Abstract
The shape and number of mitochondria respond to the metabolic needs during the cell cycle of the eukaryotic cell. In the best-studied model systems of animals and fungi, the cells contain many mitochondria, each carrying its own nucleoid. The organelles, however, mostly exist as a dynamic network, which undergoes constant cycles of division and fusion. These mitochondrial dynamics are driven by intricate protein machineries centered around dynamin-related proteins (DRPs). Here, we review recent advances on the dynamics of mitochondria and mitochondrion-related organelles (MROs) of parasitic protists. In contrast to animals and fungi, many parasitic protists from groups of Apicomplexa or Kinetoplastida carry only a single mitochondrion with a single nucleoid. In these groups, mitochondrial division is strictly coupled to the cell cycle, and the morphology of the organelle responds to the cell differentiation during the parasite life cycle. On the other hand, anaerobic parasitic protists such as Giardia, Entamoeba, and Trichomonas contain multiple MROs that have lost their organellar genomes. We discuss the function of DRPs, the occurrence of mitochondrial fusion, and mitophagy in the parasitic protists from the perspective of eukaryote evolution.
Mitochondrial dynamics in model cellular system of yeast and humans
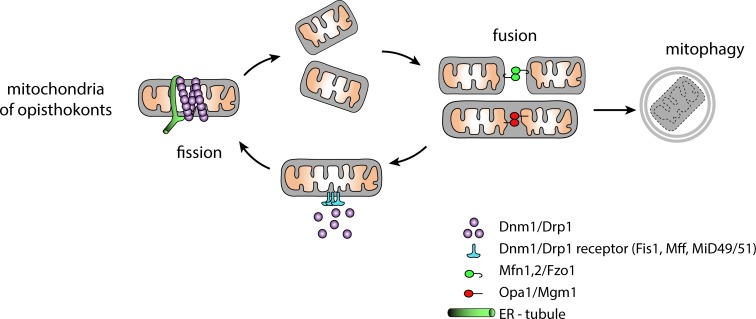
Mitochondria are semiautonomous organelles cordoned by two membranes that are not contiguous with the endomembrane system of the eukaryotic cell [1]. As studied in model cellular systems of yeast and human cells (representatives of the Opisthokonta supergroup of eukaryotes), the mitochondria constantly divide and fuse during the cell cycle. Discrete organelles are thus in a dynamic balance with the continuous and reticulate network [2]. This dynamic responds to the metabolic state of the cell, supporting the idea that mitochondrial fusion functionally complements defective organelles via the exchange of proteins, lipids, and DNA [3,4]. The organellar dynamics require precise control over the membrane fission and fusion events, which are mediated by dynamin-related proteins (DRPs) [5] (Fig 1). Fission relies on the assembly of a Drp1/Dnm1 helical oligomer (mammalian/yeast nomenclature) on the mitochondrial membrane [6], which constricts the membrane upon GTP hydrolysis [7]. The soluble cytosolic Drp1 must, however, be recruited to the mitochondrial surface by sets of receptors that are, except for Fis1, different for human and yeast systems (Mff, MiD49, MiD51, and Fis1 for human [7,8] and Mdv1, Caf4 and Fis1 [9,10] for yeast mitochondria).
Fig 1. Mitochondrial dynamics.
Mitochondria of animals and fungi undergo constant cycles of opposing fission and fusion events. For division, the molecules of DRP are recruited to the mitochondrial surface by DRP receptors. The sites of membrane fission often coincide with the ER-mitochondria connections. Membrane-anchored DRPs specific for the outer (Mfn1,2/Fzo1) and the inner (Opa1/Mgm1) mitochondrial membrane mediate the fusion. Defective mitochondria are removed from the cycle by mitophagy. DRP, dynamin-related proteins; ER, endoplasmic reticulum.
In addition to the protein machinery, mitochondrial division is assisted by the tubules of the endoplasmic reticulum (ER), which seem to wrap around the constriction sites [11,12]. In yeast, these ER-mitochondria hotspots are defined by the molecular tethering complex known as ER-mitochondria encounter structure (ERMES) [13].
The fusion of mitochondria requires two membrane-anchored DRPs specific for the two mitochondrial membranes, mitofusins (Mfn1, Mfn2/Fzo1) [14,15] in the outer membrane and Opa1/Mgm1 in the inner membrane, respectively. In yeast, the fusion of the two membranes is coordinated by Ugo1, which interacts with both Fzo1 and Mgm1 [16], and a homologous role has been proposed for mammalian SLC25A46 [17].
Finally, defective mitochondria are removed from division–fusion cycles by mitophagy, a specific autophagy pathway [18] that leads to lysosomal degradation of those organelles.
The molecular machineries and the regulatory pathways controlling mitochondrial dynamics have been dissected at a molecular level in selected opisthokont cellular models. However, the aspects of mitochondrial dynamics outside the opisthokonts remain a highly unexplored field. In parasitic protists, two opposing factors have affected mitochondrial dynamics. Parasitism has streamlined the overall cellular structure including mitochondria, whereas complex life cycles have often resulted in diverse specialized parasite stages. Here, we review current knowledge on the mitochondrial dynamics of medically important protist parasites, namely, Plasmodium spp., Toxoplasma gondii, Trypanosoma brucei, Trichomonas vaginalis, Giardia intestinalis, and Entamoeba histolytica.
Plasmodium spp.
Plasmodium parasites belong to phylum Apicomplexa, most of which are obligatory intracellular parasites with a highly specialized cellular structure for cell invasion, the apical complex [19]. Multiple Plasmodium species cause malaria in humans and other vertebrates, and these parasites go through a series of morphological transformations during their life cycle, spanning the intermediate vertebrate host and the definitive host, the mosquito [20]. In the vertebrate host, the parasite asexually reproduces first within hepatocytes, dividing to form merozoites that burst from the hepatocyte to invade erythrocytes [20]. There is a single mitochondrion in each Plasmodium cell, the energy metabolism of which is suppressed at the intraerythrocytic stage [21] as manifested by the loss of cristae [22]. Within the erythrocyte, the parasite undergoes the unusual process of schizogony, a series of nuclear divisions without cytokinesis, which gives rise to multinucleated schizonts. As the parasite grows, the mitochondrion branches massively and segregates into emerging daughter cells [23] (Fig 2). The mitochondrion associates with the apicoplast [23–25], a secondary plastid harbored by most apicomplexans [26], and this association becomes more prominent during schizogony, when both organelles divide. The actual mitochondrial division always occurs after the apicoplast has been divided (Fig 2). The coordinated division and the mutual contacts of both organelles may represent a mechanism ensuring that every daughter cell always receives one copy of each organelle [23,27]. During the initial schizogony in the liver, the divisions occur on a much larger scale generating thousands of nuclei. Nevertheless, the overall sequence of division events is analogous to the erythrocytic stage [28,29]. Here, however, the mitochondrion forms enormous branched structures intertwined with the apicoplast [28], and the association between the apicoplast and mitochondrion is not seen during schizogony until the apicoplast starts to divide [28,29]. Before the transmission to mosquito, the parasite undergoes differentiation into sexual stages (gametocytogenesis), during which the mitochondrion’s morphology changes dramatically [30]. Within the erythrocyte, the precursors of female and male gametes are formed (macrogametocyte and microgametocyte, respectively). The mitochondrion of the macrogametocyte elongates and branches, eventually forming a cluster around the apicoplast, which itself does not change [30]. Upon transmission to the mosquito, gametocytes rapidly differentiate to mature gametes, and a macrogamete and a microgamete fuse to produce a zygote that later develops into the infectious sporozoites [30]. The fusion of the gametes does not involve the fusion of their mitochondria, as only the female’s mitochondrion is retained [31]. In fact, mitochondrial fusion has not been observed in Plasmodium [28]. This corresponds to the lack of mitofusins in the genome sequences of Plasmodium species (Table 1), although an alternate molecular machinery may be in charge. Despite the observed massive synchronized mitochondrial division, there is no information on the involvement of the only two dynamin orthologues (Dyn1 and Dyn2) identified in Plasmodium genome [32–35]. While Dyn1 has been suggested to participate in the hemoglobin uptake, Dyn2 partially localizes to the endomembrane system and the apicoplast [32]. Thus, the molecular fission machinery of Plasmodium mitochondrion remains unknown.
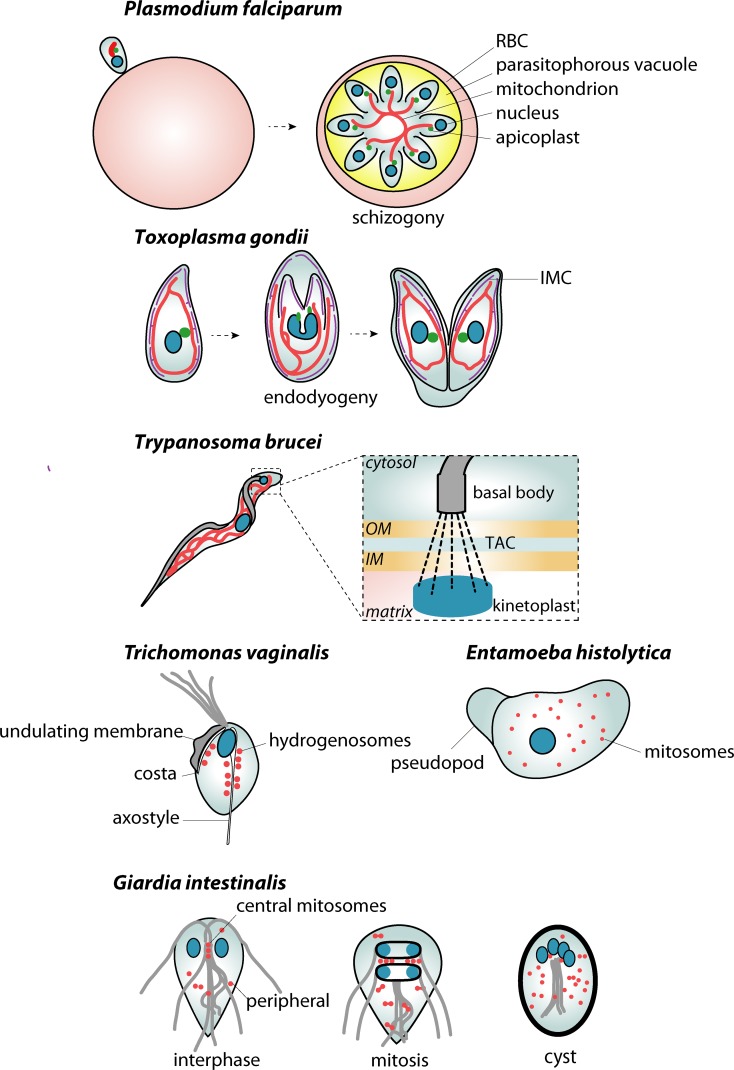
Fig 2. Key aspects of mitochondrial dynamics in parasitic protists.
Single mitochondrion of P. falciparum in synchrony with the specific cell division known as schizogony. The elongated mitochondrion branches and segregates into emerging daughter cells at the very end of the cell division. Throughout most of the cell cycle, the mitochondrion maintains the association with the apicoplast. Similarly, two daughter cells of T. gondii develop within the mother cell by a process known as endodyogeny. Here, the mitochondrion is also in contact with the IMC—a membranous compartment underneath the cytoplasmic membrane specific to Alveolata. In T. brucei, the mitochondrial genome is arranged into a kinetoplast disc, which is physically connected to the basal body of the flagellum by a TAC. The division of the single parasite mitochondrion is thus fully coordinated with the maturation of a new flagellum and the segregation of two daughter kinetoplasts. MROs of anaerobic parasitic protists are always present in higher numbers. In T. vaginalis, hydrogenosomes associate with the main cytoskeletal structures, the axostyle and costa. In G. intestinalis, central and peripheral mitosomes can be distinguished. The first are proposed to be in contact with the axonemes of flagella. Both populations divide during mitosis, which is also part of the encystation process. The mature cyst thus contains a doubled set of nuclei and mitosomes. In E. histolytica, hundreds of mitosomes are distributed all over the cell. IM, inner mitochondrial membrane; IMC, inner membrane complex; OM, outer mitochondrial membrane; MRO, mitochondrion-related organelle; RBC, red blood cell; TAC, tripartite attachment complex.
Table 1. Distribution of components involved in mitochondrial division and fusion in parasitic protists.
| Drp1/ Dnm1 | Drp1/Dnm1 receptors | Fusion | Mitofusin | ERMES | Mitophagy | |||
|---|---|---|---|---|---|---|---|---|
| Species | Fis1 | Mff MiD51 MiD49 | Caf4 Mdv1 | |||||
| Plasmodium falciparum | 32–34 | 35 | ||||||
| T. gondii | 34,40,42 | 42 | 45,46 | |||||
| Cryptosporidium sp. | 98 | |||||||
| T. brucei | 56,58,89 | 56,58,59 | 63? | 60? | 83,99,100# | |||
| Leishmania sp. | 58 | 83 | 101 | |||||
| T. vaginalis | 74 | 102 | ||||||
| G. intestinalis | 78,81,82 | |||||||
| E. histolytica | 87,88 | 89? | ||||||
White fields depict the presence of the orthologous protein; references to corresponding publications are shown. The phenotypic observation of mitochondrial fusion or mitophagy is also marked. Question mark depicts the indirect evidence.
#Homologues of ERMES components present but neither form a complex nor participate in the ER-mitochondrion contact sites.
Abbreviation: ERMES, ER-mitochondria encounter structure
Toxoplasma gondii
T. gondii is an apicomplexan parasite that infects many warm-blooded animals (including humans) as possible intermediate hosts. The definitive hosts are felids [36]. The mitochondrion of T. gondii has been described predominantly in a rapidly proliferating life stage called tachyzoite. The single mitochondrion of T. gondii clusters at the anterior part of the cell with branches spanning towards its posterior part, forming a lasso shape [37]. In a pattern analogous to Plasmodium, the mitochondrion of Toxoplasma undergoes striking extension and branching during the cellular division process known as endodyogeny (Fig 2). In contrast to schizogony of Plasmodium, only two daughter cells are formed within the mother cell [38]. The mitochondrial extensions continue to grow, ultimately surrounding the growing daughter cells. Interestingly, mitochondrial branches enter the developing daughter cells at the last possible moment of cell division, migrating along the cytoskeletal scaffolding. A small amount of mitochondrial compartment is left behind in the residual body [39].
The mechanism underlying division of the two emerging mitochondria has been partially uncovered only very recently. There are three DRPs encoded in the T. gondii genome. TgDrpA and TgDrpB are involved in the biogenesis of apicoplast and micronemes/rhoptries, respectively [34,40], and TgDrpC was shown to associate with multiple compartments, including mitochondria [41,42]. TgDrpC is specific to apicomplexans and closely related organisms [34] and is a highly divergent DRP as it contains only the N-terminal GTPase domain and lacks both the central and GED (GTPase effector) domains [41,42]. This indicates that the protein is a lineage-specific protein invention. TgDrpC forms puncta in the cytosol, some of which are in close proximity to mitochondrial membrane. By modifying the amount and primary structure of TgDrpC, prolonged mitochondria and other defects in mitochondrial morphology could be observed, suggesting its specific involvement in mitochondrial division [42]. However, TgDrpC seems to have rather pleiotropic functions, given that the morphology of other compartments like Golgi, apicoplast, and the inner membrane complex (IMC) also changed in the affected cells [41].
The genome of T. gondii also encodes a homologue of the adaptor protein, Fis1. In contrast to yeast Fis1 but similar to the human homologue, knockdown of TgFis1 did not affect mitochondrial morphology or parasite proliferation [42]. Thus, the actual role of Fis1 in the protist remains unknown. So far, no fusion between mitochondrial tubules has been directly observed in T. gondii (Table 1), yet the formation of the lasso structures indicates at least a single fusion of the mitochondrial tubules.
The mitochondrion of T. gondii was also reported to associate with the apicoplast during parasite division, but this association is not maintained throughout the whole process [39]. The mitochondrion of tachyzoites was instead shown to associate with the IMC, a unique arrangement of flattened membranes found directly below the plasma membrane of apicomplexans [43,44] (Fig 2). Mitochondrial morphology also relates to the egress of the parasite from the host cell, during which the lasso-shaped mitochondrion concentrates from the cell periphery into so-called "condensed" and "sperm-like" morphologies [44]. These structures are preserved until the induction of gliding movement and the invasion into the new host cell. Consistently, collapsed mitochondria of invading parasites were shown to re-expand and re-establish a lasso shape via the sperm-like intermediate, and only parasites that harbor lasso-shaped mitochondrion are able to divide within the host cell [44]. Interestingly, the integrity of single T. gondii mitochondria was found to be somehow controlled by autophagy [45,46]. The deletion of Atg3, an essential autophagy factor, caused specific morphological defects to the mitochondrion [46]. On the other hand, upon starvation, during which the formation of the autophagosomes is induced, the mitochondrion became irreversibly fragmented. This fragmentation could be blocked by an autophagy inhibitor, 3‐methyl adenine [45]. Thus, the exact functional connection between autophagy and mitochondrial biogenesis/dynamics must be studied further.
Trypanosoma brucei
The single mitochondrion of T. brucei, a causative agent of African trypanosomiasis [47], undergoes dramatic metabolic changes during the parasite life cycle [48]. These changes are reflected by the shape and metabolic capacity of the mitochondrion. In the tsetse fly vector, proline is the main energy source, and mitochondrion catalyzes its oxidation via the Krebs cycle [48]. Accordingly, the mitochondrion develops a highly branched network with distinct inner membrane cristae. On the other hand, the bloodstream form (BSF) of the parasite takes advantage of glucose abundance, and its mitochondrion becomes functionally repressed and acristate [48]. The recently discovered adipose tissue form of the parasite also revealed reduced mitochondrial volume [49]. The machinery responsible for the direct morphological transformation of the organelle is not understood. However, as shown in BSF, after cytokinesis the mitochondrial network gradually develops by branching of a single mitochondrial tube [50]. The most striking character of T. brucei mitochondrion is the presence of the kinetoplast, a highly ordered mitochondrial DNA [51]. The kinetoplast is physically connected to a basal body of the single flagellum via the so-called tripartite attachment complex (TAC) [52]. The division of the mitochondrion is thus fully coordinated with the maturation of a new flagellum and the segregation of two daughter kinetoplasts [53–55]. Importantly, defects in mitochondrial division result in cell cycle arrest [56], and the division of the organelle may thus serve as a cell cycle checkpoint [57]. T. brucei encodes orthologues of two mitochondrial division proteins, Drp1 and Fis1 [56,58] (Table 1). The dynamin-like protein, TbDLP, is present as two highly similar paralogues [59]. These are localized not only in the mitochondrion but also in the flagellar pocket compartment, where endocytosis occurs [56]. The ablation of TbDLP thus leads to impaired mitochondrial division as well as endocytosis [56,58]. So far, mitochondrial fusion has not been directly reported in trypanosomes. However, a candidate mitofusin (TbMNFL) homologue was identified in trypanosomes [60]. Silencing the expression of TbMNFL resulted in a mitochondrial fenestration phenotype, which is rather reminiscent of the fission defect in yeast mitochondria [60,61]. Whether the protein is dedicated to mitochondrial fusion requires further examination. Moreover, mitochondrial fusion may be limited to a specific life cycle stage of T. brucei, as haploid, gamete-like T. brucei cells that differentiate in tsetse flies undergo cellular fusion [62]. However, it was shown that mitochondria of BSF of T. brucei artificially fragmented by expression of the human proapoptotic protein Bax are capable of refusion into one single organelle after halting Bax expression [63]. Recently, frequent fusion events between mitochondrial tubules were reported in a related kinetoplastid organism, Crithidia fasciculata [64], which suggests that trypanosome mitochondrion may also fuse under physiological conditions.
Trichomonas vaginalis
T. vaginalis is a causative agent of trichomoniasis, the most common sexually-transmitted disease of nonviral origin [65]. Instead of mitochondria, T. vaginalis contains rounded organelles about 1–2 μm in diameter called hydrogenosomes, which belong to a large group of so-called mitochondrion-related organelles (MROs) [66]. Hydrogenosomes are devoid of DNA and cristae and produce ATP by substrate-level phosphorylation with concomitant production of hydrogen. However, substantial biochemical and phylogenetic evidence has demonstrated that hydrogenosomes are anaerobic adaptations of mitochondria, and the general blueprint of organelle biogenesis is shared between both types of the organelles [67,68]. Over a hundred hydrogenosomes are localized along the axostyle and costa, unique cytoskeletal structures, which may consume hydrogenosome-generated ATP [69]. Morphological studies described several types of hydrogenosomal division and their association with the ER [70,71]. However, mainly due to the lack of live imaging data [72,73], it is possible that all division types are merely different morphological manifestations of a single division process, or perhaps some of them represent distinct hydrogenosome-related pathways. There are eight DRPs in the genome of T. vaginalis. One of them was localized to the hydrogenosomes, and the expression of the dominant-negative form of the protein resulted into the presence of larger and fewer organelles [74]. This was likely a consequence of impaired hydrogenosomal division, hence strongly suggesting the role of this single DRP in the process [74]. So far, fusion between hydrogenosomes has not been observed, nor have mitofusin orthologues been identified in T. vaginalis genome.
Giardia intestinalis
Giardia intestinalis possesses some of the most reduced MROs, known as mitosomes [75]. About 40 organelles 50–200 nm in size harbor single metabolic pathway of the iron–sulfur cluster biosynthesis [76–78]. Mitosomes do not generate ATP but are still bounded by two membranes [75].
Two main populations of mitosomes are recognized in G. intestinalis: central mitosomes (CMs)—located between the two Giardia nuclei, where basal bodies of eight flagella are also localized—and the peripheral mitosomes (PMs), distributed in the rest of the cell [79,80]. Regarding the conserved position of CMs, it was proposed that CMs might be connected to the basal bodies and that their division could be coordinated with the parasite cell cycle [79].
There is only a single DRP in G. intestinalis (GlDRP), and its involvement in mitosomal dynamics remains unclear [78,81]. The main localization of the protein at the cytoplasmic membrane indicated its role in the scission of the endocytic vesicles, whereas the dominant-negative form of GlDRP interfered with the differentiation of the parasite into the cyst stage [81,82]. The mutant form of the protein also colocalized with the mitosomes, where it possibly interfered with mitosomal division [78]. However, a different study could not confirm the role of GlDRP in mitosomal division [81], and more experiments will be needed to clarify the involvement of this protein.
Interestingly, mitosomal dynamics are in synchrony with the cell cycle as both CMs and PMs divide only during mitosis [81]. This includes encystation of the parasite, during which the cell undergoes another round of mitosis without cytokinesis, and the mitosome number doubles accordingly. No fusion between mitosomes was observed in G. intestinalis even upon several hours of long observations [73,78,81], which is supported by the absence of mitofusins in the genome sequence. However, despite the obvious lack of the ERMES complex components in G. intestinalis [83], mitosomes remain in very close contact with the ER throughout the parasite cell cycle and life cycle. Moreover, the contact sites between mitosomes and the ER are enriched for long-chain acyl-CoA synthetase, which indicates synthesis and/or transfer of lipids between these two organelles [81].
Entamoeba histolytica
From a functional standpoint, the mitosomes of E. histolytica represent a uniquely redesigned mitochondrial compartment [84]. They harbor just a single pathway of sulfate activation, components of which have been obtained by lateral gene transfer from different eukaryotic and bacterial sources [85] and which is specifically needed during the encystation of the parasite [86].
Four DRPs have been identified in the E. histolytica genome [87,88]. Of these four, two proteins (EhDrpA and EhDrpB) were shown to localize to Entamoeba mitosomes [88]. They contain all necessary functional domains, and, importantly, the expression of corresponding dominant-negative forms caused dramatic elongation of the organelles, suggesting their specific involvement in the mitosomal division. Interestingly, both DRPs are functionally interdependent. They interact together and their function is not mutually substitutable [88]. These data suggest that E. histolytica requires a formation of heterooligomeric DRP complex for the mitosomal division, so far unseen in other cellular systems.
Direct fusion of E. histolytica mitosomes has not been observed so far. However, a recent study indicated fusion between endogenous and microinjected mitosomes in manipulated E. histolytica cells [89]. However, the molecular machinery and the physiological relevance of the observation remain to be tested.
Ancestral or acquired similarities?
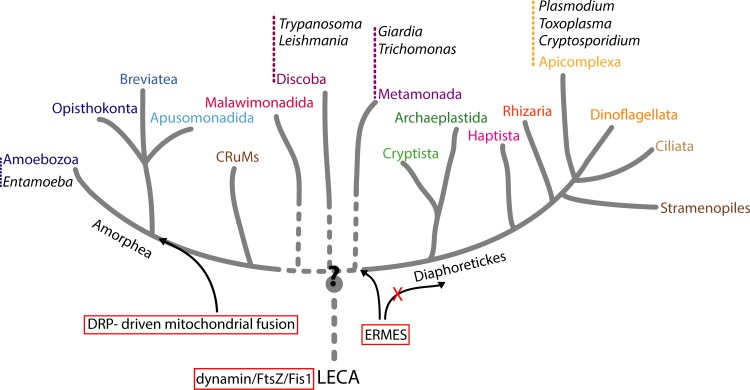
Obviously, there are multiple differences in mitochondrial morphology across the diversity of parasitic protists. Medically important parasitic protists belong to evolutionarily distinct supergroups of eukaryotes such as Stramepiles-Alveolata-Rhizaria (SAR; Plasmodium, Toxoplasma, Cryptosporidium), Discoba (Trypanosoma, Leishmania), Metamonada (Giardia, Trichomonas), and Amoebozoa (Entamoeba) (Fig 3). Our understanding of the mitochondrial dynamics comes mainly from the model organisms of the Opisthokonta group, which represent just a small piece of entire eukaryotic diversity (Fig 3). Interestingly, the comparative analyses have suggested that the mitochondrion of the last eukaryotic common ancestor (LECA) was already a complex organelle, with most of the biogenesis pathways resembling those in the current eukaryotes [90]. Its mitochondrion likely contained both the ancestral bacterial FtsZ-based machinery used for the bacterial cell division [91] and the dynamin-based membrane scission molecular machinery [92] perhaps accompanied by Fis1 receptor (Fig 3). Other complexes and proteins such as the ERMES complex or mitofusins arrived later in the evolution [83]. However, for most of the lineages of eukaryotes, we have only few functional data on the dynamics of mitochondria.
Fig 3. Parasitic protists in the eukaryotic tree of life.
The schematic tree shows the position of the discussed protist parasites across the eukaryotic diversity. Currently, two large domains of eukaryotes called Amorphea and Diaphoretickes are distinguished with several additional clades of uncertain position [103]. Plasmodium, Toxoplasma, and Cryptosporidium belong to Apicomplexa, which together with Dinoflagellata and Ciliata constitute a group of Alveolata. Alveolata further combine with Stramenopiles and Rhizaria to form SAR group [103]. Discoba and Metamonada groups, which contain Trypanosoma with Leishmania and Giardia with Trichomonas, respectively, were previously part of the Excavata supergroup of eukaryotes. According to current classification, these groups belong neither to Amorphea or Diaphoretickes and remain without clear position in the tree (dotted lines). Entamoeba is part of the Amoebozoa supergroup. The comparative analyses proposed that the LECA already contained dynamin homologue, most likely capable of dual function in both mitochondrial division and vesicle scission [92]. There was also a mitochondrial targeted FtsZ, a prokaryotic homologue of tubulin, involved in the mitochondrial division [91]. Widespread distribution of Fis1 suggests that the protein was also used by LECA, although its specific involvement in mitochondrial division is unclear. Similarly, the components of the ERMES complex described as the molecular tether of the ER and mitochondria were common to Amorphea and the former Excavata group (Discoba, Metamonada, Malawimonada), while being absent in the common ancestor of Diaphoretickes [83]. However, the presence of individual ERMES components does not automatically imply the existence of functional ERMES complex. Mitochondrial fusion mediated by a DRP was confirmed only for the members of Amorphea and CRuMs; question mark depicts the uncertain position of the root of the tree. CRuMs, Collodictyonid + Rigifilida + Mantamonas clade; DRP, dynamin-related protein; ER, endoplasmic reticulum; ERMES, ER-mitochondria encounter structure; LECA, last eukaryotic common ancestor; SAR, Stramepiles-Alveolata-Rhizaria.
In the parasitic species, independent evolutionary adaptations have resulted in different demands on the metabolic pathways harbored in mitochondria. Consequently, the metabolic workload of mitochondria has been reflected by the shape and the size of the compartment. On top of that, mitochondria of protist parasites with digenetic life cycles dramatically change during the transition between hosts. Thus, many unrelated insect vector–borne parasites modulate their mitochondrial morphology according to their available energy source, i.e., the size of their mitochondria and the cristae within increases to break down amino acids by mitochondrial metabolism during invertebrate stages. Conversely, mitochondrial size is suppressed along with the mitochondrial metabolism in the glucose-rich environment of the vertebrate host. Anoxic environments occupied by some parasitic protists have led to the most extreme mitochondrial adaptations of miniature ATP-consuming organelles such as mitosomes. In fact, the discovery of a variety of mitochondria and MROs in the parasitic protists has fundamentally influenced our general understanding of the mitochondrial evolution [90].
However, remarkable similarities can be found among the mitochondrial dynamics of evolutionarily distant protist parasites. Their mitochondria do not undergo the extensive mitochondrial fusion and fission during the cell cycle as observed in Opisthokonta. Instead, in many species, mitochondrial fission is tightly connected to cell division and usually occurs just before cytokinesis. The lack of clear mitofusin homologues outside Opisthokonta (animals and fungi) (Table 1) indicates that the importance of mitochondrial fusion may be limited in protists and/or that different molecular machinery is in charge of fusing two organellar membranes. One of the main proposed roles of mitochondrial fusion is to control the quality of mitochondrial DNA among individual mitochondria in the cell, which may be damaged by reactive oxygen species generated during respiration [3]. Given that parasites from Apicomplexa and Kinetoplastida groups contain just a single mitochondrion with one mitochondrial nucleoid [51,93,94], this role of mitochondrial fusion may not be necessary. Conversely, MROs such as hydrogenosomes or mitosomes are usually present in large numbers in protists living in anoxic environments. However, these organelles are generally devoid of the organellar genome, which removes the necessity for quality control to ensure fidelity of nucleoid inheritance.
The constantly ongoing mitochondrial fusion and fission occurring in Opisthokonta allow the stochastic segregation of the mitochondria into daughter cells during the cellular division. It is possible that the synchrony of the mitochondrial and cellular division, observed for the protist parasites with single mitochondrion and also for the multiple mitosomes of G. intestinalis, might be a consequence of absent or reduced mitochondrial dynamics.
The role of mitophagy as an ultimate aspect of mitochondrial dynamics remains largely unexplored in parasitic protists, with the exception of T. gondii [45,46]. However, the concept of mitophagy of the only mitochondrion of T. gondii is somewhat complicated, as it should lead to cell death. Thus, further research is necessary to characterize the specific role of the mitophagy pathway in protist parasite biology.
Recent discovery of the ERMES complex, which mediates the connection of the mitochondria and the ER [13], showed that mitochondrial dynamics may also be controlled by other organelles. Further connections of the ERMES with the mitochondrial nucleoid replication and in mitochondrial contact site and cristae organizing system (MICOS) suggest that processes controlling mitochondrial dynamics are much more complex than previously thought [95,96].
Studies on these complexes in parasitic protists are now emerging [83,97], already indicating that their structure and function may be quite different from the model organisms, as usual for protists. [98–102].
Acknowledgments
We would like to thank Stuart A. Ralph for his helpful comments.
Funding Statement
The project was supported by PRIMUS grant from Charles University Grant Agency PRIMUS/SCV34 https://cuni.cz/UKEN-65.html, National Sustainability Program II (Project BIOCEV-FAR, LQ1604) by the Ministry of Education, Youth and Sports of CR (MEYS) http://www.msmt.cz/?lang=2, Centre for research of pathogenicity and virulence of parasites (No. CZ.02.1.01/0.0/0.0/16_019/0000759) funded by European Regional Development Fund, and MEYS https://ec.europa.eu/regional_policy/en/funding/erdf, http://www.msmt.cz/?lang=2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505: 335–43. 10.1038/nature12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra P, Chan DC. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol. 2014;15: 634–46. 10.1038/nrm3877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337: 1062–1065. 10.1126/science.1219855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilkerson RW, Schon EA, Hernandez E, Davidson MM. Mitochondrial nucleoids maintain genetic autonomy but allow for functional complementation. J Cell Biol. 2008;181: 1117–1128. 10.1083/jcb.200712101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labbé K, Murley A, Nunnari J. Determinants and functions of mitochondrial behavior. Annu Rev Cell Dev Biol. 2014;30: 357–91. 10.1146/annurev-cellbio-101011-155756 [DOI] [PubMed] [Google Scholar]
- 6.Ingerman E, Perkins EM, Marino M, Mears JA, McCaffery JM, Hinshaw JE, et al. Dnm1 forms spirals that are structurally tailored to fit mitochondria. J Cell Biol. 2005;170: 1021–1027. 10.1083/jcb.200506078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia R, Wang RY-R, Yusuf A, Thomas PV., Agard DA, Shaw JM, et al. Structural basis of mitochondrial receptor binding and constriction by DRP1. Nature. 2018;558: 401–405. 10.1038/s41586-018-0211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Chan DC. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol Biol Cell. 2015;26: 4466–77. 10.1091/mbc.E15-08-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151: 367–80. 10.1083/jcb.151.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin EE, Graumann J, Chan DC. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J Cell Biol. 2005;170: 237–248. 10.1083/jcb.200503148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334: 358–62. 10.1126/science.1207385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murley A, Lackner LL, Osman C, West M, Voeltz GK, Walter P, et al. ER-associated mitochondrial division links the distribution of mitochondria and mitochondrial DNA in yeast. Elife. 2013;2: e00422 10.7554/eLife.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325: 477–81. 10.1126/science.1175088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160: 189–200. 10.1083/jcb.200211046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90: 121–9. 10.1016/s0092-8674(00)80319-0 [DOI] [PubMed] [Google Scholar]
- 16.Sesaki H, Jensen RE. Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J Biol Chem. 2004;279: 28298–28303. 10.1074/jbc.M401363200 [DOI] [PubMed] [Google Scholar]
- 17.Abrams AJ, Hufnagel RB, Rebelo A, Zanna C, Patel N, Gonzalez MA, et al. Mutations in SLC25A46, encoding a UGO1-like protein, cause an optic atrophy spectrum disorder. Nat Genet. 2015;47: 926–932. 10.1038/ng.3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12: 9–14. 10.1038/nrm3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowman AF, Berry D, Baum J. The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol. 2012;198: 961–71. 10.1083/jcb.201206112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: biology and disease. Cell. 2016;167: 610–624. 10.1016/j.cell.2016.07.055 [DOI] [PubMed] [Google Scholar]
- 21.Ke H, Lewis IA, Morrisey JM, McLean KJ, Ganesan SM, Painter HJ, et al. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. 2015;11: 164–74. 10.1016/j.celrep.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krungkrai J, Prapunwattana P, Krungkrai SR. Ultrastructure and function of mitochondria in gametocytic stage of Plasmodium falciparum. Parasite. 2000;7: 19–26. 10.1051/parasite/2000071019 [DOI] [PubMed] [Google Scholar]
- 23.van Dooren GG, Marti M, Tonkin CJ, Stimmler LM, Cowman AF, McFadden GI. Development of the endoplasmic reticulum, mitochondrion and apicoplast during the asexual life cycle of Plasmodium falciparum. Mol Microbiol. 2005;57: 405–419. 10.1111/j.1365-2958.2005.04699.x [DOI] [PubMed] [Google Scholar]
- 24.Kohler S, Delwiche CF, Denny PW, Tilney LG, Webster P, Wilson RJM, et al. A plastid of probable green algal origin in apicomplexan parasites. Science. 1997;275: 1485–1489. 10.1126/science.275.5305.1485 [DOI] [PubMed] [Google Scholar]
- 25.Hopkins J, Fowler R, Krishna S, Wilson I, Mitchell G, Bannister L. The plastid in Plasmodium falciparum asexual blood stages: a three-dimensional ultrastructural analysis. Protist. 1999;150: 283–295. 10.1016/S1434-4610(99)70030-1 [DOI] [PubMed] [Google Scholar]
- 26.Arisue N, Hashimoto T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol Int. 2015;64: 254–259. 10.1016/j.parint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 27.Lim L, McFadden GI. The evolution, metabolism and functions of the apicoplast. Philos Trans R Soc Lond B Biol Sci. 2010;365: 749–63. 10.1098/rstb.2009.0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanway RR, Mueller N, Zobiak B, Graewe S, Froehlke U, Zessin PJM, et al. Organelle segregation into Plasmodium liver stage merozoites. Cell Microbiol. 2011;13: 1768–1782. 10.1111/j.1462-5822.2011.01657.x [DOI] [PubMed] [Google Scholar]
- 29.Stanway RR, Witt T, Zobiak B, Aepfelbacher M, Heussler VT. GFP-targeting allows visualization of the apicoplast throughout the life cycle of live malaria parasites. Biol Cell. 2009;101: 415–435. 10.1042/BC20080202 [DOI] [PubMed] [Google Scholar]
- 30.Okamoto N, Spurck TP, Goodman CD, McFadden GI. Apicoplast and mitochondrion in gametocytogenesis of Plasmodium falciparum. Eukaryot Cell. 2009;8: 128–132. 10.1128/EC.00267-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman CD, Siregar JE, Mollard V, Vega-Rodriguez J, Syafruddin D, Matsuoka H, et al. Parasites resistant to the antimalarial atovaquone fail to transmit by mosquitoes. Science. 2016;352: 349–353. 10.1126/science.aad9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Han Z, Lu Y, Lin Y, Zhang L, Wu Y, et al. Isolation and functional characterization of a dynamin-like gene from Plasmodium falciparum. Biochem Biophys Res Commun. 2004;320: 664–671. 10.1016/j.bbrc.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 33.Charneau S, Dourado Bastos IM, Mouray E, Ribeiro BM, Santana JM, Grellier P, et al. Characterization of PfDYN2, a dynamin-like protein of Plasmodium falciparum expressed in schizonts. Microbes Infect. 2007;9: 797–805. 10.1016/j.micinf.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 34.Breinich MS, Ferguson DJP, Foth BJ, van Dooren GG, Lebrun M, Quon D V, et al. A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Curr Biol. 2009;19: 277–286. 10.1016/j.cub.2009.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dooren GG, Stimmler LM, McFadden GI. Metabolic maps and functions of the Plasmodium mitochondrion. FEMS Microbiol Rev. 2006;30: 596–630. 10.1111/j.1574-6976.2006.00027.x [DOI] [PubMed] [Google Scholar]
- 36.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30: 1217–58. 10.1016/s0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo EJL, Attias M, De Souza W. The single mitochondrion of tachyzoites of Toxoplasma gondii. J Struct Biol. 2000;130: 27–33. 10.1006/jsbi.2000.4228 [DOI] [PubMed] [Google Scholar]
- 38.Francia ME, Striepen B. Cell division in apicomplexan parasites. Nat Rev Microbiol. 2014;12: 125–136. 10.1038/nrmicro3184 [DOI] [PubMed] [Google Scholar]
- 39.Nishi M, Hu K, Murray JM, Roos DS. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci. 2008;121: 1559–68. 10.1242/jcs.021089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dooren GG, Reiff SB, Tomova C, Meissner M, Humbel BM, Striepen B. A novel dynamin-related protein has been recruited for apicoplast fission in Toxoplasma gondii. Curr Biol. 2009;19: 267–276. 10.1016/j.cub.2008.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heredero-Bermejo I, Varberg JM, Charvat R, Jacobs K, Garbuz T, Sullivan WJ, et al. TgDrpC, an atypical dynamin-related protein in Toxoplasma gondii, is associated with vesicular transport factors and parasite division. Mol Microbiol. 2019;111: 46–64. 10.1111/mmi.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melatti C, Pieperhoff M, Lemgruber L, Pohl E, Sheiner L, Meissner M. A unique dynamin-related protein is essential for mitochondrial fission in Toxoplasma gondii. PLoS Pathog. 2019;15: e1007512 10.1371/journal.ppat.1007512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding CR, Meissner M. The inner membrane complex through development of Toxoplasma gondii and Plasmodium. Cell Microbiol. 2014;16: 632–641. 10.1111/cmi.12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ovciarikova J, Lemgruber L, Stilger KL, Sullivan WJ, Sheiner L. Mitochondrial behaviour throughout the lytic cycle of Toxoplasma gondii. Sci Rep. 2017;7: 42746 10.1038/srep42746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh D, Walton JL, Roepe PD, Sinai AP. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012;14: 589–607. 10.1111/j.1462-5822.2011.01745.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Besteiro S, Brooks CF, Striepen B, Dubremetz J-F. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii. tachyzoites. PLoS Pathog. 2011;7: e1002416 10.1371/journal.ppat.1002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simarro PP, Cecchi G, Franco JR, Paone M, Diarra A, Ruiz-Postigo JA, et al. Estimating and mapping the population at risk of sleeping sickness. PLoS Negl Trop Dis. 2012;6: e1859 10.1371/journal.pntd.0001859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verner Z, Basu S, Benz C, Dixit S, Dobáková E, Faktorová D, et al. Malleable mitochondrion of Trypanosoma brucei. Int Rev Cell Mol Biol. 2015;315: 73–151. 10.1016/bs.ircmb.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 49.Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, et al. Trypanosoma brucei parasites occcupy and functionally adapt to the adipose tissue in mice. Cell Host Microbe. 2016;19: 837–848. 10.1016/j.chom.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakob M, Hoffmann A, Amodeo S, Peitsch C, Zuber B, Ochsenreiter T. Mitochondrial growth during the cell cycle of Trypanosoma brucei bloodstream forms. Sci Rep. 2016;6: 36565 10.1038/srep36565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lukes J, Guilbride DL, Votýpka J, Zíková A, Benne R, Englund PT. Kinetoplast DNA network: evolution of an improbable structure. Eukaryot Cell. 2002;1: 495–502. 10.1128/EC.1.4.495-502.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogbadoyi EO, Robinson DR, Gull K. A high-order trans-membrane structural linkage is responsible for mitochondrial genome positioning and segregation by flagellar basal bodies in trypanosomes. Mol Biol Cell. 2003;14: 1769–1779. 10.1091/mbc.E02-08-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodward R, Gull K. Timing of nuclear and kinetoplast DNA replication and early morphological events in the cell cycle of Trypanosoma brucei. J Cell Sci. 1990;95 (Pt 1): 49–57. [DOI] [PubMed] [Google Scholar]
- 54.Robinson DR, Gull K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature. 1991;352: 731–733. 10.1038/352731a0 [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann A, Käser S, Jakob M, Amodeo S, Peitsch C, Týc Í, et al. Molecular model of the mitochondrial genome segregation machinery in Trypanosoma brucei. Proc Natl Acad Sci. 2018;115: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chanez A-L, Hehl AB, Engstler M, Schneider A. Ablation of the single dynamin of T. brucei blocks mitochondrial fission and endocytosis and leads to a precise cytokinesis arrest. J Cell Sci. 2006;119: 2968–74. 10.1242/jcs.03023 [DOI] [PubMed] [Google Scholar]
- 57.Schneider A, Ochsenreiter T. Failure is not an option–mitochondrial genome segregation in trypanosomes. J Cell Sci. 2018;131: jcs221820 10.1242/jcs.221820 [DOI] [PubMed] [Google Scholar]
- 58.Morgan GW, Goulding D, Field MC. The single dynamin-like protein of Trypanosoma brucei regulates mitochondrial division and is not required for endocytosis. J Biol Chem. 2004;279: 10692–701. 10.1074/jbc.M312178200 [DOI] [PubMed] [Google Scholar]
- 59.Benz C, Stříbrná E, Hashimi H, Lukeš J. Dynamin-like proteins in Trypanosoma brucei: A division of labour between two paralogs? PLoS ONE. 2017;12: e0177200 10.1371/journal.pone.0177200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanwalleghem G, Fontaine F, Lecordier L, Tebabi P, Klewe K, Nolan DP, et al. Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1. Nat Commun. 2015;6: 8078 10.1038/ncomms9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002;12: 178–84. 10.1016/s0962-8924(01)02246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peacock L, Ferris V, Bailey M, Gibson W. Mating compatibility in the parasitic protist Trypanosoma brucei. Parasit Vectors. 2014;7: 78 10.1186/1756-3305-7-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Esseiva AC, Chanez A-L, Bochud-Allemann N, Martinou J-C, Hemphill A, Schneider A. Temporal dissection of Bax-induced events leading to fission of the single mitochondrion in Trypanosoma brucei. EMBO Rep. 2004;5: 268–273. 10.1038/sj.embor.7400095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiMaio J, Ruthel G, Cannon JJ, Malfara MF, Povelones ML. The single mitochondrion of the kinetoplastid parasite Crithidia fasciculata is a dynamic network. PLoS ONE. 2018;13: e0202711 10.1371/journal.pone.0202711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bouchemal K, Bories C, Loiseau PM. Strategies for prevention and treatment of Trichomonas vaginalis infections. Clin Microbiol Rev. 2017;30: 811–825. 10.1128/CMR.00109-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindmark DG, Müller M. Hydrogenosome, a cytoplasmic organelle of the anaerobic flagellate Tritrichomonas foetus, and its role in pyruvate metabolism. J Biol Chem. 1973;248: 7724–7729. [PubMed] [Google Scholar]
- 67.Dyall SD, Johnson PJ. Origins of hydrogenosomes and mitochondria: evolution and organelle biogenesis. Curr Opin Microbiol. 2000;3: 404–11. 10.1016/s1369-5274(00)00112-0 [DOI] [PubMed] [Google Scholar]
- 68.Bradley PJ, Lahti CJ, Plümper E, Johnson PJ. Targeting and translocation of proteins into the hydrogenosome of the protist Trichomonas: similarities with mitochondrial protein import. EMBO J. 1997;16: 3484–93. 10.1093/emboj/16.12.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29: 199–212. 10.1016/s0020-7519(98)00155-6 [DOI] [PubMed] [Google Scholar]
- 70.Benchimol M, Johnson PJ, Souza W. Morphogenesis of the hydrogenosome: An ultrastructural study. Biol Cell. 1996;87: 197–205. [PubMed] [Google Scholar]
- 71.Benchimol M. Hydrogenosomes under microscopy. Tissue Cell. 2009;41: 151–168. 10.1016/j.tice.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 72.Morin-Adeline V, Šlapeta J. The past, present and future of fluorescent protein tags in anaerobic protozoan parasites. Parasitology. 2016;143: 260–275. 10.1017/S0031182015001663 [DOI] [PubMed] [Google Scholar]
- 73.Martincová E, Voleman L, Najdrová V, De Napoli M, Eshar S, Gualdron M, et al. Live imaging of mitosomes and hydrogenosomes by HaloTag technology. PLoS ONE. 2012;7: e36314 10.1371/journal.pone.0036314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wexler-Cohen Y, Stevens GC, Barnoy E, van der Bliek AM, Johnson PJ. A dynamin-related protein contributes to Trichomonas vaginalis hydrogenosomal fission. FASEB J. 2014;28: 1113–1121. 10.1096/fj.13-235473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tovar J, León-Avila G, Sánchez LB, Sutak R, Tachezy J, van der Giezen M, et al. Mitochondrial remnant organelles of Giardia function in iron-sulphur protein maturation. Nature. 2003;426: 172–176. 10.1038/nature01945 [DOI] [PubMed] [Google Scholar]
- 76.Pyrihová E, Motyčková A, Voleman L, Wandyszewska N, Fišer R, Seydlová G, et al. A Single Tim translocase in the mitosomes of Giardia intestinalis illustrates convergence of protein import machines in anaerobic eukaryotes. Genome Biol Evol. 2018;10: 2813–2822. 10.1093/gbe/evy215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jedelský PL, Doležal P, Rada P, Pyrih J, Smíd O, Hrdý I, et al. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS ONE. 2011;6: e17285 10.1371/journal.pone.0017285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rout S, Zumthor JP, Schraner EM, Faso C, Hehl AB. An interactome-centered protein discovery approach reveals novel components involved in mitosome function and homeostasis in Giardia lamblia. PLoS Pathog. 2016;12: e1006036 10.1371/journal.ppat.1006036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Regoes A. Protein import, replication, and inheritance of a vestigial mitochondrion. J Biol Chem. 2005;280: 30557–30563. 10.1074/jbc.M500787200 [DOI] [PubMed] [Google Scholar]
- 80.Midlej V, Penha L, Silva R, de Souza W, Benchimol M. Mitosomal chaperone modulation during the life cycle of the pathogenic protist Giardia intestinalis. Eur J Cell Biol. 2016;95: 531–542. 10.1016/j.ejcb.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 81.Voleman L, Najdrová V, Ástvaldsson Á, Tůmová P, Einarsson E, Švindrych Z, et al. Giardia intestinalis mitosomes undergo synchronized fission but not fusion and are constitutively associated with the endoplasmic reticulum. BMC Biol. 2017;15: 27 10.1186/s12915-017-0361-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaechter V, Schraner E, Wild P, Hehl AB. The single dynamin family protein in the primitive protozoan Giardia lamblia is essential for stage conversion and endocytic transport. Traffic. 2008;9: 57–71. 10.1111/j.1600-0854.2007.00657.x [DOI] [PubMed] [Google Scholar]
- 83.Wideman JG, Gawryluk RMR, Gray MW, Dacks JB. The ancient and widespread nature of the ER-mitochondria encounter structure. Mol Biol Evol. 2013;30: 2044–9. 10.1093/molbev/mst120 [DOI] [PubMed] [Google Scholar]
- 84.Tovar J, Fischer A, Clark CG. The mitosome, a novel organelle related to mitochondria in the amitochondrial parasite Entamoeba histolytica. Mol Microbiol. 1999;32: 1013–21. 10.1046/j.1365-2958.1999.01414.x [DOI] [PubMed] [Google Scholar]
- 85.Mi-ichi F, Yousuf MA, Nakada-Tsukui K, Nozaki T. Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc Natl Acad Sci. 2009;106: 21731–21736. 10.1073/pnas.0907106106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mi-ichi F, Miyamoto T, Takao S, Jeelani G, Hashimoto T, Hara H, et al. Entamoeba mitosomes play an important role in encystation by association with cholesteryl sulfate synthesis. Proc Natl Acad Sci. 2015;112: E2884–E2890. 10.1073/pnas.1423718112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jain R, Shrimal S, Bhattacharya S, Bhattacharya A. Identification and partial characterization of a dynamin-like protein, EhDLP1, from the protist parasite Entamoeba histolytica. Eukaryot Cell. 2010;9: 215–223. 10.1128/EC.00214-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makiuchi T, Santos HJ, Tachibana H, Nozaki T. Hetero-oligomer of dynamin-related proteins participates in the fission of highly divergent mitochondria from Entamoeba histolytica. Sci Rep. 2017;7: 13439 10.1038/s41598-017-13721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kazama M, Ogiwara S, Makiuchi T, Yoshida K, Nakada-Tsukui K, Nozaki T, et al. Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant. Sci Rep. 2017;7: 44273 10.1038/srep44273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roger AJ, Muñoz-Gómez SA, Kamikawa R. The origin and diversification of mitochondria. Curr Biol. 2017;27: R1177–R1192. 10.1016/j.cub.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 91.Leger MM, Petrů M, Žárský V, Eme L, Vlček Č, Harding T, et al. An ancestral bacterial division system is widespread in eukaryotic mitochondria. Proc Natl Acad Sci U S A. 2015;112: 10239–46. 10.1073/pnas.1421392112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Purkanti R, Thattai M. Ancient dynamin segments capture early stages of host-mitochondrial integration. Proc Natl Acad Sci U S A. 2015;112: 2800–5. 10.1073/pnas.1407163112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maeda-Sano K, Sato S, Ueda T, Yui R, Ito K, Hata M, et al. Visualization of mitochondrial and apicoplast nucleoids in the human malaria parasite Plasmodium falciparum by SYBR green I and PicoGreen staining. Cytologia (Tokyo). 2009;74: 449–455. [Google Scholar]
- 94.Matsuzaki M, Kikuchi T, Kita K, Kojima S, Kuroiwa T. Large amounts of apicoplast nucleoid DNA and its segregation in Toxoplasma gondii. Protoplasma. 2001;218: 180–91. [DOI] [PubMed] [Google Scholar]
- 95.van der Laan M, Bohnert M, Wiedemann N, Pfanner N. Role of MINOS in mitochondrial membrane architecture and biogenesis. Trends Cell Biol. 2012;22: 185–92. 10.1016/j.tcb.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 96.Wideman JG, Muñoz-Gómez SA. The evolution of ERMIONE in mitochondrial biogenesis and lipid homeostasis: An evolutionary view from comparative cell biology. Biochim Biophys Acta. 2016; [DOI] [PubMed] [Google Scholar]
- 97.Kaurov I, Vancová M, Schimanski B, Cadena LR, Heller J, Bílý T, et al. The diverged Trypanosome MICOS complex as a hub for mitochondrial cristae shaping and protein import. Curr Biol. 2018;28: 3393–3407.e5. 10.1016/j.cub.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 98.Abrahamsen MS, Templeton TJ, Enomoto S, Abrahante JE, Zhu G, Lancto CA, et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304: 441–445. 10.1126/science.1094786 [DOI] [PubMed] [Google Scholar]
- 99.Schnarwiler F, Niemann M, Doiron N, Harsman A, Kaser S, Mani J, et al. Trypanosomal TAC40 constitutes a novel subclass of mitochondrial β-barrel proteins specialized in mitochondrial genome inheritance. Proc Natl Acad Sci. 2014;111: 7624–7629. 10.1073/pnas.1404854111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niemann M, Wiese S, Mani J, Chanfon A, Jackson C, Meisinger C, et al. Mitochondrial outer membrane proteome of Trypanosoma brucei reveals novel factors required to maintain mitochondrial morphology. Mol Cell Proteomics. 2013;12: 515–28. 10.1074/mcp.M112.023093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams RAM, Smith TK, Cull B, Mottram JC, Coombs GH. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 2012;8: e1002695 10.1371/journal.ppat.1002695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benchimol M. Hydrogenosome autophagy: An ultrastructural and cytochemical study. Biol Cell. 1999;91: 165–174. 10.1016/s0248-4900(99)80039-2 [DOI] [PubMed] [Google Scholar]
- 103.Adl SM, Bass D, Lane CE, Lukeš J, Schoch CL, Smirnov A, et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J Eukaryot Microbiol. 2019;66: 4–119. 10.1111/jeu.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]