Abstract
We demonstrate that breath holding of short durations may confound functional magnetic resonance imaging (fMRI) studies. Some subjects may hold their breath for a short time during task performance, especially if the task is challenging. Breath holding may therefore need to be considered specifically when interpreting fMRI experiments. We studied the temporal and spatial characteristics of cerebral T2*‐weighted signal during short periods of breath holding by seven individuals in a 3‐tesla MR scanner. We demonstrate that breath‐holds as short as 3 s can result in regions of significant cerebral activation. More interestingly, we show that focal activation remains present when the data is analysed in a number of different ways, including analyses that correct for motion and model the task epoch as if it were 10 times longer than the actual breath‐hold length. These findings have potential relevance for many researchers carrying out fMRI studies. Hum. Brain Mapping 24:284–290, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: magnetic resonance imaging, hypercapnia, respiration, human, adult, confounding factors
INTRODUCTION
The aim of brain mapping research is to show brain activity associated with task performance. It is well understood that oxygenation and blood flow changes are related only indirectly to neuronal activity. Furthermore, interpretation of blood oxygenation level‐dependent (BOLD) [Ogawa et al., 1990] functional magnetic resonance imaging (fMRI) studies is limited by the existence of artefacts, especially those related to motion (such as gross subject movement and regional pulsatile motion of the brain related to the cardiac and respiratory cycles). The present work is concerned with breath holding of short durations; a potential source of artefact that, to our knowledge, has not been generally recognised as such in published fMRI studies to date.
Breath holding results in a reduction of oxygen and an accumulation of carbon dioxide in the blood stream. Hypercapnia induces cerebral vasodilation and increased cerebral blood flow [e.g., Markus and Harrison, 1992]. Several investigators have studied the effects of extended breath holding on cerebral fMRI signal in human volunteers [Kastrup et al., 1998, 1999; Kwong et al., 1995; Li et al., 1999a, b; Liu et al., 2002; MacIntosh et al., 2003]. However, it is unclear from these reports what minimal duration of breath holding will result in a change in cerebral BOLD signal intensity. Only one of these studies examined breath‐holds as short as 10 s [Liu et al., 2002], another as short as 18 s [Kastrup et al., 1998], whereas all other studies were of breath‐holds of at least 30 s. It is possible that shorter or recurrent episodes of breath holding may be sufficient to induce BOLD signal changes. For example, we would expect that a 6‐s breath‐hold would result in an arterial carbon dioxide increase of 2–4 mm Hg (increasing to 10 mm Hg or more by 35 s) [Sasse et al., 1996; Stock et al., 1988], although it is unknown whether 2–4 mm Hg alone would result in observable BOLD signal change at 3 tesla. We might also expect to observe signal increase resulting from regional cerebral blood flow changes related to the neuronal processes involved in the initiation and maintenance of a breath‐hold. As a breath‐hold progresses, regions of the brain responsible for attention and motor control of respiration may well become more active. Of most concern is the interpretation of fMRI studies where brief breath‐holding periods may occur without the awareness of the investigator. This could be a significant unrecognised confound in data interpretation.
To our knowledge, a systematic study of possible confounding effects of breath holding during fMRI experiments has not been undertaken. The type of breath holding that a subject might carry out subconsciously during an fMRI experiment may be expected to vary from periods of only a few seconds up to the length of the task epoch. Importantly, breath holding may be task correlated; we would expect that it would more likely occur during a task period when greater concentration or effort is required. Task‐correlated activation could lead to misleading interpretations, particularly of individual results.
We sought to determine the possible effect of breath‐holds of various durations occurring during the task epoch of a block‐design fMRI experiment carried out at 3 tesla. Our study was not designed to reveal the underlying mechanisms of any signal change observed, rather to determine whether the act of performing a relatively short breath‐hold would produce signal changes that could potentially mislead an investigator. We hypothesised that: (1) breath‐holds as short as 6 s would result in detectable fMRI signal change; (2) longer breath‐holds would result in more widespread signal changes; and (3) breath‐hold–related signal change would be observed even if a breath‐hold as short as 6 s occurred at the start of a much longer task epoch.
SUBJECTS AND METHODS
Subjects
Seven healthy subjects (four men; three women; age range 26–45 years) participated in this study. Our institutional human research ethics committee approved the study. Written informed consent was obtained from each participant.
Paradigm
A block‐design paradigm was used for all studies. In our early studies (the first three subjects), subjects were instructed verbally either to “hold your breath” or “breathe normally.” Before scanning, these subjects were instructed that upon the “hold your breath” command they should continue their current breath until end expiration and then hold their breath in as relaxed a state as possible. The first two subjects (Subject 1 and 2) participated in studies consisting of four 39‐s breath‐hold epochs interleaved with 81‐s rest periods, whereas the third subject (Subject 3) participated in studies with three 30‐s breath‐hold epochs interleaved with 90‐s rest periods. In each of these cases, there was thus 120 s between the start of each breath‐hold. The data from these experiments were analysed to extract shorter effective breath‐hold epochs (e.g., first 6 s of each breath‐hold, first 9 s, etc.) to determine the possible effect of breath‐holds of various durations during a block‐design fMRI experiment.
To ensure the effects were consistent with stand‐alone breath‐holds of similar lengths, Subject 3 additionally carried out a series of breath‐hold studies, each with three epochs of constant duration breath‐hold (6, 9, 12, 18, 24, or 30 s) interleaved with rest such that there were 120 s between the start of each breath‐hold. The subject was made aware of the length of breath‐hold to be carried out for a particular experimental run before commencement.
After analysis of the studies above, we designed a second paradigm and carried it out on four additional subjects (Subjects 4–7). This paradigm was designed to test whether breath‐holds of 3, 6, or 9 s could result in significant activation when analysed as if the paradigm consisted of blocked 18‐s or 30‐s tasks. In these studies, we improved upon our original design by using a real‐time chest monitor observable by an operator controlling the onset of a visual cue projected onto a screen inside the scanner room. The screen initially displayed a + symbol, and upon the operator's mouse‐click this changed to an × symbol and remained an × for a predefined time. Subjects were instructed to hold their breath immediately after the symbol changed to ×, and to continue holding until the symbol changed back to a +. The duration (i.e., length of time an × was displayed) was timed by computer and preset for the particular imaging run.
Four breath‐holds of similar length were carried out during each 4.5‐min run. The start of each breath‐hold was always cued at the end of expiration. The breath‐holds were cued at the first end expiration that occurred after each of the following times: 30, 90, 150, and 210 s from the start of the run. Three runs were carried out with 3‐, 6‐, and 9‐s breath‐holds, respectively.
Image Acquisition
Multislice fMRI imaging (single‐shot gradient‐recalled echo [GRE] echo‐planar imaging [EPI], repetition time [TR] = 3.0 s, echo time [TE] = 40 ms, flip angle = 60 degrees, 128 × 128 matrix, 1.95 mm × 1.95 mm × 4 mm thick +1 mm gap) covering the whole brain was carried out on a 3‐tesla scanner (GE Signa Horizon LX; GE Medical Systems, Milwaukee, WI) using a standard birdcage quadrature head‐coil.
Data Processing and Display
Image processing and analysis was carried out using SPM99 (Wellcome Department of Cognitive Neurology, London, UK) [Frackowiak et al., 1997] and iBrain software developed in‐house [Abbott and Jackson, 2001]. Images were automatically converted to Analyze format in iBrain and then realigned in SPM99 to a target image. The target was either chosen during a rest period near the middle of the time course (first three subjects) or was the optimum image determined by iBrain (i.e., the image whose within‐brain centre‐of‐mass was closest to the median for that run) (Subjects 4–7). Images were then smoothed with a Gaussian kernel of full width at half maximum equal to twice the acquisition voxel size. Intra‐subject normalisation of global within‐brain signal intensities was not carried out. For all analyses described below, statistical parametric maps were displayed in colour in radiological convention (left is subject's right) using iBrain. Activation maps were either −log10(P) values (for analyses carried out in iBrain) or Student's t‐statistics (for analyses carried out in SPM99), and were overlaid onto a corresponding raw EPI acquisition. In regions identified as active at a threshold of P < 0.0001 (not corrected for multiple comparisons), voxel count and mean signal difference were determined in iBrain. The mean signal difference was expressed as a percentage of the mean value of the voxel:
Exploratory Statistical Analysis
For each of the first three subjects, several sets of analyses were carried out in iBrain, including analyses of subsets of the data to extract information for shorter breath‐hold durations than those actually carried out. Analyses included: (1) full duration of breath‐hold; (2a) initial portion of breath‐hold to assess the effect of breath‐holds of various lengths; (2b) 6‐s epochs extracted at various times from each subject's longest study to assess regional progression of activity; and (3) for the third subject's 6‐s breath‐hold run only, an analysis as if the task of interest were longer than the breath‐hold (analyses were carried out for 18‐ and 30‐s “task” epochs). For each of the analyses, voxel‐wise statistical analyses were carried out separately on each subject, using an unpaired t‐test. The two image acquisitions immediately after initiation of the actual breath‐hold (corresponding to an assumed haemodynamic delay of 6 s) were excluded from these analyses. For all analyses, the rest periods used were periods well after the resumption of normal breathing. For analyses 1 and 2, the rest periods used were identical 30‐s rest epochs of normal breathing that ended 6 s before the next breath‐hold instruction. For analysis 3, the rest periods were those commencing 6 s after the interval that was treated in the analysis as the “task,” bearing in mind that this “task” actually consisted of a 6‐s breath‐hold followed by 12 or 24 s of rest (normal breathing).
Analysis of Potential Confound
To verify that the potential confound observed above was robust across different analysis methods and subjects, statistical analysis of the complete time‐course (i.e., no images excluded) of the third subject's 6‐s breath‐hold run and of the studies of the further four subjects was carried out in SPM99 using a design matrix that included a boxcar function convolved with a canonical haemodynamic response function (HRF). We used the default canonical HRF of SPM99 comprising the sum of two gamma functions [Friston et al., 1998, Fig. 1]. We did not include a temporal derivative of the HRF in the model. The six realignment parameters determined during image realignment were included as covariates of no interest. Separate analyses were carried out using an 18‐s and a 30‐s boxcar model, and both positive and negative contrasts were tested (corresponding to typical block‐design fMRI analyses of activation and “deactivation”). Total counts of significant voxels were obtained for each analysis.
Figure 1.
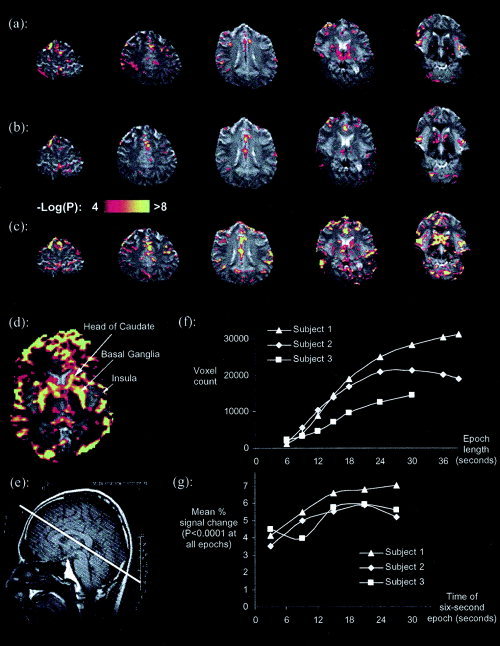
a: Activation (P uncorrected < 0.0001) present in the 6‐s breath‐hold of Subject 3. b, c: Analysis of the first 6‐s and 18‐s (respectively) epoch of the same subject's 30‐s study. d: An enlarged labeled slice of the analysis of the first 18‐s epoch of the 39‐s breath‐hold study of Subject 1. e: The position of the slice acquisition is shown by the thick white line on an anatomical scout image. f: Number of voxels above statistical significance, plotted against the length of the breath‐hold epoch extracted from each subject's longest breath‐hold study (as per analysis 2a, see text). g: Mean % signal change in those voxels that were always significant in progressive 6‐s epochs extracted from 30 s of breath holding, plotted against the time to middle of the extracted epoch (as per analysis 2b, see text). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com. Bright white replaces all color in print.]
RESULTS
Analyses of Full Duration and Early Portions of Breath‐Holds (Analyses 1 and 2)
Significant increases in BOLD signal intensity were seen after only 6 s of breath holding (Fig. 1a). These increases were observed for both the 6‐s breath‐hold study and the first 6 s of longer breath‐hold studies (Fig. 1b). Areas that had significantly increased signal intensity during the breath‐hold included the anterior and posterior cingulate, the insula, and the caudate regions. More widespread increases in signal intensity were observed with breath‐holds of longer duration (Fig. 1c–f). Activation was more extensive and included most of the caudate and basal ganglia as well as much more of the cortex.
Subject 3 carried out an ensemble of breath‐hold lengths, and the activation observed for the shorter breath‐hold experiments was remarkably consistent with that obtained when shorter time epochs were extracted from the 30‐s breath‐hold experiment and analysed independently (e.g., compare Fig. 1a and 1b). In all cases, irrespective of breath‐hold duration, the increase in signal intensity began no longer than 6 s after the onset of the breath‐hold.
In all subjects, the number of significantly activated voxels was greater the longer the breath‐hold was maintained, up to at least 30 s (Fig. 1f). To confirm that the effect seen in the analysis of different breath‐hold lengths was not due simply to an increase in study power (due to the increasing number of scans collected as the epoch length increased), we further examined the evolution of signal change and increase in number of significant voxels during an extended breath‐hold by extracting 6‐s epochs from various time points throughout the breath‐hold. The power of each of these analyses was constant because the number of task scans and rest scans remained constant. The extent of activation was seen generally to rise as the breath‐hold progressed up to at least 24 s (data not shown). The progression of mean signal change in voxels that remained significant throughout the breath‐hold (i.e., identified in this analysis as significant in the first 6‐s epoch and all other 6‐s epochs up to 30 s) tended to increase from about 4% to a plateau at about 6% during the first 18 s (Fig. 1g).
Results of Analysis Demonstrating Potential Confound
Regions of significant activation remained even when analysing the third subject's 6‐s breath‐hold study in iBrain as if it were either an 18‐ or 30‐s task, as shown in Figure 2 (a, b). This study was also analysed with SPM99, with a model including a canonical HRF and specification of motion correction parameters as covariates of no interest. Results of these analyses when modelling the response as if the task epoch were 18 or 30 s are shown in Figure 2 (c, d). Regions active were quite similar to those described earlier; however, the extent of activation in each region seemed somewhat less, particularly when modelling as a 30‐s task.
Figure 2.
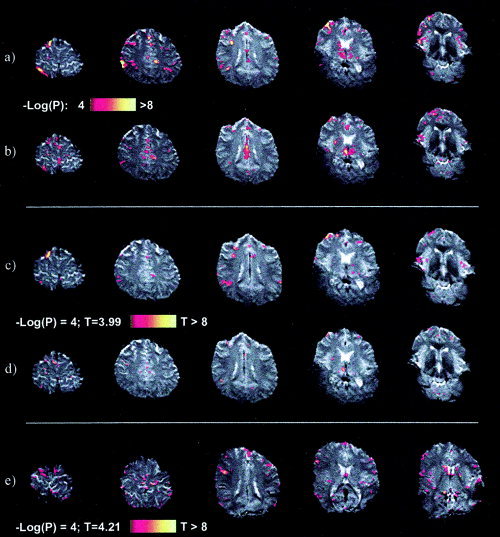
The 6‐s breath‐hold study of Subject 3 analysed using a Student's t‐test as if it were an 18‐s (a) and 30‐s (b) task. Considerable regions of significant activation (P uncorrected < 0.0001) remain evident. c, d: Analysis of the complete time‐course of the same 6‐s breath‐hold study using the general linear model as implemented in SPM99, with motion‐correction parameters included in the model as confounds, and the task modelled with a canonical HRF convolved with a boxcar of task length 18 s (c) and 30 s (d). e: Significant activity was also seen in the short breath‐hold studies of other subjects, as demonstrated here for example in the 6‐s breath‐hold study of Subject 6, modelled in SPM99 (as described above) with a task length of 30 s. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com. Bright white replaces all color in print.]
Similar SPM99 analyses (modelling as 18‐ and 30‐s tasks) were also undertaken for the studies of the additional four subjects whose breathing was monitored. Respiratory monitoring revealed that all subjects carried out the task as instructed with the exception of the first breath‐hold missed in the 6‐s run of Subject 7. Significant activity was nonetheless observed in all subjects' 6‐s breath‐hold studies, with extensive activity in Subjects 6 and 7 as shown for example in Subject 6 in Figure 2e. Subject 7 was the only subject to show considerable activity when her 3‐s breath‐hold study was analysed with an 18‐s model, although the other subjects still had some significant voxels (Table I). The negative contrasts were also examined in the four subjects to see if there was any deactivation (reduction in BOLD activity) when modelling the task as either 18 or 30 s (Table I). In most cases, there was very little if any deactivation. In the few cases where moderate deactivation was observed, the corresponding positive contrast generally showed substantially more activation.
Table I.
Summary of total number of activated voxels in SPM99 analyses of the studies of the four respiratory‐monitored subjects
| Subject no. | Model(s) | 3‐s Breath‐hold | 6‐s Breath‐hold | 9‐s Breath‐hold | |||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||
| 4 | 18 | 17 | 4 | 63 | 3 | 487 | 9 |
| 30 | 13 | 0 | 17 | 5 | 485 | 7 | |
| 5 | 18 | 4 | 6 | 26 | 35 | 516 | 60 |
| 30 | 10 | 9 | 21 | 5 | 94 | 11 | |
| 6 | 18 | 37 | 5 | 279 | 1 | 6 | 0 |
| 30 | 6 | 1 | 1,396 | 42 | 10 | 0 | |
| 7 | 18 | 79 | 3 | 383 | 16 | 849 | 53 |
| 30 | 13 | 0 | 204 | 24 | 340 | 10 | |
The actual breath‐hold length of the study (3, 6, or 9 s) shown with the length of the task in the boxcar model used in each analysis (18 or 30 s). This boxcar function was convolved with a canonical haemodynamic response function, and motion correction parameters were included in the model as covariates of no interest. Results for both positive and negative contrasts are shown (i.e., “activation” and “deactivation”). In almost all analysis pairs, there are considerably more active than de‐active voxels, confirming the presence of an effect even with breath‐holds as short as 3 s.
DISCUSSION
A major finding of this study is that breath‐holds of as little as 3–6 s can produce focal regions of activation, even when the breath‐hold occurs during an 18‐ or even a 30‐s “task” period. Subjects may hold their breath, particularly at the beginning of a demanding task. Our results therefore suggest that fMRI studies could be affected by unnoticed short breath‐holds. We have also demonstrated that longer breath‐holds can result in more widespread activation. Results from our longest breath‐hold periods are consistent with previous reports of widespread signal change accompanying breath‐holds of 30 s or more observed at 1.5 tesla [Kastrup et al., 1998, 1999; Kwong et al., 1995; Li et al., 1999a]. We have shown, however, that activation patterns are significant with breath‐holds of much shorter length. We carried out several analyses of breath‐holding with differing models and found that an effect of breath‐hold persisted.
For our measures of signal change and activation area, the 6‐s extracts from longer breath‐hold studies correspond well with the shorter breath‐hold studies, confirming that change during the initial period of breath‐holding occurs irrespective of the subsequent duration of the breath‐hold. Our SPM99 analyses included a canonical haemodynamic response, and motion correction parameters were included as covariates of no interest. Significant activation was observed for 3‐ and 6‐s breath‐holds even when the model specified in SPM99 was a 30‐s task epoch (i.e., 5–10 times that of the breath‐hold itself). The effect for 3‐s breath‐holds was subtle; however, there were generally more activated than deactivated voxels, confirming the presence of an effect (Table I).
Areas Activated
Areas that we observed to be active in a 6‐s breath‐hold included the anterior and posterior cingulate (midline systems associated with attention mechanisms), the insula (implicated in autonomic control of functions associated with breathing and swallowing, involving the mouth, throat, salivation, and breathing), and the caudate regions (primarily associated with motor control, presumably here involved in control of respiration). Our observations are consistent with the notion that many initial changes are associated with neuronal activation related to performing the breath‐hold, followed by more widespread changes likely to be associated with blood gas changes (e.g., hypercapnia), vasodilation, and other physiologic responses such as the urge to resume breathing. There may also be a slight increase in sensitivity to signal changes due to a reduction in the signal variance during the breath‐hold procedure, because the normal physiologic noise due to respiration [Windischberger et al., 2002] is absent.
Discussion of Methods
Our breath‐holding task was carried out consistently in the end expiratory phase of the respiratory cycle to avoid marked changes in intrathoracic pressure that may have occurred with breath holding in full inspiration or forced expiration. Such changes may alter cardiac output and cerebral blood flow and further influence BOLD fMRI signal [Fox et al., 1966]. In practice, it is possible that subjects may hold their breath at other points in the respiratory cycle, resulting in different time‐courses of signal change [Kastrup et al., 1998; Li et al., 1999a].
Although our study was not designed to be an exhaustive survey of the BOLD response to breath‐hold, there is nonetheless an intriguing feature of the signal time‐course that is evident in our results: that the response varies considerably with spatial location in the brain. We observed different regions of activity with several quite different time‐course models. For example, the activation maps obtained when modelling as a 30‐s block convolved with an HRF (e.g., Fig. 2d,e) show us where in the brain the time‐course of the measured response significantly correlates with this particular model. The spatial dependence of the BOLD response to breath‐hold is an issue worthy of further study in its own right.
We did not investigate the possible differences in activation patterns with consciously compared to subconsciously initiated breath holding, and for breath‐holds that occur in conjunction with tasks of interest. In practice, a controlled experiment that included subconsciously initiated breath holding would be very difficult. Nonetheless, the large and widespread signal changes we observed in the breath‐holds of 12 s or more cannot be attributed solely to the neuronal processes involved in maintaining a conscious breath‐hold, and similar patterns are therefore expected in cases of subconscious breath‐hold. Our results may also be useful in determining whether fMRI studies may be contaminated by breath holding. Since carrying out this analysis, we have identified similar patterns of “activation” in several patients undertaking fMRI language studies for clinical purposes. In these cases, the breath‐holding pattern has been recognised and the patients have been counselled and restudied, with typical language activation patterns subsequently observed. This reinforces our belief that breath holding may be a significant confound in fMRI studies.
There are many factors that may influence the confounding effects of breath holding. The length of breath‐hold and the task are important. For example, there is less likely to be a systematic change between task and rest if breath holding occurs during a short‐interstimulus, randomly ordered, event‐related study because a breath‐hold would likely extend across both task and rest events. There may still be a deleterious effect, however, such as increased variance that reduces the chance of observing the real effect of interest. The nature and seriousness of the confound are likely to be dependent on the particular paradigm used and the analysis strategy employed.
Breath holding may be more likely for difficult tasks or for subjects where, due to a clinical condition or their young age, the task to be carried out is a considerable effort. The effects of very short breath‐holds may also be more visible on high‐field MRI systems due to their increased signal‐to‐noise ratio, such as the 3T system used for this study. Normalisation of within‐brain intensities (e.g., proportional scaling) in conjunction with a breath‐hold may exacerbate the confounding effects of the breath‐hold. The net result may be a global reduction in the activation signal from the real task of interest, due to the intensity normalisation adjusting for widespread signal increases resulting from the breath‐hold.
CONCLUSIONS
We have demonstrated the potential for confound should a subject carry out brief breath‐holds during an fMRI study. The fact that breath‐hold effects have not been mentioned as confounds in published fMRI experiments to date may be due to the effects being attributed to subject motion. We suggest investigators look for signs of breath holding during prescan training of tasks, and consider instructing subjects to relax and breathe normally throughout their study. Debriefing after fMRI investigations should routinely include a question about breath holding. Furthermore, in certain experiments it may be wise to monitor breathing during the scanning session.
REFERENCES
- Abbott D, Jackson G (2001): iBrain–software for analysis and visualisation of functional MR images. Neuroimage 13(Suppl.): 59. [Google Scholar]
- Fox IJ, Crowley WP Jr, Grace JB, Wood EH (1966): Effects of the Valsalva maneuver on blood flow in the thoracic aorta in man. J Appl Physiol 21: 1553–1560. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC (1997): Human brain function. San Diego: Academic Press; 528 p. [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R (1998): Event‐related fMRI: characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Glover GH, Neumann‐Haefelin T, Moseley ME (1999): Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage 10: 675–681. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Li TQ, Takahashi A, Glover GH, Moseley ME (1998): Functional magnetic resonance imaging of regional cerebral blood oxygenation changes during breath holding. Stroke 29: 2641–2645. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Wanke I, Donahue KM, Davis TL, Rosen BR (1995): EPI imaging of global increase of brain MR signal with breath‐hold preceded by breathing O2. Magn Reson Med 33: 448–452. [DOI] [PubMed] [Google Scholar]
- Li TQ, Kastrup A, Takahashi AM, Moseley ME (1999a): Functional MRI of human brain during breath holding by BOLD and FAIR techniques. Neuroimage 9: 243–249. [DOI] [PubMed] [Google Scholar]
- Li TQ, Moseley ME, Glover G (1999b): A FAIR study of motor cortex activation under normo‐ and hypercapnia induced by breath challenge. Neuroimage 10: 562–569. [DOI] [PubMed] [Google Scholar]
- Liu HL, Huang JC, Wu CT, Hsu YY (2002): Detectability of blood oxygenation level‐dependent signal changes during short breath hold duration. Magn Reson Imaging 20: 643–648. [DOI] [PubMed] [Google Scholar]
- MacIntosh BJ, Klassen LM, Menon RS (2003): Transient hemodynamics during a breath hold challenge in a two part functional imaging study with simultaneous near‐infrared spectroscopy in adult humans. Neuroimage 20: 1246–1252. [DOI] [PubMed] [Google Scholar]
- Markus HS, Harrison MJ (1992): Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath‐holding as the vasodilatory stimulus. Stroke 23: 668–673. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW (1990): Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse SA, Berry RB, Nguyen TK, Light RW, Mahutte CK (1996): Arterial blood gas changes during breath‐holding from functional residual capacity. Chest 110: 958–964. [DOI] [PubMed] [Google Scholar]
- Stock MC, Downs JB, McDonald JS, Silver MJ, McSweeney TD, Fairley DS (1988): The carbon dioxide rate of rise in awake apneic humans. J Clin Anesth 1: 96–103. [DOI] [PubMed] [Google Scholar]
- Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager‐Mayerl G, Schmetterer L, Moser E (2002): On the origin of respiratory artifacts in BOLD‐EPI of the human brain. Magn Reson Imaging 20: 575–582. [DOI] [PubMed] [Google Scholar]


