Abstract
Aloud reading of novel words is achieved by phonological decoding, a process in which grapheme‐to‐phoneme conversion rules are applied to “sound out” a word's spoken representation. Numerous brain imaging studies have examined the neural bases of phonological decoding by contrasting pseudoword (pronounceable nonwords) to real word reading. However, only a few investigations have examined pseudoword reading under both aloud and silent conditions, task parameters that are likely to significantly alter the functional anatomy of phonological decoding. Subjects participated in an fMRI study of aloud pseudoword, aloud real word, silent pseudoword, and silent real word reading. Using this two‐by‐two design, we examined effects of word‐type (real words vs. pseudowords) and response‐modality (silent vs. aloud) and their interactions. We found 1) four regions to be invariantly active across the four reading conditions: the anterior aspect of the left precentral gyrus (Brodmann's Area (BA) 6), and three areas within the left ventral occipitotemporal cortex; 2) a main effect of word‐type (pseudowords > words) in left inferior frontal gyrus and left intraparietal sulcus; 3) a main effect of response‐modality (aloud > silent) that included bilateral motor, auditory, and extrastriate cortex; and 4) a single left hemisphere extrastriate region showing a word‐type by response‐modality interaction effect. This region, within the posterior fusiform cortex at BA 19, was uniquely modulated by varying phonological processing demands. This result suggests that when reading, word forms are subject to phonological analysis at the point they are first recognized as alphabetic stimuli and BA 19 is involved in processing the phonological properties of words. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: reading, phonological processing, decoding, words, pseudowords, fMRI
INTRODUCTION
Functional neuroimaging studies of pseudoword (pronounceable nonword) reading are a prominent part of ongoing efforts to understand the neural basis of reading [Cappa Perani et al., 1998; Fiebach et al., 2002; Fiez et al., 1999; Hagoort et al., 1999; Jobard et al., 2003; Joubert et al., 2004; Mechelli et al., 2003; Petersen et al., 1990; Price et al., 1996; Rumsey et al., 1997a] and its disorders [Brunswick et al., 1999; Rumsey et al., 1997b]. Decoding of unfamiliar nonwords necessitates sublexical phonological processing, whereby the conversion of the visual word representation into its abstract phonological code is accomplished by the use of grapheme‐to‐phoneme correspondence rules. Relative to reading familiar words, pseudoword reading places a greater demand on the phonological processing system; even though real words can be read via assembled phonology, pseudoword reading can only be accomplished via this indirect/sublexical procedure. Phonological processing problems, especially those requiring sublexical analyses, are the hallmark of some cases of acquired reading disorders [Friedman et al., 1993; Leff et al., 2001] and account for many of the difficulties encountered by children and adults with the reading disability developmental dyslexia [Vellutino et al., 2004]. Better knowledge of the neural bases of reading and sublexical phonological processing could facilitate the early identification of reading disabilities and the development of effective reading remediation strategies.
Various models and theories of word recognition offer accounts of the neural mechanisms that may underlie the transformation of orthography to phonology in reading. These theories were largely introduced to explain the different patterns of reading disabilities observed among patients with acquired dyslexias [Warrington and Shallice, 1980]. Some patients had intact word reading (regular and exception words) accompanied by difficulty in reading pseudowords (phonological dyslexia), while others could read pseudowords and regular real words, but were unable to read exception words (surface dyslexia). The dual‐route model of word reading arose to explain this double dissociation [Coltheart et al., 1993]. According to this model, known words have stored lexical representations that can be very quickly accessed via a memory‐based lexical route of reading, whereas pseudowords must undergo a more methodical process of grapheme‐to‐phoneme conversion (GPC) using a rule‐based sublexical route. A model with three routes for reading was developed based on the observation of a patient who could correctly read regular words, exception words, and pseudowords, but whose comprehension was poor [Gerhand, 2001; Southwood and Chatterjee, 2000]. Alternatively, a computational approach in the form of parallel distributed processes (PDP) describes a system of interconnected word processing units linked together in parallel [Seidenberg and McClelland, 1989]. Consequently, the same neural network is used for reading whether or not the word is familiar. Word exposure modulates the connection strengths between phonological and orthographic units such that frequently encountered words are phonologically decoded more rapidly and accurately than unknown words.
Neuroanatomical correlates of these reading models have been examined and tested using functional imaging techniques including positron emission tomography (PET), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI). The contrast of pseudoword with real word reading differs in phonological demands and elicits an increase in activity in left posterior superior (“dorsal stream”) cortical areas including the lateral inferior parietal and posterior superior temporal cortices [Hagoort et al., 1999; Herbster et al., 1997; Price et al., 1996; Pugh et al., 1996; Rumsey et al., 1997a; Simos et al., 2002]. Dyslexic readers, having impaired phonological processing abilities, show underactivation of these regions relative to typical readers during phonological processing tasks [Brunswick et al., 1999; Eden et al., 2004; Rumsey et al., 1997a; Shaywitz et al., 1998]. Based on these findings, the left inferior parietal/posterior superior temporal cortex has been purported to be the substrate subserving the assembled, sublexical, or indirect route of the dual‐route model, where GPC rules are applied to decode words. In addition, the dorsal left inferior frontal cortex has also been implicated in supporting phonological processing. This finding appears to be most consistent in studies of aloud reading [Brunswick et al., 1999; Fiez et al., 1999; Hagoort et al., 1999; Herbster et al., 1997; Poldrack et al., 1999; Pugh et al., 1996; Rumsey et al., 1997a; Zurowski et al., 2002]. The exact role of the inferior frontal cortex is likely to be more complex as semantic processing also invokes activity here, but in regions ventral and posterior to those found during phonological processing [Fiez et al., 1999; Gabrieli et al., 1998].
Familiar, frequently encountered words and irregularly spelled “sight” words that make little demand on sublexical phonological processing have been shown to activate left posterior inferior areas (inferior occipitotemporal cortex and fusiform gyrus), suggesting perhaps that this ventral stream houses the direct, lexical route of reading [Cohen et al., 2000; Fiez et al., 1999; Kiehl et al., 1999; Paulesu et al., 2000]. However, not all brain imaging studies support the idea of two routes. For example, contrary to predictions from a dual‐route model, multiple studies have shown activation of this ventral region when processing pseudowords [Brunswick et al., 1999; Paulesu et al., 2000; Price et al., 1996; Xu et al., 2001]. Consequently, it has been argued that these findings of simultaneous activity in multiple brain regions are more consistent with either a connectionist parallel distributed processing (PDP) model of reading [Seidenberg and McClelland, 1989] or other more extensive serial models.
Some of the inconsistencies reported among neuroimaging studies have been attributed to experimental parameters or statistical limitations [Mechelli et al., 2003]. Specifically, opinions regarding which cognitive model provides the best description of the neural representation have been divided, with some claiming evidence for the dual‐route model [Fiebach et al., 2002; Jobard et al., 2003; Joubert et al., 2004] and others for connectionist models [Herbster et al., 1997; Rumsey et al., 1997a]. However, in this debate surprisingly little attention has been given to the importance of aloud reading. Instead, silent and aloud reading are often assumed to be interchangeable when making cognitive inferences about the neural basis of reading or phonological processing. Further, the advent of fMRI technology has prompted a disproportionate use of tasks that avoid spoken paradigms, favoring manual responses during lexical decision or silent reading tasks because of technical constraints. As a result, silent reading studies greatly outnumber aloud reading studies due to the concern that speech‐induced movements are not compatible with the need to minimize motion‐related artifacts.
However, it has been demonstrated that neural activity underlying aloud word reading is not the equivalent of silent reading activity with the addition of related motor and auditory activity [Bookheimer et al., 1995; Huang et al., 2001]. In fact, when reading aloud vocalization is not initiated until the computation of phonology is complete [Rastle et al., 2000; Seidenberg and McClelland, 1989]. Hence, reading aloud maximally elicits activation of the phonological processing system in a way that silent reading does not [Barch et al., 1999; Huang et al., 2001]. As a consequence, the use of different response modalities (silent vs. aloud reading) will draw on different neural substrates subserving reading [Price et al., 1994; Rumsey et al., 1997a]. These findings from PET studies demonstrate the necessity to include both conditions in future brain imaging studies of reading and phonological processing. To date, only three neuroimaging studies have incorporated both aloud and silent reading to investigate phonological processing [Brunswick et al., 1999; Hagoort et al., 1999; Rumsey et al., 1997a]. Two of these studies involved overt pronunciation of single words, but the comparison task entailed decision‐making (phonological or feature) and hence did not allow for a direct comparison. A PET study by Hagoort et al. [1999] employed the same reading tasks for both the aloud and silent conditions. Native German speakers were scanned as they read real German words and pseudowords aloud and silently, but an interaction analysis of response‐type (aloud and silent) and lexicality (real words and pseudowords) was not provided. Further, the German language has a relatively transparent orthography (a regular one‐to‐one correspondence between words' spellings and their pronunciations), whereas English has an opaque (or deep) orthography. Generalization of these results from German to English may therefore be limited and underscores the need for further studies that directly address reading English words and pseudowords aloud.
Taken together, there is a need to examine brain mechanisms related to aloud and silent reading in the English language. To address this question in the context of the neural mechanisms underlying phonological processing in reading, we employed a two‐by‐two experimental paradigm that placed differential demands on phonological decoding under overt and covert reading conditions in order to elicit differential activation of their corresponding neural substrates. Pseudoword decoding puts more stress on phonological processing than real word reading, as nonwords can only be decoded using addressed phonology, whereas real words can be read either via addressed phonology or the use of the direct lexico‐semantic route. Aloud reading also puts greater demands on phonological processing than reading silently, as the phonological codes need to be represented in their entirety in order to pronounce the word. We used fMRI to detect task‐related signal changes while adult monolingual English speakers read words and pseudowords aloud and silently. An interleaved data acquisition technique was used to minimize magnetic susceptibility and motion artifacts caused by aloud word reading [Eden et al., 1999]. The aim of our study was not to verify any particular model of reading but to develop a more comprehensive understanding of the neural mechanisms of phonological processing under conditions of aloud and silent reading.
SUBJECTS AND METHODS
Subjects
Sixteen subjects (nine female, seven male; mean age 31.1 years; age range, 20.9–39.5) participated in this study. Subjects were right‐handed as determined by the Edinburgh Handedness Inventory [Oldfield, 1971] and were native speakers of English. Subject exclusion criteria included bilingualism, claustrophobia, nicotine or other drug use, a history of head injury, a known family history of psychiatric, neurological, or developmental disorders involving first‐degree relatives, presence of metal fragments in the body, or pregnancy. Subjects had normal or corrected vision, normal local and global stereopsis, and normal color vision [Ishihara, 1996]. Attention Deficit/Hyperactivity Disorder was excluded using the abbreviated Wender Utah Rating Scale [Ward et al., 1993]. Reading was evaluated by a comprehensive battery of neuropsychological tests that included real word and pseudoword reading as well as phonological awareness skills. Results of these behavioral measures were normal or above for all subjects. Subjects were paid for their participation and gave written informed consent in accordance with the Georgetown University Medical Center Institutional Review Board.
Experimental Tasks and Design
Subjects participated in two 12‐min experimental runs during which they read 1) silently (without moving the lips, tongue, or jaw), and 2) aloud. Both runs contained alternating blocks of real words and pseudowords and consisted of 10 48‐s task periods and 10 24‐s rest (fixation) periods. The two runs were pseudorandomized in order of presentation with two additional runs collected for the purpose of a different study.
To minimize any magnetic susceptibility artifacts caused by potential head and jaw movements associated with reading [Birn et al., 1998], task performance (reading) coincided with a time during the block when the gradients were turned off [Eden et al., 1999]. These trials were interleaved with periods of image acquisition, with the assumption that the 4–8‐s delay in the hemodynamic response [Bandettini et al., 1992; Kwong et al., 1992; Logothetis, 2001] would allow detection of the blood oxygenation‐level dependent (BOLD) contrast generated by the previously performed task. In this way, the use of a TR (repetition time) of 12 s yielded four brain volumes (time points) during each 48‐s task block (see Fig. 1). Within a word reading epoch, words or pseudowords were presented for 200 ms and followed by a 2450 ms response interval during which subjects viewed a fixation crosshair. Three words were presented within a trial, each trial lasting 8 s. The epoch was completed after the fixation crosshair was displayed for 4,050 ms, while a whole‐brain volume was acquired, hence resulting in each epoch lasting 12 s (equal to the TR). Three more epochs occurred, so that a total of four brain volumes were acquired within 48 s, before a 24‐s fixation block ensued, wherein subjects viewed a crosshair while two brain volumes were acquired. A total of 60 data points were collected during each 12‐min experimental run: 20 each for the word task, pseudoword task, and fixation condition.
Figure 1.

Experimental paradigm. Subjects underwent two experimental runs: reading single words (nonwords and real words) aloud and silently. No stimuli were repeated across the two runs. Each run consisted of blocks during which subjects read words (48 s) or blocks during which they only fixated (24 s). During the reading blocks three words (or nonwords) were presented during an 8‐s trial, followed by a 4‐s acquisition period. Each block contained four epochs (trial plus acquisitions) each epoch lasting 12 s, the duration of the TR. Real word or pseudoword trials consisted of a 200 ms word presentations, followed by a pause of 2450 ms in which a crosshair was presented and the subjects responded aloud or silently.
Real words were regularly spelled, single‐syllable English nouns of midrange frequency (three to five letters in length, median of four letters; mean MRC Psycholinguistic Database ratings: concreteness, 574.4 (range 270–642); familiarity, 552.1 (range 446–645); Kucera‐Francis written frequency, 60.6 (range 20–431)). Words were matched for concreteness, familiarity, and frequency across word lists. Pseudowords were generated from the real words by changing one or more letters until novel words were formed (e.g., norp, saff, janth, and kig), and were matched with real words for letter length and number of syllables. Pseudowords were reviewed by three native speakers of English and were discarded from the list if determined to be unpronounceable, were pseudohomophones of real words, or had a close orthographic resemblance to a real word. Sixty words and 60 pseudowords were presented in each experimental run. No word or pseudoword was repeated throughout the course of the entire experiment. The stimuli were displayed in white lower‐case Arial font on a black background.
fMRI Data Acquisition
All MRI data were acquired on a 1.5 T Siemens Magnetom Vision system with a circularly polarized head coil. The visual stimuli were projected onto a screen mounted on the top of the head coil. An angled mirror affixed to the head coil allowed a clear view of the screen and stimuli. Each whole‐head volume was acquired in four seconds with echo planar imaging (EPI) acquisition parameters as follows: 40 ms echo time (TE), 12‐s repetition time (TR), 64 × 64 matrix, 230 mm field of view (FOV), 46 axial slices, 3.0 mm slice thickness, 0.6 mm gap, resulting in 3.6 mm cubic voxels. For both of the runs a total of 63 frames (brain volumes) were acquired. The first three frames for each experimental run were discarded to achieve equilibrium in longitudinal relaxation.
Data Analysis
Image analysis was carried out using MEDx (Sensor Systems, Sterling, VA) and custom scripts. Image time series were motion‐corrected to the mean intensity image using the Automated Image Registration (v. 3.08) rigid body realignment algorithm [Woods et al., 1998a, b]. Gaussian spatial smoothing was applied using a low‐pass filter with a full‐width at half‐maximum of 7.2 mm (two times the voxel size) and a 9 × 9 mm convolution kernel. Global spatial variations in global image intensity were corrected with ratio normalization. To remove local low frequency signal drift, each time series was processed with a high‐pass temporal Butterworth filter with a period of 144 s (equal to two times the task period). For each subject the mean image was calculated for each task condition (word aloud, word silent, pseudoword aloud, pseudoword silent, fixation) and mean difference images were generated by subtracting the fixation condition from each task condition.
To determine regions of increased task‐related signal change for each condition relative to fixation at the group level, single‐group t‐tests were performed. These analyses resulted in t‐statistic maps that were then converted to Z‐score images and thresholded at Z > 3.1 (P < 0.001, uncorrected). The transformation matrix obtained when transforming the mean EPI image from a specific run to the EPI template (provided within Statistical Parametric Mapping, SPM96, Wellcome Department of Cognitive Neurology, London) was saved and used to shadow transform the statistical maps of various contrasts (e.g., pseudoword minus fixation) into the MNI 305 atlas space. The coordinates of the significant foci were converted from MNI space to a coordinate system corresponding with the stereotaxic atlas of Talairach and Tournoux [1988], using equations derived by Brett [1999]. For all figures displayed in the Results section, images are portrayed in the radiological convention, with the left side of the brain (L) represented on the right side of the figure and anterior towards the top of the figure. Using the GLM module within MEDx, an analysis of variance (ANOVA) was performed between the mean difference images of the four contrasts in order to identify the main effects of word‐type (pseudowords > real words) and response‐modality (aloud > silent), and the interaction of word‐type with response‐modality. The significance threshold employed was Z > 3.1 (P < 0.001).
RESULTS
Behavioral Performance
Accuracy of the pronunciation of real words and pseudowords was determined for the overt condition. Pseudowords were judged as correct if the pronunciation corresponded to English grapheme‐to‐phoneme rules; some pseudowords had more than one acceptable pronunciation. As expected, the mean accuracy for real words for the 15 subjects analyzed was 99.5%, significantly greater than the mean pseudoword accuracy of 94.5% (P < 0.003).
Functional MRI Results
Several contrasts were carried out in this study. We present results from: (1) the contrasts of each individual task (i.e., aloud pseudoword reading, aloud real word reading, silent pseudoword reading, and silent real word reading) relative to the viewing of a fixation point; (2) the conjunction of these four reading contrasts to reveal areas of activity common to all tasks; (3) the main effect of pseudoword vs. real word reading, when collapsing the aloud and silent conditions (main effect of word‐type); (4) the main effect of aloud vs. silent reading, when collapsing pseudoword and real word reading (main effect of response‐modality); and (5) the interaction of word‐type with response‐modality.
1. All Tasks vs. Fixation
We first subtracted viewing of a fixation point from each reading condition to visualize the global pattern of neural activity elicited by each reading task. Figure 2 displays representative transverse sections of the results of the single‐group t‐tests showing regions of increased task‐related signal for each condition (aloud pseudoword, aloud real word, silent pseudoword, and silent real word reading) relative to fixation. Visual inspection of the activation maps reveals the two aloud reading tasks elicited considerably more activity, both in intensity and extent, than the silent tasks, particularly in the primary motor (Brodmann's Area (BA) 4) and auditory cortices (BA 38, 21/22). Not surprisingly, for each task the overall intensity and extent of activity tended to be left hemisphere lateralized.
Figure 2.
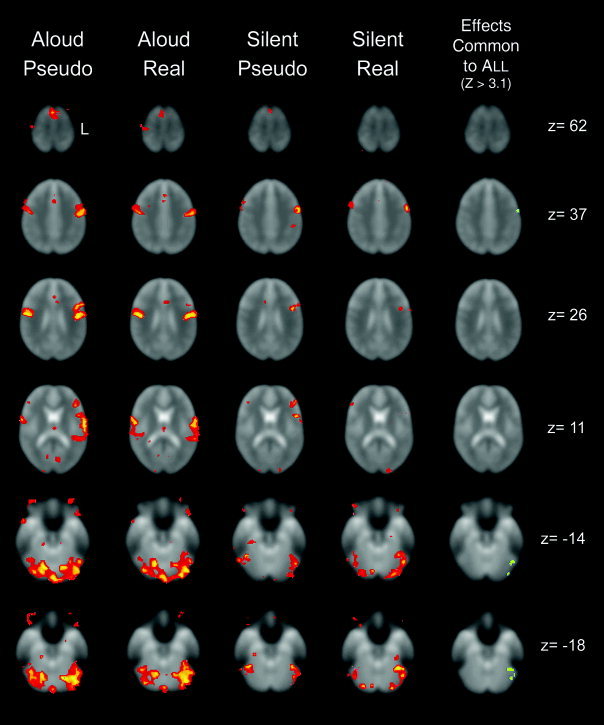
Transverse sections illustrating areas of significant activity for the four task conditions relative to the fixation baseline. From left to right: aloud pseudoword reading; aloud real word reading; silent pseudoword reading; silent real word reading; and the conjunction of all four tasks showing regions of activity common to all tasks. For all figures the z‐coordinate given is in the coordinate space of the atlas of Talairach and Tournoux [1988]. Images are portrayed in the radiological convention, with the left side of the brain (L) represented on the right side of the figure and anterior is towards to top of the figure. For visualization purposes, activity is displayed at a critical threshold of Z > 2.33 (P < 0.01, uncorrected).
2. Effects Common to All Reading Conditions: Aloud Pseudoword, Aloud Real Word, Silent Pseudoword, and Silent Real Word
The statistical maps generated as described above were submitted to a conjunction analysis to identify regions that showed increased task‐related signal change across all four reading conditions relative to their respective fixation baseline: the left anterior precentral gyrus in BA 6, and three regions within the left fusiform gyrus, one located within anterior BA 37, another in the mid‐fusiform region of BA 37, and the third posteriorly in BA 19. These are depicted in Figure 2, and Table I provides the corresponding spatial coordinates of the center of mass for each region.
Table I.
Regions of activity common to all reading tasks (vs. fixation): aloud pseudoword, aloud real word, silent pseudoword, silent real word
| BA | Cluster Center* | Cluster size (mm3) | |||
|---|---|---|---|---|---|
| x | y | z | |||
| L precentral gyrus | 6 | −54, | 1, | 36 | 224 |
| L fusiform gyrus | 37 | −39, | −44, | −20 | 368 |
| 37 | −45, | −58, | −15 | 384 | |
| 19/18 | −38, | −77, | −12 | 56 | |
Data are given in Talairach coordinates.
3. Main Effect of Word‐Type: Pseudowords > Real Words
The main effect of lexicality was determined by collapsing across aloud and silent conditions. A main effect of pseudoword reading relative to reading real words was observed in two regions within the left inferior frontal gyrus (BA 44/6 and BA44) and an area in the left intraparietal sulcus (IPS, BA 7). As can be seen in Figure 3, the left IPS region demonstrated an especially prominent effect (with corresponding spatial coordinates provided in Table II).
Figure 3.
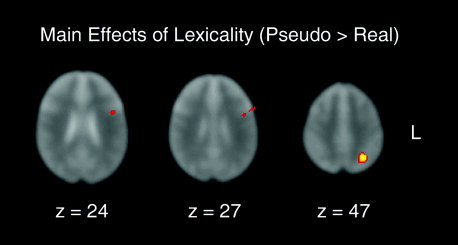
Transverse slices of the three cortical regions showing a main effect of lexicality (pseudowords > real words, collapsed across aloud and silent modalities). The z‐coordinate of each slice matches those in Table II.
Table II.
Main effect of lexicality (word‐type): pseudowords > real words
| BA | Peak voxel | Peak Z | |||
|---|---|---|---|---|---|
| x | y | z | |||
| L inferior frontal gyrus | 44/6 | −57, | 15, | 27 | 3.24 |
| L inferior frontal gyrus | 44 | −44, | 7, | 24 | 3.34 |
| L intraparietal sulcus | 7 | −28, | −54, | 47 | 4.05 |
4. Main Effect of Response‐Modality: Aloud > Silent
Table III and Figure 4 show the main effect of reading aloud relative to silent reading (with word‐type collapsed). Reading aloud elicited large increases in the BOLD‐contrast response bilaterally within the primary motor (BA 4), premotor (BA 6), presupplementary motor areas (BA 6, in the medial superior frontal gyrus), bilateral auditory cortex within the superior temporal gyri (BA 42/22), and bilateral posterior fusiform cortex (BA 19). The main effect of aloud reading also included two regions of the left hippocampal cortex (BA 36, 28), midline thalamus, and the left putamen.
Table III.
Main effect of response‐modality: aloud > silent
| BA | Peak voxel | Peak Z | |||
|---|---|---|---|---|---|
| x | y | z | |||
| R medial superior frontal gyrus | 6 | 8, | 13, | 60 | 5.41 |
| L precentral gyrus | 6 | −59, | −3, | 13 | 7.25 |
| 4 | −51, | −6, | 28 | 7.84 | |
| R precentral gyrus | 4/6 | 57, | −1, | 13 | 7.59 |
| 4 | 51, | −4, | 28 | 7.76 | |
| L superior temporal gyrus | 38 | −51, | 15, | −11 | 4.85 |
| 22/42 | −62, | −15, | 3 | 6.94 | |
| 22/42 | −61, | −27, | 7 | 7.02 | |
| R superior temporal gyrus | 22 | 55, | 6, | −4 | 6.18 |
| 22 | 57, | −27, | 3 | 6.71 | |
| L hippocampal | 36 | −18, | −6, | −33 | 6.20 |
| 28 | −12, | −28, | −10 | 5.88 | |
| L fusiform | 19 | −34, | −63, | −19 | 6.81 |
| R fusiform | 18/19 | 16, | −67, | −13 | 7.34 |
| Midline Thalamus (DM nucleus) | 4, | −19, | 6 | 5.23 | |
| L putamen | −20, | 8, | −2 | 4.26 | |
Figure 4.
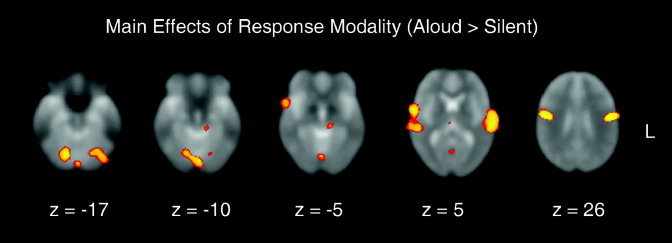
Transverse slices showing a main effect of response‐modality (aloud > silent, collapsed across pseudoword and real word‐types). The maxima of these areas are presented in Table III.
5. Interaction of Word‐Type With Response‐Modality
Using the mean difference images of the four contrasts generated as described above, an ANOVA was performed to reveal regions demonstrating an interaction effect of word‐type (pseudowords vs. real words) and response‐modality (aloud vs. silent reading). The interaction revealed a single significant locus (see Fig. 5), in the left posterior fusiform gyrus (BA 19, peak voxel –22, –73, –13; peak Z‐score = 3.59, P < 0. 0002). A post‐hoc analysis showed that the activity here for aloud pseudoword reading was greater than for aloud real word reading, silent pseudoword reading, and silent real word reading (P < 0.05). We confirmed that the area was located in the extrastriate/fusiform cortex by first registering and then overlaying each individual subjects' statistical maps with their own anatomical T1‐weighted image.
Figure 5.
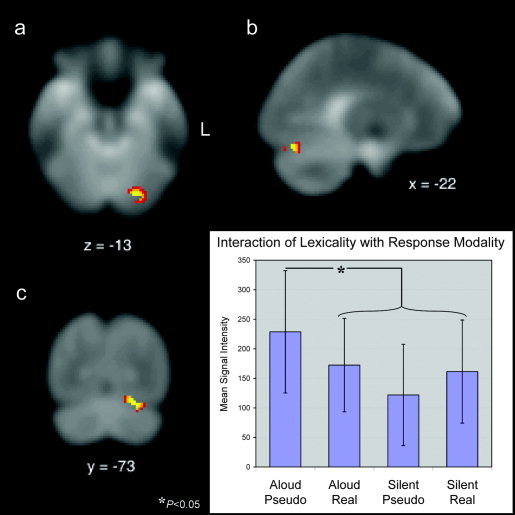
Transverse (a), sagittal (b), and coronal (c) views of the single region, located in the left posterior fusiform gyrus (BA 19), revealed by the interaction between word‐type (lexicality) and response‐modality. The bar graph illustrates the relative contribution (mean voxel intensity given in arbitrary units) of each of the four task conditions to the 142‐voxel region revealed by the interaction analysis. Post‐hoc paired t‐tests showed significantly greater activation for the aloud pseudoword reading condition relative to the aloud real word, silent pseudoword, and silent real word reading conditions (P < 0.05). There were no significant differences between the aloud and silent real word reading conditions, or between the silent pseudoword and silent real word conditions.
DISCUSSION
In this study we investigated the neural mechanisms of phonological decoding in the context of aloud and silent single word reading. Four reading conditions were used, each of which varied from the other in the demands placed on phonological processing: silent real word reading, silent pseudoword reading, aloud real word reading, and aloud pseudoword reading. The lexical unfamiliarity of pseudowords invokes addressed phonology. Phonological processing is also invoked by aloud reading, as pronunciation obligates access to the complete phonological word form. Hence, the reading of real words and reading silently served as contrasts for reading pseudowords and reading aloud, respectively. We examined 1) task‐related signal change common to the four reading conditions, 2) the main effects of word‐type and response‐modality, and 3) their interaction. Our central finding was derived from the interaction analysis and revealed a region within the left posterior fusiform cortex (ventral BA 19) that was modulated by increased stress on phonological processing. Hence, this region appears to serve a role in detecting the phonological demands associated with words. It is sensitive to a variety of aspects of phonological processing, including addressed phonology (applying sound‐correspondence rules) necessary to decode novel words in the absence of recognition‐based experience (pseudowords), and to conditions that necessitate the computation of a complete phonological representation (pronunciation). It is likely that this functional specialization for the phonological attributes of word processing is the end result of reading acquisition, much like the emergence of the “visual word form area” [McCandliss et al., 2003], a region tuned to orthographically legal word forms [Polk and Farah, 2002].
Effects Common to All Reading Conditions
Four cortical regions were active consistently across all reading conditions: the anterior aspect of the left precentral gyrus (BA 6), and three areas within the left ventral occipitotemporal cortex. This consistency, regardless of word‐type or response‐modality, suggests that these four regions play important roles in reading of regular letter combinations. The ubiquitous presence of activity within the anterior aspect of the left precentral gyrus (i.e., premotor cortex) implies that word reading in general entails some amount of subvocalization, or internal speech. Activation was observed here even for the silent reading task, for which subjects were instructed to read the words to themselves without moving their tongue or jaw, suggesting motor preparation activity regardless of whether or not the words are overtly pronounced.
Also active for all reading conditions were three regions of the left ventral occipitotemporal cortex, situated in an anterior‐posterior line along the fusiform gyrus. The most posterior region was located in the extrastriate cortex, within BA 19. Despite its early placement within the visual processing stream, this region has been shown to demonstrate a preferential response to alphabetic stimuli (words and consonant strings) over checkerboards [Cohen et al., 2003]. The second locus was within the mid fusiform gyrus at BA 37, the location of the putative visual word form area (VWFA) described by Cohen and others who have taken note of its very reliable response to orthographically legal word forms invariant of font, case, size, or position [Cohen et al., 2000, 2002]. The VWFA has been shown to respond to pseudowords and real words alike, signifying that its representation of word forms is at a prelexical level. Recent work suggests that the role of the VWFA may be quite sophisticated, serving as a repository for visual‐orthographic patterns which serve as recognition units in subsequent encounters of words [Kronbichler et al., 2004]. A region specifically sensitive to letters has been found adjacent (lateral and anterior) to this region and suggests that letters acquire a special object category with respect to BA 37 [Flowers et al., 2004]. The third fusiform region identified in the present study was located anterior to the VWFA. This area may receive afferent information from the more posterior regions of BA 37 and have a role in initiation of more complex, higher‐level word processing such as semantic access [Moore and Price, 1999].
Main Effect of Word‐Type: Pseudowords > Words
The contrast of pseudowords with words, independent of response‐modality, was performed to identify areas of the brain in which activity can be attributed to addressed phonology (necessary to decode words that cannot be read by relying on context or recall). Two foci within the left inferior frontal gyrus and an area in the left intraparietal sulcus were activated by this contrast. A great deal of evidence implicates the left inferior frontal cortex as playing a key role in phonological processing [Bookheimer et al., 1995; Burton et al., 2000; Fiez et al., 1999; Fiez and Petersen, 1998; Hagoort et al., 1999; Herbster et al., 1997; Huang et al., 2001; Newman, 2001; Rumsey et al., 1997a; Xu et al., 2001; Zatorre et al., 1996]. More in‐depth investigations of this region have led to the proposal that it has two anatomically distinct functions in reading: the ventral aspect of the inferior frontal gyrus (BA 47/45) appears to be involved with semantic processing, while the dorsal (opercular) inferior frontal gyrus (IFG, BA 44 and 46) aspect is recruited for phonologically effortful tasks [Bokde et al., 2001; Fiebach et al., 2002; Paulesu et al., 1997; Poldrack et al., 1999]. The inferior frontal cortex activity in the present study is located in BA 44, congruent with the putative phonological area. The left IFG has also been implicated in verbal working memory as the possible locus of the rehearsal loop [Paulesu et al., 1993], which could be another source of the activity we see here.
The activity observed in BA 44 for pseudowords (relative to real words) also expanded into BA 6. As noted above, left BA 6 was active during all of our reading tasks, likely reflecting the occurrence of subvocalization activity. The greater activity here for pseudowords relative to words supports the notion that motor planning is involved in the decoding of unfamiliar word forms and increases in response to heightened phonological demands. Other studies have reported increases in left precentral gyrus activity with increasing phonological demands [Hagoort et al., 1999], and cognitive models have implicated the motor system in the perception and production of the phonological features of language [Liberman and Mattingly, 1985].
Motor planning could also be a possible interpretation of the observed activity in the left intraparietal sulcus (BA 7), the third region to demonstrate a main effect of lexicality. The anterior inferior parietal lobule and posterior superior parietal lobule of the left hemisphere contribute to covert motor movement preparation or “motor attention” [Rushworth et al., 2003]. Reading of pseudowords might invoke planning of articulatory sequence movements that correspond to the pronunciation of the word. Alternatively, the greater activation of left IPS for pseudowords (relative to words) could be attributed to the increased demands unfamiliar words put on the phonological store of verbal working memory. Several PET studies have identified the left parietal cortex, including the intraparietal sulcus, as the locus of the phonological store [Becker et al., 1999; Paulesu et al., 1993; Ravizza et al., 2004] and parietal cortex has been shown to be underactivated in individuals with developmental dyslexia [Eden and Zeffiro, 1998].
Based on previous studies of phonological processing and phonological assembly, we anticipated activity in posterior aspects of the superior and middle temporal gyri [Bookheimer et al., 1995; Herbster et al., 1997; Price et al., 1996; Pugh et al., 2000; Rumsey et al., 1997a; Simos et al., 2002]. Indeed, dyslexics show a characteristic pattern of reduced activity within these regions [Brunswick et al., 1999; Paulesu et al., 1996; Rumsey et al., 1997b]. The lack of left posterior temporal activity in this study may have been due to the nature of our stimuli; perhaps our pseudowords were not challenging enough to elicit activation here. Greater discrepancy in phonological difficulty between the pseudowords and real words, such as by using polysyllabic pseudowords with infrequent letter combinations, might have revealed activity in this area. Also, as subjects were reading covertly in the silent condition, it was not possible to obtain performance accuracy. This provides some uncertainty about the demands made by this condition and future studies might address this (at least in part) by using a post‐scanning test in which subjects are asked to identify words from a list to indicate how many items they recognize from the scanning session [Turkeltaub et al., 2003]. In any case, reports of activity in the left posterior middle temporal gyrus tend to be intermittent, with some studies, including ours, reporting little or no responses here [Hagoort et al., 1999; Xu et al., 2001]. These variations across different studies raise questions about these regions' precise role in phonological processing and merits further investigation.
Main Effect of Response‐Type: Aloud Reading > Silent Reading
The analysis focusing on aloud vs. silent reading (independent of word type) revealed findings that were consistent with earlier studies of aloud word reading [Bookheimer et al., 1995; Hagoort et al., 1999; Turkeltaub et al., 2002]. We found increased activity in bilateral motor, auditory, and extrastriate visual cortices. The expansive activity underlying aloud reading attests to the fact that reading aloud involves more complex participation of multiple regions other than motor and auditory cortex, and that studies involving silent word reading cannot be substituted for aloud word reading. Our results also demonstrate the utility of interleaved fMRI data acquisition for tasks involving overt speech production to distinguish differences contributed by aloud vs. silent reading.
Interaction Effect of Word and Response Types
The interaction analysis of word‐type and response‐mode revealed a single locus in the left ventral extrastriate cortex located in posterior fusiform cortex at BA 19. This region was uniquely modulated by increased phonological processing demands invoked by a need for phonological assembly and access to the words' entire phonological code. This finding indicates a special role for left ventral BA 19 in phonological processing.
Ideas and controversies regarding the functional role of the left ventral extrastriate cortex have been evolving with a progression of neuroimaging studies of word form processing. In an early PET study by Petersen et al. [1990], activation in the left medial extrastriate cortex was detected during silent viewing of real words and pseudowords but not consonant letter or false font strings. It was concluded that this region was tuned to orthographically legitimate word forms. Although ventral extrastriate cortex had traditionally been associated with relatively low‐level visual processing such as color and form detection, this study and others that ensued supported the idea that the functions of extrastriate cortex are not limited to basic early visual processing. Specifically, left BA 19 and nearby regions have been shown to be more responsive to pseudoword compared to real word reading [Hagoort et al., 1999], pseudoword rhyming [Xu et al., 2001], and phonological working memory [Zurowski et al., 2002]. For example, Hagoort et al. [1999] report on a focus (BA 19/37; –34, –55, –11) slightly more anterior to ours that was more active during the reading of pseudowords when contrasted to real words, and more active for aloud compared to silent reading of real words (although this comparison was not significant when repeated for pseudowords). Paulesu et al. [2000] reported a similar result in a sample of English and Italian readers (their coordinates were –48, –68, –6); and Xu et al. [2001] also report a region, more lateral and anterior than the one described in the present study (46, –66, –10), which they identified when subtracting real word rhyming from pseudoword rhyming. These authors discuss the possibility of this area in mapping orthographic forms to sublexical phonological codes as the result of phonological assembly.
Taken together, these studies not only implicate the left ventral fusiform in an early process where alphabetic stimuli are distinguished from nonalphabetic stimuli [McCandliss et al., 2003; Polk and Farah, 2002], but our results go so far as to suggest the involvement of posterior areas of extrastriate cortex in the initial phonological analysis of written words. The posterior aspect of fusiform gyrus identified in the present study is 20 mm posterior and medial to the visual word form area located in mid‐fusiform cortex at BA 37 [McCandliss et al., 2003]. It has been suggested that the VWFA groups letters into “integrated perceptual units” and is sensitive to abstract information even for newly encountered words [McCandliss et al., 2003; Polk and Farah, 2002; however, see Price and Devlin, 2003]. By analogy, it seems that during aloud reading BA19 is tuned to the phonological properties of words much like the VWFA is tuned to the orthographic properties. The implication of this finding is that phonological analysis of word forms coincides with the early process of recognition of alphabetic stimuli. Our findings build on previous studies that considered phonological processing as a possible explanation for extrastriate cortex activity during reading [Buchel et al., 1998; Polk and Farah, 2002] and rhyming [Xu et al., 2001] tasks. Future studies will be necessary to elucidate the mechanisms that control left ventral BA 19 activity in response to varying levels of phonological demands. One alternative possibility to early processing of phonological information could be a top‐down mechanism from regions more directly involved in phonological processing or regions such as the prefrontal cortex (PFC) responsible for implementing task instructions (e.g., “read the presented words aloud”) [Chelazzi et al., 1993; Miller and Cohen, 2001; Wallis et al., 2001]. This scenario cannot be substantiated by the results of this study since no activity was seen within the PFC in the interaction analysis or in the comparisons of reading tasks to fixation. Temporal limitations of fMRI block design studies limit the utility of fMRI in addressing these types of questions, but magnetoencephelography (MEG) can shed more light on this matter. Studies using MEG to investigate pseudoword reading do report early activation if visual and visual association areas, although the nature of the tasks and statistical comparisons do not allow an exact interpretation with regards to the specific region of BA 19 described here [Simos et al., 2002].
CONCLUSIONS
Demands on phonological processing were modulated using a two‐by‐two design with tasks that had different phonological processing demands with reference to word‐type and response‐modality. The anterior aspect of the left precentral gyrus (BA 6) and three areas within the left ventral occipitotemporal cortex were found to be active across all reading conditions. The left inferior frontal gyrus and left intraparietal sulcus demonstrated sensitivity to word‐type, while bilateral motor, auditory, and extrastriate cortex were modulated by response mode. Activity underlying word reading in BA 19 in the left posterior fusiform cortex demonstrated a word‐type by response‐modality interaction effect, indicating that phonological processing during reading begins early in the processing stream, at the point at which words are first recognized as alphabetic stimuli.
Acknowledgements
We thank J. Agnew, P. Turkeltaub, and J. VanMeter for their help. We also thank L. Cutting, C. Vaidya, D. Howard, D.L. Flowers, two anonymous reviewers who provided constructive comments, and our subjects for their participation.
REFERENCES
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS (1992): Time course EPI of human brain function during task activation. Magn Reson Med 25: 390–397. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD (1999): Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. NeuroImage 10: 642–657. [DOI] [PubMed] [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA (1999): A comment on the functional localization of the phonological storage subsystem of working memory. Brain Cogn 41: 27–38. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R (1998): Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med 40: 55–60. [DOI] [PubMed] [Google Scholar]
- Bokde AL, Tagamets MA, Friedman RB, Horwitz B (2001): Functional interactions of the inferior frontal cortex during the processing of words and word‐like stimuli. Neuron 30: 609–617. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W (1995): Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp 3: 93–106. [Google Scholar]
- Brett M (1999): The MNI brain and The Talairach atlas. Cambridge Imagers. Available at http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html.
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U (1999): Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: a search for Wernicke's Wortschatz? Brain 122: 1901–1917. [DOI] [PubMed] [Google Scholar]
- Buchel C, Price C, Friston K (1998): A multimodal language region in the ventral visual pathway. Nature 394: 274–277. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL, Blumstein SE (2000): The role of segmentation in phonological processing: an fMRI investigation. J Cogn Neurosci 12: 679–690. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F (1998): The effects of semantic category and knowledge type on lexical‐semantic access: a PET study. Neuroimage 8: 350–359. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R (1993): A neural basis for visual search in inferior temporal cortex. Nature 363: 345–347. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene‐Lambertz G, Henaff MA, Michel F (2000): The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123: 291–307. [DOI] [PubMed] [Google Scholar]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S (2002): Language‐specific tuning of visual cortex? Functional properties of the visual word form area. Brain 125: 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, Slachevsky A, Dehaene S (2003): Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex 13: 1313–1333. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M (1993): Models of reading aloud: dual‐route and parallel‐distributed‐processing approaches. Psychol Rev 100: 589–608. [Google Scholar]
- Eden GF, Zeffiro TA (1998): Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron 21: 279–282. [DOI] [PubMed] [Google Scholar]
- Eden GF, Joseph JE, Brown HE, Brown CP, Zeffiro TA (1999): Utilizing hemodynamic delay and dispersion to detect fMRI signal change without auditory interference: the behavior interleaved gradients technique. Magn Reson Med 41: 13–20. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NA, Agnew JA, Flowers DL (2004): Neural changes following remediation in adult developmental dyslexia. Neuron 44: 411–422. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY (2002): fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE (1998): Neuroimaging studies of word reading. Proc Natl Acad Sci U S A 95: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE (1999): Effects of lexicality, frequency, and spelling‐to‐sound consistency on the functional anatomy of reading. Neuron 24: 205–218. [DOI] [PubMed] [Google Scholar]
- Flowers DL, Jones K, Noble K, VanMeter J, Zeffiro TA, Wood FB, Eden GF (2004): Attention to single letters activates left extrastriate cortex. Neuroimage 21: 829–839. [DOI] [PubMed] [Google Scholar]
- Friedman RB, Ween JE, Albert ML (1993): Alexia In: Heilman KM, Valenstein E, editors. Clinical neuropsychology, 3rd ed. New York: Oxford University Press; p 37–62. [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE (1998): The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci U S A 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhand S (2001): Routes to reading: a report of a non‐semantic reader with equivalent performance on regular and exception words. Neuropsychologia 39: 1473–1484. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ (1999): The neural circuitry involved in the reading of German words and pseudowords: a PET study. J Cogn Neurosci 11: 383–398. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT (1997): Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp 5: 84–92. [DOI] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y (2001): Comparing cortical activations for silent and overt speech using event‐related fMRI. Hum Brain Mapp 15: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara (1996): Tests for color‐deficiency. Tokyo: Kanehara. [Google Scholar]
- Jobard G, Crivello F, Tzourio‐Mazoyer N (2003): Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage 20: 693–712. [DOI] [PubMed] [Google Scholar]
- Joubert S, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux JM, Karama S, Lecours AR (2004): Neural correlates of lexical and sublexical processes in reading. Brain Lang 89: 9–20. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Smith AM, Mendrek A, Forster BB, Hare RD (1999): Neural pathways involved in the processing of concrete and abstract words. Hum Brain Mapp 7: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G (2004): The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage 21: 946–953. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H‐M, Brady TJ, Rosen BR (1992): Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 89: 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJ (2001): The functional anatomy of single‐word reading in patients with hemianopic and pure alexia. Brain 124: 510–521. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S (2003): The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci 7: 293–299. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno‐Tempini ML, Price CJ (2003): Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci 15: 260–271. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ (1999): Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage 10: 181–192. [DOI] [PubMed] [Google Scholar]
- Newman SD, Twieg D (2001): Differences in auditory processing of words and pseudowords: an fMRI study. Hum Brain Mapp 14: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD (1996): Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain 119: 143–157. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, Perani D, Fazio F (1997): Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8: 2011–2017. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U (2000): A cultural effect on brain function. Nat Neurosci 3: 91–96. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, Raichle ME (1990): Activation of extrastriate and frontal cortical areas by visual words and word‐like stimuli. Science 249: 1041–1044. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ (2002): Functional MRI evidence for an abstract, not perceptual, word‐form area. J Exp Psychol Gen 131: 65–72. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS (1994): Brain activity during reading. The effects of exposure duration and task. Brain 117: 1255–1269. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS (1996): Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex 6: 62–70. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC (1996): Cerebral organization of component processes in reading. Brain 119: 1221–1238. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA (2000): Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6: 207–213. [DOI] [PubMed] [Google Scholar]
- Rastle K, Harrington J, Coltheart M, Palethorpe S (2000): Reading aloud begins when the computation of phonology is complete. J Exp Psychol Hum Percept Perform 26: 1178–1191. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA (2004): Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P (1997a): Phonological and orthographic components of word recognition. A PET‐rCBF study. Brain 120: 739–759. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P (1997b): A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch Neurol 54: 562–573. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen‐Berg H, Gobel SM, Devlin JT (2003): The left parietal and premotor cortices: motor attention and selection. Neuroimage 20(Suppl 1): S89–100. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL (1989): A distributed, developmental model of word recognition and naming. Psychol Rev 96: 523–568. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC (1998): Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA 95: 2636–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC (2002): Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb Cortex 12: 297–305. [DOI] [PubMed] [Google Scholar]
- Southwood MH, Chatterjee A (2000): The interaction of multiple routes in oral reading: evidence from dissociations in naming and oral reading in phonological dyslexia. Brain Lang 72: 14–39. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. (Rayport M, Translator.) New York: Thieme. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. NeuroImage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM (2004): Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry 45: 2–40. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK (2001): Single neurons in prefrontal cortex encode abstract rules. Nature 411: 953–956. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW (1993): The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150: 885–890. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T (1980): Word‐form dyslexia. Brain 103: 99–112. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998a): Automated image registration. I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22: 139–152. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC (1998b): Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 22: 153–165. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega‐Bermudez F, Pietrini P, Reeves‐Tyer P, DiCamillo P, Theodore W (2001): Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb Cortex 11: 267–277. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Meyer E, Gjedde A, Evans AC (1996): PET studies of phonetic processing of speech: review, replication, and reanalysis. Cereb Cortex 6: 21–30. [DOI] [PubMed] [Google Scholar]
- Zurowski B, Gostomzyk J, Gron G, Weller R, Schirrmeister H, Neumeier B, Spitzer M, Reske SN, Walter H (2002): Dissociating a common working memory network from different neural substrates of phonological and spatial stimulus processing. NeuroImage 15: 45–57. [DOI] [PubMed] [Google Scholar]


