Abstract
A quantitative meta‐analysis using the activation likelihood estimation (ALE) method was used to investigate the brain basis of the Wisconsin Card‐Sorting Task (WCST) and two hypothesized component processes, task switching and response suppression. All three meta‐analyses revealed distributed frontoparietal activation patterns consistent with the status of the WCST as an attention‐demanding executive task. The WCST was associated with extensive bilateral clusters of reliable cross‐study activity in the lateral prefrontal cortex, anterior cingulate cortex, and inferior parietal lobule. Task switching revealed a similar, although less robust, frontoparietal pattern with additional clusters of activity in the opercular region of the ventral prefrontal cortex, bilaterally. Response‐suppression tasks, represented by studies of the go/no‐go paradigm, showed a large and highly right‐lateralized region of activity in the right prefrontal cortex. The activation patterns are interpreted as reflecting a neural fractionation of the cognitive components that must be integrated during the performance of the WCST. Hum Brain Mapp 25:35–45, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: Wisconsin Card‐Sorting Test, neuroimaging, meta‐analysis, response‐suppression, task‐switching, executive cognition
INTRODUCTION
The Wisconsin Card‐Sorting test [WCST; Grant and Berg,1948] has long been used by researchers of brain disorders as a way to assess the integrity of the functions of the frontal lobes. The task was developed specifically to provide an objective technique for measuring “human abstraction and shift of set” and can be scored according to a well‐defined set of quantitative measures. Early work at the University of Wisconsin Primate Laboratory showed that rhesus monkeys could adapt to a change in the requirement of a discrimination task in the absence of any observable clue except for a concomitant change in the stimulus object rewarded [Zable and Harlow,1946]. Subsequent research showed that monkeys with bilateral lesions to the frontal lobes lost this ability to adapt their responses according to feedback provided by the experimenter [Settlage et al.,1948]. The WCST was designed to capture and probe the very same cognitive capability, i.e., changing course on the basis of accumulating evidence, demonstrated by the nonlesioned monkeys in a form more suitable for human experimentation. Firmly linking poor performance on the WCST and lesions to the dorsolateral prefrontal cortex in humans was the work of Brenda Milner [1963], who provided strong support “for the view that the ability to shift from one mode of solution to another on a sorting task is more impaired by frontal than by posterior cerebral injury.” In addition, Milner [1963] concluded that patients with frontal lesions were more prone to “perseverative errors,” i.e., persisting in a sorting strategy despite continued negative feedback. In recent years the specificity of the WCST as an indicator of frontal lobe dysfunction has been challenged. For instance, based on a review of the WCST lesion literature, Mountain and Snow [1993] argued that there is no clear support for the utility of the WCST as a diagnostic marker of frontal lobe damage; the same conclusion was reached by Reitan and Wolfson [1994]. A more recent (and exhaustive) meta‐analysis [Demakis,2003] confirms the view that patients with frontal lobe damage achieve significantly fewer WCST categories and generate more perseverations (relative to nonperseverative errors) than do patients with posterior lesions. Nevertheless, it is now clear that lesions to the temporal and parietal lobes do lead frequently to impaired performance, including errors of perseveration, on the WCST and therefore the WCST falls short of being the “perfect test” of frontal lobe function.
The WCST From the Subject's Perspective
That a variety of brain lesions can give rise to impairments on WCST performance is not surprising given the complexity and multifactorial nature of the task itself [Andres,2003]. Succeeding at the WCST requires the subject's entire cognitive arsenal: learning, memory, attention, perceptual discrimination, and executive control. In its traditional form, the task begins with a board of four specific stimulus cards, composed of geometric elements, laid out in a row. The subject is then given a deck of 64 or 128 response cards and asked to sort them one at a time by placing each response card below one of the four stimulus cards. No instructions for how to make the sort are given. The experimenter secretly determines which of the three stimulus dimensions (color, number, or shape) constitutes a correct match for the ensuing set of response cards and after each card placement, informs the subject whether he or she has made an error. Initially unaware of the correct stimulus category, the player sorts the cards randomly or formulates a hypothesis for sorting and tests it by trial and error. After receiving some feedback, the cognitively intact and astute subject will infer the correct category and learn to sort the cards correctly. A critical part of the WCST occurs after the subject has made 10 correct sorts because, at this point, the experimenter changes the sorting criteria: what was formerly right is now wrong, and what worked before no longer seems to work. Enter the subject into a period of cognitive upheaval. The calm player will once again fall back on empiricism, the trial and error method that led him to the determination of the first correct matching criterion. The poor player will at this point continue to sort according the original rule [Berg,1948]. Negative feedback will inform this player that he or she is no longer sorting correctly, but the player will perseverate, i.e., continue to sort based on the previously correct dimension. After all response cards have been sorted, the subject can then be scored on the number of categories achieved (one category for every set of 10 correct sorts on a given stimulus dimension) and the number of perseverative errors (number of sorts that conform to the previously correct stimulus dimension even after negative feedback has indicated that a new rule has taken precedence).
It is clear that good performance on the WCST requires a certain amount of cognitive flexibility. When the relevant stimulus dimension is changed, the successful subject must do three basic things. First, the subject must recognize based on negative feedback that the current strategy is no longer appropriate and should be abandoned. Second, over the next few sorts, the subject must search for a new rule by trying each stimulus dimension until a correct match is attained. Third, once the rule has been discovered, it must be maintained in mind and applied repeatedly until negative feedback is once again encountered. If we define “cognitive set” as being those aspects or features of the perceptual milieu that are relevant to the fulfillment of current task goals, then the performance of the WCST can be described in terms of a cycle of operations or transformations upon such a cognitive set: maintaining the correct cognitive set, inhibiting the incorrect cognitive set, and switching (or shifting) cognitive set when appropriate.
Neuroimaging the Cognitive Components of the WCST
The brain basis of these operations upon cognitive set has been investigated in neuroimaging studies over the past 20 years. Because the WCST has been such an important tool for evaluating executive aspects of cognition in patients with brain damage as well as in clinical disorders such as schizophrenia [Berman et al.,1986; Goldberg et al.,1987; Kolb and Whishaw,1983; Weinberger et al.,1986] and Parkinson's disease [Monchi et al.,2004; Owen et al.,1992], there has been a great deal of interest in seeking the neural correlates of this task in the normally functioning brain. The earliest neuroimaging studies of the WCST attempted, as nearly as possible given the constraints of the scanning environment, to uncover the brain regions associated with the performance of this task when compared to a sensorimotor control [Berman et al.,1995,1986; Weinberger et al.,1986]. This approach has revealed the network of areas that are involved in the WCST when viewed as a single phenomenon, i.e., as the metabolic summation of the cognitive processes that are recruited during performance of the task as a cognitive whole. More recent work, notably that of Konishi and colleagues at the University of Tokyo [Konishi et al.,1998a,1999a,b,2002; Nagahama et al.,2001], has attempted to identify the neural basis of various presumed mental subcomponents of the WCST through creative modifications of the original task and the logic of cognitive subtraction. A considerable body of additional neuroimaging studies carried out mostly in the last 5 years has investigated directly, although not specifically within the context of the WCST itself, the neural basis of the basic cognitive processes that underlie a person's ability to succeed at the WCST. For instance, a robust literature has now emerged for studies of set shifting, a cognitive function that is clearly associated with the WCST [e.g., Braver et al.,2003; Dove et al.,2000; Dreher et al.,2002a, 2002b]. Such studies have typically used a paradigm that has been termed “task switching” because it requires subjects to alternate between the performance of two or more tasks [for a review, see Monsell,2003]. Although in the WCST there is an obligatory period between category shifts in which the subject casts about for the right rule, task‐switching paradigms focus squarely on the switching process itself by providing cues that inform the subject when to shift tasks and often by comparing brain activity between switching and nonswitching conditions. In addition, the tasks used do not require much of a learning phase and are trivial to perform. In short, then, task‐switching paradigms offer a simple and streamlined way of examining the behavioral and brain correlates of shifting cognitive set.
Another feature of cognition much studied in recent years that is important in the WCST, especially with respect to perseverative errors, is inhibitory control or response suppression. Shifting set requires at least two complementary processes, the first of which, as discussed above, involves a reconfiguration of the currently activated set of goal‐relevant attributes of the perceptual environment: e.g., I must now pay attention to the category “shape.” Implicit in this attentional shift is the corollary: I must now ignore (suppress) the category “color.” As the WCST involves extended periods of predictability (rule changes only occur after 10 correct sorts), automatic processes take over during the stretches of routine sorting that follow the cognitive tumult of a dimensional change. In the WCST, old habits thus must die hard and to ensure that they do, some form of inhibitory control is required. The most basic paradigm for the study of response suppression is the go/no‐go task [Butter,1969; Passingham,1972]. In this task subjects are instructed either to respond (go) or not to respond (no‐go) to some predefined set of stimuli that are embedded in a stream of rapidly presented targets. The stimuli are arrayed such that the go response predominates; thus, when the no‐go stimulus is encountered, subjects must overcome a predisposition to respond. As with the task‐switching paradigm, the go/no‐go test provides a way of studying, in relative isolation, an important cognitive component of the WCST.
A cognitive domain that cannot be ignored in any discussion of the mental processes necessary for success at the WCST is that of working memory [Baddeley,1992; Baddeley and Hitch,1974]. Many authors have argued [Berman et al.,1995; Goldman‐Rakic,1991; Sullivan et al.,1993] that although the WCST was not devised specifically as a test of the short‐term memory system, it nevertheless places a heavy burden on brain processes responsible for the storage and manipulation of stimulus representations no longer available through direct perception. For instance, to cite a single example, making a correct sort demands that one retain in memory the currently relevant stimulus dimension. The cognitive neuroscience of working memory is a remarkably broad topic in its own right; so broad, in fact, that it could be seen as subsuming all of the componential processes important for the WCST, including task switching and response suppression. Although we recognize the importance of information maintenance and manipulation in the performance of the WCST, we will thus focus this review on the more easily demarcated domains of task switching and response inhibition.
We first undertake a quantitative meta‐analysis of neuroimaging studies of the WCST so as to provide a global view of the network of brain areas that have been consistently associated with the performance of this task. We then conduct two additional meta‐analyses in an effort to provide a finer‐grained characterization of neural substrates of two cognitive components that are thought to be important for successful performance on the WCST: set shifting and inhibitory control. We believe that the task‐switching and go/no‐go paradigms provide the closest operational parallel for the theoretical constructs just mentioned. A further objective is to assess the degree of convergence (or lack thereof) among the brain patterns observed for each of the three meta‐analyses.
MATERIALS AND METHODS
Literature Search and Selection Criteria
We utilized the PubMed database (http://www.pubmed.org) and ISI's Web of Science to search for articles relevant for each of three meta‐analyses. Only articles that reported activation foci, derived from a cohort of normal controls, as 3D coordinates (x, y, z) in stereotactic space were included. It was our view, especially with respect to the studies of the WCST, that some degree of across‐study heterogeneity in the experimental set‐up and manipulations of interest could be accounted more a virtue than is generally assumed in conventional (e.g., without a spatial component) meta‐analyses. Insofar as the studies entered into any single meta‐analysis reflect as a whole the prevailing commonalities of the group, those inessential or extraneous differences among the set will tend not to have much of an effect on the aggregate picture. By the same token, mere heterogeneity in the composition of selected studies, in the absence of any distinguishable thread of continuity, would not likely lead to any significant findings. For the WCST analysis, we thus accepted all studies that utilized a clearly recognizable version of the task and for which the reported contrast(s) could be reasonably seen to reflect one or more of the basic cognitive processes involved in its performance. Although these rather lenient criteria obviously limit the specificity of the inferences to be drawn from the analysis, given the composite nature of the WCST, our objectives for this first meta‐analysis were necessarily modest. We were more restrictive for task switching, including only those studies for which there was included a canonical switch–repeat contrast or at least one that was intended to capture an important aspect of the switching process. Lastly, in the go/no‐go meta‐analysis, we included studies that reported either of the two contrasts (in order of preference) no‐go–go or no‐go–rest. Because it was the simplest of the three tasks, the set of go/no‐go studies resembled each other more than could be the case in either the task‐switching or WCST cohorts.
Activation Likelihood Estimation
The method for quantitative evaluation of the reliability of spatial patterns of activation foci taken from published neuroimaging studies is based on the activation likelihood estimate (ALE) technique of Turkeltaub et al. [2002]. This approach offers a principled way of measuring the degree of spatial reproducibility in Talairach coordinates reported across a set of independent neuroimaging studies. Indeed, the method yields a statistical map that indicates the set of brain voxels that are more active than would be expected by chance alone. Unlike the typical within‐study SPM analysis where every voxel in the image space is tested against a null hypothesis of no activation, the ALE method assumes necessarily that for each study of interest there is a given spatial distribution of activity and an associated set of maximal coordinates. The hypothesis test is then formulated as follows: given that for each published neuroimaging study that meets a criterion Y and has an associated set of coordinates C, to what extent are the spatial locations of the activation foci correlated across independently conducted studies? If the null hypothesis is true, then each set of coordinates (one set per study) may reasonably be assumed to have come about by way of some random spatial process. However, if there seem to be areas of increased spatial density or clustering whereby the underlying process generating the coordinates is nonrandom (or correlated across studies), the null hypothesis is rejected. The ALE method of Turkeltaub et al. [2002] provides within a permutation‐testing framework a means of evaluating such an hypothesis and thus permits one to pinpoint brain regions that show reliable activity across studies. Our implementation of the ALE method was implemented in the R statistical language (http://www.r-project.org), and the obtained ALE maps were thresholded at P < 0.001 and a minimum cluster volume of 100 mm3. As in Turkeltaub et al. [2002], activation foci were represented by a 3D Gaussian point‐spread function with a full‐width half‐maximum (FWHM) of 15 mm. Images representing task “conjunctions” were computed simply by taking the voxel‐wise intersection of any two (or all three) of the individual ALE maps.
RESULTS
Regions of reliable cross‐study activity were found for each of the three study sets: WCST, task switching, and go/no‐go. Entering into the WCST meta‐analysis were 13 studies with a total of 278 coordinates, 18 studies and 231 coordinates for task switching, and 18 studies and 224 coordinates for go/no‐go. ALE maps were computed for each of the three sets of studies and areas of significant activity along with their Talairach coordinates are reported in Table I, II, III. (A complete list of studies entering into the three meta‐analyses is provided in Table VII.)
Table I.
ALE meta‐analysis of Wisconsin Card‐Sorting Test studies
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R inferior parietal lobule | 31 | −55 | 41 | 40 | 12,995 |
| L inferior frontal gyrus | −42 | 9 | 31 | 44 | 11,664 |
| L inferior parietal lobule/precuneus | −28 | −65 | 43 | 19/7 | 10,901 |
| R inferior frontal gyrus | 43 | 11 | 28 | 44 | 9,334 |
| L medial frontal gyrus | −6 | 19 | 44 | 6/8 | 4,520 |
| Cerebellum | −38 | −70 | −17 | 1,425 | |
| L cuneus | −10 | −93 | 6 | 18 | 514 |
| L putamen | −26 | −10 | 3 | 509 | |
| R thalamus | 7 | −13 | 7 | 449 | |
| R middle/inferior occipital gyrus | 32 | −83 | −9 | 18 | 415 |
| R inferior frontal gyrus (operculum) | 32 | 21 | 0 | 47 | 412 |
| R middle frontal gyrus | 27 | 47 | −10 | 10/11 | 326 |
| L postcentral gyrus | −35 | −28 | 58 | 4/3/2/1 | 286 |
| L lingual gyrus | −6 | −92 | −9 | 17 | 262 |
| L middle frontal gyrus | −27 | 48 | −12 | 10/11 | 245 |
| L cuneus/middle occipital gyrus | −26 | −81 | 3 | 18 | 191 |
Coordinates have been converted from MNI space using the software package MRICro (online at http://www.psychology.nottingham.ac.uk/staff/cr1/mricro.html).
BA, Brodmann area.
Table II.
ALE meta‐analysis of studies of task switching
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L inferior frontal gyrus | −44 | 5 | 30 | 44/6 | 14,048 |
| L cingulate gyrus | −12 | 4 | 47 | 32 | 7,091 |
| R medial frontal gyrus | 46 | 15 | 36 | 9 | 6,276 |
| L inferior parietal lobe | −33 | −51 | 45 | 7 | 5,469 |
| R inferior parietal lobe | 33 | −61 | 44 | 7 | 4,234 |
| L middle occipital gyrus | −36 | −76 | 6 | 19 | 3,330 |
| R cingulate gyrus | 33 | 20 | 5 | 24 | 2,322 |
| L precentral gyrus | −33 | −10 | 48 | 4 | 2,313 |
| R lingual gyrus | 22 | −74 | −2 | 18/17 | 491 |
| L fusiform gyrus | −46 | −54 | −11 | 37 | 277 |
| R fusiform/inferior temporal/medial temporal gyrus | 43 | −15 | −17 | 20/21 | 251 |
| R inferior temporal gyrus | 49 | −57 | −7 | 37 | 218 |
| L cerebellum | −27 | −69 | −41 | 179 | |
| L postcentral gyrus | −37 | −29 | 53 | 3/2/1 | 153 |
| L postcentral gyrus | −44 | −24 | 35 | 3/2/1 | 100 |
BA, Brodmann area.
Table III.
ALE meta‐analysis of response suppression (go/no‐go studies)
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R middle/inferior frontal gyrus | 45 | 19 | 24 | 44/46 | 13,427 |
| R inferior parietal lobe/supramarginal gyrus | 47 | −45 | 34 | 39/40 | 3,830 |
| R superior occipital gyrus | 30 | −75 | 30 | 19 | 2,318 |
| Medial frontal gyrus | 0 | −1 | 57 | 6 | 1,312 |
| L inferior parietal lobule | −45 | −42 | 40 | 19 | 1,235 |
| L putamen | −17 | 0 | 4 | 977 | |
| R cingulate gyrus | 2 | 15 | 44 | 6/32 | 857 |
| R thalamus | 14 | −9 | 9 | 807 | |
| L precentral gyrus | −41 | −8 | 44 | 4 | 436 |
| R inferior frontal gyrus | 36 | 48 | 1 | 44/10 | 414 |
| L middle frontal gyrus | −47 | 29 | 24 | 46 | 388 |
| R inferior frontal gyrus (operculum) | 34 | 19 | 1 | 47 | 324 |
| R superior/medial frontal gyrus | 20 | 49 | 28 | 9 | 278 |
| R fusiform gyrus | −42 | −61 | −14 | 37 | 209 |
BA, Brodmann area.
Table VII.
List of studies, including date published and number of activation foci, entering into each meta‐analysis
| Author | Year | Loci (n) |
|---|---|---|
| Wisconsin Card‐Sorting | ||
| Ragland et al. | 1998 | 12 |
| Berman et al. | 1997 | 10 |
| Nagahama et al. | 1997 | 26 |
| Goldberg et al. | 1998 | 17 |
| Nagahama et al. | 2001 | 14 |
| Berman et al. | 1995 | 35 |
| Konishi et al. | 2002 | 25 |
| Monchi et al. | 2004 | 25 |
| Monchi et al. | 2001 | 48 |
| Konishi et al. | 2003 | 16 |
| Konishi et al. | 1998a | 5 |
| Jimura et al. | 2004 | 15 |
| Nagahama et al. | 1996 | 30 |
| Task switching | ||
| Smith et al. | 2004 | 10 |
| Dove et al. | 2000 | 17 |
| Sohn et al. | 2000 | 4 |
| DiGirolamo et al. | 2001 | 75 |
| Rushworth et al. | 2002 | 4 |
| Cools et al. | 2004 | 11 |
| Kimberg et al. | 2000 | 9 |
| Brass and von Cramon | 2004 | 7 |
| Braver et al. | 2003 | 8 |
| Dreher and Grafman | 2003 | 14 |
| Dreher and Berman | 2002a | 9 |
| Dreher et al. | 2002b | 12 |
| Pollmann et al. | 2000 | 6 |
| Omori et al. | 1999 | 12 |
| Brass and von Cramon | 2002 | 8 |
| Sylvester et al. | 2003 | 13 |
| Luks et al. | 2002 | 4 |
| Ruge et al. | 2003 | 13 |
| Go/no‐go | ||
| Rubia et al. | 2001 | 10 |
| Konishi et al. | 1998b | 8 |
| Mostofsky et al. | 2003 | 3 |
| Horn et al. | 2003 | 14 |
| Menon et al. | 2001 | 13 |
| Watanabe et al. | 2002 | 5 |
| Liddle et al. | 2001 | 23 |
| Asahi et al. | 2004 | 11 |
| Kelly et al. | 2004 | 23 |
| Garavan et al. | 1999 | 14 |
| Hester et al. | 2004 | 21 |
| Garavan et al. | 2002 | 16 |
| Bellgrove et al. | 2004 | 19 |
| Fassbender et al. | 2004 | 4 |
| de Zubicaray et al. | 2000 | 15 |
| Braver et al. | 2001 | 11 |
| Maguire et al. | 2001 | 5 |
| Rubia et al. | 2001 | 10 |
WCST Meta‐Analysis Results
As expected from the cognitively complex nature of the WCST, an extensive distributed pattern of activity was evident from the ALE map thresholded at P < 0.001. Several particularly prominent (cluster volume ≥ 1,000 mm3) clusters were observed in the left and right inferior parietal lobule (IPL), the left and right inferior frontal gyrus (IFG), the anterior cingulate cortex (ACC) of the medial frontal gyrus, and the cerebellum. A number of additional regions (see Fig. 1 and 2, green colors) of reliable activity were detected and are listed in Table I.
Figure 1.
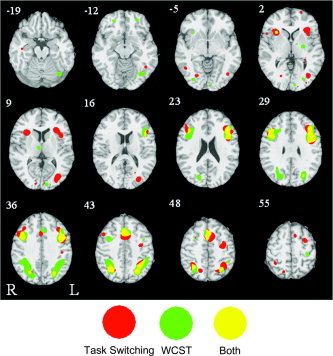
Axial slices showing significant ALE activation in WCST (green), task switching (red), and the conjunction of WCST and task switching (yellow) overlaid on the International Consortium for Brain Mapping (ICBM) single subject template.
Figure 2.
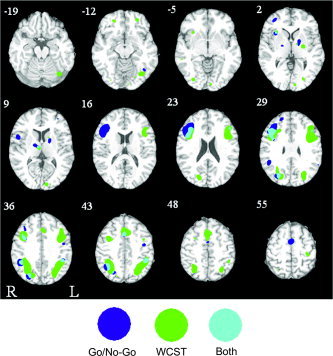
Axial slices showing significant ALE activation in WCST (green), go/no‐go (blue), and the conjunction of WCST and go/no‐go (blue) overlaid on the ICBM single subject template.
Task‐Switching Meta‐Analysis Results
The task‐switching ALE map also revealed a strong frontoparietal activation pattern with some additional clusters in occipital and inferior temporal regions (Fig. 1; Table II). As in the WCST, activation was seen in the IPL and IFG (Brodmann area [BA] 44/45) bilaterally. A region in the opercular part of the IFG (BA47), situated ventral and anterior to the frontal area noted above, showed a strong bilateral pattern that was not seen in the WCST map.
Go/No‐Go Meta‐Analysis Results
Considerably fewer significant activation clusters were detected in the go/no‐go ALE map (Fig. 2; Table III). The most prominent feature of the activation pattern was the highly lateralized cluster of activity in the right frontal cortex, which included a large portion (5,004 mm3) of the posterior part of the IFG (BA44/45) and the middle frontal gyrus (BA9/46). A single small cluster of activity was seen in the IFG left frontal lobe (388 mm3). Visible only on the right was a cluster of activity in the supramarginal gyrus located somewhat inferior to clusters observed in the IPL for the task‐switching and WCST ALE maps.
Meta‐Analysis Conjunction Results
As shown in Figure 1, 2, 3 and listed in Table IV, V, VI, a considerable degree of overlap in the regions of significant activity for the three meta‐analyses was observed. In general, all ALE maps showed a distributed frontoparietal activation pattern with foci in both the dorsolateral and dorsomedial portions of the frontal cortex. This pattern was generally more extensive in the WCST ALE map (in terms of frontal and parietal cluster volumes) than it was in the task‐switching and go/no‐go analyses. As is clear in Figure 1 and 3 (red colors), in addition to the common areas of activation in the dorsal prefrontal cortex, task switching was associated with bilateral foci in the ventrolateral prefrontal cortex. In comparison to task switching and WCST, the go/no‐go ALE map was marked by a large right dominant cluster of activation in the dorsolateral prefrontal cortex.
Figure 3.
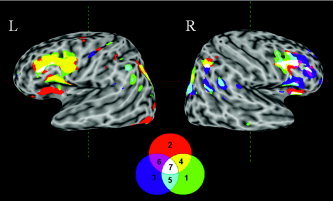
Three‐dimensional surface rendered views of all three task meta‐analyses including all possible conjunctions. 1, WCST (green); 2, task switching (red); 3, go/no‐go (blue); 4, WCST and task switching (yellow); 5, WCST and go/no‐go (cyan); 6, task switching and go/no‐go (violet); 7, WCST and task switching and go/no‐go (white). Image rendering was created with SUMA (and associated AFNI program 3dVol2Surf; [Saad et al.,2004]) using a surface representation of the ICBM single‐subject brain that was created with the FreeSurfer software package [Dale et al.,1999; Fischl et al.,1999].
Table IV.
Conjunction of Wisconsin Card‐Sorting Task and task‐switching meta‐analyses
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L middle/inferior frontal gyrus | −43 | 14 | 28 | 44/9 | 8312 |
| L precuneus/inferior parietal lobule | −31 | −60 | 42 | 19/7 | 3905 |
| R middle/inferior frontal gyrus | 43 | 15 | 31 | 44/9 | 3885 |
| Medial temporal gyrus | 0 | 17 | 45 | 6 | 3336 |
| R precuneus/inferior parietal lobule | 30 | −60 | 44 | 19/7 | 2590 |
| R inferior frontal gyrus (operculum) | 32 | 21 | 0 | 47 | 287 |
BA, Brodmann area.
Table V.
Conjunction of Wisconsin Card‐Sorting Task and go/no‐go meta‐analyses
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R middle frontal gyrus | 41 | 17 | 28 | 9 | 5,004 |
| R inferior parietal lobule | 39 | −52 | 40 | 40 | 1,664 |
| R superior occipital gyrus | 27 | −73 | 33 | 19 | 1,287 |
| L inferior parietal lobule | −45 | −42 | 41 | 40 | 1,157 |
| R medial frontal gyrus | 1 | 16 | 44 | 6/32 | 695 |
| L middle frontal gyrus | −46 | 27 | 26 | 9 | 388 |
| R thalamus | 7 | −12 | 6 | 157 | |
| R inferior frontal gyrus (operculum) | 33 | 20 | 1 | 47 | 132 |
BA, Brodmann area.
Table VI.
Conjunction of Wisconsin Card‐Sorting Task, task‐switching, and go/no‐go meta‐analyses
| Region | Talairach | BA | Volume (mm3) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R middle frontal gyrus | 42 | 17 | 29 | 9 | 2,934 |
| R medial frontal gyrus | 1 | 16 | 44 | 6 | 695 |
| R inferior parietal lobule | 35 | −58 | 44 | 7 | 532 |
| L middle frontal gyrus | −46 | 27 | 26 | 46/9 | 323 |
| L inferior parietal lobule | −41 | −43 | 41 | 40 | 245 |
| R inferior frontal gyrus (opercular) | 33 | 20 | 1 | 47 | 132 |
BA, Brodmann area.
DISCUSSION
A comparison of the ALE results shows that the three tasks of interest show a good deal of similarity, along with some notable differences, in the regional patterns of activation disclosed by probabilistic meta‐analyses of published stereotaxic coordinates. The WCST analysis showed a clear bilateral frontoparietal pattern of activation consistent with its status as an attention‐demanding and cognitively complex task of executive function and with its dependence on working memory. Task switching was also associated with a similar although less diffuse bilateral frontoparietal pattern of activation. In addition, a bilateral focus, which was not present in the WCST map, located in the ventrolateral prefrontal cortex (IFG, BA47) was reliably active across studies of task switching. Of the three analyses, the pattern of activation seen in the go/no‐go ALE map showed the greatest regional specificity and the least amount of left–right symmetry. Among the significant clusters of activity, 9 of 13 were observed in the right hemisphere, and the three largest of these were all observed on the right. Most marked was the cluster of activity seen in the right prefrontal cortex (including the IFG and middle frontal gyrus [MFG] with a volume of 13,427 mm3, more than 30 times larger than a region in a similar location on the left [388 mm3]).
If we view the three task paradigms treated in this article, WCST, task switching, and go/no‐go, as belonging to a hierarchical series with the each member of the chain constituting a cognitive superset of the task that follows,1 then we might expect the meta‐analyses reported herein to reflect this organization. That is, the significant areas of activation for the go/no‐go task should be a spatial subset of those areas seen in task switching, and likewise, the regions revealed in the task switching analysis should be a subset of those seen for the WCST. Although, broadly speaking, such a pattern was observed, each of the subsidiary tasks highlighted selected regions (bilateral ventrolateral prefrontal cortex in the case of task switching and right dorsolateral prefrontal cortex for go/no‐go) that were not as prominent in the WCST ALE map. This likely has to do with the increased cognitive specificity of the task switching and go/no‐go tasks.
In the past few years, much attention has been focused on the ventrolateral prefrontal cortex, an area corresponding closely to the activation foci seen in the task‐switching meta‐analysis. Various researchers have associated the region with different hypothetical cognitive functions, including semantic retrieval [Buckner et al.,1995; Demonet et al.,1992; Kapur et al.,1994], selection of semantic information from among competing alternatives [Thompson‐Schill et al.,1997], controlled semantic retrieval [Wagner et al.,2001], interference resolution [D'Esposito et al.,1999; Jonides et al.,1998], and overcoming residual cognitive inhibition [Dreher and Berman,2002a, 2002b; Houghton and Tipper,1996]. Although semantic function for this region has been emphasized, a more general theme linking several of these interpretations is evident, namely that of competition. In the task‐switching study of Dreher and Berman [2002a, 2002b] for instance, activity in this region was enhanced during switches to a just‐performed task when compared to switches to a task that had not been performed recently. In the semantic retrieval task in Thompson‐Schill et al. [1997], subjects either had to classify a picture according to a specific attribute of the object's representation (high selection demands) or classify the picture according to some global feature of the object (low selection demands). The ventrolateral prefrontal cortex showed greater activity when the subject had to hone in on a particular feature of the object than it did when he or she had to make a judgment based on the object's total quality. In this study, as with that of Dreher and Berman [2002a, 2002b], the subject encounters a stimulus that requires a response. When there is an unambiguous relationship between this stimulus and an associated response, a course of action is clear and a decision may proceed with little ado. When an encountered stimulus suggests a multiplicity of possible actions, either because of the semantic diversity of the object itself or because of the subject's own past predilections, some mechanism must intervene to reduce this manifold potentiality to a single course of action. In the WCST, the stimuli confronting the subject can be sorted by object, shape, or color, and each of these possibilities is eventually known to the subject. As the task proceeds, each perceptual category increasingly competes for the subject's notice and suggests several possible actions; without some way of resolving the interference presented by the different card categories, the subject will fail to sort the cards correctly. The ventrolateral prefrontal cortex seems crucial in such situations, i.e., when many behavioral outcomes are possible but only one is correct.
The go/no‐go meta‐analysis clearly pointed to an important role for the right dorsolateral prefrontal cortex. In a recent review, Aron et al. [2004b] concluded that this region is critical for inhibitory control processes. Konishi et al. [1999b], in a direct comparison of the WCST and a response‐suppression paradigm, demonstrated an area of common activity in the right frontal cortex. In addition, greater damage to the right prefrontal cortex (PFC) in a set of patients has been shown to correlate with “switch cost,” the increase in reaction time observed in trials where subjects switch from one task to another relative to trials in which the same task is repeated [Aron et al.,2004a]. There is thus evidence that response suppression is important for both the WCST and task switching. Indeed, in the conjunction of task switching and go/no‐go ALE maps, we found an area of convergence in the right frontal cortex. It is currently unclear how the function ascribed to the ventrolateral prefrontal cortex, i.e., interference resolution, can be disentangled completely from the inhibitory role of the right PFC. One suggestion is that response suppression occurs at a later processing stage than does interference resolution. That is, interference resolution can be seen as a mechanism important for arriving at a decision when many possible choices are available whereas response inhibition is a process that occurs to prevent an automatic (or prepotent) action from proceeding to fruition. Moreover, response suppression, unlike interference resolution, is important during situations that dictate highly stereotyped and repetitive responses in which little semantic deliberation is required. The sort of inhibitory control supported by the right PFC thus is critical for coping with the appearance of an unusual event that has intervened in the midst of some routine task with clear stimulus–response requirements. It will be important in future work on the brain basis of the WCST, task switching, and response suppression to clarify the relationship between inhibitory control and the process whereby a person settles on a course of action when many different possibilities present themselves.
We have examined through the use of the ALE meta‐analytic technique to what extent the WCST and two of its hypothesized component processes, task switching and response suppression, share a common neural substrate. Because each task involves a kind of executive attention, a degree of similarity was observed in their respective spatial patterns of activation. The regional profiles across the three tasks were not indistinguishable from one another, as was especially evident in the strong right PFC activation in the go/no‐go meta‐analysis. Direct statistical comparison between ALE maps would be necessary for formal confirmation of these observations, and such methods are beginning to be developed [see Laird et al.,2005]. Finally, although one cannot appreciate how the brain masters such a cognitively complex challenge such as the WCST without first uncovering the neural mechanisms that underlie its component processes, the reverse is also true, namely, that the WCST is not merely the naïve totality of its constituent parts. Taken as a whole, the WCST captures something profound about human cognition: the ability to apply a rule derived from the observation of a series of events, and then, critically, as the environment changes or new evidence surfaces, to discard (and replace) that rule as it becomes clear that its application no longer produces the effect it once did. What the WCST highlights is both the frailty and the extraordinary flexibility of inductive reasoning, that the rules derived from data are only temporary, provisional things, and that, as the philosopher David Hume famously observed, although the sun has always risen before, it may yet not rise tomorrow. In this sense, then, the WCST mirrors the scientific enterprise itself, where rules are discovered and applied only as long as they are deemed adequate, after which they are discarded and replaced by something new.
Footnotes
The proposed hierarchy breaks down somewhat if we allow that go/no‐go paradigms do contain a rudimentary form of task switching.
REFERENCES
- Andres P (2003): Frontal cortex as the central executive of working memory: time to revise our view. Cortex 39: 871–895. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW (2004a): A componential analysis of task‐switching deficits associated with lesions of left and right frontal cortex. Brain 127: 1561–1573. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA (2004b): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N (2004): Negative correlation between right prefrontal activity during response inhibition and impulsiveness: a fMRI study. Eur Arch Psychiatry Clin Neurosci 254: 245–251. [DOI] [PubMed] [Google Scholar]
- Baddeley A (1992): Working memory. Science 255: 556–559. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ (1974). Working memory In: Bower G, editor. The psychology of learning and motivation. New York: Academic Press; p 47–90. [Google Scholar]
- Bellgrove MA, Hester R, Garavan H (2004): The functional neuroanatomical correlates of response variability: evidence from a response inhibition task. Neuropsychologia 42: 1910–1916. [DOI] [PubMed] [Google Scholar]
- Berg EA (1948): A simple objective technique for measuring flexibility in thinking. J Gen Psychol 39: 15–22. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, Carson RE, Herscovitch P, Weinberger DR (1995): Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia 33: 1027–1046. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR (1997): Modulation of cognition‐specific cortical activity by gonadal steroids: a positron‐emission tomography study in women. Proc Natl Acad Sci USA 94: 8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Zec RF, Weinberger DR (1986): Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry 43: 126–135. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2002): The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2004): Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16: 609–620. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A (2001): Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, Donaldson DI (2003): Neural mechanisms of transient and sustained cognitive control during task switching. Neuron 39: 713–726. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME (1995): Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci 15: 12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butter CM (1969): Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta . Physiol Behav 4: 163–171. [Google Scholar]
- Cools R, Clark L, Robbins TW (2004): Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci 24: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- de Zubicaray GI, Andrew C, Zelaya FO, Williams SC, Dumanoir C (2000): Motor response suppression and the prepotent tendency to respond: a parametric fMRI study. Neuropsychologia 38: 1280–1291. [DOI] [PubMed] [Google Scholar]
- Demakis GJ (2003): A meta‐analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology 17: 255–264. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R (1992): The anatomy of phonological and semantic processing in normal subjects. Brain 115: 1753–1768. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Jonides J, Smith EE (1999): The neural substrate and temporal dynamics of interference effects in working memory as revealed by event‐related functional MRI. Proc Natl Acad Sci USA 96: 7514–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E (2001): General and task‐specific frontal lobe recruitment in older adults during executive processes: a fMRI investigation of task‐switching. Neuroreport 12: 2065–2071. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: an event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF (2002a): Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA 99: 14595–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Grafman J (2003): Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex 13: 329–339. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J (2002b): The roles of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H (2004): A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20: 132–143. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA (2002): Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage 17: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA (1999): Right hemispheric dominance of inhibitory control: an event‐related functional MRI study. Proc Natl Acad Sci USA 96: 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, Mattay VS, Gold JM, Weinberger DR (1998): Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage 7: 296–303. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Berman KF, Pliskin NH, Podd MH (1987): Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry 44: 1008–1014. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS (1991). Prefrontal cortical dysfunction in schizophrenia: the relevance of working memory In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. New York: Raven Press; p 1–23. [Google Scholar]
- Grant DA, Berg EA (1948): A behavioral analysis of degree of reinforcement and ease of shifting to new responses to new responses in a Weigl‐Type card‐sorting problem. J Exp Psychol 38: 404–411. [DOI] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H (2004): Predicting success: patterns of cortical activation and deactivation prior to response inhibition. J Cogn Neurosci 16: 776–785. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW (2003): Response inhibition and impulsivity: an fMRI study. Neuropsychologia 41: 1959–1966. [DOI] [PubMed] [Google Scholar]
- Houghton G, Tipper SP (1996): Inhibitory mechanisms of neural and cognitive control: applications to selective attention and sequential action. Brain Cogn 30: 20–43. [DOI] [PubMed] [Google Scholar]
- Jimura K, Konishi S, Miyashita Y (2004): Dissociable concurrent activity of lateral and medial frontal lobe during negative feedback processing. Neuroimage 22: 1578–1586. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter‐Lorenz PA (1998): Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95: 8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM (1994): Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci USA 91: 2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H (2004): Prefrontal‐subcortical dissociations underlying inhibitory control revealed by event‐related fMRI. Eur J Neurosci 19: 3105–3112. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M (2000): Modulation of task‐related neural activity in task‐switching: an fMRI study. Brain Res Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Kolb B, Whishaw IQ (1983): Performance of schizophrenic patients on tests sensitive to left or right frontal, temporal, or parietal function in neurological patients. J Nerv Ment Dis 171: 435–443. [DOI] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y (2002): Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci USA 99: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Jimura K, Asari T, Miyashita Y (2003): Transient activation of superior prefrontal cortex during inhibition of cognitive set. J Neurosci 23: 7776–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Kawazu M, Uchida I, Kikyo H, Asakura I, Miyashita Y (1999a): Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex 9: 745–753. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kameyama M, Nakahara K, Sekihara K, Miyashita Y (1998a): Transient activation of inferior prefrontal cortex during cognitive set shifting. Nat Neurosci 1: 80–84. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y (1999b): Common inhibitory mechanism in human inferior prefrontal cortex revealed by event‐related functional MRI. Brain 122: 981–991. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y (1998b): No‐go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci 10: 1209–1213. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM (2001): Event‐related fMRI study of response inhibition. Hum Brain Mapp 12: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL (2002): Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. Neuroimage 17: 792–802. [PubMed] [Google Scholar]
- Maguire RP, Broerse A, de Jong BM, Cornelissen FW, Meiners LC, Leenders KL, den Boer JA (2003): Evidence of enhancement of spatial attention during inhibition of a visuo‐motor response. Neuroimage 20: 1339–1345. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B (1963): Effects of different lesions on card sorting. Arch Neurol 9: 101–110. [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004): Neural bases of set‐shifting deficits in Parkinson's disease. J Neurosci 24: 702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001): Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 21: 7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S (2003): Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ (2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17: 419–430. [DOI] [PubMed] [Google Scholar]
- Mountain MA, Snow WG (1993): Wisconsin Card Sorting Test as a measure of frontal pathology: a review. Clin Neuropsychol 7: 108–118. [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Katsumi Y, Magata Y, Shibasaki H, Kimura J (1997): Age‐related changes in cerebral blood flow activation during a card sorting test. Exp Brain Res 114: 571–577. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Fukuyama H, Yamauchi H, Matsuzaki S, Konishi J, Shibasaki H, Kimura J (1996): Cerebral activation during performance of a card sorting test. Brain 119: 1667–1675. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H (2001): Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 11: 85–92. [DOI] [PubMed] [Google Scholar]
- Omori M, Yamada H, Murata T, Sadato N, Tanaka M, Ishii Y, Isaki K, Yonekura Y (1999): Neuronal substrates participating in attentional set‐shifting of rules for visually guided motor selection: a functional magnetic resonance imaging investigation. Neurosci Res 33: 317–323. [DOI] [PubMed] [Google Scholar]
- Owen AM, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Lange KW, Robbins TW (1992): Fronto‐striatal cognitive deficits at different stages of Parkinson's disease. Brain 115: 1727–1751. [DOI] [PubMed] [Google Scholar]
- Passingham RE (1972): Non‐reversal shifts after selective prefrontal ablations in monkeys (Macaca mulatta). Neuropsychologia 10: 41–46. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Dove A, Yves von Cramon D, Wiggins CJ (2000): Event‐related fMRI: comparison of conditions with varying BOLD overlap. Hum Brain Mapp 9: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Glahn DC, Censits DM, Smith RJ, Lazarev MG, Alavi A, Gur RE (1998): Frontotemporal cerebral blood flow change during executive and declarative memory tasks in schizophrenia: a positron emission tomography study. Neuropsychology 12: 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1994): A selective and critical review of neuropsychological deficits and the frontal lobes. Neuropsychol Rev 4: 161–198. [DOI] [PubMed] [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E (2001): Mapping motor inhibition: conjunctive brain activations across different versions of go/no‐go and stop tasks. Neuroimage 13: 250–261. [DOI] [PubMed] [Google Scholar]
- Ruge H, Brass M, Lohmann G, von Cramon DY (2003): Event‐related analysis for event types of fixed order and restricted spacing by temporal quantification of trial‐averaged fMRI time courses. J Magn Reson Imaging 18: 599–607. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002): Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Reynolds RC, Argall B, Japee S, Cox RW (2004): SUMA: an interface for surface‐based intra‐ and inter‐subject analysis with AFNI. Proceedings of the IEEE International Symposium on Biomedical Imaging, 15–18 April 2004, Arlington, VA. IEEE. p 1510–1511.
- Settlage P, Zable M, Harlow HF (1948): Problem solution by monkeys following bilateral removal of the frontal areas. VI. Performance on tests requiring contradictory reactions to similar and identical stimuli. J Exp Psychol 38: 50–65. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Rubia K (2004): Neural correlates of switching set as measured in fast, event‐related functional magnetic resonance imaging. Hum Brain Mapp 21: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000): Inaugural article: the role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen‐Tucker Z, Knight RT, Pfefferbaum A (1993): Factors of the Wisconsin Card Sorting Test as measures of frontal‐lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res 46: 175–199. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J (2003): Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41: 357–370. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 94: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare‐Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron 31: 329–338. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R (2002): The human prefrontal and parietal association cortices are involved in NO‐GO performances: an event‐related fMRI study. Neuroimage 17: 1207–1216. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF (1986): Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 43: 114–124. [DOI] [PubMed] [Google Scholar]
- Zable M, Harlow HF (1946): The performance of rhesus monkeys on series of object‐quality and positional discrimination reversals. J Comp Psychol 39: 13–23. [DOI] [PubMed] [Google Scholar]


