Abstract
After Newman and Twieg ([2001]: Hum Brain Mapp 14:39–47) and others, we used a fast event‐related functional magnetic resonance imaging (fMRI) design and contrasted the lexical processing of pseudowords and real words. Participants carried out an auditory lexical decision task on a list of randomly intermixed real and pseudo Chinese two‐character (or two‐syllable) words. The pseudowords were constructed by recombining constituent characters of the real words to control for sublexical code properties. Processing of pseudowords and real words activated a highly comparable network of brain regions, including bilateral inferior frontal gyrus, superior, middle temporal gyrus, calcarine and lingual gyrus, and left supramarginal gyrus. Mirroring a behavioral lexical effect, left inferior frontal gyrus (IFG) was significantly more activated for pseudowords than for real words. This result disconfirms a popular view that this area plays a role in grapheme‐to‐phoneme conversion, as such a conversion process was unnecessary in our task with auditory stimulus presentation. An alternative view was supported that attributes increased activity in left IFG for pseudowords to general processes in decision making, specifically in making positive versus negative responses. Activation in left supramarginal gyrus was of a much larger volume for real words than for pseudowords, suggesting a role of this region in the representation of phonological or semantic information for two‐character Chinese words at the lexical level. Hum Brain Mapp 25:212–221, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: lexical decision, auditory, inferior frontal gyrus, fMRI, pseudowords, Chinese
INTRODUCTION
Lexical decision task is a common language paradigm in the study of mental lexicon [Forster and Bednall,1976; Marslen‐Wilson,1980; Rubenstein et al.,1970]. In this task, participants are typically given a list of items to decide whether each item is a real word or not. The foils can be phonologically incorrect, meaningless nonwords or phonologically correct, meaningless pseudowords. For phonologic processing, the contrast between pseudowords and real words is of more concern as nonwords can be rejected simply based on orthography [James,1975].
Several imaging studies have compared the reading of real and pseudowords [Binder et al.,2003; Fiez,1993; Fiez et al.,1999; Hagoort et al.,1999; Mechelli et al.,2003; Price et al.,1994; Rumsey et al.,1997; Simos et al.,2002; Xu et al.,2001]. Many found that the two types of stimuli activated the same network of cortical regions, including the left inferior frontal gyrus (IFG), in which pseudowords elicited a stronger activation than did real words.
These findings have important theoretical implications, for example, for the dual‐route model, a well‐known reading model established before the imaging era [Coltheart et al.,1993; Fiebach et al.,2002]. More relevant to the present study, they also pose some difficulties to a major line of imaging research documenting a role of left IFG in semantic processing [Buckner et al.,2000; Copland et al.,2003; Demb et al.,1995; Fiez,1997; Gabrieli et al.,1998; Kapur et al.,1994; Petersen et al.,1988; Poldrack et al.,1999; Thompson‐Schill et al.,1997; Wagner et al.,2001]. Along this line, one would expect the left IFG to be more activated for real words, which have semantic representations, than for pseudowords, which do not.
It is possible that the left IFG may support multiple processes, some of which may not be specific to semantic processing. One such process is the grapheme‐to‐phoneme (G–P) conversion [Herbster et al.,1997; Price,1998; Rumsey et al.,1997]. As pseudowords have to go through the G–P conversion to access phonology whereas real words do not, this conversion process is hypothesized to produce the stronger activation observed in left IFG for pseudowords than for real words.
Newman and Twieg [2001] tested this hypothesis with a phoneme‐monitoring task. Their participants listened to blocks of real words or pseudowords and decided whether or not each stimulus ended with a /t/ sound. As the G–P conversion was not necessary with auditory stimulus presentation, the above hypothesis predicted no difference in left IFG activation across pseudoword and real word blocks, and this was indeed what Newman and Twieg [2001] found.
The study by Newman and Twieg [2001] was the first comparing the auditory processing of real and pseudowords. Kotz et al. [2002], a follow‐up study with auditory stimuli, however, located left inferior frontal sulcus, but not left IFG, with a stronger activation for pseudowords than for real words. The inconsistency may be partly due to methodologic differences, an issue that has drawn attention in reading studies [Mechelli et al.,2003]. The Newman and Twieg [2001] study used a block‐design and their phoneme‐monitoring task only implicitly probed but may not have engaged adequate lexical processing. This may account for their negative finding. The Kotz et al. [2002] study, although with an event‐related design, used a mixed version of lexical decision task that combined a semantic priming task with a lexical decision task. The target item for lexical decision was preceded by a semantically related or unrelated prime item. Their task therefore involved additional semantic processes other than lexical decision. Briefly, inconsistencies in the available results need to be resolved with more empirical studies optimizing experimental tasks.
In the present study, we followed Newman and Twieg [2001] and Kotz et al. [2002] to examine further auditory lexical processing. Although the primacy of spoken language over written language is well recognized, this research line has been given relatively little attention in the imaging field (but see Gandour et al.,2002). Using functional magnetic resonance imaging (fMRI), we imaged a lexical decision task in its pure form to explicitly contrast pseudowords and real words in the lexical access of Chinese words. Specifically, we asked whether activation in the left IFG showed a lexical effect. The involvement of other language areas would also be examined.
Words in Chinese can contain a single character or multiple characters (see Fig. 1a). In the present study, we used two‐character words, which accounts for 74% of the total Chinese vocabulary [Liu and Liang,1990]. The pseudowords used also consisted of two characters, constructed by combining the first character of one real word with the second character of another real word, provided such combination did not correspond to a real word (see Fig. 1b). In English, pseudowords share with real words their constituent syllables at the sublexical level but lack the phonological/semantic representations associated with real words at the lexical or the whole‐word level. The same relationship holds between the two‐character pseudowords and real words used here.
Figure 1.
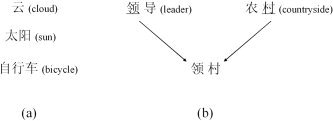
a: Chinese words can consist of a single or multiple characters, with the two‐character form being the dominant one. b: Pseudowords in the present study were constructed by combining the first character of one real word (e.g., the first character in the Chinese word for leader) with the second character of another real word (e.g., the second character of the Chinese word for countryside), provided such combination did not correspond to a real word. English words in parentheses are translations for the Chinese words.
Many individual characters in two‐character Chinese words are words themselves and have semantic meanings that may differ from that of the two‐character words they form. One therefore cannot claim that the pseudowords used in this study were totally without meaning, as for English pseudowords. Nevertheless, this should not affect comparison of the pseudoword condition with the real word condition, because the semantic information at the single‐character level was comparable across the two conditions.
Although we use “two‐character words” in the present work for easy understanding, given the present study is on speech, what we actually studied was two‐syllable words, or the phonologic form of two‐character words. All characters used in this study were single‐syllable characters, as is typically the case in Chinese.
An event‐related design was utilized so that pseudowords and real words were intermixed randomly in the same scanning session to avoid artifacts that may occur in a block design. For example, participants may adopt different response strategies if different types of trials were blocked in different scanning sessions.
SUBJECTS AND METHODS
Subjects
Fourteen native Chinese speakers (six females; mean age, 20.9 years; age range, 18–23 years) participated in the study. All had normal hearing as measured with a clinical audiometer. All were strongly right‐handed as judged by a handedness inventory [Snyder and Harris,1993]. Informed consent was obtained in accordance with guidelines from the Institute of Psychology of China, Beijing.
Experimental Design
All magnetic resonance (MR) imaging was conducted on a 1.5‐T Philips scanner at the Medical College of Shantou University, China with a standard headcoil. Twenty axial slices covering the whole brain were acquired with a T2*‐weighted gradient‐echo echo‐planar imaging (EPI) pulse sequence (repetition time [TR] = 2,000 ms; echo time [TE] = 45 ms; flip angle = 90 degrees) for the functional scans (acquisition matrix = 64 × 64; field of view [FOV] = 230 × 230 mm; slice thickness = 6 mm; no skip). Coplanar anatomic images (acquisition matrix = 256 × 256) were acquired with a T1‐weighted spin echo pulse sequence (TR = 204 ms; TE = 14 ms).
Participants lay supine inside the scanner and wore earphones and goggles specially designed for the MR environment (Resonance Techonology Company, Los Angeles, CA). Their head was restrained with padding behind the neck and in‐between the head and the headcoil. They were also told to keep their head still when doing the task inside the scanner. Participants made their responses with a button box. All auditory stimulation was delivered binaurally through the earphones and all visual stimulation through the goggles. Stimulus presentation was controlled with a PC computer using the Inquisit software package (Millisecond Software, Seattle, WA).
The design was a fast random‐interval event‐related design. Participants were first familiarized with the task and stimuli outside the scanner, and then went into the magnet and each completed five scanning sessions. The first session contained 10 practice trials and was excluded from later analysis. The remaining four sessions were for tests, each with twenty‐four trials. In each trial, a sound segment of about 1,000 ms was binaurally presented through the earphones. The segment was a digital recording of a two‐character real word or a pseudoword, spoken by a professional female broadcaster. Participants were asked to judge whether or not the segment was a real word and indicated their response with a button press. Both speed and accuracy were emphasized. Response time was measured from the onset of the presentation of the auditory stimulus.
In each session, half of the trials were pseudoword trials and the other half were real word trials, randomly intermixed. The intertrial interval (ITI) was pseudorandomized with a mean length of 10 s and a range of 6–14 s. In each testing session, participants therefore carried out the lexical decision task for 240 s (24 trials with an average length of 10 s). To avoid different eye movement patterns, all participants were required to fixate on a central dot during this period. This 4‐min period was preceded and followed by a 30‐s rest period where participants passively viewed a central cross and did not do any task. Eye‐movement monitoring with an eye‐tracking system confirmed that participants maintained visual fixation.
We first selected 120 real words from the Dictionary of Usage Frequency of Modern Chinese Words [Liu and Liang,1990] with a mean word frequency of 79.88 per million (standard error [SE] = 4.89). We then constructed the same number of pseudowords by randomly recombining the first character of one real word with the second character of another (as shown in Fig. 1b) to produce two‐character combinations that were not present in the Chinese vocabulary. Caution was taken to exclude those combinations that sounded like a real word, even though it did not exist as a real word in print. Half of the items obtained were used for the odd‐numbered participants and the other half for the even‐numbered ones. Of 60 items used for each participant, 12 were used for task familiarization and practice and 48 for testing; no item was used more than once.
Data Analysis
Image analysis was conducted with SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Functional images were slice‐acquisition corrected, motion corrected, and coregistered to the coplanar anatomic image for each participant. The T1 images were normalized to the standard statistical parametric mapping (SPM) template and the resulting transformation matrix was applied to the coregistered functional images. Such normalized functional images, interpolated to 4‐mm isotropic voxels and spatially smoothed with a Gaussian filter of 8‐mm kernel, were entered into a regression analysis using the general linear model for event‐related designs in SPM2 [Friston et al.,1995].
In regressor construction for the multiple regression, each trial was modeled with a square‐waved epoch of 1 TR, convolved with the canonical hemodynamic response function in SPM. Two regressors were constructed, one for the pseudoword trials and one for the real word trials. The rest periods and the ITIs were modeled implicitly as baseline (called rest condition for simplicity). Session‐specific effects were modeled as confound variables and low‐frequency noise in the signal was removed before regression analysis.
After the regression analysis, three linear contrasts were constructed and subject‐specific estimates of these contrasts were obtained. They were pseudo versus rest, real versus rest, and pseudo versus real contrasts.
The contrast estimates were entered into a standard SPM second‐level analysis with subject treated as a random effect, using one‐sampled t‐test (degrees of freedom [df] = 14–1). The expected mean difference value for the t‐tests was set to zero. A voxel‐wise intensity threshold (uncorrected P < 0.005) and a spatial extent threshold (cluster size greater than 20 voxels) were combined to control for multiple comparisons [Forman et al.,1995; Poline et al.,1997] in the generation of the t‐maps. All coordinates reported were in Talairach space converted from Montreal Neurological Institute (MNI) space based on an algorithm (available at http://www.mrccbu.cam.ac.uk/Imaging/mnispace.html). Percent signal change was calculated by averaging the blood oxygenation level‐dependent (BOLD) signal from all voxels in each identified region of activation, separated for different trial conditions and relative to the resting baseline.
RESULTS
Behavioral Performance
Mean accuracy and reaction time (RT) across all 14 participants were 93.1% and 1,381 ms, respectively. Incorrect trials were excluded from mean RT computation. Paired t‐tests indicated that responses to pseudowords were significantly slower and less accurate than the responses to real words (mean error rate: 9.9%, SE = 1.9 for pseudowords vs. 3.9%, SE = 1.6 for real words; t[13] = 2.48, P < 0.05; mean RT: 1,618 ms, SE = 96 for pseudowords vs. 1,143 ms, SE = 44 for real words; t[13] = 4.48, P < 0. 0001).
Imaging Data
As shown in Figure 2a and b, relative to the resting baseline both the pseudoword and the real word conditions activated an extensive set of brain regions, including bilateral superior and middle temporal cortex, bilateral inferior frontal gyrus and left precentral sulcus, supplementary motor cortex, bilateral cerebellum, middle and anterior cingulate gyrus, left insula, and left supramarginal gyrus. There were also activations in the visual areas, including bilateral calcarine and lingual gyrus, and some subcortical regions, including bilateral thalamus, left caudate, and bilateral putamen. The parahippocampal area was also activated for the pseudoword condition.
Figure 2.
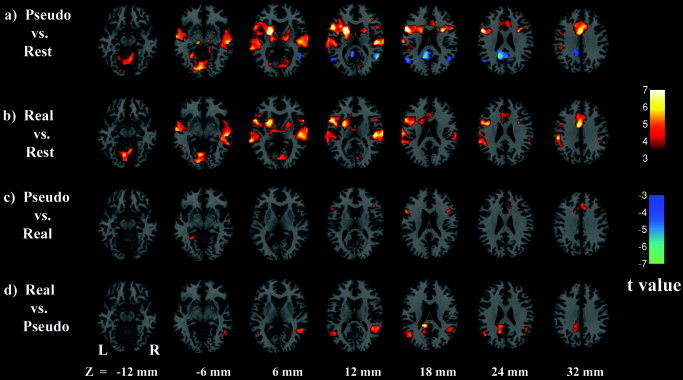
Axial t‐maps of brain activation (P < 0.005, minimum 20 contiguous voxels) for pseudoword vs. rest (a), real word vs. rest (b), pseudoword vs. real word (c), and real word vs. pseudoword (d). Deactivation in c and d are not shown to avoid redundancy. The images were superimposed on a standard SPM anatomic template brain in neurologic convention with z‐coordinate for each slice shown in Talairach space.
Whereas there was no brain region that was more active in the rest condition than in the real word condition, bilateral middle temporal cortex, middle/posterior cingulate gyrus, and left precuneus showed deactivation in the pseudoword relative to the rest condition.
Results from the direct contrast between the pseudoword condition and the real word condition are shown in Figure 2c and d. Left IFG (Brodmann area [BA]44) and right IFG (BA45) were significantly more activated for the pseudowords than for the real words, as were the supplementary motor area, middle cingulate gyrus, and right lingual gyrus. The real word condition activated more than the pseudoword condition did in bilateral middle temporal gyrus, and in a junction area between left post cingulate gyrus and left precuneus. The percentage of signal change in these areas is shown in Figure 3a and b separately for the pseudoword condition and the real word condition. Table I and Table II show the summary information for regions of activation revealed in all the above‐mentioned contrasts.
Figure 3.
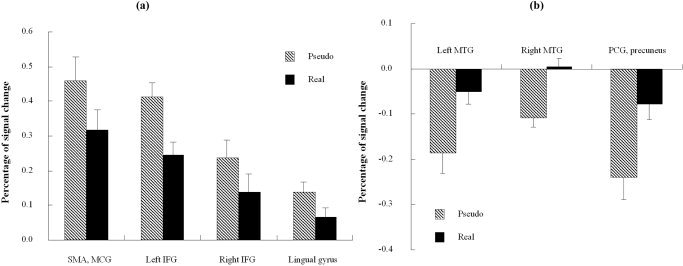
Mean percentage BOLD signal change of activated regions in the pseudoword vs. real word contrast, separated by stimulus condition. a: Regions more activated for pseudowords than for real words. b: Regions more activated (positively) for real words than for pseudowords. See Table I for abbreviations. The signal differences between the two conditions were significant for all regions shown (P = 0.001 level; two‐tail paired t‐tests.
Table I.
Summary information for activated regions revealed in statistical contrasts between pseudoword or real word versus the rest condition
| Anatomic structure | Brodmann area | Pseudoword | Real word | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Peak Z score | Volume (voxel) | Coordinates | Peak Z score | Volume (voxel) | |||||||
| Condition > rest | ||||||||||||
| IFG | Left | 45 | −32 | 32 | 9 | 3.55 | 22 | −44 | 23 | 3 | 4.00 | 88 |
| 44 | −50 | 18 | 10 | 4.47 | 21 | — | — | — | — | — | ||
| Right | 44,45 | 52 | 13 | 15 | 3.91 | 40 | 58 | 11 | 4 | 4.42 | 72 | |
| 45 | 42 | 33 | 9 | 3.89 | 23 | — | — | — | — | — | ||
| PrCS | Left | 6 | −32 | −4 | 44 | 3.58 | 22 | −29 | −5 | 43 | 3.45 | 31 |
| PrCS, IFG | Left | 6,44 | −50 | 9 | 22 | 5.06 | 125 | −44 | 5 | 24 | 4.12 | 101 |
| MCG, ACG | 24, 32 | 0 | 25 | 29 | 4.78 | 164 | 0 | 16 | 31 | 4.11 | 229 | |
| SMA Left, MCG | 24, 6, 32 | −5 | 5 | 43 | 4.60 | 159 | −3 | 8 | 42 | 4.58 | 194 | |
| STG | Left | 22, 21 | −54 | −3 | −3 | 4.16 | 205 | −58 | −7 | −4 | 4.24 | 217 |
| Right | 22, 21, 38 | 55 | −3 | −6 | 3.84 | 113 | 58 | −4 | −4 | 3.97 | 104 | |
| MTG | Left | 21, 22 | −52 | −31 | 7 | 4.23 | 166 | −63 | −34 | 7 | 4.29 | 148 |
| STG, MTG | Right | 22, 21 | 53 | −27 | 5 | 4.73 | 168 | 56 | −25 | 7 | 4.69 | 330 |
| Supramarginal gyrus | Left | 40 | −47 | −33 | 35 | 3.52 | 29 | −52 | −28 | 32 | 4.16 | 142 |
| Calcarine, lingual gyrus | Left | 17, 18 | −14 | −81 | −1 | 4.19 | 146 | −9 | −76 | 2 | 4.11 | 129 |
| Right | 17, 18 | 9 | −77 | 6 | 4.09 | 148 | 9 | −73 | 10 | 3.86 | 114 | |
| Cerebellum | Left | — | −13 | −57 | −16 | 3.99 | 155 | −6 | −67 | −14 | 4.10 | 150 |
| Right | — | 15 | −70 | −18 | 4.14 | 196 | 14 | −70 | −18 | 4.17 | 135 | |
| Thalamus | Left | — | −18 | −14 | 8 | 4.05 | 58 | −15 | −16 | 2 | 3.65 | 60 |
| — | −11 | −17 | 2 | 4.21 | 49 | — | — | — | — | — | ||
| Right | — | 19 | −11 | 6 | 3.94 | 78 | 10 | −14 | 5 | 3.45 | 43 | |
| Caudate, putamen | Left | — | −17 | 13 | 11 | 5.47 | 67 | −18 | 9 | 16 | 3.82 | 41 |
| Putamen, pallidium | Left | — | −18 | 9 | 0 | 4.81 | 60 | −17 | 9 | 0 | 4.20 | 38 |
| Putamen | Right | — | 23 | 5 | 11 | 3.78 | 46 | 30 | 2 | 5 | 3.86 | 46 |
| Insula | Left | — | −34 | 14 | −2 | 3.88 | 34 | −33 | 14 | −2 | 3.86 | 43 |
| Parahippocampus | Right | 35 | 19 | −28 | −8 | 3.71 | 43 | — | — | — | — | — |
| Rest > condition | ||||||||||||
| MTG | Left | 39 | −49 | −60 | 20 | −3.86 | 49 | — | — | — | — | — |
| Right | 22, 39 | 51 | −53 | 15 | −4.83 | 73 | — | — | — | — | — | |
| MCG, PCG | Left | 23 | −10 | −36 | 33 | −3.61 | 32 | — | — | — | — | — |
| PCG, precuneus | Left | 23 | −7 | −53 | 18 | −4.08 | 56 | — | — | — | — | — |
Coordinates shown in Talairach space for the center of mass of each activated region. IFG, inferior frontal gyrus; PrCS, precentral sulcus; SMA, supplementary motor area; MCG, ACG, PCG, middle, anterior, and posterior cingulate gyrus, respectively; STG, MTG, superior, middle temporal gyrus, respectively.
Table II.
Summary information for activated regions revealed in statistical contrasts between pseudoword and real word conditions
| Anatomic structure | Brodmann area | Pseudo > real | Real > pseudo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Peak Z‐score | Volume (voxel) | Coordinates | Peak Z‐score | Volume (voxel) | |||||||
| IFG | Left | 44 | −51 | 15 | 14 | 3.87 | 42 | — | — | — | — | — |
| IFG | Right | 45 | 49 | 17 | 18 | 3.78 | 31 | — | — | — | — | — |
| SMA, MCG | 32, 24 | 0 | 18 | 37 | 4.04 | 181 | — | — | — | — | — | |
| Lingual gyrus | Left | 19 | −26 | −50 | −3 | 3.97 | 38 | — | — | — | — | — |
| MTG | Left | 37, 21, 39 | — | — | — | — | — | −48 | −58 | 18 | 4.06 | 113 |
| Right | 21, 37 | — | — | — | — | — | 50 | −47 | 13 | 4.02 | 178 | |
| PCG, precuneus | Left | 23, 31 | — | — | — | — | — | −11 | −43 | 28 | 4.51 | 143 |
Coordinates shown in Talairach space for the center of mass of each activated region. IFG, inferior frontal gyrus; PrCS, precentral sulcus; SMA, supplementary motor area; MCG, ACG, PCG, middle, anterior, and posterior cingulate gyrus, respectively; STG, MTG, superior, middle temporal gyrus, respectively.
DISCUSSION
The behavioral performance results showed a clear lexical effect, in terms of both reaction time and response accuracy. Rejecting an item as pseudoword took longer and was more difficult for participants than accepting an item as real word. Although this finding is novel in that we have not found any previous research with a similar task setting (i.e., an auditory lexical decision task on two‐character Chinese words), it is in full accordance with the well‐established lexical effect in the lexical decision literature [see Ratcliff et al.,2004].
For imaging results, pseudowords and the real words both activated bilateral auditory cortex in the superior temporal gyrus (BA21/22), as one would expect for the present task with auditory stimulation. Activation in supplementary motor cortex, precentral sulcus, cingulate gyrus, and subcortical structures such as cerebellum and caudate mostly likely reflects the planning and execution of motor responses, which like the stimulus perception process merged with the lexical decision process in their fMRI responses. Thalamus activation may reflect the involvement of attentional processes [LaBerge,1990]. The remaining activations at middle temporal gyrus, inferior frontal gyrus, and supramarginal gyrus were all located in language‐related areas that have been well documented in imaging studies of linguistic processes [e.g., Binder et al.,1997; Levelt,2001; Poeppel et al.,2004]. There were also regions of deactivation in the pseudoword condition (Fig. 2a) to be discussed later.
Given the minimal visual processing required for this task (i.e., passively viewing a central fixation), it was surprising that significant activation in visual cortex at calcarine and lingual gyrus was found for both the pseudoword and the real word conditions, relative to the baseline. It is possible that the visual representation of Chinese characters was accessed automatically when their corresponding phonologic representation was accessed.
Overall, the pattern of activation was highly comparable for the two types of stimulus. All regions activated in the pseudoword condition except the parahippocampal gyrus were also activated in the real word condition. Most of these overlapping regions did not show up in the direct contrast between the two conditions, indicating a close match in loci, activation intensity, and spatial extent of corresponding regions. In regions where the two conditions did differ, the differences were more a matter of degree. These results replicate and support further the general finding in the lexical decision literature that pseudoword and real word processing engages the same network of cortical areas [see Mechelli et al.,2003].
The key finding from the present study came from the inferior frontal gyrus. This region of BA44/45, particularly the left IFG, which was our main focus, was activated for both the pseudoword and the real word condition relative to the baseline, and the activation was significantly stronger in the former condition than in the latter.
This finding was different from that in Newman and Twieg [2001], which did not reveal any differential left IFG activity between pseudowords and real words. It was also different from that of Kotz et al. [2002], where activity in the left inferior frontal sulcus (BA6) but not in the inferior frontal gyrus was found to depend on the lexical status of the stimulus. BA6 activation was also found in the present study but was unrelated to the pseudo/real distinction. A related study by Specht et al. [2003] did not allow for a direct comparison between pseudowords and real words because their design, a block design, was focused on the nonword/pseudoword distinction. In a contrast that was comparable between the Specht et al. [2003] and the present study, i.e., real/pseudoword judgment relative to baseline, they found activation in BA47 but not in BA44/45 whereas we found activation in BA44/45 but not in BA47. It would be premature to try to reconcile all these differences when there are only a few relevant studies. One thing that seems evident, however, is that although these few existing auditory imaging studies reported a null effect in left IFG for the differentiation of pseudowords and real words, we did find a difference. We attribute this discrepancy to methodologic differences between our study and that of Specht et al. [2003].
To the best of our knowledge, the present study is the only auditory study using an event‐related design and a pure version of the lexical decision task. The event‐related design should help reduce false activation from factors such as baseline signal shift. The random intermixing of the pseudowords and real words should encourage participants' adoption of uniform task strategies. Using the lexical decision task in its pure form should facilitate an explicit probing of lexical processing uncontaminated by other task components. In addition, in recombining real word constituent characters to construct pseudowords, the pseudowords and the real words were made highly comparable for factors such as familiarity, frequency, and phonology at the sublexical level, ensuring the pseudo/real word contrast to reveal differences only at the lexical level. These features combined help to explain why we were able to find a lexical effect in left IFG activity whereas others did not and testify to the reliability of our finding.
This finding does not support the notation that the stronger left IFG activation for pseudowords relative to real words in some previous reading lexical decision tasks was due to the grapheme‐phoneme conversion [Herbster et al.,1997; Price,1998; Rumsey et al.,1997], as such effect was found in the present study with auditory presentation, which did not involve the G–P conversion.
One thing that distinguishes pseudoword and real word conditions is that the former requires a positive (yes) response but the latter a negative (no) response in deciding whether a probe item was in one's preexisting mental representation. The lexical effect was in line with the classic finding that no responses are more effortful than yes responses [Stermberg,1966; Ratcliff,1985]. An alternative explanation of the observation that left IFG was more engaged by the processing of pseudowords than it was by processing of real words is therefore to attribute this difference to general processes not specific to lexical or semantic processing [Binder et al.,2003]. In support of this explanation, a recent imaging study with a verbal working memory task found a stronger left IFG (BA44/45) activation for no responses than for yes responses [Zhang et al.,2003]. Candidates for such general processes are likely components in the decision‐making process, such as a selection mechanism [Thompson‐Schill et al.,1997; Zhang et al.,2004a] or a guided control mechanism [Wagner et al.,2001].
According to this general process explanation, the supplementary motor area activation in the pseudo/real word comparison should reflect increased decision effort (perhaps due to more implicit articulation of the items, as in Hagoort et al., [1999]) for the pseudowords than for the real words. Similarly, the lingual gyrus activation may reflect reliance on the word‐form representations to facilitate judgment for the more difficult pseudoword items.
Binder et al. [2003] manipulated the neighborhood size of the pseudowords in a visual lexical decision task and observed the standard effect in the behavioral data, i.e., slower responses to items with larger neighborhood, as a general decision model would predict. Nearly all the brain regions sensitive to neighborhood size, however, were activated more strongly by items with no neighbors than by items with many neighbors. This pattern, the opposite of what one would expect based on the decision model, remains to be understood.
Previous imaging studies contrasting the auditory processing of pseudowords and real words have used alphabetical languages, such as English and German [Newman and Twieg,2001; Specht et al.,2003]. Results in the present study may therefore be partly specific to Chinese, a logographical language with very different linguistic features. Our recent study with two‐character Chinese words, however, has provided evidence that at least some high‐level linguistic processing can be generalized across languages [Zhang et al.,2004b], as advocated by other researchers [Bookheimer,2001; Chen et al.,2002; Matthews et al.,2002; Perfetti,2003].
The real word condition activated more than the pseudoword condition did in bilateral middle temporal gyrus, left post cingulate gyrus and left precuneus. The same set of regions were identified in a real word versus pseudoword comparison when Japanese participants articulated visually presented Kana syllables or did a phonologic lexical decision task on these materials [Ischebeck et al.,2004]. Left middle temporal gyrus and left precuneus were also found to be more activated in real word than in pseudoword conditions in Rissman et al. [2003] with a mixed version of the auditory lexical decision task.
As can be seen from Figure 2a and d, the regions that showed stronger activation for real words than for pseudowords all had deactivation in the pseudoword condition. Similar results have been noted by other researchers [Binder et al.,1999; Mechelli et al.,2003; Rissman et al.,2003]. Binder et al. [1999] examined in detail such localized, task‐induced deactivations and attributed them to interruption of ongoing semantic processing that occurs at rest. This proposal is readily applicable to our deactivation results, as semantic processing was presumably interrupted in the pseudoword condition but not in the real word condition.
Finally, the left supramarginal gyrus (BA40), although absent in the direct comparison between the two types of stimulus, showed a clear real word advantage in terms of volume of activation (148 voxels for real words and 29 for pseudowords1). Volume count, which has been used by some researchers [e.g., Newman and Twieg,2001], seems to be a more sensitive index in this case than does the t‐statistic. Given that the sublexical codes were well‐balanced across the two stimulus conditions, this activation strongly suggests that the left supramarginal gyrus is associated with the phonologic or semantic representation of two‐character Chinese words at the lexical level, consistent with current understanding of function of this language area in English studies [Levelt,2001].
Summary
There are three main results in the present study. First, replicating what has been found in the literature, processing of pseudowords and real words were found activating a highly comparable network of brain regions, including bilateral inferior frontal gyrus, superior/middle temporal gyrus, calcarine and lingual gyrus, and left supramarginal gyrus.
Second, mirroring a behavioral lexical effect, left inferior frontal gyrus was more activated for pseudowords than it was for real words. This result, in the context of the present task with auditory stimulus presentation, does not support the view that left IFG plays a role in grapheme‐to‐phoneme conversion. Rather, increased activity in this area for pseudowords is attributed to differences in general decision making processes, specifically in making positive versus negative responses.
Finally, activation in left supramarginal gyrus was of a much larger volume for real words than it was for pseudowords, suggesting a role of this region in the representation of phonologic or semantic information of two‐character Chinese words at the lexical level.
Acknowledgements
This research was supported by grants from Key International Collaboration Project from the National Natural Science Foundation of China, from the Natural Science Foundation of Guangdong, China, and from Research Grants Council Central Allocation Vote (RGC CAV) group research grant. We thank S.X. Chen, Q.L. Wu, L.F. Wu, and other members in the Molecular Imaging Research Center for their effort and contributions to this study. We also thank the three anonymous reviewers for their high quality reviews on an earlier version of this article, and the Associate Editor and Editor very much for their effort in overseeing the review process.
Footnotes
This observation was supported with a region‐of‐interest (ROI) analysis on left supramarginal cortex (BA40). Voxels significantly activated within this ROI (as determined using the false‐discovery‐rate option in SPM at the P = 0.05 level) in the pseudoword vs. rest comparison and in the real vs. rest comparison were calculated for each subject separately. The resulting 14 pairs of count values were submitted to a two‐tail, paired t‐test, which showed a larger count for the real word than for the pseudoword condition (208 voxels vs. 164 voxels, t[13] = 2.54; P = 0.025). Voxel counts in the main text are group measures from random effect analysis and therefore not subject to t‐tests.
REFERENCES
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T (1997): Human brain language areas identified by functional magnetic resonance imaging. J Neurosci 17: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci 11: 80–93. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L (2003): Neural correlates lexical access during visual word recognition. J Cogn Neurosci 15: 372–393. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2001): How the brain reads Chinese characters. Neuroreport 12: 1.11201065 [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR (2000): Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain 123: 620–640. [DOI] [PubMed] [Google Scholar]
- Chen Y, Fu S, Iversen SD, Smith SM, Matthews PM (2002): Testing for dual brain processing routes in reading: a direct contrast of Chinese character and Pinyin reading using fMRI. J Cogn Neurosci 14: 1088–1098. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M (1993): Models of reading aloud: dual‐route and parallel‐distributed‐processing approaches. Psychol Rev 100: 589–608. [Google Scholar]
- Copland DA, Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenerya HJ (2003): Brain activity during automatic semantic priming revealed by event‐related functional magnetic resonance imaging. Neuroimage 20: 302–310. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD (1995): Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach C, Friederici AD, Mueller K, von Cramon DY (2002): fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Fiez JA (1993): Functional anatomy of lexical processing: PET activation and performance studies. Diss Abstr Int 54: 662–663. [Google Scholar]
- Fiez JA (1997): Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp 5: 79–83. [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE (1999): Effects of lexicality, frequency, and spelling‐to‐sound consistency on the functional anatomy of reading. Neuron 24: 205–218. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Forster KI, Bednall ES (1976): Terminating and exhaustive search in lexical access. Mem Cognit 4: 53–61. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE (1998): The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J, Wong D, Lowe M, Dzemidzic M, Satthamnuwong N, Tong Y, Li X (2002): A cross‐linguistic fMRI study of spectral and temporal cues underlying phonological processing. J Cogn Neurosci 14: 1076–1087. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ (1999): The neural circuitry involved in the reading of German words and pseudowords: a PET study. J Cogn Neurosci 11: 383–398. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT (1997): Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp 5: 84–92. [DOI] [PubMed] [Google Scholar]
- Ischebeck A. Indefrey P, Usui N, Nose I, Hellwig F, Taira M (2004): Reading in a regular orthography: an fMRI study investigating the role of visual familiarity. J Cogn Neurosci 16: 727–741. [DOI] [PubMed] [Google Scholar]
- James CT (1975): The role of semantic information in lexical decisions. J Exp Psychol Hum Percept Perform 1: 130–136. [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, Houle S, Tulving E (1994): The role of the left prefrontal cortex in verbal processing: Semantic processing or willed action? Neuroreport 5: 2193–2196. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD (2002): Modulation of the lexical‐semantic network by auditory semantic priming: an event‐related functional MRI study. Neuroimage 17: 1761–1772. [DOI] [PubMed] [Google Scholar]
- LaBerge D (1990): Thalamic and cortical mechanisms of attention suggested by recent positron emission tomographic experiments. J Cogn Neurosci 2: 358–372. [DOI] [PubMed] [Google Scholar]
- Levelt WJ (2001): Spoken word production: a theory of lexical access. Proc Natl Acad Sci USA 98: 13464–13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang NY (1990): Dictionary of usage frequency of modern chinese words. Beijing: Yuhang Press. [Google Scholar]
- Marslen‐Wilson WD (1980): Speech understanding as a psychological process In: Simon JC, editor. Spoken language understanding and generation. Dordrecht: Reidel; p 39–67. [Google Scholar]
- Matthews PM, Fu S, Chen YP, Iversen S (2002): Functional magnetic resonance imaging: a promising tool for defining the organization of Chinese language in the brain In: Kao HSR, editor. Cognitive neuroscience studies of the Chinese language. Hong Kong: Hong Kong University Press; p 61–72. [Google Scholar]
- Mechelli A, Gorno Tempini ML, Price CJ (2003): Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci 15: 260–271. [DOI] [PubMed] [Google Scholar]
- Newman SD, Twieg D (2001): Differences in auditory processing of words and pseudowords: an fMRI study. Hum Brain Mapp 14: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA (2003): The universal grammar of reading. Sci Stud Reading 7: 3–24. [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR (2004): Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia 42: 183–200. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ (1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5: 83–96. [DOI] [PubMed] [Google Scholar]
- Price CJ (1998): The functional anatomy of word comprehension and production. Trends Cogn Sci 2: 281–288. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Watson JD, Patterson K, Howard D, Frackowiak RS (1994): Brain activity during reading. The effects of exposure duration and task. Brain 117: 1255–1269. [DOI] [PubMed] [Google Scholar]
- Ratcliff R (1985): Theoretical interpretations of the speed and accuracy of positive and negative responses. Psychol Rev 92: 212–225. [PubMed] [Google Scholar]
- Ratcliff R, Gomez P, McKoon G (2004): A diffusion model account of the lexical decision task. Psychol Rev 111: 159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE (2003) An event‐related fMRI investigation of implicit semantic priming. J Cogn Neurosci 15: 1160–1175. [DOI] [PubMed] [Google Scholar]
- Rubenstein H, Garfield L, Millikan JA (1970): Homographic entries in the internal lexicon. J Verbal Learn Verbal Behav 9: 487–494. [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P (1997): A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch Neurol 54: 562–573. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC (2002): Brain mechanisms for reading words and pseudowords: an integrated approach. Cereb Cortex 12: 297–305. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ (1993): Handedness, sex and familiar sinistrality effects on spatial tasks. Cortex 29: 115–134. [DOI] [PubMed] [Google Scholar]
- Specht K, Holtel C, Zahn R, Herzog H, Krause BJ, Mottaghy FM, Radermacher I, Schmidt D, Tellmann L, Weis S, Willmes K, Huber W (2003): Lexical decision of nonwords and pseudowords in humans: a positron emission tomography study. Neurosci Lett 345: 177–181. [DOI] [PubMed] [Google Scholar]
- Sternberg S (1966): High‐speed scanning in human memory. Science 153: 652–654. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Pare Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega‐Bermudez F, Pietrini P, Reeves‐Tyler P, DiCamillo P, Theodore W (2001): Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb Cortex 11: 267–277. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Feng CM, Fox P, Gao JH, Tan LH (2004a): Is left inferior frontal gyrus (LIFG) a general mechanism for working memory selection? Neuroimage 23: 596–603. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhuang J, Ma L, Yu W, Peng D, Ding G, Zhang Z, Weng X (2004b): Semantic processing of Chinese in left inferior prefrontal cortex studied with reversible words. Neuroimage 23: 975–982. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Leung HC, Johnson MK (2003): Frontal activations associated with accessing and evaluating information in working memory: an fMRI study. Neuroimage 20: 1531–1539. [DOI] [PubMed] [Google Scholar]


