Abstract
The neural systems sustaining object naming were examined using the activation likelihood estimation (ALE) meta‐analysis approach on the results of 16 previously published studies. The activation task in each study required subjects to name pictures of objects or animals, but the baseline tasks varied. Separate meta‐analyses were carried out on studies that used: (1) high‐level baselines to control for speech processing and visual input; and (2) low‐level baselines that did not control for speech or complex visual processing. The results of the two meta‐analyses were then compared directly, revealing a double dissociation in the activation pattern for studies using high and low baselines. To interpret the differential activations, we report two new functional imaging experiments. The aim of the first was to characterize activation differences associated with visual stimuli that are typically used in baseline conditions (complex visual features, simple structures, or fixation). The aim of the second was to classify object‐naming regions in terms of whether they were engaged preferentially by semantic or phonological processes. The results reveal a remarkably precise correspondence between the areas identified by the meta‐analyses as affected differentially by baseline and the areas that are affected differentially by non‐object structure, semantics or phonology. As expected, high‐level baselines reduced object‐naming activation in areas associated with the processing of complex visual features and speech production. In addition, high‐level baselines increased sensitivity to activation in areas associated with semantic processing, visual‐speech integration and response selection. For example, activation in the anterior temporal areas that neuropsychological studies have associated with semantic processing was more strongly activated in the context of high‐level baselines. These results therefore have implications for understanding the convergence of functional imaging and neuropsychological findings. Hum Brain Mapp 25:70–82, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: picture naming, object recognition, baselines, semantics, phonology, functional imaging, meta‐analysis
INTRODUCTION
The ability to name pictures of objects is used routinely as a test of language function in children and adults with neurological impairments. In children, object naming is one of the earliest milestones in linguistic development and object naming in preschool children reflects future reading ability [Swan and Goswami,1997]. Like reading, object naming is based on the production of the speech sounds associated with a visual stimulus. In cognitive terms, this requires the integration of perceptual, semantic, and phonological processes. Critically, disruption to any of these processes will result in object‐naming deficits. Object‐naming ability can therefore be used to assist in the diagnosis of a range of different cognitive syndromes such as agnosia (a perceptual deficit with relatively normal speech perception and production), anomia (a speech production deficit with relatively normal perception, semantics and speech fluency), and semantic dementia (a semantic impairment with relatively normal perception and speech fluency).
Given the important role that object naming plays in neuropsychological assessment, it is surprising that relatively few functional imaging studies have investigated the neural system that sustains it. Previous studies have compared object‐naming activation to reading [Bookheimer et al.,1995; Moore and Price,1999b], color naming [Price et al.,1996], verbal fluency [Etard et al.,2000], semantic categorization [Tyler et al.,2004], or a range of baselines [Murtha et al.,1999]. Other studies have investigated how the activation pattern changes for overt and covert naming [Zelkowicz et al.,1998], for object category [Chao et al.,1999,2002; Chao and Martin,2000; Damasio et al.,1996; Grabowski et al.,1998; Kawashima et al.,2001; Martin et al.,1996; Moore and Price,1999a; Smith et al.,2001], by scanning modality [Votaw et al.,1999], across languages [Vingerhoets et al.,2003], with name agreement [Kan and Thompson‐Schill,2004], with gender of subjects [Grabowski et al.,2003], and during object learning [van Turennout et al.,2000,2003]. To our knowledge, no previous study has segregated the object‐naming system into its perceptual, semantic, and phonological components. We believe this would be useful for understanding the different ways in which object naming can be disturbed.
The aim of our meta‐analyses was to identify the set of regions engaged by object naming and to categorize these areas in terms of how they were affected by the baseline task. We distinguish two types of baselines: (1) low‐level baselines that do not attempt to control for speech production or perceptual processes, thereby enabling all the object‐naming regions to be identified irrespective of whether these regions are associated with perceptual, semantic, or phonological processing; and (2) high‐level baselines that attempt to control for speech production and perceptual processes with the aim of identifying areas associated with object recognition and semantic associations. Object naming relative to high‐level baselines is therefore likely to reveal less activation in perceptual and speech production areas than would object naming relative to low‐level baselines.
To complement and validate the meta‐analyses, we also report the results of two new experiments. Experiment 1 identified areas involved in perceptual processing by comparing activation evoked by pictures of 3‐D meaningless non‐objects, 2‐D geometric shapes, and fixation. Experiment 2 identified areas involved in semantic and phonological processing by comparing activation to pictures of familiar objects when subjects were engaged in different tasks. Our prediction was that the meta‐analyses would reveal greater object‐naming activation when the baseline was low than it would when the baseline was high. Furthermore, these areas should correspond to those associated with perceptual processing in Experiment 1 and phonological processing in Experiment 2; however, it is also possible that high‐level baselines affect processes other than those engaged by perception and speech production. If so, the meta‐analyses may reveal areas where object‐naming activation was identified relative to high‐level baselines but not relative to low‐level baselines. For example, baselines that require subjects to say “OK” to meaningless visual stimuli may increase demands on working memory and executive task processes while decreasing attention to perceptual processing. Likewise, there may be unpredicted processing that occurs during low‐level baselines more than that during high‐level baselines [Binder et al.,1999]. Our comparison of high‐ and low‐level baselines might then reveal activation in unexpected regions. Such findings would provide further insights into the ideal paradigms for investigating the object‐naming system in neurologically normal and brain‐damaged patients.
MATERIALS AND METHODS
ALE Meta‐Analysis
Details of the ALE meta‐analysis are reported in Laird et al. [2005]. Data from 18 different contrasts were entered into the analyses (see Table I). All used positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) to identify object‐naming responses, over the whole brain, relative to either low (L), or high (H) level baselines (Table I). We report the main effect of all studies (L + H); and the effect of baseline (H vs. L). Several previous object‐naming studies were excluded from the meta‐analyses. The exclusion criteria were: no non‐object baseline [Chao et al.,1999,2002; Chao and Martin,2000]; results were only reported in regions of interest rather than for the whole brain [Grabowski et al.,1998; Grafton et al.,1997; Heim et al.,2002; Kan and Thompson‐Schill,2004]; the task was action rather than object naming [Damasio et al.,2001]; and the task was scene identification rather than object naming [Renvall et al.,2003].
Table I.
Object‐naming studies
| Reference | Scanner | Task | Stimulus category | Baseline stimulus | Baseline response |
|---|---|---|---|---|---|
| L Bookheimer et al.,1995 | PET | Overt | Animals and objects | Random lines | — |
| L Etard et al.,2000 | PET | Overt | Animals and objects | None (rest) | — |
| L Murtha et al.,1999 | PET | Overt | Animals only | Plus signs | — |
| L Martin et al.,1996 | PET | Covert | Animals and objects | Nonsense objects | — |
| L Smith et al.,2001 | fMRI | Covert | Animals and objects | Gray squares | — |
| L Vingerhoets et al.,2003 | fMRI | Covert | Animals and objects | Scrambled lines | — |
| L Tyler et al.,2004 | fMRI | Covert | Animals and objects | Fixation | — |
| L van Turennout et al.,2000 | fMRI | Covert | Not specified | Visual noise | — |
| L van Turennout et al.,2003 | fMRI | Covert | Animals and objects | Visual noise | — |
| H Damasio et al.,1996 | PET | Overt | Animals tools | Inverted faces | Say “up/down” |
| H Price et al.,1996 | PET | Overt | Objects only | Colored squares. | Say name color |
| H Zelkowicz et al.,1998 | PET | Overt | Not specified | Non‐objects | Say “hiya” |
| H Murtha et al.,1999 | PET | Overt | Animals only | Abstract pattern | Say “yes” |
| H Moore and Price,1999a | PET | Overt | Animals and objects | Non‐objects | Say “OK” |
| H Moore and Price,1999b | PET | Overt | Animals and objects | Non‐objects | Say “OK” |
| H Votaw et al.,1999 | PET | Overt | Animals only | Abstract figure | Say “large/small” |
| H Votaw et al.,1999 | fMRI | Covert | Animals only | Abstract figure | Say “large/small” |
| H Kawashima et al.,2001 | PET | Covert | Animals and objects | Digits | Read number |
A summary of the previously published functional imaging studies of object naming that were included in the meta‐analyses. The two subdivisions refer to object‐naming relative to low level baselines (L) and object‐naming relative to high (speaking) baselines (H).
Experiment 1: Perceptual Processing of Non‐objects
The aim of Experiment 1 was to identify areas that showed differential responses to the types of visual stimuli that are typically used in high‐level baselines. There were two stimulus conditions (Fig. 1): (1) photographs of 3‐D meaningless non‐objects; and (2) drawings of 2‐D geometric shapes. Each trial entailed the simultaneous presentation of four stimuli for 3 s followed by 500‐ms fixation before presentation of the next stimulus. As data were collected using PET, the conditions were blocked, with 22 different stimuli per block (77 s). The task was the same for both conditions, and involved a four‐choice button press to indicate the odd man out. Specifically, the first (index) finger was used to indicate that the odd man out was the stimulus on the left, the little finger was used to indicate that the odd man out was the stimulus on the right etc. For example, in Figure 1, the middle finger would make the correct response for both the non‐object and shape examples. The third condition was continuous fixation, with no additional stimuli.
Figure 1.
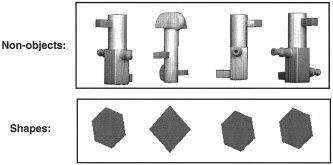
Examples of the stimuli used in Experiments 1.
In total, 12 subjects (11 males, 1 female; mean age, 44.5 years; age range, 24–65 years) participated in two PET scans for conditions 1 and 2 and one PET scan for condition 3. In other words, 60 scans were entered into the data analysis (see below). These data have not been reported previously.
Experiment 2: Semantic and Phonological Processing
The aim of Experiment 2 was to dissociate areas involved in the phonological and semantic processing of objects. There were three stimulus conditions, which each presented a series of outline drawings of common objects or animals at a rate of one per 3.5 s (500‐ms onset duration followed by 3,000‐ms fixation). The tasks for each of these conditions (Fig. 2) were as follows: (1) phonological (Does the object name have two syllables?); (2) semantic (Is the object a natural [living] item?); and (3) perceptual (Is there a line above or below the object?). The fourth condition was a baseline measurement (resting with eyes closed). The order of the four conditions was counterbalanced within and between subjects. Subjects were instructed to make a manual key press with their right index finger if the object met the task criteria (Fig. 2).
Figure 2.
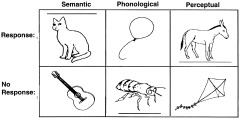
Examples of the stimuli used in Experiments 2.
In total, six subjects (five males, one female; mean age 39 years; age range, 20–68 years) participated in 12 different PET scans, 3 for each of 4 conditions (72 scans in total). There were 87 different stimuli in total, divided into 3 sets of 29 with 14 targets for each task within a set. Each set was repeated in each of the three task conditions to avoid stimulus confounds between conditions. The order of repetition of a stimulus set across tasks was counterbalanced across subjects, and the order of stimuli within a set was randomly ordered for each presentation to reduce expectation in the subjects.
PET Acquisition and Analysis for Experiments 1 and 2
Regional cerebral blood flow (rCBF) was measured using a CTI Siemens Ecat HR+ PET scanner. Standard procedures were adopted with the intravenous administration of 5 mSv of 15O‐labeled water at the constant rate of 10 ml/min. The activation task was started approximately 10 s before the onset of the 90‐s acquisition period. The protocol conformed to guidelines established by ARSAC UK and was approved by the joint Ethics Committee of the National Hospital for Neurological Diseases and the Institute of Neurology, London.
The PET data were analyzed using SPM99. Data were realigned to correct for head motion, spatially normalized to the Montreal Neurological Institute (MNI) template with 2 × 2 × 2 mm voxels and spatially smoothed (8 mm for Experiment 1; 10 mm for Experiment 2) to account for individual differences in functional anatomy and improve signal to noise. Statistical analysis involved ANCOVA with subject effects modeled and global activity included as a subject‐specific covariate. The condition and subject effects were estimated according to the general linear model at each voxel. To test hypotheses about regionally specific condition effects, the estimates were compared using linear contrasts. The resulting set of voxel values constitutes an SPM of the t statistic (SPMt). The SPMt values were transformed to the unit normal distribution (SPMZ).
For Experiment 1, our comparisons of interest were as follows: (1) 3‐D non‐objects versus fixation (to identify all visual processing areas); and (2) 3‐D non‐objects versus 2‐D shapes (to highlight the areas involved in complex structural analysis). For Experiment 2, our comparisons of interest were as follows: (1) phonological > semantic and perceptual and rest (to identify areas more activated by phonological decisions than by any other condition); and (2) semantic > phonological and perceptual and rest (to identify areas more activated by semantic decisions than by any other condition).
Our regions of interest, for both experiments, were the areas affected differentially by baseline in the meta‐analyses. In these regions of interest, effects are reported at P < 0.001 uncorrected.
RESULTS
Meta‐Analyses of Different Baselines During Object Naming
The meta‐analysis of all object‐naming studies, summed over high and low baselines, is illustrated in Figure 3. The effects of differing baselines are illustrated in Figure 4. Activations fell into three distinct classes: (1) unaffected by the baseline manipulation; (2) greater when the baseline was low level (i.e., did not involve speaking); and (3) greater when the baseline was high level (i.e., did involve speaking) (Table II). Critically, this highlights the double dissociation in the activation pattern for studies using high and low baselines. Twelve areas were more activated during object naming when the baseline was low relative to high. These were distributed primarily in ventral occipital, posterior temporal, inferior frontal, and cerebellar regions Nine areas were more activated during object naming when the baseline was high relative to low. These were identified in anterior temporal and dorsal occipital areas. To interpret this double dissociation, we consider the results of Experiments 1 and 2.
Figure 3.
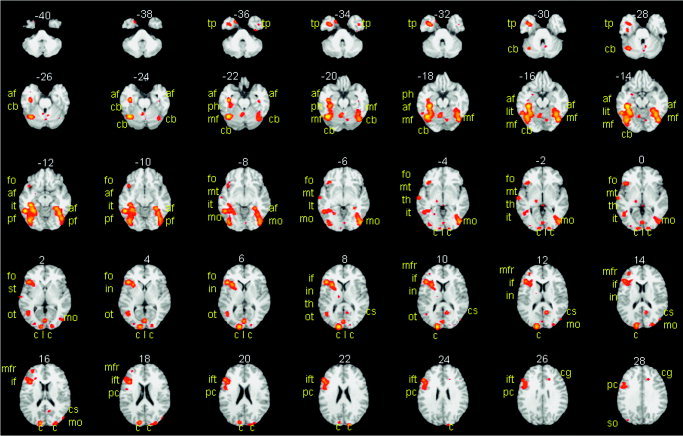
The results of the meta‐analysis of all studies. Activation when all 18 contrasts were entered into the meta‐analysis (see Table I) is highlighted in yellow and orange on axial slices of the brain from −40 mm below the AC–PC line to 28 mm above the AC–PC line (2‐mm increments). Activation more than 28 mm above the AC–PC line is not shown but was identified in the supplementary motor area (SMA) and dorsal aspect of the precentral gyrus irrespective of baseline. The slice number is indicated above each slice in white digits. The name of the activated brain area is indicated in yellow letters; af, anterior fusiform; mo, middle occipital; tp, temporal pole; ph, parahippocampal; cs, calcarine sulcus; dc, dorsal cuneus; ift, inferior frontal (triangularis); cb, cerebellum; it, inferior temporal.
Figure 4.
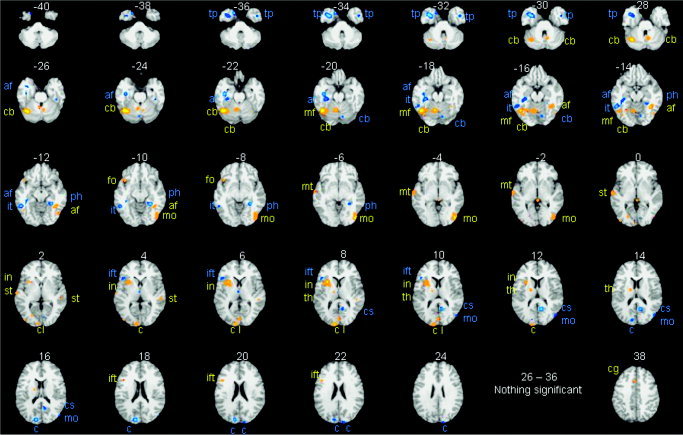
The results of the comparison of the meta‐analyses with high and low baselines. Object‐naming activation that was greater when the baseline was low relative to high is shown and labeled in yellow and orange. Greater activation when the baseline was high relative to low is shown and labeled in blue. Axial slices are as in Figure 3, with an additional effect 38 mm above the AC–PC line (see Table II for further details); af, anterior fusiform; mo, middle occipital; tp, temporal pole; ph, parahippocampal; cs, calcarine sulcus; dc, dorsal cuneus; ift, inferior frontal (triangularis); cb, cerebellum; it, inferior temporal.
Table II.
Object‐naming activation
| Label | Anatomical name | z slices | Side | Function/contrast | x | y | z | Z score |
|---|---|---|---|---|---|---|---|---|
| Irrespective of baseline a | ||||||||
| pf | Posterior fusiform | −12 to −10 | Left | Visual cortices | ||||
| Right | Visual cortices | |||||||
| mf | Middle fusiform | −20 to −8 | Right | Visual cortices | ||||
| ot | Occipitotemporal | +2 to +8 | Left | Visual cortices | ||||
| so | Superior occipital | 32 to 36 | Left | Visual cortices | ||||
| mfr | Middle frontal | 12 to 20 | Left | Response area | ||||
| pc | Precentral gyrus | 20 to 38 | Left | Response area | ||||
| sma | Supplementary motor area | 44 to 48 | Mid | Response area | ||||
| [Picture naming relative to low baseline] > [Picture naming relative to high baseline] b | ||||||||
| Meta‐analysis | Experiment 1 | |||||||
| mo | Inferior occipital | −10 to −2 | Right | Non > shapes | 44 | −78 | 0 | 6.2 |
| Non > shapes | 48 | −70 | −10 | 6.0 | ||||
| cu | Ventral cuneus (BA17) | 2 to 14 | Left | Non > fixation | −20 | −98 | 6 | 5.7 |
| l | Lingual gyrus | 6 to 12 | Left | Non > fixation | 0 | −94 | 12 | 4.6 |
| mf | Mid‐fusiform | −20 to −14 | Left | Non > fixation | −42 | −62 | −14 | 4.7 |
| Meta‐analysis | Experiment 2 | |||||||
| fo | Frontal operculum | −10 to −8 | Left | Ph > S & Pe & R | −32 | 18 | −8 | 4.7 |
| if | Inferior frontal | 20 to 26 | Left | Ph > S & Pe & R | −38 | 6 | 22 | 4.3 |
| cb | Cerebellum | −32 to −14 | Left | Ph > S & Pe & R | −42 | −68 | −26 | 4.1 |
| in | Insula | 2 to 14 | Left | Ph > S & Pe & R | −44 | 12 | 10 | 3.7 |
| th | Thalamus | 8 to 16 | Left | Ph > S & Pe & R | −4 | −18 | 8 | 4.1 |
| −12 | −8 | 10 | 3.4 | |||||
| cg | Cingulate gyrus | 38 | Left | Ph > S & Pe & R | 6 | 28 | 32 | 5.3 |
| mt | Middle temporal | −6 to 0 | Left | Auditory processing area | ||||
| st | Superior temporal | 2 to 6 | Bilateral | Auditory processing area | ||||
| [Picture naming relative to high baseline] > [picture naming relative to low baseline] c | ||||||||
| Meta‐analysis | Experiment 2 | |||||||
| af | Anterior fusiform | −26 to −12 | Left | S > Ph & Pe & R | −34 | −20 | −24 | 3.4 |
| S > Ph & Pe & R | −40 | −26 | −16 | 3.2 | ||||
| mo | Middle occipital | 12 to 18 | Right | S > Ph & Pe & R | 46 | −78 | 8 | 4.5 |
| [46 | −80 | 14 | 3.6] | |||||
| tp | Temporal pole | −38 to −28 | Left | S & R > Ph & Pe | −44 | 8 | −30 | 3.9 |
| Left | S & R > Ph & Pe | −48 | 10 | −20 | 3.8 | |||
| Right | S & R > Ph & Pe | 42 | 18 | −32 | 4.2 | |||
| ph | Parahippocampal | −14 to −6 | Right | S & R > Ph & Pe | 32 | −28 | −18 | 3.1 |
| cs | Calcarine sulcus | 8 to 16 | Right | S & R > Ph & Pe | 10 | −58 | 10 | 5.3 |
| c | Dorsal cuneus (BA18) | 14 to 24 | Right | S & Pe > Ph & R | 6 | −92 | 20 | 4.1 |
| Left | S & Pe > Ph & R | [−6 | −92 | 18 | 2.7] | |||
| ift | Inferior frontal (triangularis) | 4 to 12 | Left | S & Ph > Pe & R | −50 | 26 | 6 | 4.3 |
| cb | Cerebellum | −20 to −16 | Right | S & Ph > Pe & R | 16 | −80 | −22 | 4.3 |
| it | Inferior temporal | −18 to −8 | Left | S & Ph > Pe & R | [−56 | −52 | −18 | 3.6] |
Comparison of areas activated in meta‐analysis with results of Experiments 1 and 2. Column 1, abbreviations used to label the regions in Figures 3 and 4; column 2, full name of each area; column 3, location (in mm relative to the anterior–posterior commissure line) of axial slices where activation was observed in the meta‐analyses; column 4, other conditions that engage the area. Coordinates x, y, z refer to the location of the axial, coronal, and sagittal slice of peak activation according to the atlas of Talaraich and Tournoux [1988]. Non, 3‐D non‐objects; Shapes, 2‐D geometric shapes (Experiment 1). Bil, bilateral; Ph, phonological task; S, semantic task; Pe, perceptual task; R, resting with eyes closed (Experiment 2). Z scores of all effects reported from Experiments 1 and 2 were significant at P < 0.001 uncorrected (Z > 3.1) or P < 0.05 corrected (Z > 4.5). Coordinates and Z scores for the left dorsal cuneus and the left inferior temporal cortex (Table 2c) are in square brackets to indicate that they were part of a more extensive activation rather than the center of the activation.
Areas that were identified as activated in the meta‐analysis of all studies (A+B+C+D) but not in the comparison of different baselines (A+B vs. C+D).
Areas identified as activated during object naming when the baseline was low more than when it was high.
Areas identified as activated when the baseline was high more than when it was low.
Experiment 1: Visual Processing of 3‐D Non‐objects
Relative to fixation, 3‐D non‐objects activated bilateral mid‐fusiform, inferior and middle occipital lobe regions, the lingual gyrus, and cuneus. With the exception of the dorsal aspect of the cuneus, the same regions were activated by 3‐D non‐objects relative to 2‐D shapes (see Table III). Critically, activation for 3‐D non‐objects included 4 of 12 areas that the object‐naming meta‐analysis identified as more activated when the baseline was low relative to high (Table II, III; Fig. 5). As predicted, activation in these four areas for object naming relative to low‐level baselines thus can be explained in terms of differences in visual structure (complex visual processing is not as well controlled with low‐level baselines as it is with high‐level baselines).
Table III.
Results of Experiment 1
| Lobe activated/region | Non > fixation | Non > shapes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | Peak coordinates* | Z score | Peak coordinates** | Z score | |||||
| Temporal | |||||||||
| Mid‐fusiform | R | 44 | −68 | −18 | 5.3 | 44 | −68 | −18 | 5.8 |
| Mid‐fusiform | R | 36 | −58 | −18 | 5.3 | 38 | −58 | −14 | 4.1 |
| Mid‐fusiforma | L | −42 | −62 | −14 | 4.7 | −42 | −66 | −14 | 4.3 |
| Occipital | |||||||||
| Lingual | L | −10 | −88 | −12 | 7.1 | −10 | −92 | −10 | 4.8 |
| Lingual | L | −14 | −96 | −4 | 7.1 | −14 | −96 | −6 | 4.8 |
| Lingual | L | −8 | −86 | −2 | 7.1 | 0 | −80 | 2 | 3.7 |
| Lingual | R | 12 | −86 | −16 | 6.3 | 14 | −100 | −8 | 4.6 |
| Cuneus | L | −28 | −96 | 2 | 5.9 | −22 | −96 | 4 | 4.0 |
| Cuneusa | L | −20 | −98 | 6 | 5.9 | −16 | −84 | 10 | 3.5 |
| Cuneus | R | 10 | −92 | 2 | 6.7 | 12 | −94 | 0 | 4.5 |
| Cuneus | R | 20 | −96 | 4 | 5.9 | 24 | −92 | 2 | 4.4 |
| Cuneus | R | 16 | −92 | 6 | 6.2 | NS | NS | NS | NS |
| Cuneus | R | 20 | −88 | 10 | 6.2 | NS | NS | NS | NS |
| Cuneusa | 0 | −94 | 12 | 4.6 | NS | NS | NS | NS | |
| Inferior | L | −22 | −80 | −14 | 5.1 | −28 | −94 | −10 | 5.9 |
| Inferior | L | −34 | −84 | −12 | 6.5 | −34 | −86 | −10 | 5.9 |
| Inferior | L | −42 | −72 | −12 | 5.9 | −42 | −72 | −14 | 5.6 |
| Inferior | L | −40 | −86 | −2 | 5.6 | −40 | −80 | 4 | 5.5 |
| Inferior | L | −36 | −88 | 2 | 5.4 | −38 | −90 | 4 | 5.4 |
| Inferior | R | 36 | −86 | −22 | 5.0 | 32 | −92 | 0 | 5.6 |
| Inferior | R | 32 | −70 | −14 | 6.0 | 30 | −66 | −14 | 4.7 |
| Inferior | R | 28 | −78 | −14 | 6.3 | 28 | −86 | −14 | 5.3 |
| Inferior | R | 34 | −88 | −10 | 5.0 | 30 | −96 | −6 | 5.5 |
| Middlea | R | 48 | −72 | −8 | 5.6 | 48 | −70 | −10 | 6.0 |
| Middle | R | 34 | −90 | 6 | 5.9 | 32 | −90 | 6 | 5.2 |
| Middlea | R | 42 | −78 | 2 | 5.7 | 44 | −78 | 0 | 6.2 |
| Middle | R | 46 | −80 | 10 | 5.6 | −40 | −80 | 8 | 4.4 |
| Middle | R | 30 | −82 | 28 | 5.0 | 34 | −80 | 22 | 5.0 |
| Middle | R | −26 | −86 | 10 | 5.4 | −28 | −88 | 10 | 3.8 |
| Middle | R | −20 | −96 | 14 | 5.2 | −22 | −96 | 14 | 3.5 |
| Middle | R | −32 | −80 | 20 | 5.7 | −34 | −82 | 20 | 3.5 |
Peak coordinates of activation in the comparison of 3‐D non‐objects (Non) to fixation.
Peak coordinates of activation in the comparison of 3‐D non‐objects (Non) to 2‐D shapes.
Differentially affected by baseline in the meta‐analysis.
NS, not significant.
Figure 5.
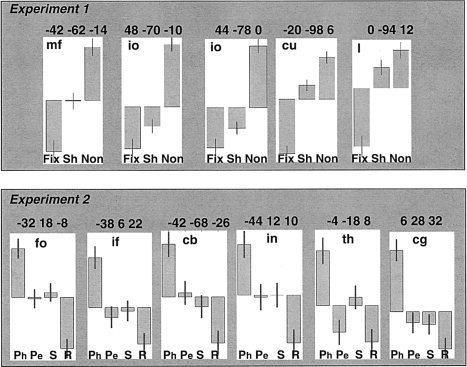
The relative effect sizes for Experiment 1 and 2 in areas more activated by object naming when the baseline was low than high. Mean centered plots showing relative effect sizes for Fix (fixation), Sh (2‐D geometric shapes); and Non (3‐D non‐objects) in Experiment 1; and Ph (phonological task); Pe (perceptual task); S (semantic task), and R (resting with eyes closed) in Experiment 2. Each plot is labeled with the peak co‐ordinates of the effects reported in Table II; af, anterior fusiform; mo, middle occipital; tp, temporal pole; ph, parahippocampal; cs, calcarine sulcus; dc, dorsal cuneus; ift, inferior frontal (triangularis); cb, cerebellum; it, inferior temporal.
Also consistent with our predictions, none of the areas that the object‐naming meta‐analysis identified as more activated for high‐ > low‐level baselines corresponded to non‐object activation in Experiment 1. For example, activation in the middle occipital, cuneus, and calcarine sulcus areas for object naming relative to high‐level baselines was located more dorsally (+12 to +24 mm above the anterior–posterior commissure [AC–PC] line), than activation observed for non‐objects relative to fixation or shapes (−2 to +14mm relative to the AC–PC line).
Experiment 2: Phonological and Semantic Processing of Real Objects
Phonological activation
Consistent with previous studies reporting activation in response to phonological tasks with heard and written words [Burton et al.,2003; Devlin et al.,2003; Fujimaki et al.,1999; Gold et al.,2005; Gold and Buckner,2002; McDermott et al.,2003; Mummery et al.,1998; Poldrack et al.,1999; Price et al.,1997; Roskies et al.,2001; Shivde and Thompson‐Schill,2004], phonological decisions on pictures of objects (relative to semantic, perceptual, and resting conditions) activated bilateral inferior frontal, insula, precentral, and posterior lateral inferior temporal cortices, supramarginal gyri, anterior cingulate, supplementary motor area (SMA), cerebellum, and the left thalamus (Table IV). Critically, these phonological activations included 6 of 12 areas that were more activated in the object‐naming meta‐analysis for low > high baselines (all in the left hemisphere): frontal operculum (fo); inferior frontal (if‐t); cerebellum (cb), insula (in); thalamus (th); and cingulate gyrus (cg). Figure 5 shows relative effect sizes across tasks in each of these regions. Greater activation in these areas for object naming when the baseline is low is therefore likely to reflect differences in phonological processing. In other words, the high‐level baselines controlled for speech output more than the low‐level baselines did.
Table IV.
Activation for phonological and semantic decisions in Experiment 2
| Lobe activated/region | Hemisphere | Peak coordinates* | Z score | Voxels* | ||
|---|---|---|---|---|---|---|
| Phonological > semantics, perceptual and rest | ||||||
| Temporal | ||||||
| Posterior inferior | L | −48 | −56 | −18 | 4.1 | 194 |
| Posterior inferior | R | 60 | −66 | −20 | 3.6 | 18 |
| Parietal | ||||||
| Supramarginal | R | 64 | −40 | 48 | 4.0 | 33 |
| Supramarginal | L | −56 | −40 | 42 | 3.6 | 55 |
| Frontal | ||||||
| Insulaa | L | −32 | 18 | −8 | 4.7 | 221 |
| Insula | L | −36 | 26 | −2 | 4.5 | — |
| Insula | R | 38 | 22 | −6 | 4.2 | 257 |
| Insula | R | 44 | 16 | 4 | 4.0 | — |
| Inferiora | L | −38 | 6 | 22 | 4.3 | — |
| Inferiora | L | −44 | 12 | 10 | 3.7 | 55 |
| Inferior | L | −58 | 20 | 24 | 3.6 | 14 |
| Inferior | R | 56 | 12 | 32 | 4.7 | 317 |
| Middle dorsal | L | −40 | 40 | 24 | 3.7 | 70 |
| Middle ventral | L | −46 | 40 | 0 | 3.7 | 48 |
| Middle ventral | L | −34 | 40 | −18 | 3.5 | 26 |
| Middle dorsal | R | 42 | 42 | 14 | 3.3 | 13 |
| Middle ventral | R | 30 | 48 | −20 | 4.5 | 239 |
| Superior | R | 48 | 52 | −16 | 4.0 | 32 |
| Superior | L | −24 | 52 | −18 | 3.6 | 91 |
| Precentral | R | 58 | 8 | 40 | 3.9 | 317 |
| Precentral | R | 54 | −2 | 42 | 3.4 | — |
| Precentral | L | −46 | 2 | 40 | 4.5 | 266 |
| Anterior cingulatea | R | 6 | 28 | 32 | 5.3 | 321 |
| Anterior cingulate | L | −8 | 14 | 46 | 4.0 | 89 |
| Supplementary motor area | L | −38 | 0 | 64 | 4.2 | 51 |
| Supplementary motor area | L | −24 | 16 | 63 | 3.4 | 13 |
| Cerebellum | ||||||
| Medial | L | −2 | −62 | −30 | 5.4 | 362 |
| Medial | R | 4 | −80 | −24 | 4.2 | — |
| Medial | L | 0 | −76 | −40 | 3.4 | — |
| Lateral | R | 30 | −76 | −26 | 4.5 | 252 |
| Lateral | R | 46 | −72 | −28 | 4.1 | — |
| Laterala | L | −42 | −68 | −26 | 4.1 | 194 |
| Subcortical | ||||||
| Thalamusa | L | −4 | −18 | 8 | 4.1 | 83 |
| Thalamusa | L | −12 | −8 | 10 | 3.4 | — |
| Semantics > phonological, perceptual and rest | ||||||
| Temporal | ||||||
| Medial pole | R | 26 | −4 | −44 | 4.0 | 105 |
| Lateral pole | R | 56 | −16 | −40 | 3.9 | 30 |
| Lateral inferior | L | −62 | −16 | −34 | 3.5 | 30 |
| Anterior fusiforma | L | −34 | −20 | −24 | 3.4 | 13 |
| Posterior middle | L | −52 | −50 | 8 | 3.3 | 7 |
| Posterior middle | R | 62 | −48 | 8 | 3.3 | 19 |
| Parietal | ||||||
| Angular gyrus | L | −54 | −60 | 40 | 3.5 | 21 |
| Frontal | ||||||
| Inferior | R | 48 | 6 | 26 | 3.9 | 43 |
| Inferior | R | 42 | 34 | 4 | 3.5 | 9 |
| Cerebellum | ||||||
| Lateral | R | 50 | −60 | −26 | 4.3 | 115 |
| Lateral | L | −40 | −36 | −34 | 3.7 | 29 |
| Medial | L | −22 | −46 | −44 | 3.7 | 14 |
| Occipital | ||||||
| Middle dorsala | R | 46 | −78 | 8 | 4.5 | 88 |
| Middle ventral | L | −48 | −74 | −8 | 3.7 | 16 |
| Cuneus | L | −22 | −96 | 12 | 3.9 | 56 |
Number of voxels activated at P < 0.001, uncorrected.
Differentially affected by baseline in the meta‐analysis.
Semantic activation
Semantic decisions, relative to the phonological, perceptual, and rest conditions, activated bilateral anterior and posterior middle temporal regions, middle occipital cortex, the cerebellum, left angular gyrus, left cuneus and right inferior frontal cortex (Table IV). Notably, the right dorsal middle occipital activation and the left anterior fusiform activation corresponded to that observed in the object‐naming meta‐analysis for high more than low‐level baselines. In addition, all other regions associated with object naming when the baseline was high relative to low, were also activated by semantic decisions relative to at least two of the other conditions. In other words, their association with semantic processing depended on the baseline context. Specifically, as illustrated in Figure 6, bilateral temporal poles and the right calcarine sulcus and parahippocampal gyrus were activated by semantic decisions and rest (an implicit conceptual processing state) relative to phonological and perceptual decisions, the dorsal cuneus was activated by semantic and perceptual decisions relative to phonological decisions and rest, and the left inferior frontal (triangularis), cerebellum, and left posterior inferior temporal cortex were activated by semantic and phonological decisions relative to perceptual decisions and rest.
Figure 6.
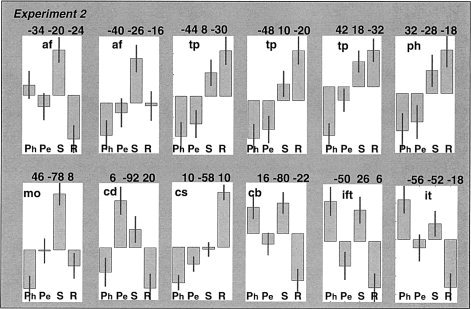
The relative effect sizes for Experiment 1 and 2 in areas that more activated by object naming when the baseline was high than low. Mean centered plots showing relative effect sizes for Ph (phonological task); Pe (perceptual task); S (semantic task), and R (resting with eyes closed) conditions in Experiment 2. Each plot is labeled with the peak co‐ordinates of the effects reported in Table II; af, anterior fusiform; mo, middle occipital; tp, temporal pole; ph, parahippocampal; cs, calcarine sulcus; dc, dorsal cuneus; ift, inferior frontal (triangularis); cb, cerebellum; it, inferior temporal.
Summary
Of 12 areas that the object‐naming meta‐analysis identified as more activated during low‐level baselines, 4 were associated with perceptual processing (Experiment 1), and 6 were associated with phonological processing/speech production (Experiment 2). The two remaining activations (for object naming when the baseline was low relative to high) were in bilateral superior and left middle temporal areas. These areas are likely to reflect auditory processing of the spoken response because they correspond to classic speech perception areas [Crinion et al.,2003; Scott et al.,2000]. In contrast, all nine areas that the object‐naming meta‐analysis identified as more activated during high than low‐level baselines were also activated during semantic decisions in Experiment 2. Activation depended on what semantic decisions were being compared to (see Table II, III, IV for details of peak activation; Fig. 5, 6 for the relative size of the effects across tasks).
To illustrate this remarkable consistency, Figure 7 summarizes the effects observed in the meta‐analyses and Experiments 1 and 2. The top row shows the main effect of all studies entered into the meta‐analyses and the second row shows areas where the object‐naming meta‐analysis found higher activation for low (yellow) or high (blue) baselines. The third row shows activation for non‐objects relative to shapes and fixation (red), the phonological task (yellow), and semantic task (blue). As can be seen, the orange areas in the second row (low baselines) correspond to the red (perceptual) and yellow (phonological) areas in the third row. Likewise, the blue areas in the second row (high baselines) correspond to the blue (semantic) areas in the third row. On slice 10, for example, a left inferior frontal area responds to semantic tasks in Experiment 2 and for object naming relative to high‐level baselines in the meta‐analyses. Conversely, an adjacent area in the insula cortex responds to phonological tasks in Experiment 2 and object naming relative to low‐level baselines in the meta‐analyses. On the same slice, the close correspondence between the meta‐analysis and new experiments can also be observed in the left thalamus and the right calcarine sulcus.
Figure 7.
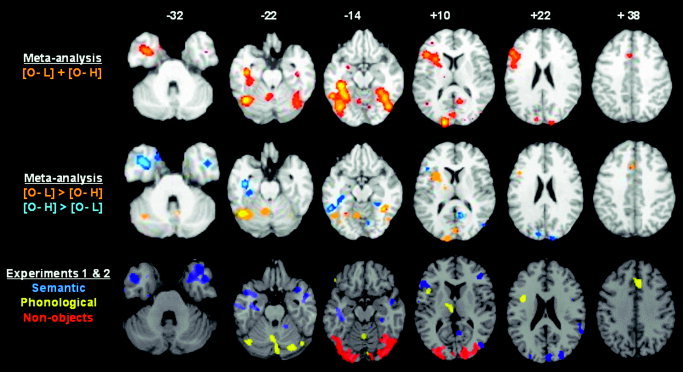
Comparison of the effects observed in the meta‐analyses, Experiments 1 and 2. The top row shows the main effect of all studies entered into the meta‐analyses on axial slices of the brain −32 mm, −22 mm, −14 mm, +10 mm, +22 mm, and +38 mm relative to the AC–PC line. The second row shows the difference in the meta‐analyses for object naming with low (orange) and high (blue) baselines (on the same slices as the top line). O, object naming; L, low‐level baseline; H, high‐level baseline. The third row shows activation in red for non‐objects relative to shapes and fixation (Experiment 1), in yellow for the phonological task (Experiment 2), and in blue for the semantic task (Experiment 2). Differences in the shape of the brain reflect the different templates used in Experiments 1 and 2 (MNI template from SPM99); and in the meta‐analysis. Likewise, differences in the color scale reflect the different analysis packages. The threshold for activation was P < 0.05 in the meta‐analyses and P < 0.001 for non‐objects and the phonological task. For semantic activation, the threshold was set at P < 0.001 in slices 10 and 22 but P < 0.05 in slices −32, −22 and −14 (to highlight the effects in the temporal poles and anterior fusiform). As can be seen, there is a high correspondence between the orange areas in the second row and the red and yellow areas in the third row, and in the blue areas in the second and third rows. Details of listing all activations can be found in Table II.
DISCUSSION
Previous functional imaging studies of object naming can be divided broadly in terms of whether or not attempts were made to control for visual input and speech output. We distinguish between high‐level baselines that require a speech response to meaningless but visually complex figures; and low‐level baselines that do not control for speech production or complex visual processing. As high‐level baselines control for more than low‐level baselines do, more activation was expected for object naming in the context of low‐level baselines, particularly in areas associated with speech production and visual processing. Our object‐naming meta‐analysis confirmed this prediction, but also revealed areas where activation was observed more when the baseline was high than when it was low. Although this reverse effect was less expected, it is consistent with the findings of Binder et al. [1999], who demonstrated that resting baselines activate areas associated with semantic relative to phonological tasks.
Inclusion of several different experimental conditions in Experiment 2 allowed us to characterize further object‐naming activations relative to high‐level baselines. It was not simply the case that high‐level baselines enhanced sensitivity to semantic activation. Some areas were activated by rest more than semantic tasks (e.g., an area in the right calcarine sulcus and to a lesser extent the temporal poles; see Fig. 6). Other regions were engaged by phonological as well as semantic processing (left inferior frontal, right cerebellum, and left inferior temporal). The latter areas therefore seem to play a role in both semantic and phonological processing. There are three possible interpretations of enhanced object‐naming activation with high‐level baselines. The first interpretation is that the areas do indeed play a critical role in object naming. In addition, they are also activated during low‐level baselines that allow inner speech, imagery, and semantic associations to be engaged. Such processes may be suppressed during high‐level baselines. The second interpretation is that the areas do not play a critical role in object naming. Instead, activation for object naming is only a reflection of artifactual differences between baseline tasks. Finally, the third interpretation is that object‐naming activation for high‐ relative to low‐level activation is an artifact of the multiple studies entered into the meta‐analyses and the assumption that subjects in different studies will show the same effects.
Evidence that activation plays a critical role in object naming (option 1) is provided by reference to neuropsychological studies. It is well known that left temporal or frontal damage impairs object‐naming ability. Specifically, Lambon Ralph et al. [2001] have proposed that object‐naming difficulties after anterior temporal lobe damage (including temporal poles, anterior fusiform, and parahippocampal cortices) can be explained solely in terms of a semantic impairment, and De Renzi et al. [1987] have proposed that object‐naming difficulties after left posterior inferior temporal damage can be explained in terms of deficits transmitting visual information to the speech areas. Thompson‐Schill et al. [1998] have proposed that the left posterior inferior frontal cortex is necessary for a nonsemantic selection process that is engaged when there are many sources of competing information [see Kan and Thompson‐Schill,2004]. In addition, right cerebellar metabolism [Feeney and Baron,1986] and structure [Price et al.,1999] are known to be intricately connected to that in the left inferior frontal cortex and therefore may also play a role in response selection. In other words, neuropsychological data suggest that most areas that were activated for object naming when the baseline was high relative to low are indeed important for object naming, and reflect the contribution of semantic processing, visual‐speech integration, and response selection. The differential effect of baseline in these regions must therefore reflect either increased activation during low‐level baselines (e.g., semantic associations during resting conditions) or decreased activation during high‐level baselines. For example, it may be necessary to suppress visual‐speech integration and response selection during high‐level baselines because these tasks require the same speech response to each stimulus (e.g., “OK” or “hiya”).
The other areas associated with object naming, in the context of high‐level baselines, were the right middle occipital, right calcarine sulcus, and the dorsal cuneus. Although these areas are in visual cortex, they were not activated differentially by non‐objects relative to fixation in Experiment 1. Furthermore, the meta‐analyses did not reveal activation in these regions for object naming relative to low‐level baselines. Activation in these areas for object naming relative to high‐level baselines is, therefore, not interpreted easily in terms of differences in visual input (unlike the occipital areas that were more activated for low‐level baselines). Examination of Figure 6 illustrates that the right middle occipital area was activated by semantic decisions more than it was by phonological, perceptual, and rest conditions (Experiment 2). In contrast, the dorsal cuneus was most activated during perceptual decisions and the calcarine sulcus was most activated during rest (with eyes closed). This suggests that each of these areas may have a different function. For example, the calcarine sulcus, may play a role in visual imagery [Lambert et al.,2002], which is higher when subjects rest with eyes closed than when they are viewing stimuli and higher for low‐level baselines than for high‐level baselines. Without reference to neuropsychological data, we cannot determine whether activation in the right middle occipital cortex, calcarine sulcus, or dorsal cuneus plays a critical role in object naming. Future neuropsychological (or TMS) studies are therefore required to compare the effect of lesions in dorsal (Brodmann area [BA] 18) and ventral (BA17) regions of the cuneus, and in the middle and inferior occipital areas that are affected differentially by low and high baselines.
Finally, Experiment 2 allows us to compare the distributed set of activation differences between semantic and phonological decisions in response to pictures of objects. To our knowledge, no previous study has reported such a comparison, even though there are numerous functional imaging reports that have compared semantic and phonological tasks when the stimuli are written words [Burton et al.,2003; Devlin et al.,2003; Fujimaki et al.,1999; Gold et al.,2005; Gold and Buckner,2002; McDermott et al.,2003; Mummery et al.,1998; Poldrack et al.,1999; Price et al.,1997; Roskies et al.,2001; Shivde and Thompson‐Schill,2004]. These previous studies of written word processing have shown consistently that activation is enhanced for phonological relative to semantic decisions in posterior inferior frontal and supramarginal gyri (BA40), and semantic relative to phonological decisions in anterior inferior frontal, the angular gyrus, and posterior and middle temporal areas.
We observed the same effects when the stimuli were pictures of objects in Experiment 2 (see Table IV); with the exception that we did not observe greater activation for semantic decisions in the left anterior inferior frontal cortex. Although further studies are needed to statistically validate this potential difference between object and word processing, we believe there is a simple explanation. It is well established that phonological retrieval is more dependent on semantic retrieval when stimuli are pictures than when they are words, because, unlike words, pictures cannot be named based on direct links between orthography to phonology [Glaser and Glaser,1989]. If left anterior inferior frontal activation reflects semantic retrieval, it may thus be engaged equally during semantic and phonological tasks when the stimuli are pictures, but more engaged during semantic than phonological tasks when the stimuli are written words.
Conclusions
The meta‐analyses identified a complex set of differences between object‐naming paradigms that used low‐ or high‐level baselines. Investigation of these effects revealed that they could be explained in terms of differential demands on perceptual, semantic, or phonological processing. As expected, high‐level baselines reduced activation associated with the processing of complex visual features and speech production. In addition, high‐level baselines increased sensitivity to activation in areas associated with semantic processing, visual‐speech integration and response selection. These results lead us to the following conclusions: (1) the validity of the ALE meta‐analyses is verified in remarkable detail by reference to new experiments (see Fig. 7); (2) contrary to expectation, high‐level baselines may provide better sensitivity to semantic processing, visual‐speech integration, and response selection while controlling for visual and phonological processing; and (3) the correspondence between functional imaging and neuropsychological results is critically dependent on the baseline task used in the imaging study.
REFERENCES
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11: 80–95. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Zeffiro TA, Blaxton T, Gaillard W, Theodore W (1995): Regional cerebral blood flow during object naming and word reading. Hum Brain Mapp 3: 93–106. [Google Scholar]
- Burton H, Diamond JB, McDermott KB (2003): Dissociating cortical regions activated by semantic and phonological tasks: a FMRI study in blind and sighted people. J Neurophysiol 90: 1965–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Haxby JV, Martin A (1999): Attribute‐based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci 2: 913–919. [DOI] [PubMed] [Google Scholar]
- Chao L, Weisberg J, Martin A (2002): Experience dependent modulation of category‐related cortical activity. Cereb Cortex 12: 545–551. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A (2000): Representation of manipulable man‐made objects in the dorsal stream. Neuroimage 12: 478–484. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon‐Ralph MA, Warburton EA, Howard D, Wise RJ (2003): Temporal lobe regions engaged during normal speech comprehension. Brain 126: 1193–1201. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Tranel D, Hichwa R, Damasio A (1996): A neural basis for lexical retrieval. Nature 380: 499–505. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LL, Hichwa RD, Damasio AR (2001): Neural correlates of naming actions and of naming spatial relations. Neuroimage 13: 1053–1064. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Zambolin A, Crisi G (1987): The pattern of neuropsychological impairment associated with left posterior cerebral artery infarcts. Brain 110: 1099–1116. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF (2003): Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 15: 71–84. [DOI] [PubMed] [Google Scholar]
- Etard O, Mellet E, Papathanassiou D, Benali K, Houde O, Mazoyer B, Tzourio‐Mazoyer N (2000): Picture naming without Broca's and Wernicke's area. Neuroreport 11: 617–622. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Baron JC (1986): Diaschisis. Stroke 17: 817–830. [DOI] [PubMed] [Google Scholar]
- Fujimaki N, Miyauchi S, Putz B, Sasaki Y, Takino R, Sakai K, Tamada T (1999): Functional magnetic resonance imaging of neural activity related to orthographic, phonological, and lexico‐semantic judgments of visually presented characters and words. Hum Brain Mapp 8: 44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO (1989): Context effects in Stroop‐like word and picture processing. J Exp Psychol Gen 118: 13–42. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Kirchhoff BA, Buckner RL (2005): Common and dissociable activation patterns associated with controlled semantic and phonological processing: evidence from fMRI adaptation. Cereb Cortex 10.1093/cercor/bhi024. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL (2002): Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 35: 803–812. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR (1998): Premotor and prefrontal correlates of category‐related lexical retrieval. Neuroimage 7: 232–243. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Eichhorn GR, Tranel D (2003): Effects of gender on blood flow correlates of naming concrete entities. Neuroimage 20: 940–254. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rozzolatti G (1997): Premotor cortex activation during observation and naming of familiar tools. Neuroimage 6: 231–236. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD (2002): Broca's area in the human brain is involved in the selection of grammatical gender for language production: evidence from event‐related functional magnetic resonance imaging. Neurosci Lett 328: 101–104. [DOI] [PubMed] [Google Scholar]
- Kan IP, Thompson‐Schill SL (2004): Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn Affect Behav Neurosci 4: 43–57. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Hatano G, Oizumi K, Sugiura M, Fukuda H, Itoh K, Kato T, Nakamura A, Hatano K, Kojima S (2001): Different neural systems for recognizing plants, animals, and artifacts. Brain Res Bull 54: 313–317. [DOI] [PubMed] [Google Scholar]
- Lambert S, Sampaio E, Scheiber C, Mauss Y (2002): Neural substrates of animal mental imagery: calcarine sulcus and dorsal pathway involvement—an fMRI study. Brain Res 924: 176–183. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR (2001): Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry 70: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Wiggs C, Ungerleider L, Haxby J (1996): Neural correlates of category‐specific knowledge. Nature 379: 649–652. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG (2003): A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia 41: 293–303. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ (1999a): A functional neuroimaging study of the variables that generate category specific object processing differences. Brain 122: 943–962. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ (1999b): Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage 10: 181–192. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ (1998): Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci 10: 766–777. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Evans A (1999): The neural substrate of picture naming. J Cogn Neurosci 11: 399–423. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Frackowiak RSJ, Friston KJ (1996): The neural regions sustaining object recognition and naming. Proc R Soc Lond B Biol Sci 263: 1501–1507. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJ (1997): Segregating semantic from phonological processes during reading. J Cogn Neurosci 9: 727–733. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frackowiak RS, Friston KJ (1999): Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci 11: 371–382. [DOI] [PubMed] [Google Scholar]
- Renvall K, Laine M, Hiltunen J, Rinne JO, Kaasinen V, Sipila H, Cornelissen K, Martin N (2003): Naming multiple objects: neural correlates as measured by positron emission tomography. Appl Neuropsychol 10: 224–233. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle MA, Petersen SE (2001): Task‐dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci 13: 829–843. [DOI] [PubMed] [Google Scholar]
- Scott SK, Holmes A, Friston KJ, Wise RJ (2000): A thalamo‐prefrontal system for representation in executive response choice. Neuroreport 11: 1523–1527. [PubMed] [Google Scholar]
- Shivde G, Thompson‐Schill SL (2004): Dissociating semantic and phonological maintenance using fMRI. Cogn Affect Behav Neurosci 4: 10–19. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ (2001): Differences in functional magnetic resonance imaging activation by category in a visual confrontation naming task. J Neuroimaging 11: 165–170. [DOI] [PubMed] [Google Scholar]
- Swan D, Goswami U (1997): Picture naming deficits in developmental dyslexia: the phonological representation hypothesis. Brain Lang 56: 334–353. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Swick D, Farah MJ, D'Esposito M, Kan IP, Knight RT (1998): Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA 95: 15855–15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE (2004): Processing objects at different levels of specificity. J Cogn Neurosci 16: 351–362. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Bielamowicz L, Martin A (2003): Modulation of neural activity during object naming: effects of time and practice. Cereb Cortex 13: 381–391. [DOI] [PubMed] [Google Scholar]
- van Turennout M, Ellmore T, Martin A (2000): Long lasting cortical plasticity in the object naming system. Nat Neurosci 3: 1329–1334. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Van Borsel J, Tesink C, van den Noort M, Deblaere K, Seurinck R, Vandemaele P, Achten E (2003): Multilingualism: an fMRI study. Neuroimage 20: 2181–2196. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Faber TL, Popp CA, Henry TR, Trudeau JD, Woodard JL, Mao H, Hoffman JM, Song AW (1999): A confrontational naming task produces congruent increases and decreases in PET and fMRI. Neuroimage 10: 347–356. [DOI] [PubMed] [Google Scholar]
- Zelkowicz BJ, Herbster AN, Nebes RD, Mintun MA, Becker JT (1998): An examination of regional cerebral blood flow during object naming tasks. J Int Neuropsychol Soc 4: 160–166. [DOI] [PubMed] [Google Scholar]


