Abstract
Bimanual interference emerges when spatial features, such as movement direction or amplitude, differ between limbs, as indicated by a mutual bias of limb trajectories. Although first insights into the neural basis of directional interference have been revealed recently, little is known about the neural network associated with amplitude interference. We investigated whether amplitude versus directional interference activates differential networks. Functional magnetic resonance imaging (fMRI) was applied while subjects performed cyclical, bimanual joystick movements with either the same vs. different amplitudes, directions, or both. The kinematic analysis confirmed that subjects experienced amplitude interference when they moved with different as compared to the same amplitude, and directional interference when they moved along different as compared to the same direction. On the brain level, amplitude and directional interference both resulted in activation of a bilateral superior parietal‐premotor network, which is known to contribute to sensorimotor transformations during goal‐directed movements. Interestingly, amplitude but not directional interference exclusively activated a bilateral network containing the dorsolateral prefrontal cortex, anterior cingulate, and supramarginal gyrus, which was shown previously to contribute to executive functions. Even though the encoding of amplitude and directional information converged and activated the same neural substrate, our data thus show that additional and partly independent mechanisms are involved in bimanual amplitude as compared to that in directional control. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: dorsolateral prefrontal cortex, anterior cingulate cortex, sensorimotor transformation, executive function, response inhibition, interhemispheric information interchange
INTRODUCTION
Bimanual movements require continuous interhemispheric communication between the contributing cortical areas [Serrien et al., 2001; Serrien and Brown, 2002], such as the primary motor cortex (M1), the supplementary motor area (SMA), the dorsolateral premotor cortex, and the parietal cortex [for reviews see Swinnen, 2002; Wenderoth et al., 2004c]. This information exchange between the hemispheres can be beneficial when both limbs execute the same movements because it may reinforce bilateral neuronal activity. In this case, bimanual actions are highly stable and are sometimes even performed better than their unimanual components executed in isolation [Helmuth and Ivry, 1996]. However, a tight interhemispheric coupling can also be detrimental when the arms have to be moved simultaneously along different trajectories. In this case, unwanted information overflow gives rise to spatial interference, as reflected by mutual assimilation effects with respect to movement direction [Franz, 1997; Franz et al., 2000; Franz and Ramachandran, 1998; Swinnen et al., 2001, 2002; Wenderoth et al., 2003] or movement amplitude [Franz, 1997; Sherwood, 1994; Sherwood and Nishimura, 1999; Spijkers and Heuer, 1995; Swinnen et al., 2001; Walter et al., 2001].
Recently, neurophysiological results in monkeys [Donchin et al., 1998; Rokni et al., 2003; Steinberg et al., 2002] and humans [Wenderoth et al., 2004a] have provided first insights into how directional information is processed during bimanual actions. Single‐cell recordings in monkeys were used to identify population vectors that represent the planned movement direction. Analyzing these population vectors in both hemispheres, it was found that by default there is a strong interhemispheric interchange of directional information between the primary motor and the premotor areas. This became even evident for unimanual movements, such that movement direction was not only represented in the hemisphere contralateral but, surprisingly, also in the hemisphere ipsilateral to the moving arm [Steinberg et al., 2002]. However, for bimanual movements along different directions, this interhemispheric default coupling needs to be suppressed, such that the movement direction of each arm can be encoded relatively independently [Rokni et al., 2003]. In humans, a previous functional magnetic resonance imaging (fMRI) study [Wenderoth et al., 2004a] revealed that bimanual movements along different directions evoked increased activation of a superior parietal‐dorsal premotor network that was located mainly within the right hemisphere. Interestingly, most of the right hemispheric areas were also activated for the unimanual submovements, irrespective of whether the left or right hand was used. In summary, these data suggest that directional information is exchanged between hemispheres during bimanual movements, in agreement with results obtained in monkeys.
Although the neurophysiological basis for directional control during bimanual actions has been studied occasionally, virtually nothing is known about the neural mechanisms underlying bimanual amplitude control. Based on behavioral results for unimanual movements, it has generally been assumed that direction and amplitude are two critical parameters for goal‐directed movements that are processed separately by independent channels [Bock and Arnold, 1992; Favilla et al., 1989; Gordon et al., 1994; Messier and Kalaska, 1997; Rosenbaum, 1980; Soechting and Flanders, 1989; Soechting and Ross, 1984]. However, neurophysiological studies in monkeys have suggested that both parameters are encoded by the same neural substrate [Fu et al., 1993, 1995; Kurata 1993; Messier and Kalaska, 2000; Riehle and Requin, 1989]. More specifically, single‐cell recordings in superior parietal, premotor, and primary motor cortex indicate that few cells modulate their activity as a function of the planned amplitude only. Instead, most cells representing amplitude requirements also encode movement direction [Fu et al., 1993, 1995; Kurata, 1993; Messier and Kalaska, 2000; Riehle and Requin, 1989].
Behavioral studies of bimanual actions have shown that simultaneous movements with different amplitudes give rise to interference effects, such that the larger amplitude is slightly reduced whereas the smaller amplitude is increased compared to that for unimanual task execution [Franz, 1997; Sherwood, 1994; Sherwood and Nishimura, 1999; Spijkers and Heuer, 1995; Swinnen et al., 2001; Walter et al., 2001]. Only a few studies have addressed whether directional and amplitude interference are interdependent and these yielded inconsistent results. Franz [1997] studied spatial assimilation effects for rhythmical drawings of two orthogonal lines or a line and a circle with different amplitudes. They revealed that amplitude interference emerged only during the tracing of parallel but not orthogonal directions. By contrast, Swinnen et al. [2001] employed a rhythmical star and line drawing task such that the movement directions between the left and right limb differed by 0, 45, or 90 degrees. Additionally, subjects produced either the same or different amplitudes. The data suggested some level of interdependence such that directional interference depended on the amplitude produced and amplitude interference was more pronounced when orthogonal versus parallel directions had to be traced.
We used fMRI to identify the brain regions that reflect amplitude interference during bimanual actions. Moreover, we explored whether the identified areas are specific for amplitude interference only, or whether the same areas respond also to directional interference. Subjects were therefore required to perform rhythmical bimanual movements with either the same or different amplitudes and along either the same or different directions. This factorial design allowed us to address whether the simultaneous specification of movement amplitude versus direction during bimanual actions engages the same or different neural substrates.
SUBJECTS AND METHODS
Subjects
Twelve subjects (four males, eight females; age range, 19–33 years) participated in the experiment. All were right‐handed [Oldfield, 1971], naive with respect to the task, and had normal vision. None participated in regular musical training, had a history of neurological or psychiatric disease, or exhibited overt sensorimotor deficits. All subjects gave written informed consent before participating in the experiment, which was approved by the local ethical committee of K.U.Leuven. Subjects were paid for their services.
Experimental Setup
Subjects lay supine in the MR scanner with their upper arms next to the body and the forearms in nearly vertical position, i.e., with an elbow angle between 90 and 135 degrees. In this position they operated with each wrist a 2‐D joystick (Fig. 1A). The joysticks were manufactured in‐house, each utilizing two optical encoders (spatial resolution, 0.18 degrees; Hewlett‐Packard, Malaysia) to register movements along the vertical and horizontal dimension with a sampling rate of 100 Hz and without causing interference with image acquisition. Subjects could not see their hands but looked at a screen, which was used to provide visual instructions, back‐projected by a LCD projector (1,280 × 1,024 pixels; Barco 6300) and viewed via a mirror inside the scanner. Head movements were restricted by a bite‐bar as well as two cushions mounted to the left and right side of the head. Subjects were trained to avoid eye movements by looking at a fixation cross displayed in front of them at all times.
Figure 1.
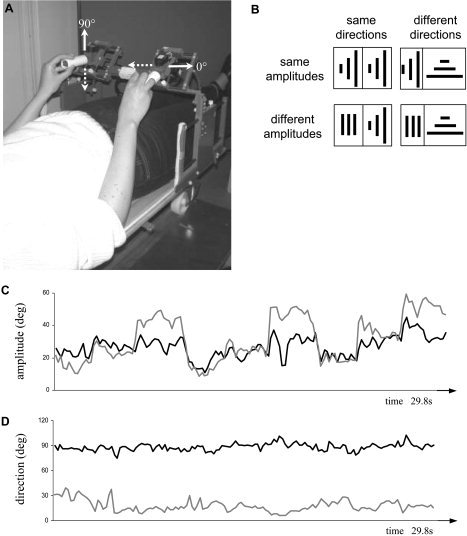
A: Experimental setup showing exemplary movements along orthogonal directions. B: Amplitude and direction requirements during the four bimanual conditions as symbolized by pictograms. The three lines indicate the amplitude/direction requirements for the first, second, and third interval for the left (left part) and right wrist (right part). The length of the lines reflects whether 30, 60, or 90% of the maximal amplitude were required. C: Exemplary time course of the produced amplitudes and produced directions (D) of a typical subject for the right (gray) and left hand (black) when movements with different amplitudes and different directions were required (Amp&DirInterf condition). The left‐hand amplitude (C, black trace) exhibits clear deviations from the required constant medium amplitude.
Behavioral Tasks
Before scanning, subjects practiced the required bimanual movement conditions. During this pretraining, they familiarized themselves with the joystick and performed cyclical drawing movements along the vertical as well as the horizontal direction, which were paced by a metronome at 2.4 Hz. For both directions, they produced small, medium, or large amplitudes, corresponding to approximately 30, 60, and 90% of their individual maximal amplitude.
The spatial requirements of the left and the right wrist movements were varied such that subjects moved with the same versus different amplitudes and along the same versus different directions. In the same amplitude condition, subjects switched between small, medium, and large movements but importantly always produced identical amplitudes with both wrists. Each amplitude (small, medium, large) was maintained for eight full drawing cycles. During one trial, this sequence was repeated three times (i.e., the subject restarted the sequence by changing from large‐ to small‐amplitude movements). Switching to the subsequent amplitude was indicated by a stressed beat of the metronome, which was clearly distinguishable from low beeps indicating the movement rhythm. In the different amplitude condition, subjects were required to switch between the three different amplitudes with the right wrist, while they were to maintain a constant medium amplitude with their left wrist at all times (Fig. 1C). With respect to the directional requirements, subjects moved either along the same directions, i.e., tracing vertical lines with both wrists, or along different directions, requiring vertical movements with the left and horizontal movements with the right limb. During scanning, these amplitude and direction requirements were combined in a factorial design (Fig. 1B), such that subjects performed bimanual movements under four conditions: (1) the same amplitudes and directions, i.e., no interference (NoInterf); (2) different amplitudes but same directions, i.e., amplitude interference (AmpInterf); (3) the same amplitudes but different directions, i.e., directional interference (DirInterf); and (4) different amplitudes and different directions, i.e., amplitude and directional interference (Amp&DirInterf). The average amplitude remained constant across hands and conditions.
Kinematic Analysis
Using interactive software (MATLAB 5.3), all drawing movements were divided into nine intervals, each lasting 3.34 s (i.e., the imposed time to complete eight movement cycles with a given amplitude). For each interval, a peak picking algorithm was applied to detect turning points in the x‐ and y‐directions. Movement amplitude was determined as the Euclidean distance between the x‐ and y‐coordinates of two successive turning points. From these data, mean amplitude (meanAmp) as well as its standard deviation (sdAmp) were determined. Movement direction was calculated by γ = arctan ((y2 − y1)/(x2 − x1)) and (x1,y1), (x2,y2) indicating the coordinates of two consecutive turning points. From these data, mean and the standard deviation (sdDir) of γ were determined and the directional error (errorDir) was calculated as the absolute difference between the average of the produced and required orientation (90 degrees for vertical and 0 degrees for horizontal movements). The four parameters meanAmp, sdAmp, errorDir, and sdDir were subjected to analyses of variance for repeated measurements (repeated‐measures ANOVA). The α‐level was set to 0.05 and significant effects were explored further by planned comparisons.
Additionally, cycle duration (determined as the time interval between two successive turning points) was compared among the four movement conditions. Mean cycle durations varied between 404.7 ms (Amp&DirInterf) and 408.7 ms (DirInterf). No significant differences across conditions were found, indicating that subjects complied well with the timing requirements.
Scanning Procedures
The fMRI measurements were executed on a 1.5‐T MR Siemens Sonata scanner using a quadrature head coil. Each scan session began with the acquisition of a 3D high‐resolution T1‐weighted image (magnetization prepared rapid acquisition gradient echo [MPRAGE]; repetition time/echo time [TR/TE] = 11.4/4.4 ms, inversion recovery delay (TI) = 300 ms, field of view = 256 mm, matrix = 256 × 256, slab thickness = 160 mm, and 160 slices) for anatomical details. Afterwards, subjects performed eight scanning runs, each containing 224 gradient‐echo echoplanar T2‐weighted functional images (TR/TE = 2,840/50 ms, field of view = 192 mm, matrix = 64 × 64, slice thickness = 4 mm, and 36 sagittal slices; preceded by three dummy scans). Each run consisted of three blocks of the five conditions: (1) NoInterf; (2) AmpInterf; (3) DirInterf; (4) Amp&DirInterf; and (5) rest not requiring any movements. Each condition lasted 9.4 scans. During the scan session, the upcoming condition was indicated by a template that appeared 1.5 s before task initiation and remained visible for 3 s. Conditions were ordered randomly across runs and subjects. Between the runs, there was a short break of approximately 3 min.
Imaging Analysis
Imaging data were analyzed with the Statistical Parametric Mapping software (SPM99; Wellcome Department of Cognitive Neurology, London, UK; online at http://www.fil.ion.ucl.ac.uk/spm) [Friston et al., 1995a, b]. The functional images were realigned to the first volume of each run to correct for head movements and slice timing was applied to correct for differences in acquisition time during scanning. After coregistering the functional images to the anatomical image, they were spatially normalized into a standard reference frame [Talairach and Tournoux, 1988], using a representative brain (Montreal Neurological Institute [MNI]) as a template. All functional images were subsampled to a voxel size of 2 × 2 × 2 mm and smoothed with a Gaussian kernel of 10 mm full width at half maximum (FWHM). For the first‐level analysis, a general linear model was used, containing for each condition a boxcar function convolved with the standard SPM99 hemodynamic response function. Additionally, six movement parameters derived from realignment (translation and rotation in x, y, and z dimensions) were added as covariates of no interest.
Contrasts of interest were calculated for each subject and run individually. Subsequently, these contrasts were entered into a second‐level mixed‐effects analysis. Areas reflecting spatial interference were determined by calculating the amplitude interference (AmpInterf + Amp&DirInterf > NoInterf + DirInterf) and the directional interference main effects (DirInterf + Amp&DirInterf > NoInterf + AmpInterf), as well as their conjunction in accordance to the method suggested by Brett et al. [2004] to determine areas responding similarly to both experimental manipulations. For each main effect, a correction for multiple comparisons was applied by means of the false discovery rate (FDR) ensuring an error probability of P < 0.05 [Nichols and Hayasaka, 2003]. However, due to the adaptive nature of the FDR correction to the signal extent, the t thresholds were 3.47, 3.25, and 3.13 for the amplitude interference, directional interference, and the conjunction of both main effects, respectively. To ensure consistency between the main effects and the conjunction, we used a fixed t threshold of 3.47 for all comparisons (this procedure guarantees for all contrasts a P < 0.05).
Within the areas responding either to amplitude or directional interference (main effect network), we determined the Amplitude interference × Directional interference interaction (AmpInterf − NoInterf vs. Amp&DirInterf − DirInterf). Significance was determined after applying a FDR‐correction (t > 3.75) for the reduced search volume of the main effect network with P < 0.05. For all comparisons, the minimum cluster size was set to 10 voxels.
RESULTS
Behavioral Results
Exemplary amplitude and direction data of a typical subject performing the Amp&DirInterf condition (i.e., moving with different amplitudes and along different directions) are shown in Figure 1C and 1D. With respect to the movement amplitude of the right hand (Fig. 1C, gray), it can be seen that the subject switched across small, medium, and large amplitudes, as required. However, the movements of the left wrist (Fig. 1C, black) deviated substantially from the required medium amplitude and instead tended to mirror the right hand's movements.
With respect to movement direction (Fig. 1D), the subject complied well with the requirements for the vertical line drawing of the left wrist (black; target = 90 degrees), but produced a marked error for the horizontal movements with the right hand (gray; target = 0 degrees).
Amplitude interference
Focusing first on the conditions requiring the same amplitudes for both wrists (Fig. 2A), we confirmed using a 2 × 2 × 3 repeated‐measures ANOVA with the factors direction (same or different), hand (left or right), and size (small, medium, or large) that subjects switched with both wrist across small, medium, and large amplitudes, as required (ANOVA revealed a significant size effect; F[2,22] > 100.5, P < 0.001). Moreover, subjects produced similar meanAmps with the left and right wrist. For the different amplitude conditions (Fig. 2B), we subjected the meanAmp data of each hand to a separate Direction × Size repeated‐measures ANOVA and subsequent post‐hoc tests. With the right wrist (Fig. 2B, right panel), subjects complied well with the requirements to switch between the different amplitudes, as indicated by a significant size effect (F[2,22] > 92.8, P < 0.0001).1 Additionally, movement direction influenced the produced amplitudes of the right hand such that meanAmp was around 4 degrees larger when subjects moved along the horizontal as compared to the vertical direction (significant direction effect; F[1,11] > 6.62, P < 0.05). With the left wrist (Fig. 2B, left panel), subjects were required to maintain a constant amplitude irrespective of whether the right hand produced small, medium, or large movements. However, the Size × Direction ANOVA revealed that meanAmp of the left hand was significantly smaller when the right hand produced the small as compared to the large amplitude (significant size effect and post‐hoc test; F[2,22] > 6.83, P < 0.05). This involuntary amplitude modulation of the left hand was pronounced particularly when subjects were to move additionally along different directions (Fig. 2B, gray; significant Size × Direction interaction; F[2,22] > 10.3, P < 0.001).
Figure 2.
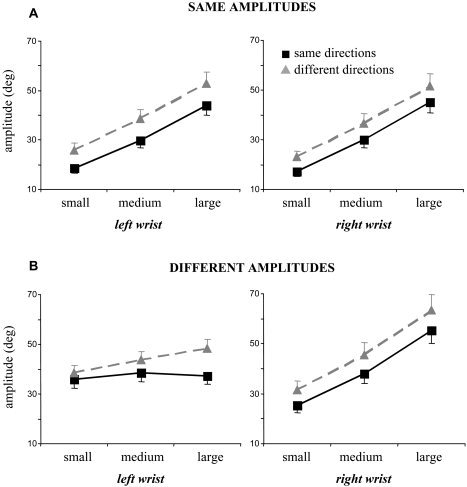
Mean amplitude (meanAmp) data and standard errors on group level for conditions requiring the same (A) or different amplitudes (B). Data for movements along the same (black squares) and different directions (gray triangles) are shown for the left and the right wrist as well as the small, medium, and large amplitude requirements (corresponding to 30, 60, and 90% of the individual maximal amplitude) separately. Note that when subjects were to move simultaneously along different amplitudes (B), the mean amplitude of the left wrist exhibited clear modulations, even though subjects were required to move with a constant medium amplitude.
The consistency of the produced amplitudes was quantified by sdAmp (Fig. 3A) and was subjected to a 2 × 2 Amplitude (same or different) × Direction (same or different) repeated‐measures ANOVA. sdAmp was significantly larger when subjects moved along different as compared to the same amplitudes (significant amplitude effect; F[1,11] > 9.78, P < 0.01). Additionally, sdAmp was increased slightly when subjects moved along different (gray) as compared to the same directions (black), but neither the direction main effect nor the Amplitude × Direction interaction reached significance (P > 0.1).
Figure 3.
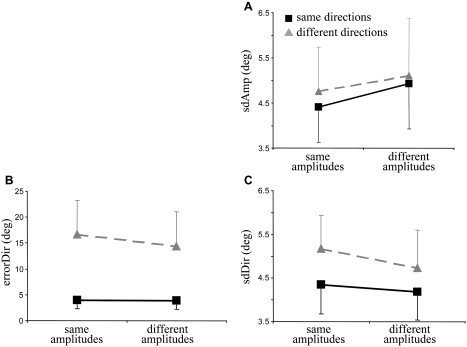
Mean group results and standard errors of the amplitude standard deviation (sdAmp; A), directional error (errorDir; B), and directional standard deviation (sdDir; C). Data are shown for the same vs. different amplitudes (abscissa) and for movements along the same (black squares) vs. different directions (gray triangles). Each data point was yielded by averaging across the three amplitude requirements (i.e., small, medium, and large) and both wrists.
Directional interference
Subjects made a larger directional error (errorDir) when moving along different than when moving along the same directions (Fig. 3B). This was confirmed by a 2 × 2 Amplitude (same or different) × Direction (same or different) repeated‐measures ANOVA, revealing a significant direction effect (F[1,11] > 10.22; P < 0.01). Interestingly, errorDir was influenced additionally by the amplitude requirements. Surprisingly, the rise of errorDir evoked by interfering directions was smaller when subjects produced different than when subjects produced the same amplitudes with both hands. This was confirmed by a significant main effect of amplitude (F[1,11] > 7.15; P < 0.05), as well as a significant Direction × Amplitude interaction (F[2,22] > 15.15; P < 0.005).
Consistency of the produced movement direction was quantified by sdDir, which increased significantly when subjects moved along different versus the same directions (Fig. 3C), as indicated by an Amplitude × Direction ANOVA (direction main effect; F[1,11] > 12.86, P < 0.005). Similar to errorDir, sdDir was influenced additionally by the compatibility of movement amplitudes, such that sdDir was significantly smaller for different than for the same amplitudes (amplitude main effect; F[1,11] > 21.68, P < 0.001). The Direction × Amplitude interaction did not reach significance (P > 0.09).
In summary, our behavioral results revealed that amplitude error as well as variability was higher when subjects moved along different versus the same amplitude. The same result pattern was yielded with respect to the directional error and variability, such that both parameters were increased when subjects traced different versus the same direction in parallel.
Moreover, the effect of amplitude interference was more pronounced when subjects had to move additionally along different versus the same direction. By contrast, the effect of directional incompatibility was surprisingly reduced when subjects moved with different versus the same amplitude.
Brain Activation Results
Bimanual movements as compared to rest activated a typical bilateral motor network including the dorsal and ventral premotor cortex (PMd and PMv, respectively), supplementary motor area (SMA), primary sensorimotor cortex (SM1), superior parietal cortex, basal ganglia, and cerebellum, as well as several frontal and temporal areas. We were particularly interested to identify brain areas reflecting amplitude interference, directional interference, or both.
Figure 4 shows an overview of the cortical areas responding significantly to amplitude interference (red), directional interference (blue), or both (purple), printed on top of a rendered brain (Fig. 4A) as well as superimposed onto selected slices within the premotor cortex (Fig. 4B).
Figure 4.
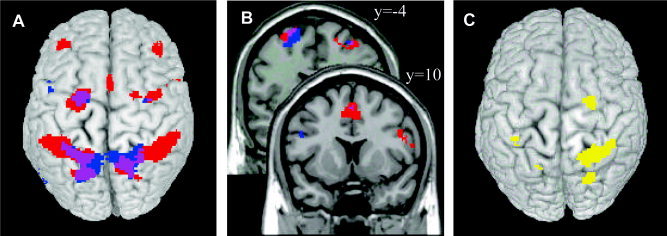
A: Top view of areas exhibiting an amplitude interference main effect (red), a directional interference main effect (blue), or both (purple). B: More detailed view of the interference main effects as in A within the dorsal (upper slice) and ventral premotor cortex (lower slice). C: Network exhibiting a significant amplitude interference × directional interference interaction. Activation is superimposed on top of a rendered brain shown in neurological convention (right is right). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Regions that were activated significantly by the amplitude interference main effect but did not reach significance for the conjunction or interaction analysis were considered involved predominantly in the control of the bimanual amplitude requirements (Fig. 5A–E; Table I). These areas were located bilaterally in the middle frontal gyrus corresponding to the dorsolateral prefrontal cortex (DLPFC; Fig. 5A), the right superior and inferior precentral gyrus corresponding to PMd and PMv, respectively (Fig. 5B,C), the anterior cingulate (Fig. 5D), the right supramarginal gyrus (Fig. 5D) and the superior parietal cortex, more specifically around the left junction of the postcentral and the intraparietal sulcus (Fig. 5E). However, inspecting the blood oxygenation level‐dependent (BOLD) response for the superior parietal spot (Fig. 5E, line plot), it can be seen that this area tended to be activated considerably when subjects moved along different directions, even when the amplitude requirements were identical for both limbs.
Figure 5.
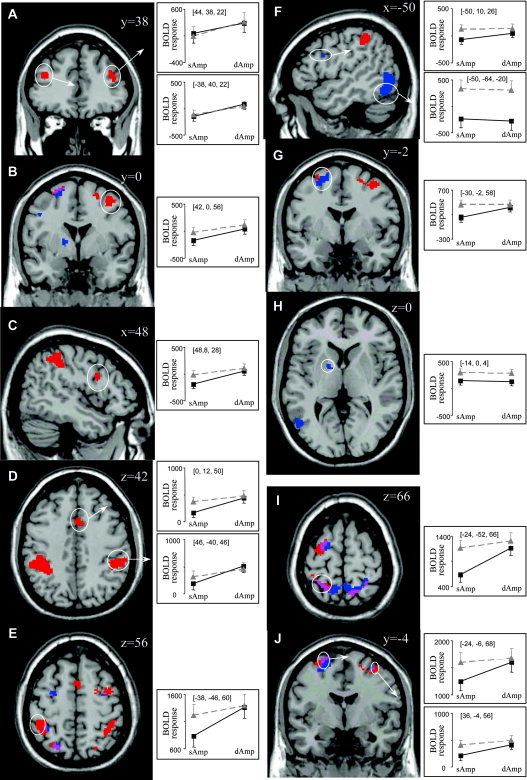
Brain slices showing areas that exhibited a significant amplitude interference main effect (A–E), a significant directional interference main effect (F–H), and a significant conjunction effect (I, J). For selected regions line plots are shown displaying the blood oxygenation level‐dependent (BOLD) response in arbitrary units for movements with the same (sAmp) vs. different amplitudes (dAmp) and along the same (black squares) and different (gray triangles) directions. All ordinates of the line plots are scaled to the same range of 1,000 units. Slices are shown in neurological convention (right is right) and coordinates are reported with respect to the Montreal Neurological Institute (MNI) reference brain. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Amplitude interference main effect
| Brain region | MNI coordinates | t |
|---|---|---|
| x, y, z | ||
| L mid. frontal gyrus (inferior part, DLPFC)a | −38, 40, 22 | 4.84 |
| R mid. frontal gyrus (inferior part, DLPFC)a | 44, 38, 22 | 4.29 |
| L sup. precentral gyrus/sup. frontal gyrus (PMd)b | −24, −6, 70 | 5.27 |
| R sup. precentral gyrus/sup. frontal gyrus (PMd)a | 24, 0, 60 | 3.74 |
| R sup. precentral gyrus/sup. frontal gyrus (PMd)a | 42, 0, 56 | 4.11 |
| R inf. precentral gyrus (PMv)a | 48, 8, 28 | 3.74 |
| Anterior cingulate (paralimbic cortex)a | 0, 12, 50 | 4.70 |
| L supramarginal gyrusc | −34, −42, 44 | 5.99 |
| R supramarginal gyrusa | 46, −39, 46 | 4.49 |
| L junction postcentral sulcus/intraparietal sulcusa | −38, −46, 60 | 6.13 |
| R junction postcentral sulcus/intraparietal sulcusc | 36, −46, 60 | 4.71 |
| L superior parietal gyrus/transverse sulcusb | −16, −52, 70 | 5.10 |
| R superior parietal gyrus/transverse sulcusb | 14, −60, 66 | 4.88 |
False discovery rate corrected P < 0.05, t > 3.47, cluster size >10.
MNI, Montreal Neurological Institute; L, left; R, right; mid., middle; DLPFC, dorsolateral prefrontal cortex; sup., superior; PMd, dorsal premotor cortex; inf., inferior; PMv, ventral premotor cortex.
Coordinates reaching significance only for the amplitude interference main contrast but not for other comparisons.
Areas also exhibiting a significant conjunction effect.
Areas also exhibiting a significant interaction effect.
Regions that were activated significantly by the directional interference main effect but did not reach significance for the conjunction or interaction analysis were considered involved predominantly in the control of the bimanual directional requirements (Fig. 5F–H; Table II). These areas were found in the left superior and inferior precentral gyrus corresponding to PMd and PMv, respectively (Fig. 5F,G), the left inferior temporal gyrus (Fig. 5G), and subcortically in the globus pallidus (Fig. 5H). However, the dorsal premotor region (Fig. 5F) tended to be activated as well when only the amplitude requirements differed between the wrists, as indicated by the line plots.
Table II.
Directional interference main effect
| Brain region | MNI coordinates | t |
|---|---|---|
| x, y, z | ||
| L sup. precentral gyrus/sup. frontal gyus (PMd)a | −30, −2, 58 | 3.67 |
| R sup. precentral gyrus/sup. frontal gyus (PMd)b | 36, −4, 56 | 3.74 |
| L inf. precentral gyrus (PMv)a | −50, 10, 26 | 3.79 |
| L inf. temporal gyrus (posterior part)a | −50, −64, −20 | 5.54 |
| L sup. parietal gyrus/transverse sulcusc | −18, −64, 62 | 4.89 |
| R sup. parietal gyrus/transverse sulcusc | 20, −56, 66 | 5.53 |
| L globus pallidusa | −14, 0, 4 | 4.00 |
False discovery rate corrected P < 0.05, t > 3.47, cluster size >10.
Coordinates reaching significance only for the directional interference main contrast but not for other comparisons.
Areas also exhibiting a significant conjunction effect.
Areas also exhibiting a significant interaction effect.
MNI, Montreal Neurological Institute; L, left; R, right; sup., superior; PMd, dorsal premotor cortex; inf., inferior; PMv, ventral premotor cortex.
A conjunction analysis between these main effects was carried out, revealing areas that responded similarly to amplitude and directional interference (Table III). This analysis revealed a largely bilateral superior parietal‐premotor network, including the bilateral superior precentral/frontal gyrus (PMd; Fig. 5J) and the bilateral superior parietal gyrus (Fig. 5I) with extending activation to the left transverse sulcus and the left supramarginal gyrus. Inspecting the BOLD signal for the four different conditions, it can be seen that these areas increased their activity when either amplitude (AmpInterf) or direction (DirInterf) differed between limbs, as compared to producing the same movements with both limbs (NoInterf). However, when both amplitude and direction were incompatible between the wrists (Amp&DirInterf) only a slight additional rise of the hemodynamic response was observed.
Table III.
Conjunction: amplitude and directional interference
| Brain region | MNI coordinates | t |
|---|---|---|
| x, y, z | ||
| L sup. precentral gyrus/sup. frontal gyus (PMd) | −24, −6, 68 | 4.37 |
| R sup. precentral gyrus/sup. frontal gyus (PMd) | 36, −4, 56 | 3.64 |
| L sup. parietal gyrus | −24, −52, 66 | 4.84 |
| R sup. parietal gyrusa | 28, −52, 64 | 4.20 |
| L transverse sulcus | 14, −60, 66 | 4.46 |
| L supramarginal gyrus | −34, −42, 48 | 4.02 |
False discovery rate corrected P < 0.05, t > 3.47, cluster size >10.
Areas also exhibiting a significant interaction effect.
MNI, Montreal Neurological Institute; L, left; R, right; sup., superior; PMd, dorsal premotor cortex.
Finally, the amplitude interference × directional interference interaction (AmpInterf − NoInterf > Amp&DirInterf − DirInterf) was calculated. The identified areas were located mainly within the right cerebral hemisphere (Fig. 4C, Table IV), including large clusters in the right superior precentral/frontal gyrus corresponding to PMd (Fig. 6A), the right superior parietal gyrus (Fig. 6A), the bilateral ascending intraparietal sulcus (Fig. 6B), the right descending intraparietal sulcus, the left transverse sulcus (Fig. 6A), and the right cerebellum (lobule VI/crus I; Fig. 6C). Exploring the hemodynamic response of the cortical regions in further detail (see line plots), it is obvious that these areas were activated only moderately when subjects moved with the same amplitude and along the same direction, but increased their activity to a similar extent when amplitude, direction, or both parameters differed between limbs. Finally, the cerebellum was the only area that was activated less strongly when both amplitude and direction differed between wrists compared to that when only one parameter was incompatible.
Table IV.
Interaction: Directional interference × Amplitude interference
| Brain region | MNI coordinates | t |
|---|---|---|
| x, y, z | ||
| R sup. precentral gyrus/sup. frontal gyus (PMd) | 24, −8, 62 | 4.64 |
| R sup. parietal gyrus | 28, −52, 64 | 6.60 |
| R junction postcentral sulcus/intraparietal sulcus | 38, −46, 58 | 5.15 |
| R descending intraparietal sulcus | 24, −70, 54 | 4.48 |
| L transverse sulcus | −18, −64, 62 | 4.24 |
| L ascending intraparietal sulcus | −36, −38, 42 | 4.39 |
| Cerebellum lobule VI/crus I | 10, −80, −22 | 4.44 |
False discovery rate corrected P < 0.05, t > 3.75, cluster size >10.
MNI, Montreal Neurological Institute; L, left; R, right; sup., superior; PMd, dorsal premotor cortex.
Figure 6.
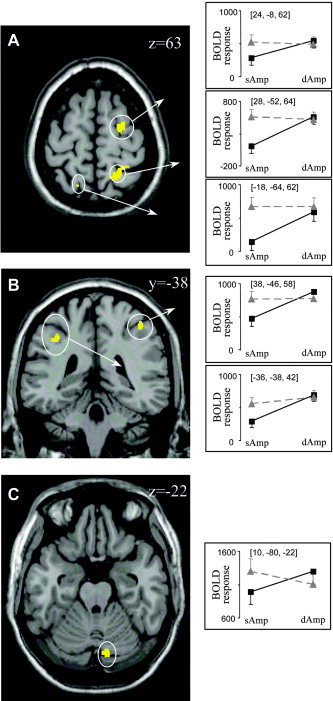
A–C: Brain slices showing areas that exhibit a significant Amplitude interference × Directional interference interaction effect. The same conventions are used as in Figure 5. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The converse interaction (i.e., Amp&DirInterf − DirInterf > AmpInterf − NoInterf) did not reveal any significant results.
DISCUSSION
We used fMRI to compare whether amplitude and directional interference during bimanual actions activate the same or specialized neural circuits. Bimanual movements requiring the same versus different amplitudes and executed along the same versus different directions were therefore combined in a factorial design. Our results provide new insights into the control of bimanual movements and into the more general concepts of motor control, such as the encoding of movement amplitude versus direction.
Behavioral Results
From the online kinematic registrations of subjects' movements in the scanner, we quantified the accuracy and consistency of the produced amplitudes and directions. In accordance with previous results, these measurements revealed that subjects experienced substantial interference for either type of spatial incompatibility between limbs, in good agreement with earlier behavioral results [Franz, 1997; Franz et al., 2000; Franz and Ramachandran, 1998; Sherwood, 1994; Sherwood and Nishimura, 1999; Spijkers and Heuer, 1995; Swinnen et al., 2001, 2002; Walter et al., 2001; Wenderoth et al., 2003]. As such, our paradigm ensured that subjects had to continuously process either amplitude or directional information, because otherwise marked interference effects (such as a transition to mirror‐symmetric movements) would have automatically emerged.
Moreover, directional interference decreased when subjects moved with different amplitude and along different directions, as compared that with to the different directions‐only condition (Fig. 3B,C). Conversely, amplitude interference further increased when subjects moved with different amplitudes and along different directions, as compared to that with the different amplitudes‐only condition (Fig. 2B, left panel and Fig. 3A).2 This suggests that amplitude interference and directional interference are not only interacting but also partly independent phenomena because they exhibit different result patterns.
Other movement parameters such as cycle duration remained largely unchanged across the four movement conditions. In addition, the overall amplitudes produced (i.e., averaged within conditions and hands) as well as the level of cognitive complexity (such as moving on the beat, estimating and switching between different amplitudes, etc.) were matched across all bimanual conditions. Based on the observed behavior, we can thus interpret changes in BOLD response as a genuine index for increasing processing loads resulting either from amplitude or from directional interference during bimanual movements.
We first discuss areas that were commonly activated by both interference types and then focus on those that were specifically involved during either amplitude or directional interference. Finally, we pay specific attention to the dorsal versus ventral premotor cortex, which exhibited differential response patterns to amplitude and directional interference, respectively.
Areas Commonly Activated by Amplitude and Directional Interference: Superior Parietal‐Dorsal Premotor Circuits
As identified by the conjunction analysis, commonly activated areas were found mainly within a bilateral superior parietal‐dorsal premotor network including PMd, the medial and lateral superior parietal gyrus, as well as the adjacent supramarginal gyrus (Fig. 4A, purple; Table IV). Single‐cell recordings in monkeys [Caminiti et al., 1998; Johnson et al., 1996; Kalaska et al., 1997; Lacquaniti and Caminiti, 1998; Rizzolatti et al., 1998; Wise et al., 1997] and functional imaging studies in humans [Astafiev et al., 2003; Connolly et al., 2003; Ellermann et al., 1998; Grafton et al., 1996; Kawashima et al., 1996; Kertzman et al., 1997; Lacquaniti and Caminiti, 1998] have shown that these areas are crucially involved in sensorimotor transformations for guiding motor actions. However, in most of these experiments only movement direction was manipulated. Our results extend this view by suggesting that the same areas contribute also to amplitude control. This is consistent with findings from single‐cell recordings in the premotor and primary motor cortex, which revealed that direction and amplitude are represented by largely overlapping cell populations [Fu et al., 1993, 1995; Kurata, 1993; Messier and Kalaska, 2000; Riehle and Requin, 1989]. Moreover, it supports the idea that neuronal pools of the parieto‐premotor circuits that process distinct aspects of sensory information are distributed across different cortical areas but are highly interconnected. This network architecture allows the matching between different neural representations of space in a rather general and flexible way, connecting the sensory and the motor domain [Burnod et al., 1999; Caminiti et al., 1991]. Consequently, the same neural circuits are activated by many different tasks relying on sensorimotor transformations to guide behavior in space.
Furthermore, the amplitude interference × directional interference interaction analysis revealed areas that were activated only moderately when spatial requirements were identical but exhibited a substantial increase if amplitude, direction, or both parameters differed between limbs. This analysis revealed a mainly right hemispheric superior parietal‐dorsal premotor circuit that responded strongly to directional interference, confirming earlier results [Wenderoth et al., 2004a, d]. Amplitude interference or the combination of both interference types activated this area as well, but did not evoke a further increase of the BOLD response. Our present results thus indicate that this mainly right hemispheric network is activated substantially by either kind of spatial incompatibility between the limbs, supporting the general view that the right hemisphere is particularly involved in processing spatial information. This putative lateralization is relative rather than absolute because the left hemisphere also was sensitive to amplitude interference and, to a somewhat lesser extent, to directional interference only, as discussed in the following section.
Areas Specifically Reflecting Amplitude Interference: Dorsolateral Prefrontal Cortex‐Anterior Cingulate‐Supramarginal Gyrus Circuit
Amplitude interference activated a much more extended network than did directional interference (Fig. 4A), including a dorsolateral prefrontal cortex (DLPFC)‐anterior cingulate cortex (ACC)‐supramarginal gyrus circuit as well as some spots in the dorsal premotor and superior parietal cortex. Particularly, the DLPFC is considered to be a part of the so‐called central executive [Collette and Van der Linden, 2002], which serves several high‐level cognitive functions such as the continuous updating of working memory, inhibition of irrelevant information or inappropriate responses, and the shifting between environmental stimuli or several cognitive operations [Baddeley, 1986; Miyake et al., 2000]. Importantly, the DLPFC, ACC, and supramarginal gyrus seem to be involved particularly in the inhibition of unwanted yet competing responses such as those during conflict situations, for example [Botvinick et al., 1999; Carter et al., 1998; Hester et al., 2004; Kerns et al., 2004; Sylvester et al., 2003]. In accordance with this hypothesis, the ACC was shown to contribute also to bimanual movements that deviate from the naturally preferred mirror‐symmetric coordination mode [Stephan et al., 1999; Wenderoth et al., 2004a]. Importantly, in the present study, the DLPFC‐ACC‐supramarginal gyrus involvement was observed only when subjects moved with different amplitudes but not when subjects moved along different versus the same direction.
One might argue that this differential activation for amplitude versus directional interference mainly reflects higher cognitive demands, imposed by the switching between different amplitudes or by estimating 30, 60, and 90% of the maximal amplitude, whereas directions were kept constant throughout the whole condition. However, all bimanual conditions required subjects to switch between different estimated amplitudes, such that these requirements per se should not represent a confound. Moreover, in previous studies [Wenderoth et al., 2004a,b, d], we investigated directional interference using a more complicated design, requiring also the switching and the estimation of different directions. When bimanual conditions were compared (i.e., directional incompatible movements > directional compatible movements), we mainly observed activation within the superior parietal‐dorsal premotor cortex [Wenderoth et al., 2004a, d]. Only when bimanual incompatible movements were contrasted with unimanual movements, anterior cingulate activation was found [Wenderoth et al., 2005]. In summary, our data indicate that amplitude but not directional interference during bimanual movements activated a DLPFC‐ACC‐supramarginal gyrus network involved in the control of executive functions. Perhaps, this difference emerged from distinct mechanisms controlling the interhemispheric information exchange of amplitude versus direction requirements. More specifically, these areas might play an important role during response inhibition. Alternatively, subjects may have used a different cognitive strategy to control the relative amplitudes between the hands as compared to that for the directional specifications.
Areas Specifically Reflecting Directional Interference: Inferior Temporal Gyrus and Globus Pallidus
Regions exhibiting a clear directional interference main effect were the posterior part of the inferior temporal gyrus (Fig. 5F), which extended to the middle temporal gyrus as well as the middle occipital gyrus. It has been shown that these areas are involved in mental imagery [Farah, 1989; Ganis et al., 2004; Ishai et al., 2000], suggesting that subjects used some form of visualization to estimate orthogonal directions.
Moreover, the globus pallidus (Fig. 5H) responded specifically to directional interference, confirming our earlier result that in humans, the globus pallidus is activated particularly when bimanual movements along incompatible directions are performed under somatosensory guidance only [Wenderoth et al., 2004d]. However, single‐cell responses in monkeys suggest that the globus pallidus is unlikely to encode extrinsic directions per se, but rather reflects some covarying factors [Turner and Anderson, 1997]. In particular, neurophysiological findings and modeling studies point to a more general function of the basal ganglia, such that subthalamic nucleus‐pallidal circuits facilitate selected motor program whereas competing motor actions are suppressed to avoid interference [for an overview, see Alexander and Crutcher, 1990; Mink, 1996; Redgrave et al., 1999; Rubchinsky et al., 2003].
Lateralized Activation of the Premotor Cortex
The premotor cortex is known to be strongly involved in sensorimotor transformations. In the present study, regions of the left as well as the right dorsal premotor cortex (PMd) responded commonly to both amplitude as well as directional interference (see discussion above). Other regions tended to be activated more strongly by either amplitude or directional interference. More specifically, the left PMd contained a patchwork of adjacent regions exhibiting slightly different levels of specialization (see the red‐to‐blue gradient in Fig. 4B, upper slice, and Fig. 5I) such that some neurons seemed associated more with amplitude interference (red), others with directional interference (blue), and again others with both interference types (purple). In addition, the right PMd responded to either type of spatial incompatibility, even though amplitude interference evoked a much more extended activation. Unlike the dorsal premotor cortex, the ventral premotor cortex (PMv) responded in a more lateralized fashion (Fig. 3B lower slice), such that the left PMv was activated mainly by directional interference and the right PMv by amplitude interference (this hemispheric lateralization is relative, rather than absolute). In general, it is believed the right PM is engaged more frequently in spatial tasks and that it is concerned more strongly with global as compared to local processing of perceptual information than is the left PM [Schubotz and von Cramon, 2003]. It has been argued that the control of complex tasks with high attentional demands may become divided between hemispheres to access separate resource pools [for a review see Banich, 1998]. It is thus tempting to speculate that amplitude control, which required frequent switches to new amplitude requirements, was processed mainly by the right hemisphere specialized for spatial control, whereas the less demanding directional control, requiring only to maintain orthogonal directions, was somewhat more processed by the left hemisphere. This multiple‐task strategy hypothesis deserves further investigation.
SUMMARY AND CONCLUSIONS
Our study revealed that amplitude interference and directional interference during bimanual movements commonly activate bilateral superior parietal‐dorsal premotor areas, which were shown previously to contribute to sensorimotor transformations during goal‐directed actions. Whereas the dorsal premotor cortex seemed to be involved in processing both amplitude and directional information, the ventral premotor cortex showed some level of hemispheric specialization, responding preferentially to either amplitude (right hemisphere) or directional interference (left hemisphere).
Interestingly, amplitude interference but not directional interference was associated with activation of a DLPFC‐ACC‐supramarginal gyrus network, which contributes to executive functions. It is likely that these areas are not concerned with encoding amplitude per se, but are activated due to the specific requirements of our bimanual setup. Based on this finding, it is hypothesized that compared to directional information, amplitude information during bimanual movements is processed at least partly by other areas. In general, our data thus support the view that even though the encoding of amplitude and directional information converge at one point and activate the same neural substrate (here, superior parietal‐dorsal premotor areas), additional independent mechanisms are involved in bimanual amplitude as compared to that in direction control.
Footnotes
Interestingly, amplitude modulation (i.e., the difference between the large and small amplitude), was even larger for the different (31.67 degrees) than for the same amplitude conditions (29.76 degrees).
One might argue that this increase in amplitude interference results from a confound, i.e., when subjects moved with their right hand along the horizontal, directional interference induced a deviation toward the vertical. Consequently, the Euclidean distance between the turning points would increase, whereas it is reasonable that subjects predominantly controlled the amplitude of the horizontal component, i.e., along the required movement direction. However, testing this possibility by analyzing only the amplitudes along the main movement direction revealed the same result pattern as using the Euclidean distance, indicating that the observed interaction between amplitude and directional interference is a psychophysical phenomenon rather than an artifact.
REFERENCES
- Alexander GE, Crutcher MD (1990): Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13: 266–271. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M (2003): Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD (1986): Working memory. Oxford: Clarendon Press. [Google Scholar]
- Banich MT (1998): The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn 36: 128–157. [DOI] [PubMed] [Google Scholar]
- Bock O, Arnold K (1992): Motor control prior to movement onset: preparatory mechanisms for pointing at visual targets. Exp Brain Res 90: 209–216. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD (1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Brett M, Nichols T, Andersson J, Wager T, Poline JB (2004): When is a conjunction not a conjunction? (Poster WE 137). Presented at the 10th Annual Meeting of the Organization for Human Brain Mapping, June 13–17, Budapest, Hungary.
- Burnod Y, Baraduc P, Battaglia‐Mayer A, Guigon E, Koechlin E, Ferraina S, Lacquaniti F, Caminiti R (1999): Parieto‐frontal coding of reaching: an integrated framework. Exp Brain Res 129: 325–346. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Ferraina S, Mayer AB (1998): Visuomotor transformations: early cortical mechanisms of reaching. Curr Opin Neurobiol 8: 753–761. [DOI] [PubMed] [Google Scholar]
- Caminiti R, Johnson PB, Galli C, Ferraina S, Burnod Y (1991): Making arm movements within different parts of space: the premotor and motor cortical representation of a coordinate system for reaching to visual targets. J Neurosci 11: 1182–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD (1998): Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M (2002): Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev 26: 105–125. [DOI] [PubMed] [Google Scholar]
- Connolly JD, Andersen RA, Goodale MA (2003): FMRI evidence for a “parietal reach region” in the human brain. Exp Brain Res 153: 140–145. [DOI] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E (1998): Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278. [DOI] [PubMed] [Google Scholar]
- Ellermann JM, Siegal JD, Strupp JP, Ebner TJ, Ugurbil K (1998): Activation of visuomotor systems during visually guided movements: a functional MRI study. J Magn Reson 131: 272–285. [DOI] [PubMed] [Google Scholar]
- Farah MJ (1989): The neural basis of mental imagery. Trends Neurosci 12: 395–399. [DOI] [PubMed] [Google Scholar]
- Favilla M, Hening W, Ghez C (1989): Trajectory control in targeted force impulses. VI. Independent specification of response amplitude and direction. Exp Brain Res 75: 280–294. [DOI] [PubMed] [Google Scholar]
- Franz EA (1997): Spatial coupling in the coordination of complex actions. Q J Exp Psychol A 50: 684–704. [DOI] [PubMed] [Google Scholar]
- Franz EA, Ramachandran VS (1998): Bimanual coupling in amputees with phantom limbs. Nat Neurosci 1: 443–444. [DOI] [PubMed] [Google Scholar]
- Franz EA, Waldie KE, Smith MJ (2000): The effect of callosotomy on novel versus familiar bimanual actions: A neural dissociation between controlled and automatic processes? Psychol Sci 11: 82–85. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Heather JD, Frackowiak RS (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–188. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995b): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Fu QG, Flament D, Coltz JD, Ebner TJ (1995): Temporal encoding of movement kinematics in the discharge of primate primary motor and premotor neurons. J Neurophysiol 73: 836–854. [DOI] [PubMed] [Google Scholar]
- Fu QG, Suarez JI, Ebner TJ (1993): Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol 70: 2097–2116. [DOI] [PubMed] [Google Scholar]
- Ganis G, Thompson WL, Kosslyn SM (2004): Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res 20: 226–241. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Ghez C (1994): Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res 99: 97–111. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Woods RP, Arbib MA (1996): Functional anatomy of pointing and grasping in humans. Cereb Cortex 6: 226–237. [DOI] [PubMed] [Google Scholar]
- Helmuth LL, Ivry RB (1996): When two hands are better than one: reduced timing variability during bimanual movements. J Exp Psychol Hum Percept Perform 22: 278–293. [DOI] [PubMed] [Google Scholar]
- Hester R, Murphy K, Garavan H (2004): Beyond common resources: the cortical basis for resolving task interference. Neuroimage 23: 202–212. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV (2000): Distributed neural systems for the generation of visual images. Neuron 28: 979–990. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R (1996): Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex 6: 102–119. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE (1997): Cortical control of reaching movements. Curr Opin Neurobiol 7: 849–859. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Naitoh E, Matsumura M, Itoh H, Ono S, Satoh K, Gotoh R, Koyama M, Inoue K, Yoshioka S (1996): Topographic representation in human intraparietal sulcus of reaching and saccade. Neuroreport 7: 1253–1256. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS (2004): Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kertzman C, Schwarz U, Zeffiro TA, Hallett M (1997): The role of posterior parietal cortex in visually guided reaching movements in humans. Exp Brain Res 114: 170–183. [DOI] [PubMed] [Google Scholar]
- Kurata K (1993): Premotor cortex of monkeys: set‐ and movement‐related activity reflecting amplitude and direction of wrist movements. J Neurophysiol 69: 187–200. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Caminiti R (1998): Visuo‐motor transformations for arm reaching. Eur J Neurosci 10: 195–203. [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF (1997): Differential effect of task conditions on errors of direction and extent of reaching movements. Exp Brain Res 115: 469–478. [DOI] [PubMed] [Google Scholar]
- Messier J, Kalaska JF (2000): Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized‐delay reaching task. J Neurophysiol 84: 152–165. [DOI] [PubMed] [Google Scholar]
- Mink JW (1996): The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognit Psychol 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S (2003): Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 12: 419–446. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K (1999): The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89: 1009–1023. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J (1989): Monkey primary motor and premotor cortex: single‐cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol 61: 534–549. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998): The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283–296. [DOI] [PubMed] [Google Scholar]
- Rokni U, Steinberg O, Vaadia E, Sompolinsky H (2003): Cortical representation of bimanual movements. J Neurosci 23: 11577–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA (1980): Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109: 444–474. [DOI] [PubMed] [Google Scholar]
- Rubchinsky LL, Kopell N, Sigvardt KA (2003): Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus‐pallidal circuits. Proc Natl Acad Sci USA 100: 14427–14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY (2003): Functional‐anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage 20(Suppl): 120–131. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Brown P (2002): The functional role of interhemispheric synchronization in the control of bimanual timing tasks. Exp Brain Res 147: 268–272. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Wiesendanger M (2001): Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci 14: 1897–1905. [DOI] [PubMed] [Google Scholar]
- Sherwood DE (1994): Hand preference, practice order, and spatial assimilations in rapid bimanual movement. J Mot Behav 26: 123–134. [DOI] [PubMed] [Google Scholar]
- Sherwood DE, Nishimura KN (1999): Spatial error detection and assimilation effects in rapid single and bimanual aiming movements. J Mot Behav 31: 381–393. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Flanders M (1989): Sensorimotor representations for pointing to targets in three‐dimensional space. J Neurophysiol 62: 582–594. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Ross B (1984): Psychophysical determination of coordinate representation of human arm orientation. Neuroscience 13: 595–604. [DOI] [PubMed] [Google Scholar]
- Spijkers W, Heuer H (1995): Structural constraints on the performance of symmetrical bimanual movements with different amplitudes. Q J Exp Psychol A 48: 716–740. [Google Scholar]
- Steinberg O, Donchin O, Gribova A, Cardosa de Oliveira S, Bergman H, Vaadia E (2002): Neuronal populations in primary motor cortex encode bimanual arm movements. Eur J Neurosci 15: 1371–1380. [DOI] [PubMed] [Google Scholar]
- Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse S, Herzog H, Sturm V, Zilles K, Seitz RJ, Freund HJ (1999): The role of ventral medial wall motor areas in bimanual co‐ordination. A combined lesion and activation study. Brain 122: 351–368. [DOI] [PubMed] [Google Scholar]
- Swinnen SP (2002): Intermanual coordination: from behavioural principles to neural‐network interactions. Nat Rev Neurosci 3: 348–359. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Dounskaia N, Duysens J (2002): Patterns of bimanual interference reveal movement encoding within a radial egocentric reference frame. J Cogn Neurosci 14: 463–471. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Dounskaia N, Levin O, Duysens J (2001): Constraints during bimanual coordination: the role of direction in relation to amplitude and force requirements. Behav Brain Res 123: 201–218. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J (2003): Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 41: 357–370. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Turner RS, Anderson ME (1997): Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol 77: 1051–1074. [DOI] [PubMed] [Google Scholar]
- Walter CB, Swinnen SP, Dounskaia N, Van Langendonk H (2001): Systematic error in the organization of physical action. Cogn Sci 25: 393–422. [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Hecke PP, Swinnen SP (2004a): Parieto‐premotor areas mediate directional interference during bimanual movements. Cereb Cortex 14: 1153–1163. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, van Hecke P, Swinnen SP (2005): The role of anterior cingulate cortex and precuneus in the coordination of motor behavior. Eur J Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- Wenderoth, Debaere F, Swinnen SP (2004c): Neural networks involved in cyclical interlimb coordination as revealed by medical imaging techniques In: Swinnen SP, Duysens J, editors. Neuro‐behavioral determinants of interlimb coordination. Norwell: Kluwer Academic Publishers; p 187–221. [Google Scholar]
- Wenderoth N, Puttemans V, Vangheluwe S, Swinnen SP (2003): Bimanual training reduces spatial interference. J Mot Behav 35: 296–308. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Toni I, Bedeleem S, Debaere F, Sunaert S, van Hecke P, Swinnen SP (2004d): Information processing in human parieto‐frontal circuits during goal‐directed movements. Cereb Cortex (submitted). [DOI] [PubMed]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R (1997): Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42. [DOI] [PubMed] [Google Scholar]


