Abstract
Motor practice induces plastic changes within the cortical motor system. Whereas rapidly evolving changes of cortical motor representations were the subject of a number of recent studies, effects of long‐term practice on the motor system are so far poorly understood. In the present study pianists and nonmusicians were investigated using functional magnetic resonance imaging. Both groups performed simple and complex movement sequences on a keyboard with the right hand, the tasks requiring different levels of ordinal complexity. The aim of this study was to characterize motor representations related to sequence complexity and to long‐term motor practice. In nonmusicians, complex motor sequences showed higher fMRI activations of the presupplementary motor area (pre‐SMA) and the rostral part of the dorsal premotor cortex (PMd) compared to simple motor sequences, whereas musicians showed no differential activations. These results may reflect the higher level of visuomotor integration required in the complex task in nonmusicians, whereas in musicians this rostral premotor network was employed during both tasks. Comparison of subject groups revealed increased activation of a more caudal premotor network in nonmusicians comprising the caudal part of the PMd and the supplementary motor area. This supports recent results suggesting a specialization within PMd. Furthermore, we conclude that plasticity due to long‐term practice mainly occurs in caudal motor areas directly related to motor execution. The slowly evolving changes in M1 during motor skill learning may extend to adjacent areas, leading to more effective motor representations in pianists. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: motor cortex, fMRI, motor skill, premotor cortex, musician
INTRODUCTION
Sightreading and music performance requires processing very complex visuomotor transformation, motor sequences, and motor skills [Palmer, 1997]. Previous studies using functional imaging revealed that an occipito‐parieto‐frontal network is mainly involved in the process of sightreading and music performance [Sergent, 1992]. Professional musicians are highly trained in these domains. As a result, learning new complex motor sequences for them is easier than in nonmusicians and requires a smaller cortical network [Hund‐Georgiadis and von Cramon, 1999]. This finding is in line with recent results which support the view that changes to the cortical auditory and the visuomotor system in musicians provide a model of long‐term plasticity [Münte et al., 2002].
The present study focused on differential activation patterns of the motor system during execution of previously trained simple and complex movement sequences on a piano keyboard. In a previous pilot study, the extent of activation in the primary and secondary motor and parietal areas was investigated, revealing a significantly smaller cortical network for an overtrained complex movement sequence in professional piano players [Krings et al., 2000].
Studies in monkeys and in humans using functional imaging indicate that there is a rostrocaudal gradient in the cortical motor system concerning movement complexity and degree of practice [Geyer et al., 2000]. However, the whole system of primary and secondary cortical motor areas is activated in simple finger movements [Kollias et al., 2001] as well, suggesting that an increasing task complexity mainly leads to a higher degree of activation in cortical motor areas.
The aim of the present study was to compare the cerebral activation in musicians and nonmusicians who had to perform previously trained simple and complex motor sequences.
SUBJECTS AND METHODS
Subjects
Twelve students of the Cologne School of Music (musicians: 10 female, two male; mean age 26.6 years) and 12 subjects without any experience in music performance (nonmusicians: seven female, five male; mean age 25.4 years) were investigated. All subjects were right‐handed according to the Edinburgh Handedness Inventory. All musicians had piano as their principal instrument; on average, they played piano for 18.4 years and practiced weekly for 22 hours. Their musical training had started on average at the age of eight. Written informed consent was obtained from all subjects in accordance with the guidelines of the local ethics committee.
Task
Subjects were asked to perform two different movement sequences on a keyboard, a simple and a complex sequence, which were presented in musical notation on a computer screen. Before scanning, the nonmusicians learned the simple musical notation of the sequences until they were familiar with it. The simple task consisted of pressing sequential keys beginning with the thumb on different keys (1‐2‐3‐4‐5). During the complex task, subjects had to omit varying fingers of the sequential movement and to start each sequence with a different key (2‐3‐4‐5, 1‐3‐4‐5, etc.), thus demanding a higher level of ordinal complexity with equal interval properties as the simple task.
The keyboard was a commercially available electronic keyboard synthesizer which was stripped of all electronic components to make it suitable for MRI. Playing the keyboard did not, therefore, elicit any sound. Before MRI scanning, the subject had to play the sequences until their performance no longer improved. In the MRI scanner the subjects lay in a prone position with the keyboard placed on their lap. The notes of the sequences were presented via a mirror placed in front of the subjects head. As baseline, the note “a” was presented as a crotchet in musical notation as many times as the total number of notes in the simple and complex task. The subjects were instructed to read the notes of the baseline condition similar to the movement sequences, but not to play it.
There were two experimental conditions during “on” periods of an epoch paradigm, alternated by the baseline condition (“off” periods). The baseline condition during the “off” period consisted of reading the notes of the baseline paradigm without moving the hand or imagining music. The performance of the movement sequences and the lack of hand movement during “off” periods was controlled by monitoring the hand movements of the subjects with a videocamera during the fMRI session. The videotapes were used for analysis of regularity (number of errors) and speed (number of keystrokes during the on periods), which were compared between subject groups using Student's t‐test.
fMRI Procedure
The cerebral activation was studied with fMRI employing the blood oxygen level‐dependent (BOLD) contrast on a 1.5 T Philips Gyroscan (Philips, Best, The Netherlands) scanner in a standard headcoil. An epoch design was used with two sessions with three each active conditions (simple or complex task) and three baseline conditions (reading the note “a”). The fMRI sessions comprised four dummy scans followed by 72 whole‐brain scans using single‐shot gradient‐refocused echo‐planar imaging (EPI) (TR = 3.587 s, TE = 50 ms, flip angle = 90°, slice thickness = 5 mm, 22 slices). Thus, the duration of each epoch was 43 s.
Data Analysis
The fMRI data were analyzed using Statistical Parametric Mapping software (SPM99, online at http://www.fil.ion.ac.uk/spm). The dummy scans were discarded. The remaining scans were realigned and spatially normalized to the standard stereotactic space using the EPI‐template of the Montreal Neurological Institute (MNI). The voxel size was 3 × 3 × 3 mm. Subsequently, the normalized data were smoothed using a Gaussian kernel = 9 × 9 × 9 mm in order to improve the signal‐to‐noise ratio. Hemodynamic fluctuation was then estimated using an appropriate design matrix with a boxcar function as reference waveform. The voxel‐by‐voxel parameter estimation for the smoothed data was carried out according to the general linear model. The resulting set of voxel values constituted a map of t‐statistic values (SPM(t)‐map). In order to take interindividual variation into account, a random effect model was applied [Friston et al., 1999] comparing the raw data of the subjects with a one‐sample t‐test (P = 0.001). Furthermore, the statistical values of the two subject groups were compared using the two‐sample t‐test and a two‐way ANOVA. The resulting activations were corrected within boxes of 20 × 20 × 20mm around voxels found to be active in a pilot study [Krings et al., 2000]. The resulting SPM(t)‐maps were transformed into Talairach space [Talairach and Tournoux, 1988].
RESULTS
Behavioral Data
Analysis of the videotapes recorded during the fMRI session revealed that all subjects performed the music piece correctly and did not move their hands during the “off” section of the epoch paradigm. Analysis of the videotapes revealed that there were no significant differences regarding speed or regularity between the tasks or the groups using Student's t‐test. The nonmusicians on average played 67.4 ± 3.4 (SE) notes per epoch during the simple task and 64.9 ± 3.2 notes during the complex task. The musicians played 72.4 ± 3 (simple task) and 69.8 ± 3.6 notes (complex task).
fMRI Data
In the analysis of the fMRI data a predominantly frontoparietal cortical network was found to be active during execution of both simple and complex motor tasks (Fig. 1). This network included the primary sensorimotor cortex in the left hemisphere and the premotor cortex bilaterally. In the parietal cortex, secondary sensory areas in the left hemisphere were activated. In addition, a bilateral activation of the precuneus (Brodmann area (BA) 7) and the medial part of BA 40 was observed. There was a small activated cluster in the left occipital region (BA 37) (Tables 1 and 2). Furthermore, there was bilateral activation of cerebellar areas, with a large cluster in the right hemisphere, and of the left thalamus.
Figure 1.
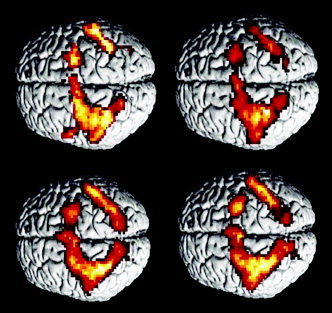
Overview on fMRI activations. Above left, musicians performing the simple motor sequence; right, musicians performing the complex movement sequence. The bottom pictures show activations for nonmusicians during execution of simple (left) and complex (right) movement sequences. For all conditions essentially the same bilateral parieto‐frontal network was found.
Table I.
fMRI activitations in non‐musicians for the simple and complex task, and comparison between the tasks
| Lobe | Brodmann area | Coordinates | t | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Non‐musicians, simple | |||||
| L frontal | 4 | −36 | −20 | 56 | 15.45 |
| 6 | −30 | −6 | 56 | 10.16 | |
| 6 | −27 | 0 | 55 | 9.57 | |
| 6 | −6 | −9 | 47 | 11.28 | |
| R frontal | 6 | 24 | −6 | 61 | 9.91 |
| L parietal | 2 | −33 | −35 | 60 | 15.78 |
| 3 | −33 | −32 | 51 | 14.34 | |
| 19 Prec | −24 | −71 | 34 | 11.75 | |
| 7 Prec | −24 | −56 | 50 | 12.76 | |
| 7 SPL | −30 | −47 | 58 | 14.4 | |
| 5 IPL | −33 | −44 | 55 | 12.49 | |
| 40 IPL | −33 | −42 | 38 | 4.72 | |
| R parietal | 7 SPL | 33 | −47 | 49 | 7.6 |
| 7 Prec | 21 | −60 | 42 | 5.24 | |
| 40 IPL | 36 | −42 | 44 | 5.99 | |
| Non‐musicians, complex | |||||
| L frontal | 4 | −39 | −18 | 53 | 14.98 |
| 6 | −27 | −3 | 53 | 11.76 | |
| 6 | −9 | −3 | 53 | 8.71 | |
| 32 | 0 | 8 | 47 | 9.21 | |
| R frontal | 6 | 24 | −3 | 64 | 6.74 |
| L parietal | 3 | −36 | −32 | 57 | 14.16 |
| 2 | −33 | −38 | 63 | 11.9 | |
| 40 IPL | −39 | −38 | 54 | 13.78 | |
| 7 SPL | −30 | −50 | 58 | 23.1 | |
| 7 Prec | −9 | −58 | 55 | 12.83 | |
| 19 Prec | −24 | −71 | 34 | 5.11 | |
| R parietal | 7 SPL | 15 | −58 | 61 | 10.16 |
| 7 Prec | 12 | −70 | 50 | 4.84 | |
| 40 IPL | 36 | −41 | 49 | 6.39 | |
| Non‐musicians, complex–simple | |||||
| L frontal | 6 | −27 | 8 | 52 | 5.28 |
| 6 | −3 | 6 | 60 | 6.8 | |
| R frontal | 6 | 3 | 6 | 60 | 6.6 |
Prec, precuneus; SPL, superior parietal lobe; IPL, inferior parietal lobe.
Table II.
fMRI activitations in musicians for the simple and complex task
| Lobe | Brodmann area | Coordinates | t | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Musicians, simple | |||||
| L frontal | 4 | −36 | −23 | 59 | 9.96 |
| 6 | −21 | −6 | 61 | 11.35 | |
| 6 | −6 | −3 | 55 | 11.26 | |
| R frontal | 6 | 24 | 3 | 61 | 5.27 |
| 6 | 3 | 0 | 61 | 8.75 | |
| L parietal | 2 | −51 | −27 | 48 | 7.59 |
| 3 | −30 | −33 | 49 | 14.76 | |
| 7 SPL | −24 | −52 | 58 | 8.1 | |
| 7 Prec | −15 | −58 | 53 | 10.48 | |
| 40 | −30 | −35 | 57 | 19.63 | |
| 5 | −21 | −43 | 66 | 4.53 | |
| R parietal | 40 | 39 | −51 | 51 | 9.43 |
| 7 SPL | 21 | −61 | 56 | 7.49 | |
| 7 Prec | 12 | −65 | 50 | 4.63 | |
| Limbic lobe | 24 | −9 | 2 | 41 | 4.64 |
| Musicians, complex | |||||
| L frontal | 4 | −36 | −4 | 42 | 5.84 |
| 6 | −18 | −3 | 61 | 12.15 | |
| R frontal | 6 | 6 | −3 | 58 | 7.2 |
| 6 | 27 | −4 | 53 | 9.36 | |
| 32 | 6 | 11 | 44 | 5.74 | |
| L parietal | 2 | −36 | −26 | 40 | 7 |
| 3 | −39 | −26 | 54 | 6.87 | |
| 5 | −30 | −41 | 63 | 4.68 | |
| 7 SPL | −25 | −53 | 47 | 6.53 | |
| 40 IPL | −39 | −47 | 44 | 5.92 | |
| R parietal | 40 IPL | 39 | −47 | 52 | 7.33 |
| 7 | 15 | −49 | 61 | 5.04 | |
| R angular | 39 | 36 | −57 | 33 | 6.68 |
The statistical comparison of simple and complex sequences did not reveal any significant activation.
Prec, precuneus; SPL, superior parietal lobe; IPL, inferior parietal lobe.
Comparison of cortical activation in nonmusicians revealed a significantly greater activation of the left dorsal premotor area (PMd) and bilateral presupplementary motor area (pre‐SMA) during the complex motor task compared to the simple motor task (Fig. 2). In the reverse contrast, no significant activation was found. In musicians, there was no differential activation for complex and simple motor tasks.
Figure 2.
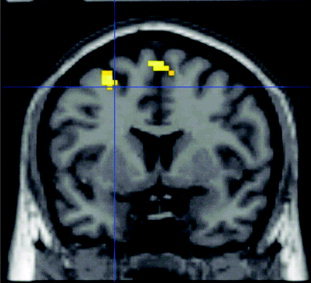
The two regions that showed increased fMRI activation in nonmusicians for performance of the complex vs. the simple movement sequence. On the lateral convexity, the rostral part of the dorsal premotor cortex, on the medial wall, the pre‐SMA are related to movement complexity. The same contrast in musicians showed no differential activation.
Statistical comparison between subject groups revealed a significantly higher activation of the PMd and the supplementary motor area (SMA) of the left hemisphere in nonmusicians compared to musicians for both simple and complex motor task (Fig. 3 and Table 3). The reverse contrast (musicians vs. nonmusicians) did not reveal any significant activations for either task.
Figure 3.
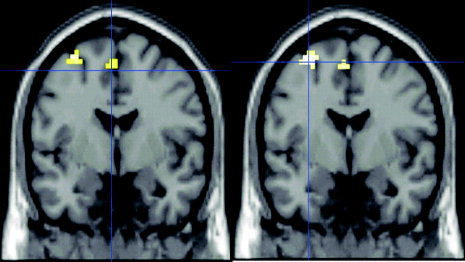
Areas with stronger activation for simple (left) and complex (right) movement sequences in nonmusicians compared to musicians. Note that these areas (caudal part of PMd and SMA) are located posterior to the regions related to task complexity in nonmusicians (y = −3 in this figure vs. y = 6 in Fig. 2). This suggests that changes of cortical motor representations due to long‐term practice mainly occur within caudal motor areas directly related to execution. A possible greater anticipatory behavior and a higher level of abstract motor planning in musicians was not mirrored by changes in fMRI activity in our study.
Table III.
fMRI activations for the two‐sample t‐test between musicians and non‐musicians for both tasks
| Lobe | Brodmann area | Coordinates | t | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Non‐musicians vs. musicians, simple | |||||
| L frontal | 6 | −33 | 0 | 58 | 4.33 |
| 6 | −3 | −3 | 55 | 4.72 | |
| Non‐musicians vs. musicians, complex | |||||
| L frontal | 6 | −30 | 0 | 55 | 4.44 |
| 6 | −6 | −3 | 55 | 4.43 | |
Note that the activated areas are essentially the same, independent of task complexity.
An interesting observation was made regarding the exact localization of the PMd activations of the two complex fMRI contrasts above: The fMRI activity in PMd related to increased task complexity in nonmusicians was located entirely rostral to the VCA line. In contrast, the increased fMRI activity in nonmusicians compared to musicians for both the simple and the complex task was found entirely caudal to the VCA line. The finding that the main factors task and group led to differential activations in separate regions is supported by the result that there was no significant task × group interaction in the two‐way ANOVA. This result may correspond to changes of neuronal activation within different parts of the lateral premotor cortex, the rostral dorsal premotor cortex (PMdr), and the caudal premotor cortex (PMdc). These two areas are assumed to represent distinct parts of the cortical motor system [Rizzolatti et al., 1998; Geyer et al., 2000] and recent fMRI studies have found differential activation patterns within these regions [Simon et al., 2002; Boussaoud, 2001].
DISCUSSION
In the present study the effect of task complexity and motor skills were investigated in musicians and nonmusicians. The results demonstrate an involvement of the dorsal premotor area in experience‐related changes of the motor system; the activation of this area is also related to increasing complexity of motor tasks in subjects lacking long‐term motor training. However, we noted a functional distinction within the PMdr: whereas the rostral part of the PMd was related to task complexity, the caudal part of this area was related to long‐term motor practice. On the medial wall, an effect of long‐term practice was found in the SMA proper, whereas the activation of the pre‐SMA was related to task complexity.
Motor Areas Related to Task Complexity
Having shown fMRI activation increases that were related both to task complexity and motor practice, the PMd appears to play a key role in the organization of movement sequences. Furthermore, the present results suggest a functional segregation within PMd which is supported by research in monkeys on the organization of the motor system and by recent fMRI findings [Fujii et al., 2000; Boussaoud et al., 2001; Simon et al., 2002].
In the present study the complex task and the simple task differed in ordinal properties: for every sequence there was a different finger omitted within the sequence of tapping the keyboard with the right hand. Thus, the difference in complexity between the two motor tasks is mainly related to attentional and motor planning demands, which is in line with the characteristics of the regions engaged more strongly in the complex task in nonmusicians. In musicians, no differential fMRI activity was found when comparing performance of complex and simple sequences. This possibly reflects the reorganization of the motor system in this group, allowing a higher level of complexity in movement planning without recruiting of additional neuronal resources. This is supported by behavioral data which indicate that long‐term musical practice leads to increased anticipatory behavior and a greater range of motor planning [Drake and Palmer, 2000].
Whereas in musicians no differential fMRI activation was noted for the simple and the complex motor task, in nonmusicians the activation of the rostral part of the PMdr was related to task complexity. In primates, the rostrocaudal gradient within PMd is well characterized: PMdr is mainly connected to area MIP integrating somatosensory and visual stimuli [Wise et al., 1997] without clear somatotopy. There are recent studies indicating that there is a similar gradient within PMd in the human brain, as well. The rostral part of PMd seems to be involved in coordination of oculomotor and limb motor behavior [Fujii et al., 2000] and mediate a multisensory representation of limb position [Lloyd et al., 2003]. It has been suggested that in humans PMdr is related to movement coding through a set of extrinsic kinematic attributes [Rijntjes et al., 1999]. In contrast to the caudal part of PMd, PMDr is connected to prefrontal cortex, mediating orientation of attention and maintaining of visuospatial information for action [Simon et al., 2002; Boussaoud, 2001].
The second region related to task complexity was the part of the medial BA 6 rostral to the VCA line which denotes the border of the supplementary motor area. This area, F6 in macaque monkey and pre‐SMA in humans, is assumed to play a key role in integration of external stimuli during motor planning [Akkal et al., 2002; Shima et al., 1996]. It was shown that activity in pre‐SMA precedes SMA and M1 activity [Ball et al., 1999] and is related to motor preparation but not to motor execution [Lee et al., 1999]. In the present study the activity of pre‐SMA may reflect the different levels of complexity of the numerical order among sequence components [Clower and Alexander, 1998].
Interpretation of our data in the context of a recently proposed model of motor control [Sakai et al., 1998, 1999, 2000] assigns the increased attentional and abstract motor planning level of the complex motor sequences to the PMdr region, whereas the pre‐SMA activation reflects the increased demands of visuomotor association.
Motor Areas Related to Long‐Term Practice
In contrast to the areas related to task complexity, the two regions engaged more strongly in nonmusicians than in musicians were found more caudally. The caudal part of the dorsal premotor cortex (PMdc) on the lateral convexity, and the supplementary motor area (SMA proper) on the medial wall were related to long‐term motor practice. The activation in these areas showed no difference regarding task complexity. This is in line with previous findings [Sadato et al., 1996] suggesting that the main difference in complexity between the tasks consisted in different levels of attention, planning, and visuomotor association with a constant level of activity in more caudal areas mediating motor execution.
The increased fMRI activity of the supplementary motor area in nonmusicians compared to musicians is in line with previous results on motor learning [Hund‐Georgiadis and von Cramon, 1999]. This region is organized somatotopically [Indovina and Sanes, 2001; Mayer et al., 2001] and is mainly involved in execution of movements [Lee et al., 1999], but also plays a role in motor planning [Tanji, 1996]. Its activation during performance of movement sequences reflects mainly processing of ordinal properties [Schubotz and von Cramon, 2001; Ullen et al., 2003], which corresponds to the demands of the task used in the present study.
In addition to SMA, the caudal part of PMd was related to long‐term practice both during execution of the simple and the complex motor task. Like SMA, this area is densely connected with M1 [Geyer et al., 2000]. In monkeys, the distinct connections of PMdr and PMdc are well characterized [Wise et al., 1997]: whereas the rostral part of PMd is much more strongly connected to the frontal cortex than the caudal portion of PMd, the latter, in contrast to the former, projects directly to the spinal cord. Input to PMdc is mainly somatosensory, whereas PMdr also receives visual information from the parieto‐occipital visual area. Our results, in line with recent findings from other studies [Boussaoud, 2001; Simon et al., 2002], support the concept of an analogous functional segregation within PMd in humans, with the more rostral part attributed mainly to attention and the more caudal PMdc related to coding of limb movement parameters. Both SMA and PMdc are adjacent to M1. Together with M1, these areas directly contribute to motor execution. A study investigating changes in motor system activity during visuomotor adaptation in monkeys [Wise et al., 1998] noted that changes in neuronal activity in PMdc and M1 concurred.
The results of the present study suggest that changes in the motor system due to long‐term practice mainly involve caudal motor regions directly related to execution. M1 did not show effects related to long‐term practice which is likely to be caused by the different performance speed for both tasks in the two subject groups. In a pilot study from our group [Krings et al., 2000], M1 was recruited to a lesser extent in musicians as well. Since fMRI activity in M1 increases roughly linearly with tapping frequency [Kastrup et al., 2002], we speculate that the higher performance speed of the musicians lead to equal levels of neuronal activity in M1 like in nonmusicians, though neuronal motor representation in M1 is more effective in pianists as well. The decreased fMRI activity within the motor systems in musicians appears to be the result of a slowly evolving reorganization of the motor system due to practice. There are different mechanisms of motor learning [Ungerleider et al., 2002], comprising rapid changes [Classen et al., 1998] followed by a reorganization over the course of weeks [Karni et al., 1995]. These mechanisms lead to a larger representation of the cortical motor hand area; in contrast, the results of the present study in concurrence with previous findings [Krings et al., 2000; Jaencke et al., 2000] indicate that long‐term practice induces a smaller area of fMRI activation for a given task within motor areas directly related to execution. Similar mechanisms were found for conditional motor learning [Deiber et al., 1997]. A recent study in monkeys examined SMA neurons during learning of a visuomotor task and found short‐term modulations of activity possibly due to priming and more slowly evolving changes in firing pattern due to practice [Lee and Quessy, 2003]. It seems that both mechanisms lead to changes on the synaptic level. Possibly there is a mutual dependence between the anatomical extension of a given motor representation and the utilization of networks subserved by the anatomical structures. The results obtained in musicians suggest that decreased network utilization in professional piano players is associated with extended motor representation [Amunts et al., 1997]. This is supported by a recent morphometric study which found that the gray matter volume in premotor areas among amateur and professional musicians and nonmusicians correlates positively with musician status [Gaser and Schlaug, 2003].
One potential bias of this study is the fact that the gender ratio between the groups is different. A morphometric study [Amunts et al., 2000] investigated asymmetries within the motor cortex related to handedness and gender reporting stronger asymmetries in men than in women. However, in this study only the depth of the central sulcus was studied. It is unknown if there are gender‐specific differences within the premotor region.
In conclusion, we have shown that in execution of movement sequences, task complexity is related to more rostral areas of the premotor cortex, whereas effects of long‐term practice tend to occur within the caudal part of the motor system. This possibly reflects the properties of the underlying mechanisms of cortical reorganization due to motor skill learning which are mainly related to motor execution, whereas attention and abstract planning possibly do not exhibit the same potential of long‐term reorganization.
Acknowledgements
We thank Dr. Stuart Fellows and two anonymous reviewers for valuable comments on the manuscript.
REFERENCES
- Akkal D, Bioulac B, Audin J, Burbaud P (2002): Comparison of neuronal activity in the rostral supplementary and cingulate motor areas during a task with cognitive and motor demands. Eur J Neurosci 15: 887–904. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K (1997): Motor cortex and hand motor skills: structural compliance in the human brain. Hum Brain Mapp 5: 206–215. [DOI] [PubMed] [Google Scholar]
- Amunts K, Jancke L, Mohlberg H, Steinmetz H, Zilles K (2000): Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38: 304–312. [DOI] [PubMed] [Google Scholar]
- Ball T, Schreiber A, Feige B, Wagner M, Lucking CH, Kristeva‐Feige R (1999): The role of higher‐order motor areas in voluntary movement as revealed by high‐resolution EEG and fMRI. Neuroimage 10: 682–694. [DOI] [PubMed] [Google Scholar]
- Boussaoud D (2001): Attention versus intention in the primate premotor cortex. Neuroimage 14: S40–S45. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998): Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79: 1117–1123. [DOI] [PubMed] [Google Scholar]
- Clower WT, Alexander GE (1998): Movement sequence‐related activity reflecting numerical order of components in supplementary and presupplementary motor areas. J Neurophysiol 80: 1562–1566. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M (1997): Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol 78: 977–991. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M (1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81: 3065–3077. [DOI] [PubMed] [Google Scholar]
- Drake C, Palmer C (2000): Skill acquisition in music performance: relations between planning and temporal control. Cognition 74: 1–32. [DOI] [PubMed] [Google Scholar]
- Fujii N, Mushiake H, Tanji J (2000): Rostrocaudal distinction of the dorsal premotor area based on oculomotor involvement. J Neurophysiol 83: 1764–1769. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23: 9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Matelli M, Luppino G, Zilles K (2000): Functional neuroanatomy of the primate isocortical motor system. Anat Embryol (Berl) 202: 443–474. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Rao SM, Haaland KY, Bobholz JA, Mayer AR, Binder JR, Cox RW (2000): Specialized neural systems underlying representations of sequential movements. J Cogn Neurosci 12: 56–77. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J (2000): Integration of target and body‐part information in the premotor cortex when planning action. Nature 408: 466–470. [DOI] [PubMed] [Google Scholar]
- Hund‐Georgiadis M, von Cramon DY (1999): Motor‐learning‐related changes in piano players and non‐musicians revealed by functional magnetic‐resonance signals. Exp Brain Res 125: 417–425. [DOI] [PubMed] [Google Scholar]
- Indovina I, Sanes JN (2001): On somatotopic representation centers for finger movements in human primary motor cortex and supplementary motor area. Neuroimage 13: 1027–1034. [DOI] [PubMed] [Google Scholar]
- Jaencke L, Shah NJ, Peters M (2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cogn Brain Res 10: 177–183. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Kruger G, Neumann‐Haefelin T, Glover GH, Moseley ME (2002): Changes of cerebral blood flow, oxygenation, and oxidative metabolism during graded motor activation. Neuroimage 15: 74–82. [DOI] [PubMed] [Google Scholar]
- Kollias SS, Alkadhi H, Jaermann T, Crelier G, Hepp‐Reymond MC (2001): Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res Brain Res Rev 36: 185–195. [DOI] [PubMed] [Google Scholar]
- Krings T, Topper R, Foltys H, Erberich S, Sparing R, Willmes K, Thron A (2000): Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett 278: 189–193. [DOI] [PubMed] [Google Scholar]
- Lee D, Quessy S (2003): Activity in the supplementary motor area related to learning and performance during a sequential visuomotor task. J Neurophysiol 89: 1039–1056. [DOI] [PubMed] [Google Scholar]
- Lee KM, Chang KH, Roh JK (1999): Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 9: 117–123. [DOI] [PubMed] [Google Scholar]
- Lloyd DM, Shore DI, Spence C, Calvert GA (2003): Multisensory representation of limb position in human premotor cortex. Nat Neurosci 6: 17–18. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Zimbelman JL, Watanabe Y, Rao SM (2001): Somatotopic organization of the medial wall of the cerebral hemispheres: a 3 Tesla fMRI study. Neuroreport 12: 3811–3814. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L (2002): The musicians brain as a model of neuroplasticity. Nat Rev Neurosci 3: 473–478. [DOI] [PubMed] [Google Scholar]
- Palmer C (1997): Music performance. Annu Rev Psychol 48: 115–138. [DOI] [PubMed] [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C (1999): A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci 19: 8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G (2001): The cortical motor system. Neuron 31: 889–901. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Sadato N, Campbell G, Ibanez V, Deiber M, Hallett M (1996): Complexity affects regional cerebral blood flow change during sequential finger movements. J Neurosci 16: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Putz B (1998): Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci 18: 1827–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B (1999): Presupplementary motor area activation during sequence learning reflects visuo‐motor association. J Neurosci 19: RC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Takino R, Miyauchi S, Nielsen M, Tamada T (2000): What and when: parallel and convergent processing in motor control. J Neurosci 20: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2001): Interval and ordinal properties are associated with distinct premotor areas. Cereb Cortex 11: 210–222. [DOI] [PubMed] [Google Scholar]
- Sergent J, Zuck E, Terriah S, MacDonald B (1992): Distributed neural network underlying musical sight‐reading and keyboard performance. Science 257: 106–109. [DOI] [PubMed] [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J (1996): Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci U S A 93: 8694–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D (2002): Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol 88: 2047–2057. [DOI] [PubMed] [Google Scholar]
- Tanji J (1996): New concepts of the supplementary motor area. Curr Opin Neurobiol 6: 782–787. [DOI] [PubMed] [Google Scholar]
- Ullen F, Forssberg H, Ehrsson HH (2003): Neural networks for the coordination of the hands in time. J Neurophysiol 89: 1126–1135. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A (2002): Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78: 553–564. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R (1997): Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42. [DOI] [PubMed] [Google Scholar]
- Wise SP, Moody SL, Blomstrom KJ, Mitz AR (1998): Changes in motor cortical activity during visuomotor adaptation. Exp Brain Res 121: 285–299. [DOI] [PubMed] [Google Scholar]


