Abstract
Psychological studies of deductive reasoning have shown that subjects' performance is affected significantly by the content of the presented stimuli. Specifically, subjects find it easier to reason about contexts and situations with a social content. In the present study, the effect of content on brain activation was investigated with functional magnetic resonance imaging (fMRI) while subjects were solving two versions of the Wason selection task, which previous behavioral studies have shown to elicit a significant content effect. One version described an arbitrary relation between two actions (Descriptive: “If someone does …, then he does …”), whereas the other described an exchange of goods between two persons (Social‐Exchange: “If you give me …, then I give you …”). Random‐effect statistical analyses showed that compared to baseline, both tasks activated frontal medial cortex and left dorsolateral frontal and parietal regions, confirming the major role of the left hemisphere in deductive reasoning. In addition, although the two reasoning conditions were identical in logical form, the social‐exchange task was also associated with right frontal and parietal activations, mirroring the left‐sided activations common to both reasoning tasks. These results suggest that the recruitment of the right hemisphere is dependent on the content of the stimuli presented. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: deductive reasoning, Wason selection task, fMRI, conditional rules, social exchange, content effect
INTRODUCTION
Reasoning can be defined as a combination of cognitive processes that allows us to draw inferences from a given set of information and reach conclusions that are not explicitly available, providing new knowledge. Reasoning is the central nucleus of thinking and is essential in almost every aspect of mental activity, from text comprehension to problem solving and decision making.
Although philosophical and psychological aspects of reasoning have been largely investigated over the years, its functional neuroanatomy remains poorly understood. The contribution of traditional, lesion‐based neuropsychology has led to controversial results [Shuren and Grafman, 2002; Wharton and Grafman, 1998], and only recently the development of functional neuroimaging techniques has opened new possibilities for the investigation of the neural correlates of reasoning.
Neuroimaging studies on reasoning have adopted two different approaches. One approach investigates the neural basis of distinct normative models of reasoning (i.e., deductive vs. inductive) [Goel et al., 1998; Goel and Dolan, 2000; Knauff et al., 2002] and the differences between them. [Goel et al., 1997; Osherson et al., 1998; Parsons and Osherson, 2001]. The other approach explores mental processes that underlie reasoning (linguistic or spatial manipulation) using different kinds of stimuli (such as semantic vs. non‐semantic [Goel et al., 2000], concrete vs. abstract [Goel and Dolan, 2001], or belief‐laden vs. belief‐neutral [Goel and Dolan, 2003]). The pattern of cerebral activation associated with logical reasoning seems to be influenced strongly by the content of the stimuli. In fact, arbitrary material without semantic content (e.g., “All P are Q”) activates frontal and parietal regions (including Brodmann areas [BA] 6, 7, 44 in both hemispheres, with a left‐sided prevalence), whereas semantically meaningful material (e.g., “All dogs are animals”) activates a left frontotemporal system (including BA44, 8, 9, 21, 22). Taken together, these studies have highlighted the crucial role of the prefrontal cortex, with activation seen predominantly in the left hemisphere.
The psychological analysis of human reasoning has demonstrated clearly that our inferential abilities are influenced considerably not only by the presence or the absence of a semantic content per se, but also by the specific content of the stimuli and the context in which they are presented. In fact, the psychological reality of the so‐called “content and context effects” is well documented both in inductive–probabilistic [Tversky and Kahneman, 1981] and in deductive reasoning [Wason, 1969; Wason and Johnson‐Laird, 1972].
To test for the presence of such content effects and to probe the structure of human reasoning, for over 30 years psychologists have been using an experimental paradigm known as the Wason Selection Task [Wason, 1966]. This reasoning task, originally developed to investigate the (presumed) logical nature of human inferential abilities, quickly became a classic task for the study of conditional reasoning (i.e., the activity of drawing inferences from situations in which the occurrence of one event is conditioned on the occurrence of a second event). In the original version of the task, subjects are shown a conditional rule in the form “If P then Q,” followed by four cards. Each card represents a separate occurrence that might satisfy or violate the rule. Each card has two sides, one side showing information about the truth or falsity of the antecedent (e.g., P or not P) and the other side showing information about the truth or falsity of the consequent (e.g., Q or not Q) of the rule. The four cards represent all four possible logical categories (P, not P, Q, and not Q). Only one side of each card is shown to the subjects, but they are allowed to turn over some cards to see the hidden side, and their task is to indicate all and only the cards that, if turned, will reveal a violation of the conditional rule. Due to the nature of the cards, the conditional rule is true unless there are cards combining the P and the not‐Q features. The correct logical answer therefore would be to choose the P card (because it could reveal a not Q) and the not‐Q card (because it could reveal a P). Despite the apparent simplicity of the task, a number of studies [Wason and Shapiro, 1971; for review see Evans, 1982] have demonstrated that subjects fail to perform according to the norms of formal logic when they reason about abstract rules (e.g., rules that arbitrarily pair letters and numbers, such as “If there is an A on one side of the card, then there is a 3 on the other side”), descriptive rules (rules that describe an arbitrary relationship between two events, such as “If a person goes to Boston, then he takes the subway) or causal rules (e.g., ”If a person eats hot chili peppers, then he will drink a cold beer“). With such conditional rules, in fact, subjects usually perform below 20% accuracy, typically choosing the P and Q cards, or the P card alone.
Many authors have observed a marked improvement of subjects' performance using conditional rules with different contents. In fact, most subjects (about 65%) correctly choose the P and the not‐Q cards when presented with conditional rules with a social content [Manktelow and Over, 1991]. A typical example is represented by rules that express “social contracts” (i.e., rules that describe situations in which to obtain a benefit P, an individual is obligated to satisfy a requirement Q, such as “If a person is drinking beer, then he must be over 20 years old”).
These observations have raised a long‐standing debate about the origin of the content effect in the Selection Task and, indirectly, about the nature of human cognitive architecture [Cosmides and Tooby, 2000]. To date, various interpretations of these results have been proposed. According to one of the most influential accounts, throughout evolution the social nature of humans facilitated the development of different adaptive reasoning mechanisms specialized for representing and making inferences in very specific contexts and situations [Cosmides, 1989]. In this perspective, the human cognitive architecture contains a number of domain‐specific representations and inference systems activated by specific kinds of conditional rules and related to specific situations (e.g., “social‐exchange” and “hazardous” contexts, which would automatically activate “social contract” and “precaution” reasoning algorithms, respectively) [Cosmides and Tooby, 2000]. In support of this hypothesis, the Fiddick et al. [2000] subjects achieved high performance on the selection task with both kinds of conditional rules. According to the authors, these results provide evidence for evolved mechanisms for reasoning about social contexts. (There is an on‐going debate on how these data should be interpreted. For different perspectives see Fodor [2000], Girotto et al. [2001], Sperber et al. [1995], and Sperber and Girotto [2002].)
Embracing the recent advancements in the neurobiological approach to the study of social cognition [for review see Adolphs, 2003], in recent years Cosmides and Tooby [2000] and Duchaine et al. [2001] have proposed that each of these reasoning mechanisms is implemented in specific cerebral regions. In support of this hypothesis, Stone et al. [2002] have reported recently the case of R.M., a patient with focal lesions in the limbic system (affecting orbitofrontal cortex, amygdala, and temporal poles) who shows specific impairment in the task involving social contracts, with relative normal performance on the task involving precaution rules. Other authors have observed significantly worse performance on the task involving social contracts, relative to that on the task with descriptive rules, in patients with lesions in the dorsolateral and ventromedial frontal cortex, contrary to what is typically observed in normal subjects [Adolphs, 2001]. Goel et al. [2004] have examined recently the role of the frontal lobes in reasoning about social situations, testing patients with frontal lobe lesions and healthy subjects on the selection task with arbitrary (e.g., “If a card has an A on one side, then it must have a 4 on the other side”) versus social‐contract (e.g., “If a person drinks alcohol, then he must be at least 21”) content. Although both patients and controls performed poorly on the arbitrary condition, only controls performed significantly better on the social condition. Frontal patients failed to show this facilitation. Moreover, this effect was more marked for left‐hemisphere patients, suggesting an asymmetrical involvement of the frontal lobes in social reasoning.
The possible existence of different neural correlates for conditional reasoning is also supported by two recent positron emission tomography (PET) studies investigating the effect of a “logic‐emotional training” on the activated areas during the solution of a modified version of the selection task. A shift from posterior to anterior prefrontal areas, between the no‐training and training conditions, was observed across different subject groups [Houdè et al., 2000], and before and after the same training within the same subject group [Houdè et al., 2001].
None of these studies has explored directly the effects of content of the conditional rule on neural activation. In the present study, we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of reasoning processes involved in the solution of the selection task and the effect on cerebral activation of conditional rules differing in their content. Participants were asked to solve the selection task with descriptive (e.g., “If one cracks walnut shells, then he drinks pond water”) and social‐exchange (e.g., “If you give me sunflower‐seeds, then I give you poppy petals”) conditional rules. The imaging study was preceded by a behavioral experiment, aiming to replicate the results obtained by Cosmides [1989], using an experimental procedure that required multiple repetitions of the same task.
SUBJECTS AND METHODS
Behavioral Experiment
Participants
In total, 55 volunteers (29 males, 26 females; mean age, 22.65 years; age range, 19–26 years) participated in the behavioral study. All reported having had little or no training in formal logic.
Experimental tasks
All experimental stimuli were presented in Italian. Subjects were administered two reasoning tasks, descriptive (DES) and social‐exchange (SE) tasks, according to the terminology adopted by Cosmides [1989]. Both tasks had exactly the same structure as the original version of the selection task, differing only in the content of the conditional rules. In the DES task, subjects were presented with conditional statements in the form “If P, then Q,” which described an arbitrary relation between two actions carried out by a hypothetical member of an unknown tribe (e.g., “If one cracks walnut shells, then he drinks pond water”). In the SE task, subjects were presented with a conditional statement in the form “If P, then Q,” which described an exchange of goods proposed by Big Kiku, the head of an unknown tribe, to four members of the neighboring tribe of Nabars (e.g., “If you give me sunflower‐seeds, then I give you poppy petals”). As in the original studies [Cosmides, 1989; Fiddick et al., 2000], in both tasks subjects were then shown four cards corresponding to the logical categories P, not P, Q, and not Q, and asked to indicate all and only those that, if turned, would reveal whether the rule had been broken. For both tasks, the correct answer consists of choosing the P and the not‐Q cards because whatever the content, only these cards can reveal a falsification of the descriptive or a violation of the social‐exchange conditional rule.
The conditional rules were created using unfamiliar stimuli and impersonal situations, pertinent to characters and objects proper to fictitious tribes, to ensure that subjects had not had any experience with those kinds of contexts. Very similar stimuli were used in the two tasks, with the only difference among the experimental conditions being the kind of reasoning required of the subjects with reference to the content of the conditional rule. In fact, in the DES and SE tasks, subjects had to reason in terms of a possible violation of an arbitrary conditional rule or an agreement between two persons, respectively.
Procedure and materials
Subjects were tested on a laptop PC (Acer Travelmate 514TXV). Stimuli were presented and subjects' answers and response times were recorded using the SuperLab software (Cedrus Corporation, San Pedro, CA).
Each task included four conditional rules, each of which was followed immediately by its corresponding group of four cards. The left–right order of the cards corresponding to the four possible logical categories (P, not P, Q, not Q) was randomly ordered in each trial and the order of the two tasks was counterbalanced across subjects.
Each task was preceded by a screen with specific instructions, consisting of a brief story to introduce the following four conditional rules and an example of the task. Each story was phrased to activate a “detective set” [Van Duyne and Scanlan, 1974], i.e., they created a context suitable for the following rules. When the four cards appeared, subjects indicated those selected by pressing one or more of four keys on the keyboard, and decided by themselves when to advance to the next screen by pressing the spacebar. Subjects were given as much time as they needed to complete the experiment but they were explicitly instructed to answer as fast as possible.
Imaging Experiment
Participants
The results of the behavioral study were used to select subjects for the imaging study. To eliminate possible effects due to differences in performance, only those subjects who scored at or above 50% on both tasks were asked to participate in the fMRI study. In total, 12 right‐handed monolingual native speakers of Italian (7 females, 5 males; mean age, 23.5 years; age range, 21–26 years) took part in the experiment. Handedness was verified using the Edinburgh Inventory [Oldfield, 1971]. All participants declared that they had little or no training in formal logic and none had a history of neurologic or psychiatric disorders. Subjects gave informed written consent to the experimental procedure, which was approved by the local Ethics Committee.
Experimental tasks
The same reasoning tasks used in the behavioral study (DES and SE) were administered in the imaging experiment. A matching task was used as a baseline, in which a conditional rule was presented (e.g., “If one cracks walnut shells, then he drinks pond water”), followed by four cards. Two of them showed the name of objects actually mentioned in the sentence (e.g., walnut shells and pond water) whereas the other two cards showed the name of objects not present in the sentence (e.g., granite rocks and carrot roots; see Fig. 1). The task was to select all and only the cards of the former kind. Each baseline task consisted of exactly the same conditional sentences presented in the corresponding reasoning task. According to a classic “cognitive subtraction” logic, the aim of the baseline was to control for visuoperceptual and linguistic processing, as well as for motor response requirements.
Figure 1.
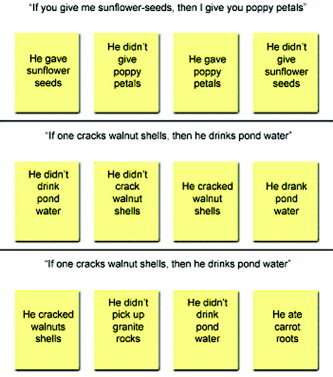
Examples of the stimuli presented in the SE (top), DES (middle), and baseline (bottom) tasks (translated from Italian). The first two tasks differ only in propositional content, whereas their logical structures are identical. In the baseline task, subjects have to indicate all and only the cards that mention the objects whose names are shown in the conditional sentence.
Procedure and materials
A block‐design paradigm was used in which the two reasoning tasks were repeated four times (one for each scanning sequence). The response times from the behavioral experiment were used to calibrate the presentation time of the stimuli during functional scanning.
Each task consisted of four trials presented sequentially with no interstimulus interval. In each trial, a conditional rule was presented for 5 s followed by a group of four cards, which remained on the screen for 20 s, during which subjects could select the cards by pressing a four‐button keyboard. Each reasoning task was followed immediately by the baseline task, which had the same structure except that subjects had only 10 s to select the cards. Specific instructions were shown for 12 s at the beginning of each task (scans corresponding to the instructions were not included in the functional analysis). The order of presentation of the tasks was varied across the four scanning sequences, and the order of the sequences was randomly ordered individually for each subject.
Before being positioned in the scanner, subjects received a brief training to ensure that they had understood the instructions and remembered the brief stories we used to introduce the tasks. In addition, they were instructed to perform the task throughout its 20‐s period and double‐check their answers to ensure accuracy if they finished before the cards were removed from view.
Stimuli were displayed by a RGB projector (800 × 600 pixels) connected to a laptop on a screen located at the back of the camera, and a mirror was placed in front of the subjects' eyes to allow them to see the projected images. Stimuli were presented and subjects' answers were recorded using the SuperLab software.
Image Acquisition
Anatomic T1‐weighted and functional T2*‐weighted magnetic resonance (MR) images were acquired with a 1.5‐T whole‐body scanner (General Electric Medical Systems, Milwaukee, WI) equipped with a standard quadrature head coil for signal reception and transmission. Functional images were acquired using a T2*‐weighted gradient‐echo, echo planar pulse sequence (30 contiguous slices parallel to the anterior–posterior commissure [AC–PC] line covering the whole brain, repetition time [TR] = 4,000 ms, echo time [TE] = 60 ms, flip angle = 90 degrees, field of view [FOV] = 280 × 280 mm2, matrix = 64 × 64, slice thickness = 4 mm, and in‐plane resolution 4.38 × 4.38 mm). Each scanning sequence comprised 143 sequential volumes, for 572 volumes in total for each subject. Immediately after the functional scanning, a high‐resolution T1‐weighted anatomic scan (3D, spoiled gradient recalled (SPGR) pulse sequence, 124 slices, TR = 600 ms, TE = 20 ms, slice thickness = 1.5 mm, and in‐plane resolution 0.78 × 0.78 mm) was acquired for each subject.
fMRI Data Preprocessing
Image preprocessing and statistical analysis were carried out using SPM99 (Wellcome Department of Cognitive Neurology; http://www.fil.ion.ucl.ac.uk/spm) implemented on MATLAB v6.5 (The MathWorks, Natick, MA). Data from one subject were discarded due to the presence of artifacts in the functional images. The first five volumes of each subject were discarded to allow for T1 equilibration effects. For each subject, all volumes were spatially realigned to the first volume of the first session to correct for between‐scan motion, and a mean image from the realigned volumes was created. This image was spatially normalized to the Montreal Neurological Institute (MNI) brain template [Evans et al., 1993] using a 12‐parameter affine normalization and 12 nonlinear iterations with 7 × 8 × 7 basis functions [Ashburner and Friston, 1999]. The derived spatial transformation was then applied to the realigned T2*‐weighted volumes, which after normalization were resampled in 2 × 2 × 4‐mm voxels using sinc interpolation in space. All functional volumes were then spatially smoothed with an 8‐mm full‐width half‐maximum (FWHM) isotropic Gaussian kernel to compensate for residual between‐subject variability after spatial normalization (to make comparisons across subjects) and to permit application of Gaussian random field theory for corrected statistical inference [Worsley and Friston, 1995]. The resulting time series across each voxel were then high‐pass filtered with an upper cut‐off of 120 s, using cosine functions to remove section‐specific low‐frequency drifts in the blood oxygenation level‐dependent (BOLD) signal. Global means were normalized to a grand mean of 100 to remove effects due to global intensity fluctuations in the signal, and the time series temporally smoothed with a low‐pass filter to remove effects due to physiologic noise.
Random‐Effect Statistical Data Analysis
The statistical maps of the simple main effects (each of the reasoning tasks minus the baseline) were first computed. Data were analyzed using a random‐effect model to generalize results to the population from which subjects were extracted [Friston et al., 1999], implemented in a two‐level procedure. In the first level, for each subject condition effects at each voxel were estimated according to the general linear model (GLM) as implemented in SPM99 [Friston et al., 1995] and regionally specific condition effects were evaluated using linear contrasts to produce a contrast image. At the second level, the resulting contrast images from all subjects were entered into a single sample t test to assess the population mean effects. The entire process produced for each comparison of interest (DES–baseline and SE–baseline) a statistical parametric map of the t statistics for each voxel. Maxima were reported in MNI stereotaxic coordinates for foci exceeding a height threshold of P < 0.001, uncorrected for multiple comparisons (t > 4.14). To avoid false positives, only clusters bigger than 20 voxels were considered [Forman et al., 1995].
To investigate the cerebral activations preferentially evoked by the two reasoning conditions, direct statistical comparisons between the two tasks were computed at the second level (random effect), masked by the main effect at P < 0.001. A paired t test on the individual subjects' contrast images obtained from the first‐level analysis was used. Based on a priori hypotheses of the involvement of right hemispheric frontal and parietal foci resulting from the task‐related differences in the simple main effects, the statistical maps were thresholded at P < 0.005, uncorrected for multiple comparisons (t > 3.17).
Conjunction Analysis
To test for areas activated by both reasoning tasks, we carried out a conjunction analysis at the second level (random effect), and applied a threshold of P < 0.01, corrected for multiple comparisons (t > 4.02) to the resulting statistical map. Because the probability reported by such an analysis can pass a certain statistical threshold even if one of the contrasts would not be significant if tested alone, the results of the conjunction analysis were masked with the results of the individual t test for the two reasoning tasks at P < 0.01.
Localization of Activation
For visualization purposes, the foci of maximum activation were superimposed on a high‐resolution anatomic image created by averaging the individual subjects' normalized T1 images with SPM99 and sliced with MRIcro [Rorden and Brett, 2000; see also http://www.mricro.com]. The location of these foci in terms of Brodmann areas was determined using the nomenclature given by Talairach and Tournoux [1988] after correction for differences between the MNI and Talairach coordinate systems by means of a nonlinear transformation (see http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
RESULTS
Behavioral Results
In agreement with previous studies [Cosmides, 1989; Sperber et al., 1995], a significant effect of the content of the conditional rule was present in the behavioral experiment preceding the functional study. To test for significant effects of the task (DES or SE) or of the order of task presentation, a 2 × 2 simple factorial analysis of variance (ANOVA) was carried out. This analysis showed that the percentage of correct answers in the DES task (mean = 26.81; standard deviation [SD] = 24.93) was significantly lower than that in the SE task (mean = 65.45; SD = 32.8; F[1,106] = 47.61; P < 0. 0001). In addition, no significant main effect of the order of task presentation (F[1,106] = 0.368; P > 0.05) nor significant interaction between the task and the order of presentation (F[1,106] = 0.353; P > 0.05) was observed. Despite the fact that our subjects carried out multiple repetitions of the same task as required by the fMRI acquisition, no learning effect was observed. The analysis of the response times showed that subjects took an average of 4.2 s to read and memorize the conditional rules and an average of 17.72 (SD = 4.09) and 16.47 (SD = 3.56) s to select the cards in the DES and the SE tasks respectively, with no significant difference between them (t[1,54] = 1.631; P > 0.05).
The behavioral results during functional scanning showed no significant difference between the mean of correct answers in the DES (mean = 71.02; SD = 34.49) and in the SE (mean = 81.25; SD = 30.07) tasks (F[1,80] = 2.143; P > 0.05). This is in agreement with participants' selection criteria, because only those who reported a percentage of correct answers larger than 50% in both reasoning tasks were selected for the functional study. Again, neither a significant main effect of the order of task presentation throughout the four scanning sequences (F[3,80] = 1.041; P > 0.05) nor significant interaction between the task and the presentation order (F[3, 80] = 0.256; P > 0.05) was observed, indicating that no learning occurred during the experiment. The analysis of the response times during functional scanning showed no significant differences between the DES (mean = 14.70; SD = 1.18) and the SE (mean = 14.47; SD = 1.39) tasks (F[1,1] = 0.175; P > 0.05). Moreover, no significant main effect of the order of task presentation (F[1,3] = 0.719; P > 0.05) nor a significant interaction between the task and the presentation order (F[1,3] = 0.831; P > 0.05) was observed.
Two separate statistical analyses (2 × 2 simple factorial ANOVA) were carried out on the subjects who took part in the functional study to compare reaction times and performance for both reasoning conditions (task: DES or SE) in the behavioral study and during the functional scanning (session: behavioral or functional). The comparison of the reaction times showed no significant main effect of task (F[1,1] = 2.56; P > 0.05). The main effect of the session approached statistical significance (F[1,1] = 4.08; P = 0.068), but the interaction between task and session was not significant (F[1,1] = 0.785; P > 0.05). When comparing the percentages of correct answers, no significant main effect of task (F[1,1] = 1.40; P > 0.05) or of session (F[1,1] = 0.999; P > 0.05) was observed, nor was there significant effect of the interaction between the two factors (F[1,1] = 0.417; P > 0.05).
Imaging Results
The analysis of simple main effects showed that a common cerebral network was recruited by the two reasoning conditions (Figs. 2, 3; Table I). In fact, both tasks activated on the left the inferior parietal lobule (angular gyrus, BA39, and the supramarginal gyrus, BA40), the posterior part of the dorsolateral prefrontal cortex (middle frontal gyrus, BA9/8), the cingulate gyrus (BA32), and the frontal medial cortex, including the medial (BA9/8) and superior (BA8/6) frontal gyri. In addition, the DES task activated a cluster localized in the medial portion of the parietooccipital sulcus, at the border between the precuneus (BA7) and the cuneus (BA19), and the right anterior dorsolateral prefrontal cortex (middle frontal gyrus, BA46, 10). However, the SE task activated the left anterior prefrontal cortex (middle frontal gyrus, BA46), the left caudate nucleus. In the right hemisphere, the SE task activated two frontal and parietal regions approximately mirroring those that in the DES task were activated only in the left hemisphere: the inferior parietal lobule comprising the angular gyrus (BA39) and supramarginal gyrus (BA40), and a dorsolateral frontal cluster within the middle frontal gyrus (BA9).
Figure 2.
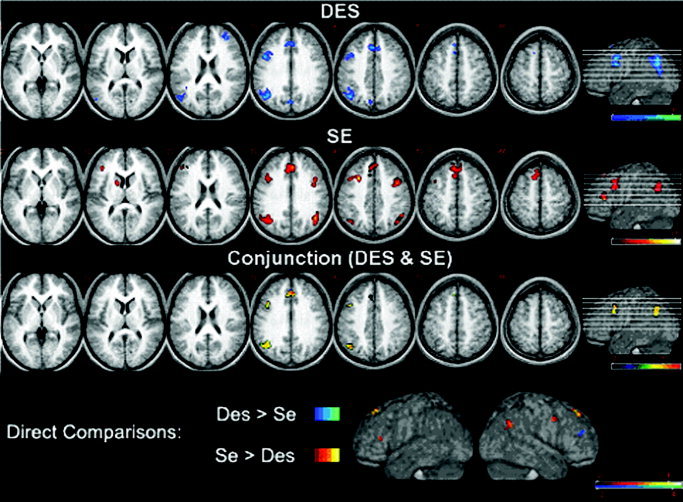
From top to bottom, activation foci for the DES and the SE tasks (P < 0.001, uncorrected for multiple comparisons; t > 4.14), for the conjunction analysis (P < 0.01, corrected for multiple comparisons; t > 4.02), and for the direct comparisons between the two tasks (red, SE > DES; blue, DES > SE; P < 0.005, uncorrected for multiple comparisons; t > 3.17). Areas of increased activation were superimposed on seven representative axial slices of the group mean anatomic image, derived from the T1‐weighted images of the participants. The number above each slice represents its distance (in mm) from the AC–PC plane. The height of the individual slices is also shown, in the rightmost part of the figure, on a rendering of the same average brain.
Figure 3.
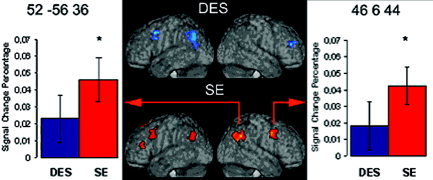
In the center of the figure, lateral views of renderings derived from the subjects' individual normalized T1 images are shown, with superimposed clusters of activation in DES (top) and SE (bottom) reasoning tasks. The colored arrows link the right‐hemispheric frontal and parietal clusters activated in the SE task with histograms indicating BOLD signal change percentage (amplitude of the hemodynamic response curve) in both reasoning tasks (blue, DES; red, SE), after baseline subtraction. For each effect, standard error bars are indicated. Asterisks above histogram bars indicate a significant effect. MNI stereotactic coordinates of the maxima of the clusters (as reported in Table I) are shown in the superior part of each graph.
Table I.
Stereotactic coordinates and t‐values of the foci of maximum activation for DES and SE reasoning tasks
| Region | Hemisphere | BA | x | y | z | t |
|---|---|---|---|---|---|---|
| DES vs. baseline | ||||||
| Cingulate gyrus | L | 32 | −10 | 32 | 32 | 5.60 |
| R | 32 | 2 | 36 | 32 | 5.35 | |
| Medial frontal gyrus | L | 8 | −4 | 28 | 44 | 7.89 |
| R | 8 | 10 | 26 | 40 | 5.61 | |
| Superior frontal gyrus | L | 8 | −4 | 16 | 56 | 4.84 |
| Middle frontal gyrus | L | 8 | −44 | 14 | 44 | 9.78 |
| L | 9 | −42 | 18 | 32 | 5.68 | |
| Superior frontal gyrus | L | 6 | −8 | 14 | 64 | 6.78 |
| Middle frontal gyrus | R | 46 | 38 | 46 | 20 | 9.37 |
| R | 10 | 34 | 56 | 24 | 8.26 | |
| Parietooccipital junction | L | 39/19 | −54 | −70 | 16 | 5.41 |
| Angular gyrus | L | 39 | −38 | −48 | 24 | 4.46 |
| L | 39 | −48 | −68 | 36 | 7.50 | |
| Supramarginal gyrus | L | 40 | −46 | −66 | 40 | 7.36 |
| L | 40 | −46 | −52 | 44 | 4.94 | |
| Precuneus | L | 7 | −6 | −78 | 40 | 7.18 |
| R | 7 | 2 | −68 | 36 | 4.88 | |
| Cuneus | L | 19 | −4 | −80 | 36 | 6.23 |
| SE vs. baseline | ||||||
| Cingulate gyrus | L | 32 | −4 | 28 | 32 | 6.45 |
| R | 32 | 4 | 36 | 32 | 8.07 | |
| Medial frontal gyrus | L | 9 | −4 | 40 | 36 | 6.24 |
| R | 9 | 4 | 42 | 44 | 5.63 | |
| L | 8 | −6 | 30 | 44 | 5.07 | |
| R | 8 | 6 | 32 | 52 | 5.78 | |
| Superior frontal gyrus | L | 6 | −6 | 14 | 68 | 6.55 |
| R | 8/6 | 10 | 16 | 56 | 4.93 | |
| Middle frontal gyrus | L | 9 | −42 | 20 | 36 | 6.31 |
| L | 8 | −28 | 18 | 44 | 11.63 | |
| L | 6 | −42 | 6 | 56 | 4.27 | |
| R | 9 | 46 | 6 | 44 | 8.01 | |
| R | 9 | 48 | 14 | 32 | 5.73 | |
| L | 46 | −40 | 36 | 12 | 7.58 | |
| L | 46 | −50 | 38 | 20 | 6.23 | |
| Parietooccipital junction | L | 39/19 | −52 | −62 | 28 | 4.90 |
| Angular gyrus | L | 39 | −46 | −64 | 32 | 5.49 |
| Supramarginal gyrus | L | 40 | −44 | −64 | 40 | 5.46 |
| Parietooccipital junction | R | 39/19 | 46 | −64 | 28 | 7.72 |
| Angular gyrus | R | 39 | 46 | −58 | 32 | 7.90 |
| R | 39 | 52 | −56 | 36 | 8.47 | |
| Supramarginal gyrus | R | 40 | 46 | −68 | 40 | 7.39 |
| Caudate nucleus | L | −14 | 8 | 12 | 6.76 |
Stereotactic coordinates and t‐values of the foci of maximum activation for the DES and the SE reasoning tasks (P < 0.001, uncorrected for multiple comparisons; t > 4.14). Coordinates (x, y, and z) are expressed in MNI space adopted by SPM99, in terms of distance (in mm) from the anterior commissure. The foci were anatomically localized on the standard stereotactic brain atlas developed by Talairach and Tournoux [1988] after correcting for differences between the MNI and Talairach coordinate systems using a nonlinear transformation.
DES, descriptive; SE, social‐exchange; BA, Brodmann area; L, left; R, right.
The direct comparisons between the two tasks confirmed the differences observed in the simple main effects (Figs. 2, 3; Table II). The comparison of the DES task with the SE task resulted in activation of the right anterior prefrontal cortex (middle frontal gyrus, BA46). The reverse comparison revealed bilateral activations in the medial portions of the superior frontal gyrus (BA8), the left anterior prefrontal cortex (middle frontal gyrus, BA46), the left caudate nucleus, the right posterior prefrontal cortex (middle frontal gyrus, BA9), and the right inferior parietal lobule (BA39).
Table II.
Results of direct comparisons between BOLD signals evoked by SE and DES reasoning tasks
| Region | Hemisphere | BA | x | y | z | t |
|---|---|---|---|---|---|---|
| SE > DES | ||||||
| Superior frontal gyrus | L | 8 | −8 | 44 | 52 | 3.29 |
| R | 8 | 8 | 44 | 52 | 3.54 | |
| L | 8 | −8 | 40 | 56 | 4.35 | |
| R | 8 | 8 | 38 | 56 | 3.81 | |
| Middle frontal gyrus | L | 46 | −40 | 32 | 16 | 3.35 |
| R | 9 | 40 | 6 | 40 | 3.97 | |
| Angular gyrus | R | 39 | 46 | −60 | 32 | 3.44 |
| R | 39 | 50 | −60 | 36 | 3.42 | |
| Caudate nucleus | L | −16 | 10 | 12 | 3.28 | |
| DES > SE | ||||||
| Middle frontal gyrus | R | 46 | 40 | 46 | 20 | 4.22 |
| Conjunction analysis | ||||||
| Cingulate/medial frontal gyrus | R | 32/9 | 4 | 38 | 32 | 5.87 |
| Medial frontal gyrus | L | 8 | −6 | 30 | 44 | 5.74 |
| Superior frontal gyrus | L | 8 | −6 | 34 | 56 | 4.41 |
| Middle frontal gyrus | L | 8 | −44 | 14 | 44 | 5.97 |
| L | 9 | −42 | 18 | 32 | 5.03 | |
| Angular gyrus | L | 39 | −44 | −58 | 32 | 4.80 |
| L | 39 | −50 | −66 | 36 | 5.09 | |
| Supramarginal gyrus | L | 40 | −44 | −64 | 40 | 5.50 |
| L | 40 | −42 | −66 | 44 | 5.51 |
Results of direct comparisons between the BOLD signal evoked by SE vs. DES (masked by SE; top) and DES vs. SE (masked by DES; middle) reasoning tasks (P < 0.005, uncorrected for multiple comparisons; t > 3.17) and for conjunction analysis (bottom; P < 0.01, corrected for multiple comparisons; t > 4.02). Stereotactic coordinates (x, y, and z) in MNI space and maximal t‐value of clusters are reported.
DES, descriptive; SE, social‐exchange; BA, Brodmann area; L, left; R, right.
The commonly activated regions were revealed also by the results of the conjunction analysis (Fig. 2; Table II). In fact, the statistical map showed a cerebral network confined almost entirely in the left hemisphere, comprising three main functional clusters: a temporoparietal cluster including the angular gyrus (BA39) and the supramarginal gyrus (BA40), a frontal lateral cluster mainly localized in the posterior portion of the dorsolateral prefrontal cortex within the middle frontal gyrus (BA9/8), and a frontal medial cluster, which extended dorsally from cingulate sulcus (BA32/9) through the medial frontal gyrus (BA9/8), up to the left superior frontal gyrus (BA8).
The commonly activated regions in the left hemisphere differed in their spatial extent according to the reasoning condition. The left temporoparietal cluster was wider in the DES (354 voxels, volume = 5,664 mm3) than it was in the SE (207 voxels, volume = 3,312 mm3) task. An opposite pattern was observed for both the left frontal lateral and the frontal medial clusters, which were more extensive in the SE than in the DES task (respectively, 211 [volume = 3,376 mm3] vs. 120 voxels [volume = 1,920 mm3] and 482 [7,712 mm3] vs. 175 voxels [2,800 mm3]). In particular, in the SE task compared to the DES task, the frontal lateral cluster extended more dorsally, up to the superior frontal sulcus (BA6), whereas the frontal medial cluster was more extended both rostrally and dorsally, reaching the superior frontal gyrus (BA6).
DISCUSSION
The aim of this study was to investigate whether the pattern of brain activation associated with solving the selection task is affected by the content of the conditional rule. The first step was to demonstrate with a behavioral experiment the existence of a significant content effect in a population of healthy subjects. In line with previous studies, the behavioral results showed that solving the selection task is easier when it is based on rules with a social content [see Manktelow and Over, 1991]. To prevent possible problems of interpretation, only those subjects who performed at or above 50% on both behavioral tasks were selected for participation in the imaging study. We did this to avoid difficulty with interpreting activation data associated with different performance levels. This is an issue that has been discussed extensively in relation to clinical studies [Price and Friston, 1999], but is also relevant to normal subjects. For example, it is well known that differences in performance due to differential task difficulty can result in significant changes in brain activity [Barch et al., 1997]. In the present experiment, in agreement with participants' selection criteria, behavioral results during functional scanning showed no significant difference in the percentage of correct answers between the two tasks (P > 0.05). The observed differences in brain activation between the two reasoning conditions therefore can be attributed to differences in the content of the stimuli and not to differences in difficulty or performance level between the two versions of the task.
The imaging results indicated that although the patterns of activation largely overlap in the two tasks, some cerebral regions are activated preferentially based on the content of the stimuli. We first discuss the results of the conjunction analysis and then consider the regions of activation that differ across the two tasks.
The conjunction analysis shows the regions activated during both reasoning tasks, i.e., independently of the specific content of the conditional rule. The DES and SE tasks both activated a left‐sided network, including three main functional clusters.
The first, more posterior cluster is located near the temporoparietal junction and comprises portions of the angular gyrus (BA39) and supramarginal gyrus (BA40). This is an area that has been linked traditionally to logicogrammatical reasoning. Luria [1973] associated damage to this region with the clinical syndrome of “semantic aphasia,” characterized by disorders of “quasi‐spatial” reasoning involving linguistic as well as mathematical tasks. More recently, functional imaging data have considered this area to be a crucial component of the network activated during semantic tasks [Demonet et al., 1994; Vandenberghe et al., 1996] and during mental manipulation of numerical quantities [Dehaene and Cohen, 1997; Lee, 2000].
The second cluster is located in the posterior part of the dorsolateral frontal cortex (BA9, 8), a region that has been implicated previously in the inspection and manipulation of information already maintained in memory [Fletcher and Henson, 2001] and in executive functioning. In particular, according to Petrides [1995], BA9 is involved typically in cognitive processes concerning monitoring and manipulation of information in working memory. Consistent with these hypotheses, some studies have shown this region to be implicated in rule‐governed tasks, such as the Wisconsin Card‐Sorting Test [Tulving et al., 1994]. For example, the same region in the left posterior prefrontal cortex (with activation peak at coordinates −44, 16, 30) was observed to be active during the solution of the Wisconsin Card‐Sorting Test by Monchi et al. [2001]. According to other authors, dorsolateral frontal cortex is involved in relational integration, which is necessary for reasoning processes that require one to consider multiple relations simultaneously [Robin and Holyoak, 1995] and to integrate information from various sources [Christoff et al., 2001; Kroger et al., 2002]. In particular, a specific activation of the posterior part of the left prefrontal cortex (with peak coordinates at −44, 4, 30) was observed by Christoff et al. [2001] during two‐relational but not during one‐relational and zero‐relational reasoning problems. Moreover, the activation of the dorsolateral prefrontal cortex (with peak coordinates at −50, 22, 26, but comprising the region described in the present experiment) was reported by Kroger et al. [2002] for reasoning problems with high levels of relational complexity. These results are also supported by studies on patients with the frontal variant of frontotemporal dementia, using both deductive and inductive reasoning tasks, showing specific impairments at two‐relational but not at zero‐ and one‐relational reasoning problems [Waltz et al., 1999].
The third cluster is located in the medial prefrontal cortex, including the medial and superior frontal gyri (BA8) and portions of the cingulate cortex (BA32/9), regions whose activity has been associated with executive control [Posner and Dehaene, 1994] and selection and coordination of subgoals, irrespective of the content of the material held and manipulated in working memory [Fletcher and Henson, 2001]. Moreover, activation of this region has been observed in social reasoning situations [Fletcher et al., 1995; Frith and Frith, 2003]. For example, Goel et al. [1995] found the same region in the medial prefrontal cortex (−6, 46, 28) to be activated in a cognitive task requiring subjects to draw inferences based on others' knowledge states. In addition, activation of cingulate and medial frontal gyri (with peak coordinates at −12, 28, 28) was associated by Goel et al. [1997] with the ability to make generalizations and abstractions about world knowledge.
The activation of this left‐sided network is consistent with previous literature indicating a major role of the left hemisphere in the processes underlying logical reasoning [Wharton and Grafman, 1998]. Interestingly, Noveck et al. [2004] have observed recently that a similar left‐lateralized frontoparietal network is involved during conditional reasoning, in particular while solving modus tollens (“If P, then Q,” “not Q,” then “not P”), which is a critical component of the reasoning processes involved in the selection task. The latter is more complex, in that it requires participants to produce counterexamples to the conditional rule by generating hypotheses and verifying them deductively by means of modus tollens. Basically, the same left‐lateralized cerebral network, including the inferior parietal lobule (−39, −54, 39), the cingulate and medial frontal gyri (−3, 18, 42), and the dorsolateral prefrontal cortex (−45, 15, 39) was in fact activated in the direct comparison between modus tollens and modus ponens.
Similar supporting evidence also comes from lesion‐based neuropsychologic studies using a variety of experimental paradigms and different kinds of reasoning tasks. For example, Read [1981] observed that the performance of left temporal lobectomy patients on three‐term relational problems with semantic content (e.g., “John is taller than Mark. Mark is taller than Robert. Who is the tallest?”) was significantly inferior to that of both right lobectomy patients and normal controls, even though the task involved easily imaginable dimensions (e.g., taller than, fatter than) and subjects were encouraged to use visual strategies.
The idea that reasoning is a left‐hemisphere phenomenon is also supported by split‐brain investigations. Gazzaniga and Smylie [1984] explored the inferential abilities of the two disconnected hemispheres in split‐brain patients, observing that only the left hemisphere could make inferences from the experimental stimuli, despite the fact that the concept to be inferred was available to the right hemisphere. More recently, Wolford et al. [2000] investigated the different abilities of the separated hemispheres in a probability guessing task, which suggested that the left hemisphere has a dominant role in hypothesis generation, which is another critical component of the processes engaged in solving the selection task. In their view, the left hemisphere, particularly the frontal and prefrontal areas, houses a mechanism (the so‐called “interpreter”) that uses all available information to search for and posit causal relationships among events [see also Gazzaniga, 1989].
Finally, in a recent study using matched verbal and spatial reasoning tasks, Langdon and Warrington [2000] found that only left‐hemisphere patients failed at the verbal tasks, whereas both left‐ and right‐hemisphere patients failed at the spatial tasks, suggesting a critical role of the left hemisphere in both verbal and spatial logical reasoning.
Cerebral regions preferentially activated by either reasoning task were also observed, supporting a long‐standing literature that shows significant differences in the way subjects solve these and, presumably, in the way in which the brain is activated during such activity [see Rodick et al., 2000].
In the DES task, activation in the medial portion of the occipitoparietal sulcus was observed, comprising parts of the precuneus (BA7) and cuneus (BA19). This region has been implicated in visuospatial processing and imaginative operations, and its activation has been observed previously in multiple reasoning tasks [Acuna et al., 2002; Goel and Dolan, 2001; Nichelli et al., 1994; Knauff et al., 2002, 2003; Kroger et al., 2002; Paulus et al., 2001], frequently in association with activity in the inferior parietal lobe and prefrontal cortex [Baker et al., 1996; Dagher et al., 1999]. In particular, activation of the same region was reported by Osherson et al. [1998] in the comparison between a deductive reasoning task and baseline (peak coordinates −10, −86, 24) and in the direct comparison between the same deductive reasoning task and a probabilistic reasoning task (peak coordinates −10, −80, 44). The same region was also observed to be activated by a wide array of cognitive tasks, including hypothesis testing and response selection [Elliott and Dolan, 1998], planning [Lazeron et al., 2000], category shifting [Nagahama et al., 1998], and generation of response sequences [de Zubicaray et al., 1998]. Moreover, recent findings have associated activity in the precuneus and in the superior temporal gyrus with subprocesses involved in the maintenance of strategies in the presence of uncertainty [Jessen et al., 1999; Opitz et al., 1999].
Activations in the DES task were also observed in the anterior part of the right middle frontal gyrus (BA46, 10), a region that recent studies using multiple‐choice gambling tasks have shown to be involved in resolving conflicting decisions [Paulus et al., 2001; Rogers et al., 1999]. According to these findings, in the presence of equally conflicting response alternatives this region of the prefrontal cortex provides important modulatory information that guides the selection of the responses. Moreover, Kroger et al. [2002] suggested that the anterior part of the dorsolateral prefrontal cortex (extending from BA46 to BA10 up to the frontal pole) is activated by particularly difficult reasoning and problem‐solving tasks. Activation of this area has been observed during highly complex tasks across a wide range of domains [for a review, see Christoff and Gabrieli, 2000]. According to Christoff et al. [2001], this area may be involved selectively in active processing, such as manipulation or evaluation, performed upon self‐generated information.
The innovative result of the present study was the preferential activation of the right cerebral hemisphere during the SE task. Indeed, the social content led to the activation in the right hemisphere of those frontal lateral and parietal areas that in the DES task were confined to the left hemisphere. These activation differences could not be attributed to a difference in familiarity with the stimuli, because all the sentences in both reasoning tasks referred to the same kind of unfamiliar objects. This finding indicates that the claim of left‐hemispheric dominance for reasoning processes, based on the evidence reviewed above, is in need of some qualifications.
A first aspect to consider is the differential involvement of the two hemispheres in relation to different contents. In fact, previous studies comparing the performance between right‐ and left‐brain damaged patients on a variety of reasoning tasks yielded ambiguous results. Some studies showed that content‐independent reasoning processes are mediated primarily by the left hemisphere, whereas content‐dependent reasoning is mediated by regions in the right hemisphere [Wharton and Grafman, 1998]. Other recent results cast some doubts on this claim, suggesting a dominant role of the left hemisphere in reasoning about social material [Goel et al., 2004]. In this case, there is also some convergent evidence from other lines of investigation that needs to be considered.
In the case of localized lesions, Whitaker et al. [1991] observed specific kinds of deficits consequent to left‐ or right‐hemisphere lesions during the solution of deductive tasks such as modus ponens and modus tollens. Patients with left temporal lesions performed worse overall compared to healthy subjects and patients with right lesions; however, right‐hemisphere lesioned patients still retained world knowledge. For example, given the conditional “If it rains, the streets will be dry,” and the claim “It rains,” most right‐lesioned patients concluded that “The streets will be wet.”
The differential role of the two hemispheres in solving syllogisms with familiar or unfamiliar content was also investigated by Deglin and Kinsbourne [1996] using the transient effects of electroconvulsive therapy (ECT). This technique allows one to suppress the activity of one hemisphere for 30–40 min (simulating a brain lesion) with the simultaneous facilitation of the opposite hemisphere. Their results suggest a dominant role of the left hemisphere in the logical processing of deduction and a reciprocal inhibition between the typical reasoning style of each hemisphere in the normal brain. Indeed, the authors concluded that the left hemisphere reasons in isolation, in terms of formal logic operations and “indifferent to the nature of the material operated on”; on the other hand, right hemisphere activity “is characterized by the tendency to incorporate already existing knowledge,” and “seems incapable of the willing suspension of the disbelief” [Deglin and Kinsbourne, 1996: p. 303].
This hypothesis is in agreement with the results obtained previously by Golding [1981], who compared the performance of left‐hemisphere lesioned patients, right‐hemisphere lesioned patients, and normal controls on the selection task with an abstract‐descriptive content. He observed that only one of the left‐hemisphere lesioned patients (1.7%) and no control subjects correctly selected the P and not‐Q cards, whereas 10 of 20 right‐lesioned patients selected them correctly. The results were interpreted in terms of interhemispheric inhibition and of a dominant role of the left hemisphere in the formal‐logical processes of reasoning required to solve the task, which would be released fully after a lesion in the right hemisphere.
There is also independent evidence for a crucial role of the right hemisphere in social cognition. Tranel et al. [2002] have observed recently a marked deficit in social behavior, emotional functioning, and decision making in patients with lesions confined to the prefrontal regions of the right hemisphere, but not in patients with similar lesions in the left hemisphere.
Minor differences were also observed in the left hemisphere. The SE task activated the caudate nucleus, a structure that is related anatomically to the prefrontal cortex through multiple frontostriatal connections [Alexander et al., 1986]. Converging evidence indicate that the prefrontal cortex and the caudate are major components of a neural system mediating complex reasoning [Christoff et al., 2001]. In particular, caudate nucleus activation was observed for multiple reasoning tasks [Dagher et al., 1999; Goel et al., 2000; Goldberg et al., 1998; Owen et al., 1996; Osherson et al., 1998; Rao et al., 1997]. Moreover, from the clinical and neuropsychologic literature, caudate lesions are known to produce specific impairments in complex reasoning processes, such as planning, organizing, and sequencing [Mendez et al., 1989; Petty et al., 1996]. Similar deficits in complex reasoning also occur in caudate‐lesioned animals [Divac et al., 1967] and in early Huntington's disease, which involves the caudate nuclei. Finally, the SE task also activated a cluster located in the left middle frontal gyrus (BA46).
In general, frontal lateral and frontal medial activations were both more extensive in the SE than in the DES task, in line with results obtained by Houdè et al. [2000, 2001], who observed a shift from posterior to frontal activations in association with training on a modified version of the selection task, which resulted in improved performance. In the present study, this difference was particularly evident in the medial prefrontal cortex, which other studies observed to be active in social reasoning situations [Fletcher et al., 1995; Frith and Frith, 2003; Goel et al., 1995]. On the contrary, parietal activations were more extensive in the DES than in the SE task. More generally, the present results are in line with the claim that frontal and parietal regions are activated preferentially by familiar and abstract content, respectively [Goel et al., 2000; Goel and Dolan, 2001]. It is worth noting that the observation of right hemispheric areas that are preferentially activated by social content is, in general, consistent with the hypothesis of the existence of cerebral specializations for reasoning about the social sphere [Adolphs, 2003; Cosmides and Tooby, 2000; Duchaine et al., 2001]. Because only one kind of social content (namely, social‐exchange) was investigated in the present experiment; however, our data do not seem sufficient to support the hypothesis of the existence of cerebral specializations for specific contents within the social domain, as opposed to the hypothesis of a more general specialization for the entire social domain.
CONCLUSIONS
In conclusion, the activation of a left‐hemispheric frontoparietal network independent of content, observed in the present study, is consistent with the hypothesis of a major role of the left hemisphere in deductive reasoning. As for the effect of content on cerebral activation, social content, relative to descriptive, more abstract material, resulted in more extensive frontal activations and in the involvement of frontal and parietal regions in the right hemisphere. The latter finding is consistent with studies showing a major role of the right hemisphere in the processing of world knowledge during reasoning [Deglin and Kinsbourne, 1996; Whitaker, 1991] and in decision making involving the social sphere [Tranel et al., 2002]. Future studies will help clarify whether this involvement is associated with the entire social domain, or if different cerebral regions are recruited by specific types of social content.
Acknowledgements
This work was supported by MIUR (Ministero Instruzione Università e Ricerca; grant PRIN‐COFIN R0146). We thank Prof. Kevin McCabe for his help in preparing the study, and all of the participants for their infinite patience. Special thanks to Prof. Vittorio Girotto for his valuable input in all stages of this study, and to Analia Arevalo for correcting the English.
REFERENCES
- Acuna BD, Eliassen JC, Donoghue JP, Sanes JN (2002): Frontal and parietal lobe activation during transitive inference in humans. Cereb Cortex 12: 1312–1321. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2001): The neurobiology of social cognition. Curr Opin Neurobiol 11: 231–239. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2003): Cognitive neuroscience of human social behaviour. Nat Rev Neurosci 4: 165–178. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9: 357–381. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW (1996): Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia 34: 515–526. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE (2000): The frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology 28: 168–186. [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD (2001): Rostrolateral prefrontal cortex involvement in relational integration during reasoning. Neuroimage 14: 1136–1149. [DOI] [PubMed] [Google Scholar]
- Cosmides L (1989): The logic of social exchange: has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition 31: 187–276. [DOI] [PubMed] [Google Scholar]
- Cosmides L, Tooby J (2000): The cognitive neuroscience of social reasoning In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; p 1259–1270. [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ (1999): Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain 122: 1973–1987. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Williams SC, Wilson SJ, Rose SE, Brammer MJ, Bullmore ET, Simmons A, Chalk JB, Semple J, Brown AP, Smith GA, Ashton R, Doddrell DM (1998): Prefrontal cortex involvement in selective letter generation: a functional magnetic resonance imaging study. Cortex 34: 389–401. [DOI] [PubMed] [Google Scholar]
- Deglin VL, Kinsbourne M (1996): Divergent thinking styles of the hemispheres: how syllogisms are solved during transitory hemisphere suppression. Brain Cogn 31: 285–307. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L (1997): Cerebral pathways for calculation: double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex 33: 219–250. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Price C, Wise R, Frackowiak RS (1994): Differential activation of right and left posterior sylvian regions by semantic and phonological tasks: a positron‐emission tomography study in normal human subjects. Neurosci Lett 182: 25–28. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK (1967): Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol 63: 184–190. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Cosmides L, Tooby J (2001): Evolutionary psychology and the brain. Curr Opin Neurobiol 11: 225–230. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ (1998): Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage 8: 17–29. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM (1993): 3D Statistical neuroanatomical model from 305 MRI volumes. IEEE Conference Record, Nuclear Science Symposium and Medical Imaging Conference (San Francisco). p 1813–1817.
- Evans JSBT. 1982. The psychology of deductive reasoning. Boston: Routledge and Kegan Paul; 277 p. [Google Scholar]
- Fiddick L, Cosmides L, Tooby J (2000): No interpretation without representation: the role of domain‐specific representations and inferences in the Wason selection task. Cognition 77: 1–79. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ (1995): Brain systems for encoding and retrieval of auditory‐verbal memory. An in vivo study in humans. Brain 118: 401–416. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN (2001): Frontal lobes and human memory: insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Fodor J (2000): Why we are so good at catching cheaters. Cognition 75: 29–32. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ (1999): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD (2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358: 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaniga MS, Smylie CS (1984): Dissociation of language and cognition. A psychological profile of two disconnected right hemispheres. Brain 107: 145–153. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS (1989): Organization of the human brain. Science 245: 947–952. [DOI] [PubMed] [Google Scholar]
- Girotto V, Kemmelmeier M, Sperber D, van der Henst JB (2001): Inept reasoners or pragmatic virtuosos? Relevance and the deontic selection task. Cognition 81: 69–76. [DOI] [PubMed] [Google Scholar]
- Goel V, Buchel C, Frith C, Dolan RJ (2000): Dissociation of mechanisms underlying syllogistic reasoning. Neuroimage 12: 504–514. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ (2000): Anatomical segregation of component processes in an inductive inference task. J Cogn Neurosci 12: 110–119. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ (2001): Functional neuroanatomy of three‐term relational reasoning. Neuropsychologia 39: 901–909. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ (2003): Explaining modulation of reasoning by belief. Cognition 87: 11–22. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S (1997): The seats of reason? An imaging study of deductive and inductive reasoning. Neuroreport 8: 1305–1310. [DOI] [PubMed] [Google Scholar]
- Goel V, Gold B, Kapur S, Houle S (1998): Neuroanatomical correlates of human reasoning. J Cogn Neurosci 10: 293–302. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M (1995): Modeling other minds. Neuroreport 6: 1741–1746. [DOI] [PubMed] [Google Scholar]
- Goel V, Shuren JE, Sheesley L, Grafman J (2004): Asymmetrical involvement of frontal lobes in social reasoning. Brain 127: 783–790. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, Mattay VS, Gold JM, Weinberger DR (1998): Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage 7: 296–303. [DOI] [PubMed] [Google Scholar]
- Golding E (1981): The effect of unilateral brain lesion on reasoning. Cortex 17: 31–40. [DOI] [PubMed] [Google Scholar]
- Houdè O, Zago L, Crivello F, Moutier S, Pineau A, Mazoyer B, Tzourio‐Mazoyer N (2001): Access to deductive logic depends on a right ventromedial prefrontal area devoted to emotion and feeling: evidence from a training paradigm. Neuroimage 14: 1486–1492. [DOI] [PubMed] [Google Scholar]
- Houdè O, Zago L, Mellet E, Moutier S, Pineau A, Mazoyer B, Tzourio‐Mazoyer N (2000): Shifting from the perceptual brain to the logical brain: the neural impact of cognitive inhibition training. J Cogn Neurosci 12: 721–728. [DOI] [PubMed] [Google Scholar]
- Jessen F, Erb M, Klose U, Lotze M, Grodd W, Heun R (1999): Activation of human language processing brain regions after the presentation of random letter strings demonstrated with event‐related functional magnetic resonance imaging. Neurosci Lett 270: 13–16. [DOI] [PubMed] [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson‐Laird PN (2003): Reasoning, models, and images: behavioral measures and cortical activity. J Cogn Neurosci 15: 559–573. [DOI] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW (2002): Spatial imagery in deductive reasoning: a functional MRI study. Brain Res Cogn Brain Res 13: 203–212. [DOI] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ (2002): Recruitment of anterior dorsolateral prefrontal cortex in human reasoning: a parametric study of relational complexity. Cereb Cortex 12: 477–485. [DOI] [PubMed] [Google Scholar]
- Langdon D, Warrington EK (2000): The role of the left hemisphere in verbal and spatial reasoning tasks. Cortex 36: 691–702. [DOI] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts SA, Machielsen WC, Scheltens P, Witter MP, Uylings HB, Barkhof F (2000): Visualizing brain activation during planning: the Tower of London test adapted for functional MR imaging. AJNR Am J Neuroradiol 21: 1407–1414. [PMC free article] [PubMed] [Google Scholar]
- Lee KM (2000). Cortical areas differentially involved in multiplication and subtraction: a functional magnetic resonance imaging study and correlation with a case of selective acalculia. Ann Neurol 48: 657–661. [PubMed] [Google Scholar]
- Luria AR (1973): Towards the mechanisms of naming disturbance. Neuropsychologia 11: 417–421. [DOI] [PubMed] [Google Scholar]
- Manktelow KI, Over DE (1991): Social roles and utilities in reasoning with deontic conditionals. Cognition 39: 85–105. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Adams NL, Lewandowski KS (1989): Neurobehavioral changes associated with caudate lesions. Neurology 39: 349–354. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001): Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Sadato N, Yamauchi H, Katsumi Y, Hayashi T, Fukuyama H, Kimura J, Shibasaki H, Yonekura Y (1998): Neural activity during attention shifts between object features. Neuroreport 9: 2633–2638. [DOI] [PubMed] [Google Scholar]
- Nichelli P, Grafman J, Pietrini P, Alway D, Carton JC, Miletich R (1994): Brain activity in chess playing. Nature 369: 191. [DOI] [PubMed] [Google Scholar]
- Noveck IA, Goel V, Smith KW (2004): The neural basis of conditional reasoning with arbitrary content. Cortex 40: 613–622. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, Friederici AD, von Cramon DY (1999): The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cereb Cortex 9: 379–391. [DOI] [PubMed] [Google Scholar]
- Osherson D, Perani D, Cappa S, Schnur T, Grassi F, Fazio F (1998): Distinct brain loci in deductive versus probabilistic reasoning. Neuropsychologia 36: 369–376. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC (1996): Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci 8: 353–364. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Osherson D (2001): New evidence for distinct right and left brain systems for deductive versus probabilistic reasoning. Cereb Cortex 11: 954–965. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL (2001): Prefrontal, parietal, and temporal cortex networks underlie decision‐making in the presence of uncertainty. Neuroimage 13: 91–100. [DOI] [PubMed] [Google Scholar]
- Petrides M (1995): Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci 769: 85–96. [DOI] [PubMed] [Google Scholar]
- Petty RG, Bonner D, Mouratoglou V, Silverman M (1996): Acute frontal lobe syndrome and dyscontrol associated with bilateral caudate nucleus infarctions. Br J Psychiatry 168: 237–240. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S (1994): Attentional networks. Trends Neurosci 17: 75–79. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1999): Scanning patients with tasks they can perform. Hum Brain Mapp 8: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Bobholz JA, Hammeke TA, Rosen AC, Woodley SJ, Cunningham JM, Cox RW, Stein EA, Binder JR (1997): Functional MRI evidence for subcortical participation in conceptual reasoning skills. Neuroreport 8: 1987–1993. [DOI] [PubMed] [Google Scholar]
- Read DE (1981): Solving deductive‐reasoning problems after unilateral temporal lobectomy. Brain Lang 12: 116–127. [DOI] [PubMed] [Google Scholar]
- Robin N, Holyoak KJ (1995): Relational complexity and the functions of prefrontal cortex In: Gazzaniga MS, Bizzi E, editors. The cognitive neuroscience (1st ed.). Cambridge, MA: MIT Press; p 987–997. [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999): Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19: 9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Brett M (2000): Stereotaxic display of brain lesions. Behav Neurol 12: 191–200. [DOI] [PubMed] [Google Scholar]
- Shuren JE, Grafman J (2002): The neurology of reasoning. Arch Neurol 59: 916–919. [DOI] [PubMed] [Google Scholar]
- Sperber D, Cara F, Girotto V (1995): Relevance theory explains the selection task. Cognition 57: 31–95. [DOI] [PubMed] [Google Scholar]
- Sperber D, Girotto V (2002): Use or misuse of the selection task? Rejoinder to Fiddick, Cosmides, and Tooby. Cognition 85: 277–290. [DOI] [PubMed] [Google Scholar]
- Stone VE, Cosmides L, Tooby J, Kroll N, Knight RT (2002): Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proc Natl Acad Sci USA 99: 11531–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotactic atlas of the human brain. Stuttgart: Thieme; 122 p. [Google Scholar]
- Tranel D, Bechara A, Denburg NL (2002): Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision‐making, and emotional processing. Cortex 38: 589–612. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S (1994): Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D (1981): The framing of decisions and the psychology of choice. Science 211: 453–458. [DOI] [PubMed] [Google Scholar]
- Van Duyne HJ, Scanlan D (1974): Left‐right ear differences in auditory perception of verbal instruction for nonverbal behavior: a preliminary report. Neuropsychologia 12: 545–548. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS (1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone K, Mishkin FS, de Menezes Santos M, Thomas CR, Miller BL (1999): A system for relational reasoning in human prefrontal cortex. Psychol Sci 10: 119–125. [Google Scholar]
- Wason PC (1966): Reasoning In: Foss B, editor. New horizons in psychology. Harmondsworth: Penguin; 448 p. [Google Scholar]
- Wason PC (1969): Regression in reasoning? Br J Psychol 60: 471–480. [DOI] [PubMed] [Google Scholar]
- Wason PC, Johnson‐Laird PN (1972): Psychology of reasoning: structure and content. Cambridge, MA: Harvard University Press; 264 p. [Google Scholar]
- Wason PC, Shapiro DA (1971): Natural and contrived experience in a reasoning problem. Q J Exp Psychol 23: 63–71. [Google Scholar]
- Wharton CM, Grafman J (1998): Deductive reasoning and the brain. Trends Cogn Sci 2: 54–59. [DOI] [PubMed] [Google Scholar]
- Whitaker H, Markovits H, Savary F, Grou C, Braun C (1991): Inference deficits after brain damage. J Clin Exp Neuropsychol 13: 38. [Google Scholar]
- Wolford G, Miller MB, Gazzaniga M (2000): The left hemisphere's role in hypothesis formation. J Neurosci 20: RC64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995): Analysis of fMRI time‐series revisited‐again. Neuroimage 2: 173–181. [DOI] [PubMed] [Google Scholar]
- Zorrilla LT, Aguirre GK, Zarahn E, Cannon TD, D'Esposito M (1996): Activation of the prefrontal cortex during judgments of recency: a functional MRI study. Neuroreport 7: 2803–2806. [DOI] [PubMed] [Google Scholar]


