI] [PubMed] [Google Scholar]
- Anthony BJ (1985): In the blink of an eye: implications of reflex modification for information processing In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in psychophysiology. Vol I London: Jessica Kingsley Publishers; p 167–218. [Google Scholar]
- Baumgart F, Kaulisch T, Tempelmann C, Gaschler‐Markefski B, Tegeler C, Schindler F, Stiller D, Scheich H (1998): Electrodynamic headphones and woofers for application in magnetic resonance imaging scanners. Med Phys 25: 2068–2070. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR (1995): Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Berg WK, Balaban MT (1999): Startle elicitation: stimulus parameters, recording techniques, and quantification In: Dawson ME, Schell AE, Boehmelt AH, editors. Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge, UK: Cambridge University Press; p 21–50. [Google Scholar]
- Bradley MM, Lang PJ (1994): Measuring emotion: the self‐assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Bradley, MM , Vrana, SR (1993): The startle probe in the study of emotion and emotional disorders In: Birbaumer N, Öhman A, editors. The structure of emotion. Toronto: Hogrefe and Huber Publishers; p 21–50. [Google Scholar]
- Cacioppo JT, Berntson GG (1994): Relationships between attitudes and evaluative space: a critical review with emphasis on the separability of positive and negative substrates. Psychol Bull 115: 401–423. [Google Scholar]
- Carl JR, Gellman RS (1987): Human smooth pursuit: stimulus‐dependent responses. J Neurophysiol 57: 1446–1463. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ (2000): Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci 20: 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002): Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33: 653–663. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD (2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- Davis KD, Pope GE, Crawley AP, Mikulis DJ (2002): Neural correlates of prickle sensation: a percept‐related fMRI study. Nat Neurosci 5: 1121–1122. [DOI] [PubMed] [Google Scholar]
- Davis M (1992): The role of the amygdala in conditioned fear In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley‐Liss; p 255–306. [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD (1996): Cerebral representation of one's own past: neural networks involved in autobiographical memory. J Neurosci 16: 4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Cook EW III, Lang PJ (1989): Affective judgment and psychophysiological response: dimensional covariation in the evaluation of pictorial stimuli. J Psychophysiol 3: 51–64. [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC (2000): Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11: 43–48. [DOI] [PubMed] [Google Scholar]
- Heilman KM (2000): Emotional experience: a neurological model In: Lane RD, Nadel L, editor. Cognitive neuroscience of emotion. New York: Oxford University Press; p 328–344. [Google Scholar]
- Hitchcock J, Davis M (1986): Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci 100: 11–22. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M (1991): Efferent pathway of the amygdala involved in conditioned fear as measured with the fear‐potentiated startle paradigm. Behav Neurosci 105: 826–842. [DOI] [PubMed] [Google Scholar]
- Klose U, Erb M, Wildgruber D, Muller E, Grodd W (1999): Improvement of the acquisition of a large amount of MR images on a conventional whole body system. Magn Reson Imaging 17: 471–474. [DOI] [PubMed] [Google Scholar]
- Koch M, Ebert U (1993): Enhancement of the acoustic startle response by stimulation of an excitatory pathway from the central amygdala/basal nucleus of Meynert to the pontine reticular formation. Exp Brain Res 93: 231–241. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ (1997a): Neural activation during selective attention to subjective emotional responses. Neuroreport 8: 3969–3972. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ (1997b): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE (1997c): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Lang PJ (1980): Behavioral treatment and bio‐behavioral assessment: computer applications In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. New Jersey: Alex Publishing; p 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (1997): International affective picture system (IAPS): technical manual and affective ratings. Gainesville: The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO (1993): Looking at pictures: affective, facial, visceral and behavioral reactions. Psychophysiology 30: 261–273. [DOI] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, Gray JA, Phillips ML (2003): Task instructions modulate neural responses to fearful facial expressions. Biol Psychiatry 53: 226–232. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000): Cognitive‐emotional interactions: listening to the brain In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; p 129–155. [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988): Differential projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian A, Russel JA (1974): An approach to environmental psychology. Cambridge, MA: MIT Press. [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ (2001): Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain 124: 1241–1252. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ (1998): Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE (1997): Anatomic localization and quantitative analysis of gradient refocused echo‐planar fMRI susceptibility artifacts. Neuroimage 6: 156–167. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Andreasen NC, Downhill JE, Davidson RJ, Kirchner PT, Watkins GL, Ponto LL, Hichwa RD (1997): Emotional activation of limbic circuitry in elderly normal subjects in a PET study. Am J Psychiatry 154: 384–389. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SCR, Gray JA, David AS (1997): A specific neural substrate for perceiving facial expressions of disgust. Nature 389: 495–497. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992): Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD (1998): A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K (1997): Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925. [DOI] [PubMed] [Google Scholar]
- Rosner B (1994): Fundamentals of biostatistics. Belmont, CA: Wadsworth Publishing Company. [Google Scholar]
- Tranel D (2000): Electrodermal activity in cognitive neuroscience: neuroanatomical and neuropsychological correlates In: Lane RD, Nadel L, editors. Cognitive neuroscience of emotion. New York: Oxford University Press; p 192–224. [Google Scholar]
- Tranel D, Damasio H (1989): Intact electrodermal skin conductance responses after bilateral amygdala damage. Neuropsychologia 27: 381–390. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H (1994): Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology 31: 427–438. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR (1995): Double dissociation between overt and covert face recognition. J Cogn Neurosci 7: 425–432. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, Skerrett D, Lagopoulos J, Rennie C, Bahramali H, Olivieri G, David AS, Peduto A, Gordon E (2001): Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage 14: 1070–1079. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Wundt W (1874): Grundriss der Psychologie. Leipzig: Engelmann. [Google Scholar]


