Abstract
This cortical stimulation mapping study investigates the neural representation of action and object naming. Data from 13 neurosurgical subjects undergoing awake cortical mapping is presented. Our findings indicate clear evidence of differential disruption of noun and verb naming in the context of this naming task. At the individual level, evidence was found for punctuate regions of perisylvian cortex subserving noun and verb function. Across subjects, however, the location of these sites varied. This finding may help explain discrepancies between lesion and functional imaging studies of noun and verb naming. In addition, an alternative coding of these data served to highlight the grammatical class vulnerability of the target response. The use of this coding scheme implicates a role for the supramarginal gyrus in verb‐naming behavior. These data are discussed with respect to a functional–anatomical pathway underlying verb naming. Hum. Brain Mapping 24:1–10, 2005. © 2004 Wiley‐Liss, Inc.
Keywords: language, semantic, verb, noun, category‐specific, temporal lobe epilepsy, electric stimulation
INTRODUCTION
The identification of dedicated cortical regions necessary for specific language behaviors has a long, well‐documented history. This pursuit has given rise to well‐accepted neuropsychologic constructs such as the eloquent language areas. Given this tradition, it is perhaps surprising that after the nearly 150 years since the discoveries of P. Broca and his contemporaries, a cogent characterization of fundamental structure–function relationships of language processes remains elusive. One example of this quandary is illustrated by the lack of neurolinguistic characterization of noun and verb processing, specifically whether the grammatical categories of noun and verb are represented differentially within the neural system for language. The grammatical distinction between noun and verb is ubiquitous in the world's languages, and as some have argued, may be a reflection of an even more basic conceptual parcellation, such as abstract representations dividing those things that are static from those that change state [Givón, 1979, 1984]. Whether there are identifiable anatomically distinct neural regions honoring this linguistic distinction remains a matter of debate. This question has been addressed extensively in the lesion literature [Berndt et al., 2002; Bird et al., 2000; Damasio and Tranel, 1993; Daniele et al., 1994; Gainotti et al., 1995; Kemmerer and Tranel, 2000; Lu et al., 2002; Miceli et al., 1988; Miozzo, 2003; Tranel et al., 2001], and more recently in neuroimaging studies [Kellenbach et al., 2002; Khader et al., 2003; Perani et al., 1999; Tyler et al., 2001]. One generalization made from the lesion studies is that deficits in verb naming are associated with prefrontal damage whereas noun‐naming deficits are more common after damage to anterior temporal regions. Although there is abundant evidence from lesion studies for differential disruptions of nouns and verbs, neuroimaging studies have been far less successful in illuminating anatomic differences.
Studies of positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have given rise to conflicting results. For example, using PET technique, Tyler et al. [ 2001] reported no difference between nouns and verbs on tasks of semantic categorization and lexical decision; in contrast, Perani et al. [ 1999], also using a lexical decision task, did report verb‐ (but not noun‐) specific activations in Broca's area and left middle temporal gyrus. Studies using magnetoencephalographic (MEG) technique have failed to establish noun and verb differences in normal subjects [Soros et al., 2003]. In addition, there have been few reports of event‐related potential (ERP) studies that document distinct noun and verb differences. In a recent study, Khader et al. [ 2003] used a semantic priming task and found no topographic differences in the N‐400 component as a function of grammatical class. However, in a contrast of voltage differences in the 300–800‐msec time window for verbs versus nouns, some differences were observed with greater positivity over central to frontal areas and more negativity over occipital and temporal‐parietal regions.
The differences between the results of lesion studies and those of functional imaging studies are not entirely surprising. Language imaging typically reveals a wide assortment of cortical regions. These patterns of activations reflect not only necessary regions, but also supporting, competing, and nonsufficient but complementary systems, and thus may obscure subtle differences of grammatical class. This situation highlights the importance of using converging methods in the study of language function.
In addition to the issues of anatomic localization, further questions arise with respect to functional characteristic of verb‐ and noun‐naming deficits. Specifically, it remains unclear whether the factors that underlie a differential disruption of nouns and verbs reflect lexical or grammatical disruptions.
One hypothesis holds that differential noun and verb impairments reflect lexical‐semantic differences. Differences in the composition and distribution of semantic features may thus lead to cases of double dissociation, in a fashion conceptually similar to that which has been proposed for category‐specific naming deficits [Breedin et al., 1998]. Nouns and verbs differ on a wide range of lexical, semantic, and usage dimensions, thus making it difficult to attribute any observed differences to grammatical class per se. Several recent attempts to control for various confounds include the inclusion of both abstract and concrete nouns and verbs [Brendt and Haendiges, 2000], equating for imageability [Bird et al., 2000, 2001], and controlling for argument structure [Kim and Thompson, 2000] (for a recent review of these issues, see Shapiro and Caramazza [ 2003] and Druks and Masterson [ 2003]).
Alternatively, differential verb and noun errors may reflect the fact that verbs do more of the grammatical work. Verbs are pivotal components within a syntactic structure, and it may be this representational armature that sets them apart from nouns. In this view, verb disruption is associated with deficits in syntactic form, as in the case of patients with agrammatism [see Silveri et al., 2003].
When differential disruption of nouns and verbs are found, methodologic concerns factor significantly in the interpretation of results, often limiting theoretical claims. These include issues related to task requirements, stimulus properties, and quantification of errors.
The use of static line drawings to elicit both object and action naming is a common practice [Cappa et al., 2002; Tranel et al., 2001; Vigliocco et al., 2002]. One concern with the use of static pictures is that it requires subjects to make inferences about the actions being carried out, a process not required for the comparison condition of object naming. This difference may lead to errors based on difference in pictorial complexity rather than linguistic pathology. In addition, often a completely separate set of pictures may be used to elicit noun responses, further complicating the equating of stimulus complexity.
One factor that limits the inferences that can be drawn from action and object naming data with respect to grammatical class differences (i.e., nouns and verbs) concerns the nature of the error coding schemes used. A common error‐coding scheme judges responses as semantically incorrect, incomplete, or no response [e.g., Cappa et al., 2002]. This coarse coding may miss important generalizations. For example, consider two hypothetical patients who make errors naming pictures that depict the actions “mowing,” “hitting,” and “running.” Patient A responds, “grass,” “ball,” and “shoe,” whereas Patient B responds, “sweeping,” “clapping,” and “sliding.” Common error‐coding schemes treat Patient A's and Patient B's answers as wrong and based on these data, conclude evidence for a common verb naming deficits. It should be clear, however, that there may be major differences between patients. Patient B seems able to access and produce verbs in the context of this task, but simply produces the wrong ones. Patient A's errors reveal a pattern of only reporting nouns, which may imply, among other things, an inability to access and produce verbs as a class. It remains unknown whether such distinctions factor in patterns of object and action naming, as to our knowledge this error dimension has surprisingly not been examined. Explication of errors involving category maintenance or category switching would be useful in relating these deficits to models of normal language processing. For example, Fay and Cutler [ 1977] found that 99% of semantic errors in naming in normal subjects maintained grammatical class.
Cortical Stimulation Mapping
Studies of patients undergoing direct cortical stimulation during presurgical treatments for intractable epilepsy have provided valuable insight into the localization of language‐related cortex. In these studies, electrical current applied directly to cortex results in temporary functional lesions, and the patterns of deficits observed have been found highly predictive of postsurgical outcome. Past studies of this population have typically utilized object‐naming tasks in the clinical setting to assess language function. In a classic study, Ojemann et al. [ 1989] described 117 patients' naming errors observed in extensive regions of language‐dominant cortex, even well outside classically defined language areas.
When considered at the group level, cortical stimulation studies, such as that of Ojemann et al. [ 1989], indicate widespread disruption; however, individual subjects may exhibit exquisite functional specializations. These include specializations for language in first and second languages [Ojemann and Whitaker, 1978], semantic category [Ilmberger et al., 2002], and conditions of word retrieval, such as reading versus picture naming [Ojemann et al., 1989].
We make use of cortical stimulation mapping technique in presurgical patients to explore whether specific cortical regions are associated with actions and objects at the oral‐naming level through an investigation during the naming of transitive action vignettes. The use of moving transitive action stimuli (e.g., a person cracking an egg or peeling a banana) has several advantages. The stimuli are not static, and thus the images to be named are a more natural depiction of actions. Also, the same stimulus is used for the elicitation of a noun and a verb (e.g., the noun “egg” and the action “cracking” are elicited using the same vignette), thus stimulus complexity is kept constant. In addition, we adopt two error‐coding schemes to differentiate the hypothetical patterns of responses of Patients A and B described above. The first scheme is accepted more commonly in the literature, whereby any mislabeling of the stimulus is considered incorrect. The second scheme uses an alternative criterion that excludes within‐class naming errors. That is, when eliciting the verb “bouncing,” a subject who provides the incorrect verb label “running” evidences an ability to a produce a lexical element (albeit the wrong target) from within the grammatical category of verbs. In this second coding, these within‐class errors are treated as evidence for preserved grammatical class information.
In this way, we are able to make some inferences about general processes of lexical production in the face of action and object naming as well as to identify impairments that preclude the ability to generate items from within a specific grammatical category.
In summary, this study seeks to examine the distribution of naming errors produced in response to stimulation of sites within the temporal lobe under conditions of action and object naming. The approach adopted in this study is motivated by methodologic and theoretical considerations. The use of naturalistic moving transitive action stimuli permits elicitation of both object and actions names, thus controlling for stimulus complexity. Naming disruption is evaluated along two error measurements, one highlighting lexical disruption and the other grammatical class involvement. If under the condition of cortical stimulation, error‐naming patterns follow tendencies of normal speech errors, we would expect that substitution errors would not cross grammatical boundaries. On the other hand, the extent to which the errors produced switch grammatical category may indicate a deeper level of impairment, e.g., an inability to access lexical items within a grammatical class.
Based on data from lesions studies, we hypothesize that a preponderance of verb errors will be associated with stimulation to the anterior temporal lobe. Based on previous cortical stimulation studies, however, we expect individual variation in action‐ and object‐naming sites across subjects and within subjects, evidence for a well‐delineated separation of function.
MATERIALS AND METHODS
Subjects
Subjects comprised 13 patients (8 female, 5 male; age range 19–32 years) undergoing resection treatment at the University of Washington Medical Center for chronic epilepsy (n = 12) and for tuberous sclerosis (n = 1; Patient 12). Eleven patients were right‐handed and two were left‐handed. All cortical stimulation occurred in the subject's language‐dominant hemisphere (which corresponded to handedness in all subjects) determined by presurgery WADA testing (Table I).
Table I.
Subject demographics
| Subject | Age (yr) | Gender | Language‐dominant hemisphere | Stimulation sites (n) | Grid placement |
|---|---|---|---|---|---|
| 1 | 42 | Female | Right | 3 | Inferior frontal to anterior temporal |
| 2 | 36 | Female | Right | 4 | Inferior frontal to anterior temporal |
| 3 | 19 | Male | Right | 5 | Inferior parietal to posterior temporal |
| 4 | 28 | Female | Right | 3 | Inferior frontal to posterior temporal |
| 5 | 31 | Female | Right | 3 | Inferior frontal to anterior temporal |
| 6 | 32 | Male | Right | 5 | Inferior frontal to anterior temporal |
| 7 | 41 | Female | Right | 3 | Inferior parietal to posterior temporal |
| 8 | 34 | Male | Right | 1 | Inferior frontal to anterior temporal |
| 9 | 25 | Male | Right | 4 | Inferior parietal to posterior temporal |
| 10 | 25 | Female | Left | 3 | Inferior frontal to anterior temporal |
| 11 | 31 | Male | Right | 4 | Inferior frontal to anterior temporal |
| 12 | 49 | Female | Left | 1 | Inferior parietal to posterior temporal |
| 13 | 34 | Female | Right | 3 | Inferior frontal to anterior temporal |
All subjects were implanted with subdural grids of electrodes (16 × 16) approximately 1 week before resection. The grid permitted direct monitoring of electroencephalograph (EEG), allowing for identification of epileptic foci. In addition, pair‐wise electrodes could be stimulated with small currents (2–8 mA), resulting in a transient functional lesion lasting approximately the duration of the current (1–2 sec). Stimulating grid sites preoperatively allowed the neurosurgeon (G.A.O.) to map the functional topography of the cortex, thus informing surgical decisions.
Prior clinical mapping identified motor and sensory cortices in these patients using indwelling grids that spanned frontal, temporal, and parietal cortices. In addition, language mapping using static line drawings of objects was employed to locate cortical areas important for object naming. For each subject, one to six sites in overlapping and adjacent cortex were chosen by the neurosurgeon to conduct the action‐ and object‐naming experiment. These sites were limited to anterior middle and posterior superior temporal gyrus, middle and posterior middle temporal gyrus, the supramarginal gyrus, and ventral lateral occipital gyrus. The ability to sample equally across all cortical regions is limited by clinical factors (i.e. grid placement). The data presented here are those obtained only from the action and object measure. The labels for the sites (21, A, etc.) are part of a local system developed by the neurosurgeon for clinical purposes.
Protocol
Great care was used to develop ecologically valid naming stimuli to examine processing of human actions [Corina, 1998]. Vignettes of actors carrying out a variety of common transitive actions and intransitive actions (e.g., bouncing a ball, peeling a banana, sneezing) were filmed. Norming data from 140 undergraduate students provided a consistency measure for the labeling of these stimuli. The norming study facilitated the evaluation of whether a subject's answer was a possible, although infrequently used, correct name (e.g., “limb” for “stick”), or was an incorrect response (e.g., “cucumber” for “telephone”).
Twenty‐six transitive actions were chosen for the present experiment. A list of stimulus pairs used is given in Table II. The items chosen included both high‐ and low‐frequency items from both grammatical categories. The transitive nature of the stimuli served to elicit either a description of the common action being carried out (e.g., “bouncing”) or the common concrete object involved in the action (e.g., “ball”). A 4‐sec instruction screen with “Name the object” or “Name the action” displayed in a large, light blue font on a black background preceded each 2‐sec vignette. There were two versions of each of 26 items, one for each instruction screen.
Table II.
Stimulus pairs
| Stimulus pairs | |
|---|---|
| Bite pear | Light match |
| Blow feather | Open door |
| Bounce ball | Peel banana |
| Braid hair | Play guitar |
| Break stick | Pop balloon |
| Button shirt | Pull wagon |
| Crack egg | Push wheelchair |
| Crush can | Read book |
| Cut paper | Sit chair |
| Deal cards | Shine apple |
| Dial phone | Smell flower |
| Fold towel | Smoke cigarette |
| Lift weight | Squeeze lemon |
Subjects were asked to view and name three unique sets of stimuli. Each set contained two blocks of action‐object trials, and 12 unique items were represented in each set. At separate times, subjects were required to name the action and the object for each stimulus item. Half of these items were designated as stimulation trials, and the other half as control trials.
During the stimulation procedure, three separate examiners transcribed the subject's responses. Responses were scored for two separate analyses. A general disruption measure was obtained by counting all off‐target, anomic, delayed, and paraphasic responses (Type A). This measure was used to assess general effects of stimulation during action and object naming.
A second measure was used to examine whether the errors produced followed expected patterns of semantic substitution found in normals (i.e., errors maintained grammatical class) or whether errors included production of lexical items from another grammatical class. Under this coding, semantic paraphasic errors that nevertheless maintained the target grammatical category were excluded (Type B). For instance, if under stimulation at site 23, the stimulus was ACTION “peel banana,” and the subject responded, “dialing,” this would be scored as a Type A error only. Stimulation at site 23 cannot be said to disrupt the class of actions because the subject successfully retrieved and uttered a verb. If in the same situation, however, a subject had replied, “banana” or “apple” (providing an object response when the target is an action), this may indicate that retrieval or production of the class of verbs may be vulnerable. Type B errors therefore provide an opportunity to chart cortical regions that exhibit grammatical class vulnerability, regardless of the semantic proximity of the response.
The location of grid sites was determined using the cortical parcellation system (CPS), which uses the Foundational Model of Anatomy NeuroNames terminology [Martin et al., 2001, 2003] (Fig. 1). This system divides the lateral surface of cortex into 37 regions using landmarks and projections from these landmarks to make general statements about the location of sites stimulated during surgery. Sites were located in the system through the use of landmarks on 3D reconstructions created from structural magnetic resonance imaging (MRI), intraoperative photographs, intraoperative schematic drawings of grid placement, and with guidance from functional information as determined by the neurosurgeon (primarily sites associated with motor and sensory cortices). The localization of sites was directed by the primary author of the CPS, who was unaware of the sites that had errors associated with them. All localization endeavors were given a confidence rating on a scale from 1 to 5, where 1 is “not at all confident” and 5 is “very confident.” The rating was determined by amount and quality of images and descriptions. All stimulated sites except one received a confidence rating of 3 or above. Site 21 for Subject 11 did not receive the required rating, and was omitted from the localization analysis.
Figure 1.
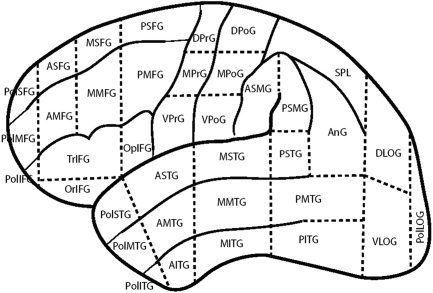
Cortical parcellation system (CPS).
RESULTS
To determine whether naming disruption at a site determined by the neurosurgeon was an effect of stimulation or attributable to the baseline naming error rate of the subject, a within‐subject analysis of naming errors was carried out. Fischer's exact test (P < 0.05) was used to compare each subject's baseline performance, derived from the naming error rate in each control trial associated with the site, regardless of target, and performance under stimulation at that site. This definition of baseline, restricted to the controls associated with a certain site, was established to eliminate variation in performance due to fatigue, inattention, and other physical factors experienced by the subject during the procedure. The results of this analysis are shown in Table III. It should be understood that although the entries in this table represent the number of errors observed over the total number of stimulations, the P values represent the reliability that an error is observed under stimulation relative to the unstimulated baseline for each individual site.
Table III.
Results of analysis
| Subject | Site | Baseline | Objects | Actions | Subject | Site | Baseline | Objects | Actions |
|---|---|---|---|---|---|---|---|---|---|
| Type A | Type B | ||||||||
| 1 | 32 | 2/12 | 3/6 | 5/6 | 1 | 32 | 1/12 | 2/6 | 5/6a |
| 30 | 5/12 | 2/6 | 4/6 | 30 | 5/12 | 1/6 | 4/6 | ||
| 31 | 7/12 | 2/6 | 6/6 | 31 | 5/12 | 1/6 | 6/6 | ||
| 2 | 25 | 0/12 | 1/6 | 6/6b | 2 | 25 | 0/12 | 1/6 | 6/6b |
| 27 | 1/12 | 2/6 | 6/6a | 27 | 1/12 | 2/6 | 6/6a | ||
| 28 | 1/12 | 1/6 | 2/6 | 28 | 1/12 | 1/6 | 2/6 | ||
| 26 | 0/12 | 0/6 | 3/6 | 26 | 0/12 | 0/6 | 3/6 | ||
| 3 | 21 | 1/15 | 6/9a | 1/6 | 3 | 21 | 1/15 | 4/9 | 1/6 |
| B | 1/12 | 2/6 | 1/6 | B | 0/12 | 2/6 | 0/6 | ||
| D | 1/6 | 0/3 | 0/3 | D | 1/6 | 0/3 | 0/3 | ||
| 31 | 0/6 | 0/3 | 0/3 | 31 | 0/6 | 0/3 | 0/3 | ||
| 20 | 1/12 | 6/6a | 0/6 | 20 | 0/12 | 6/6b | 0/6 | ||
| 4 | 22/23 | 4/18 | 4/9 | 7/9 | 4 | 22/23 | 3/18 | 4/9 | 7/9a |
| 24 | 5/12 | 0/6 | 5/6 | 24 | 5/12 | 0/6 | 5/6 | ||
| A | 0/12 | 2/6 | 2/6 | A | 0/12 | 1/6 | 2/6 | ||
| 5 | 30 | 2/12 | 6/6 | 6/6 | 5 | 30 | 2/12 | 6/6 | 6/6 |
| 31 | 2/12 | 0/6 | 5/6 | 31 | 2/12 | 0/6 | 5/6 | ||
| 32 | 0/12 | 2/6 | 6/6b | 32 | 0/12 | 2/6 | 6/6b | ||
| 6 | 22 | 2/18 | 3/9 | 5/9 | 6 | 22 | 1/18 | 3/9 | 5/9a |
| 35 | 2/12 | 2/6 | 0/6 | 35 | 0/12 | 2/6 | 0/6 | ||
| 20 | 1/12 | 6/6a | 2/6 | 20 | 0/12 | 6/6b | 2/6 | ||
| 36/37 | 2/12 | 1/6 | 3/6 | 36/37 | 2/12 | 1/6 | 3/6 | ||
| 21 | 2/12 | 5/6 | 1/6 | 21 | 2/12 | 5/6 | 1/6 | ||
| 7 | 31 | 0/13 | 0/6 | 0/5 | 7 | 31 | 0/13 | 0/6 | 0/5 |
| 21 | 0/12 | 4/6a | 2/6 | 21 | 0/12 | 0/6 | 0/6 | ||
| 33 | 0/18 | 2/9 | 6/9b | 33 | 0/18 | 2/9 | 6/9b | ||
| 8 | 22 | 0/12 | 0/6 | 4/6a | 8 | 22 | 0/12 | 0/6 | 4/6a |
| 9 | 36 | 1/18 | 1/9 | 0/9 | 9 | 36 | 1/18 | 1/9 | 0/9 |
| 35 | 2/6 | 1/3 | 0/3 | 35 | 1/6 | 1/3 | 0/3 | ||
| 30 | 0/12 | 4/6a | 4/6a | 30 | 0/12 | 4/6a | 4/6a | ||
| 20 | 0/12 | 6/6b | 5/6a | 20 | 0/12 | 6/6b | 4/6a | ||
| 10 | A | 2/12 | 0/6 | 4/6 | 10 | A | 1/12 | 0/6 | 1/6 |
| 20 | 0/12 | 5/6a | 4/6a | 20 | 0/12 | 4/6a | 3/6 | ||
| 21 | 5/12 | 6/6 | 6/6 | 21 | 3/12 | 5/6 | 6/6 | ||
| 11 | 30 | 1/12 | 0/6 | 1/6 | 11 | 30 | 1/12 | 0/6 | 1/6 |
| 32 | 0/12 | 1/6 | 4/6a | 32 | 0/12 | 0/6 | 4/6a | ||
| 31 | 4/13 | 2/5 | 1/6 | 31 | 3/13 | 2/5 | 0/6 | ||
| 21 | 1/24 | 6/12a | 2/12 | 21 | 1/24 | 4/12 | 1/12 | ||
| 12 | 30 | 1/25 | 5/11a | 4/12 | 12 | 30 | 1/25 | 4/11a | 4/12 |
| 13 | 30 | 0/12 | 0/6 | 1/6 | 13 | 30 | 0/12 | 0/6 | 0/6 |
| 20 | 1/12 | 3/6 | 0/6 | 20 | 1/12 | 3/6 | 0/6 | ||
| 21 | 0/18 | 8/9b | 2/9 | 21 | 0/18 | 2/9 | 0/9 |
Baseline error rate and object‐naming and action‐naming error rates under stimulation are listed as number of errors over number of opportunities for errors.
P < 0.05
P < 0.01
All subjects showed significant disruption during stimulation at one or more tested sites. Two subjects (Subjects 9, 10) displayed nonspecific disruption affecting both action and object naming. Five subjects (Subjects 1, 2, 4, 5, 8) showed significant disruption for action naming at one or more sites. For these subjects, stimulation at these same sites did not produce significant errors in object naming. Three subjects (Subjects 3, 12, 13) showed the reverse pattern, i.e., significant disruption in object naming with a sparing of action naming at the same sites. Finally, three subjects (Subjects 6, 7, 11) displayed double dissociations between action and object naming, with anatomically distinct sites giving rise to specific errors in action or object naming but not in both.
When the sites were plotted in relation to the CPS, statistically significant Type A errors in object and action naming largely overlapped (Fig. 2). As indicated in the figure, naming disruption was evident in widespread perisylvian region areas, but particularly vulnerable were cortical regions in the middle and posterior superior temporal and both anterior and posterior banks of the supramarginal gyrus.
Figure 2.
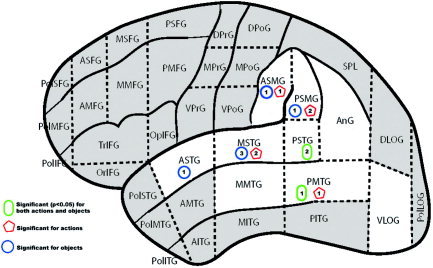
Significant sites under Type A analysis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In the three subjects who exhibited double dissociation, the site responsible for significant disruption in object naming (site 20 for Subject 6, site 21 for Subject 7, and site 21 for Subject 11) was located anterior and proximal to the site responsible for significant disruption of action naming (sites 22, 33, and 32). In Subject 7, a double dissociation was localized to the supramarginal gyrus, with stimulation to the anterior portion (site 21) leading to significant object‐naming errors, whereas at a distance of approximately 1 cm posterior (site 33), we observed significant action‐naming errors (Fig. 3). In Subject 6, a double dissociation was observed in the middle superior temporal gyrus, with anterior stimulation leading to significant object‐naming errors and stimulation in the posterior portion (approximately 1 cm posterior) leading to significant action‐naming errors. In Subject 11, stimulation to the middle superior temporal gyrus led to significant action errors, and stimulation within the field of a previously resected anterior inferior temporal lobe region led to significant object‐naming errors.
Figure 3.
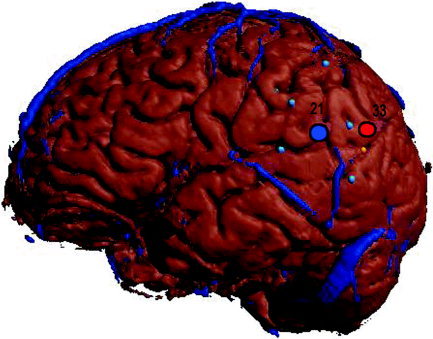
Double dissociation of action (site 33) and object (site 21) naming in Subject 7. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Assessments of function utilizing the Type B coding provided an opportunity to ascertain better whether these naming deficits arose due to factors of lexical selection and production of specific items or rather represented a more profound problem in accessing and producing lexical items of a specific grammatical class under conditions of cortical stimulation. Recall that under this coding, semantic paraphasias in which the grammatical category was maintained were excluded from the error count. In other words, these were sites where subjects were unable to provide a response or where subjects' errors switched category (i.e., the intended target was a verb, but the response given was a noun).
The results of this analysis revealed that there were sites at which, when stimulated, a subject was significantly more likely to generate words that belonged to the opposite grammatical category of the intended target or no target at all. These errors were seen for both verb and noun targets, although they were seen more commonly under conditions in which a verb was to be named (resulting in a noun response). Three sites, one in the anterior superior temporal gyrus and two in the middle temporal gyrus, resulted in the inability to name nouns. Nine sites were encountered in which stimulation resulted in an inability to name verbs. These sites were located in the middle superior temporal gyrus (5), the supramarginal gyrus (3), and the posterior middle temporal gyrus (1).
DISCUSSION
Several important findings emerge from this study. Our data show selective disruptions in action and object naming and further evidence for impairments in the retrieval and production of lexical items within a grammatical category. We discuss each of these findings in turn.
In this study, stimulation of several temporal lobe regions within the language‐dominant hemisphere resulted in deficits in action and object naming, and some of these regions selectively impaired one class of naming behaviors but not the other. Aggregation of these vulnerable sites, however, does not support strongly a uniform regional specialization for these naming behaviors. Within the temporal lobe regions, we observe both action‐ and object‐naming errors. This finding runs counter to the generalization gleaned from lesion studies, suggesting a propensity for noun‐naming deficits to be associated with anterior temporal lobe regions.
Nevertheless, it is clear that within individuals, there are regions that when disrupted produce highly selective deficits in these naming behaviors. We observed numerous instances where stimulation of a discrete location led to errors in action naming, for example, but stimulation at this exact same area had no effect on object naming. In addition, we have presented three cases of double dissociation of action and object naming. In these cases, two cortical regions separated by as little as 1 cm can take on differential naming functions. Moreover, in all of these cases, the region giving rise to object‐naming disruption lay anterior to that implicated in action‐naming disruption.
These findings have implications for the apparent inconsistency in the neurolinguistic literature regarding selective deficits of action and object naming. Specifically, there is ample evidence for disruptions in patient studies, whereas neuroimaging techniques have failed to observe frank regional differences in action and object naming. Our data would indicate that there is highly individual specialization of these functions across temporal‐parietal cortex, and those neuroimaging techniques that require averaging of subject responses will therefore fail to illuminate punctate regions. In addition, selective regions may be quite close spatially, further hampering imaging efforts to capture these differences.
These data also have implications for the representational level of underlying impairment. It is well known that in naturally occurring speech that semantic errors in naming in normal subjects maintained grammatical class [Fay and Cutler, 1977]. In this study, we have taken care to provide a coding of stimulation errors that helps differentiate between this normal pattern of lexical disruption and a more profound inability to access and produce lexical items from a specific grammatical category. When applied, this coding scheme continued to reveal discrete regions where category‐specific naming errors were attested.1
Errors in verb naming were observed with stimulation to the supramarginal gyrus, in the posterior middle temporal gyrus, and in the middle superior temporal gyrus. Errors in noun naming were seen with stimulation to the anterior and middle superior temporal gyrus. It is noteworthy that with stimulation to the supramarginal gyrus, subjects were able to generate noun forms, albeit incorrect ones. In contrast, our subjects were unable to produce verb forms with stimulation to this same region. This finding suggests a key role of the supramarginal gyrus in the mediation of verb forms.
As mentioned previously, a generalization made from lesion studies is that deficits in verb naming are associated with prefrontal damage whereas noun‐naming deficits are more common after anterior temporal lesions. This generalization has many exceptions, however, especially with respect to the anatomic region resulting in verb‐naming disruption. It is particularly interesting to note that although the left prefrontal cortex is implicated often in the selective deficits of verb naming, numerous case studies have also reported select verb‐naming deficits after damage to posterior parietal regions [Daniele et al., 1994]. For example, in a recent study, Silveri et al. [ 2003] present a case study of a patient with left parietal lesion with only minimal impairment in object naming but severe difficulties in action naming. Interestingly, the prevalent error in verb naming was the production of nouns in place of verbs, which Silveri et al. [ 2003] state “as if the patient were virtually unable to produce verbs.” This patient's naming pattern is highly similar to that observed in our study, where with stimulation to the supramarginal gyrus, subjects were able to generate noun forms (albeit incorrect ones) but were unable to produce verb forms. This pattern may factor significantly in our understanding of the mechanics of language. There is growing evidence for differentiation of cortical networks underlying language. Several findings point to intimate relationships between left inferior frontal cortex (Broca's area) and inferior parietal regions (supramarginal gyrus) in language functions, including articulatory motor mapping [Hickok and Poppell, 2000], verbal working memory [Smith and Jonides, 1998], and phonological‐semantic binding [Corina et al., 1999]. In addition, this circuitry has been implicated in studies of imagination of complex movements [Jancke et al., 2001]. Damasio and Tranel [ 1993] suggested a relationship between the anatomic systems that mediate access to verbs and those that support concepts of movement. Moreover, they speculated that networks in the dorsal component of temporooccipital and parietal cortices, which project to premotor and prefrontal regions, are likely substrates for conceptual representations of verbs. Damasio and Tranel [ 1993] concluded that anatomic damage in regions at the end of this processing stream in the frontal lobe is associated frequently with specific disruption of verbs. Our data further suggest that impairments may be seen in earlier points within this route.
The present coding illustrates that action naming under stimulation is more often likely to lead to the lack of a response or an object substitution. The anatomic distribution of these error responses further suggests an anterior‐to‐posterior dimension with pure object‐naming errors observed in middle and anterior portions of the temporal lobe and action‐naming deficits situated along a dorsal‐to‐ventral band in the vicinity of the inferior parietal lobule and posterior temporal lobe (Fig. 4).
Figure 4.
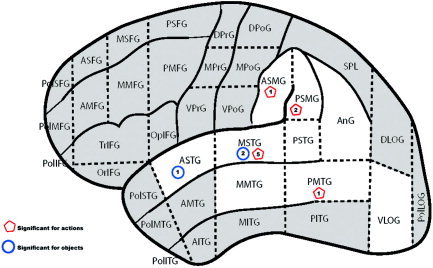
Significant sites under Type B analysis. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Taken together, these data indicate that sites supporting action and object naming may be well distributed across perisylvian regions. At an individual level, however, naming errors may be highly selective. In addition, the data from the three subjects who showed double dissociation provide an indication of individual subspecialization whereby object naming lies more anterior to sites involved in action naming. Consistent with previous reports of cortical specificity, these data show that highly punctate regions of cortex may be critical for processes involved in naming that honor the grammatical class distinction of nouns and verbs. Finally, as seen in the present study, consideration of error patterns in which subjects are unable to produce any targets within the desired grammatical class may provide a more concise delineation of regions necessary for grammatical form‐specific language production.
Footnotes
Under this coding scheme, the most common errors are omissions; however, switching errors are attested. A potential concern with this coding is whether category‐switching errors are motivated along word‐frequency dimension, such that the intended target was lower in frequency than was the response (i.e., what would seem to be an avoidance of a grammatical category is simply a preference for higher frequency words). To investigate this, we calculated word frequency ratings for each target and response based on the Kucera–Francis written frequency count [Kucera and Francis, 1967]. The log transform of these ratings were subjected to a two‐tailed t‐test but were not significantly different (P = 0.6).
REFERENCES
- Berndt RS, Haendiges AN (2000): Grammatical class in word and sentence production: Evidence from an aphasic patient. J Mem Lang 43: 249–273. [Google Scholar]
- Berndt RS, Haendiges AN, Burton MW, Mitchum CC (2002): Grammatical class and imageability in aphasic word production: their effects are independent. J Neurolinguist 15: 353–371. [Google Scholar]
- Bird H, Howard D, Franklin S (2001): Noun‐verb differences? A question of semantics: a response to Shapiro and Caramazza. Brain Lang 76: 213–222. [DOI] [PubMed] [Google Scholar]
- Bird H, Ralph MAL, Patterson K, Hodges JR (2000): The rise and fall of frequency and imageability: noun and verb production in semantic dementia. Brain Lang 73: 17–49. [DOI] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Schwartz MF (1998): Semantic factors in verb retrieval: an effect of complexity. Brain Lang 63: 1–31. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D (2003): The neural correlates of noun and verb processing. J Neurolinguist 16: 183–189. [Google Scholar]
- Cappa SF, Sandrini M, Rossini PM, Sosta K, Miniusii C (2002): The role of the left frontal lobe in action naming: rTMS evidence. Neurology 59: 720–723. [DOI] [PubMed] [Google Scholar]
- Corina DP (1998): Video database of human actions with rating norms. Seattle, WA: Laboratory for Cognitive Neuropsychology, University of Washington. [Google Scholar]
- Corina DP, McBurney SL, Dodrill C, Hinshaw K, Brinkley J, Ojemann G (1999): Functional roles of Broca's area and SMG: evidence from cortical stimulation mapping in a deaf signer. Neuroimage 10: 570–581. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D (1993): Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA 90: 4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, Gainotti G (1994): Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia 32: 1325–1341. [DOI] [PubMed] [Google Scholar]
- Druks J, Masterson J (2003): Objects vs. actions and nouns vs. verbs. J Neurolinguist 16: 59–65. [Google Scholar]
- Fay D, Cutler A (1977): Malapropisms and structure of mental lexicon. Linguist Inq 8: 505–520. [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L (1995): Neuroanatomical correlates of category‐specific semantic disorders: a critical survey. Memory 3: 247–264. [DOI] [PubMed] [Google Scholar]
- Givón T (1979): On understanding grammar. New York: Academic Press. [Google Scholar]
- Givón T (1984): Syntax: a functional‐typological introduction. Amsterdam: Benjamins. [Google Scholar]
- Hickok G, Poepel D (2000): Towards a functional neuroanatomy of speech perception. Trends Cogn Sci 4: 131–138. [DOI] [PubMed] [Google Scholar]
- Ilmberger J, Rau S, Noachtar S, Arnold S, Winkler P (2002): Naming tools and animals: asymmetries observed during direct electrical cortical stimulation. Neuropsychologia 40: 695–700. [DOI] [PubMed] [Google Scholar]
- Jancke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ (2001): The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb Cortex 11: 114–121. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Wijers AA, Hovius M, Mulder J, Multer G (2002): Neural differentiation of lexico‐syntactic categories or semantic features? Event‐related potential evidence for both. J Cogn Neurosci 14: 561–577. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Tranel D (2000): Verb retrieval in brain‐damaged subjects: 1. Analysis of stimulus, lexical, and conceptual factors. Brain Lang 73: 347–392. [DOI] [PubMed] [Google Scholar]
- Khader P, Scherag A, Streb J, Rosler F (2003): Differences between noun and verb processing in a minimal phrase context: A semantic priming study using event‐related brain potentials. Brain Res Cogn Brain Res 17: 293–313. [DOI] [PubMed] [Google Scholar]
- Kim M, Thompson CK (2000): Patterns of comprehension and production of nouns and verbs in agrammatism: Implications for lexical organization. Brain Lang 74: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera H, Francis WN (1967): Computational analysis of present‐day American English. Providence: Brown University Press. [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez‐Rothi LJ, Raymer A, Gilmore RL, Bauer RM, Roper SN (2002): Category‐specific naming deficits for objects and actions: semantic attribute and grammatical role hypothesis. Neuropsychologia 40: 1608f2 –1621. [DOI] [PubMed] [Google Scholar]
- Martin RF, Mejino JLV, Bowden DM, Brinkley JF, Rosse C (2001): Foundational model of neuroanatomy: implications for the human brain project. Proc AMIA Symp 438–442. [PMC free article] [PubMed] [Google Scholar]
- Martin RF, Brinkley JF, Hertzenberg X, Poliakov A, Corina DP, Ojemann GA (2004): Anatomical parcellation of cortical language sites. Abstract under review for MEDINFO 2004.
- Miceli G, Silveri MC, Nocentini U, Caramazza A (1988): Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology 2: 351–358. [Google Scholar]
- Miozzo M (2003): On the processing of regular and irregular forms of nouns and verbs: evidence from neuropsychology. Cognition 87: 101–127. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Ojemann JG, Lettich E, Berger M (1989): Cortical language localization in left‐dominant hemisphere. J Neurosurg 71: 316–326. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Whitaker HA (1978): The bilingual brain. Arch Neurol 35: 409–412. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio F (1999): The neural correlates of verb and noun processing: a PET study. Brain 122: 2337–2344. [DOI] [PubMed] [Google Scholar]
- Silveri MC, Perri R, Cappa A (2003): Grammatical class effects in brain‐damaged patients: functional locus of noun and verb deficit. Brain Lang 85: 49–66. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A (2003): The representation of grammatical categories in the brain. Trends Cogn Sci 7: 201–206. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J (1998): Neuroimaging analyses of human working memory. Proc Natl Acad Sci 29: 12061–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soros P, Cornelissen K, Laine M, Salmelin R (2003): Naming actions and objects: cortical dynamics in healthy adults and in an anomic patient with a dissociation in action/object naming. Neuroimage 19: 1787–1801. [DOI] [PubMed] [Google Scholar]
- Tranel D, Adolphs R, Damasio H, Damasio AR (2001): A neural basis for the retrieval of words for actions. Cogn Neuropsychol 18: 655–670. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Russell R, Fadili J, Moss HE (2001): The neural representation of nouns and verbs: PET studies. Brain 124: 1619–1634. [DOI] [PubMed] [Google Scholar]
- Vigilocco G, Vinson DP, Damian MF, Levelt W (2002): Semantic distance effects on object and action naming. Cognition 85: 61–69. [DOI] [PubMed] [Google Scholar]


