Abstract
The changes in the cerebellar activation pattern of two successive fMRI scanning runs were determined for visually guided to‐and‐fro saccades in 12 healthy volunteers familiar with the study paradigm. Group and single subject‐analyses revealed a constant activation of the paramedian cerebellar vermis (uvula, tonsils, tuber, folium/declive), which reflects constant ocular motor activity in both runs. A significant decrease in activation of the cerebellar hemispheres found in the second run is best explained by either a decrease in attention or the effects of motor optimization and learning. The significant, systematic changes of the cerebellar activation pattern in two successive runs were not expected, because the ocular motor task was simple, familiar, and highly automated. These findings indicate that similar effects may bias other cerebellar activation studies, in which sensorimotor tasks are repeated in a single session. Hum. Brain Mapping 16:63–70, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: cerebellum, saccades, functional magnetic resonance imaging (fMRI), ocular motor, human
INTRODUCTION
There are a few brain activation studies that include analysis of cerebellar structures during various ocular motor tasks [Desmurget et al., 2001; Dieterich et al., 2000; Luna et al., 2001; Miall et al., 2000; O'Driscoll et al., 2000]. Only two of the fMRI studies available focus on the cerebellum: one on smooth‐pursuit eye movements and hand tracking [Miall et al., 2000], and the other on optokinetic nystagmus and saccades [Dieterich et al., 2000]. In the latter cerebellar study using FLASH sequences for higher in‐plane resolution, the differential effects of to‐and‐fro horizontal saccades were analyzed. Various signal changes were found in the cerebellar hemispheres (superior semilunar lobule, simple lobule, quadrangular lobule, and inferior semilunar lobule) as well as in the cerebellar vermis (uvula, culmen), the dentate nucleus, and the middle cerebellar peduncle [Dieterich et al., 2000]. The hemispherical signal changes were attributed to attention and timing, whereas the areas identified in the vermal structures were ascribed to ocular motor control. Activity in the dentate nuclei and the cerebellar peduncles were attributed to both.
To further delineate these areas and to attribute their functional significance as regards ocular motor control and attention or timing, a subsequent study was designed in which a visually guided saccadic task was compared to a second one that required more attention. In this second task, subjects had to signify that they detected a color change of the target by pressing a button. This setup was flawed, however, by: a) the subjects' readiness to press the button as well as the actual execution that induced motor‐related activations; and b) a consistently decreasing activation pattern in the second run that was independent of the random presentation of the stimulus conditions. The latter finding prompted us to design an even more simple experiment: a comparison of activation changes in two successive runs of horizontal saccades. This experiment may have an impact on all studies in which identical sensorimotor tasks are performed repeatedly in an fMRI series. Only subjects familiar with the stimulus conditions and the experimental setup, who had already participated in several fMRI studies with visual and ocular motor tasks, were recruited. Our intention was to avoid the major effects of familiarization to an unfamiliar and therefore unadapted visual sensorimotor task. The main goal was to compare the differential effects on the cerebellar activation pattern of two familiar and identical sensorimotor tasks, performed successively in one scanning session.
MATERIALS AND METHODS
Subjects
Twelve healthy volunteers (7 females and 5 males), mean age 29.8 years (range 24–51 years), participated in the experiment. Data from one subject had to be excluded from the analysis due to a technical failure. Subjects had no history of visual or central nervous system disorders except for corrected refraction anomalies. The laterality quotient for handedness according to the 10‐item inventory of the Edinburgh test [Oldfield, 1971; Salmaso and Longoni, 1985] was +100. The study was approved by the local ethics committee; all subjects gave their informed consent to participate.
Visual Stimulation
During MRI data acquisition, subjects lay supine wearing MRI‐compatible video glasses (Resonance Technology Inc., Northridge, CA) that allowed presentation of computer‐generated animations. The size of the visual field was 30° in the horizontal and 20° in the vertical dimension.
Subjects were scanned under two experimental conditions: fixation of a stationary target straight ahead (diameter 1.2°; rest condition) and visually guided voluntary saccades with an amplitude of 24° at a frequency of 1 Hz (saccadic stimulation). During saccadic stimulation, the target dot was presented at two alternating positions, 12° to the left or right on the horizontal axis.
Subjects were instructed to fixate the target during rest conditions and during saccadic stimulation periods. Rest and stimulation periods alternated every 25 sec (i.e., 25 saccades per stimulation period), starting with rest. Total presentation time was 2 × 300 sec, with a break of about 2 min in between. Subjects had been trained to maintain fixation on the target during both stimulus conditions. The possibility of doing this had been tested with electro‐oculography under comparable laboratory conditions.
MRI Data Acquisition
Functional images were acquired on a standard clinical scanner (Siemens Vision, Erlangen, Germany) at a magnetic field strength of 1.5 Tesla using a circularly polarized head coil and echo planar imaging (EPI) with a T2*‐weighted gradient‐echo multislice sequence (TE = 60 msec, voxel size 3.75 × 3.75 × 4 mm3, matrix 64 × 64, interscan interval 2.5 sec). Twenty transversal slices were positioned to cover the whole cerebellum and tilted backward so as to be parallel to the tentorium cerebelli.
Data Preprocessing
The first 10 image volumes (25 sec) of each run were discarded because of spin saturation effects. Further data processing was performed using SPM99 [Friston et al., 1995b] on an UltraSPARC workstation (Sun Microsystems). All volumes were realigned to the first one of each scanning session to correct for subject motion and were spatially normalized [Friston et al., 1995a] to the standard space defined by the Montreal Neurological Institute template. After normalization, the image volumes had a resolution of 2 × 2 × 2 mm3. Image data were smoothed with a 3D isotropic (6 mm FWHM) Gaussian filter. To decide whether global intensity scaling should be applied to the image data, we calculated the degree of correlation between the experimental paradigm and the global signal as proposed by Aguirre et al. [1998]. Global scaling was only applied, if no significant correlation was found (P < 0.05).
Statistical Analysis
The statistical significance of signal changes evoked by saccadic stimulation was computed independently for each subject in each of the two scanning runs. Statistical parametric maps were calculated on a voxel‐by‐voxel basis for each imaging run using the general linear model [Friston et al., 1995b] with a hemodynamic model of the two states of the experiment. After the single‐subject evaluation, statistical parametric maps for the whole group of subjects were created by entering the results from the first‐level statistics into a t‐test, according to the random effects model [Frison and Pocock, 1992; Woods, 1996].
For an additional direct comparison (run 1–run 2) of the activation patterns across the two runs (session effects), we used a paired t‐test for the second level. This yielded a statistical parametric map showing significant differences in BOLD signal between the two imaging runs.
For further statistical analysis, image masks were created to separate three different infratentorial regions of interest (ROIs). These were: 1) the cerebellar vermis and the tonsils; 2) the cerebellar hemispheres; and 3) the caudal brainstem and the cerebellar peduncles. This separation was performed to identify differential effects of ocular motor function (vermis), attention, and timing (hemispheres). In each run, the numbers of activated voxels in the three ROIs were calculated for the individual subjects and used to evaluate significant differences between the two runs (paired t‐test).
RESULTS
Electro‐oculography revealed that the mean velocities of the saccades in the first (370 ± 12°/sec) and the second run (365 ± 8°/sec) were not significantly different.
Group analysis showed significant activations during both runs in the cerebellar hemispheres (simple lobule, inferior semilunar lobule, superior semilunar lobule) and the cerebellar vermis (uvula, tuber, folium, declive) (Table I, Figs. 1, 2). In addition, activation of the pons and the cerebellar peduncles was seen at various locations in 10 of 11 subjects in the single‐subject evaluation. The activation pattern of the second run differed from that of the first run by four features: 1) significantly fewer activated voxels in the cerebellar hemispheres (single subject evaluation, P < 0.05, Fig. 3), which is reflected by the reduced size of the activated areas in the simple lobule, superior and inferior semilunar lobules; 2) no activation of the biventer lobule and parts of the simple lobule; 3) additional bilateral activations in the superior semilunar lobule; and 4) an increase in t‐values of the activated areas in the vermis, the locations of which remained constant (e.g., uvula 5.43 vs. 8.32, Table I).
Table I.
Cerebellar activation areas associated with visually guided saccades from two identical, consecutive imaging runs*
| Area | Run 1 | Run 2 | Run 1 > Run 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t‐value | x | y | z | t‐value | x | y | z | t‐value | x | y | z | |
| Cerebellar hemispheres | ||||||||||||
| Biventer lobule | 5.54 | −32 | −58 | −48 | — | 3.99 | −20 | −62 | −46 | |||
| Inferior semilunar lobule | 4.47 | 8 | −82 | −42 | 5.20 | 10 | −76 | −38 | — | |||
| 4.09 | −10 | −78 | −42 | 7.07 | −8 | −78 | −36 | 4.49 | −52 | −56 | −40 | |
| — | — | 3.52 | −24 | −84 | −42 | |||||||
| Superior semilunar lobule | 4.58 | −40 | −56 | −40 | — | 3.60 | −36 | −42 | −42 | |||
| — | 6.60 | 30 | −84 | −22 | — | |||||||
| — | 5.50 | −16 | −84 | −26 | — | |||||||
| — | 9.02 | −46 | −64 | −28 | — | |||||||
| — | — | 3.56 | 44 | −48 | −38 | |||||||
| Simple lobule | 4.18 | 36 | −48 | −36 | — | — | ||||||
| 5.51 | 40 | −60 | −26 | 6.15 | 40 | −58 | −30 | — | ||||
| 9.98 | −36 | −60 | −26 | — | 4.02 | −32 | −56 | −30 | ||||
| Vermal structures | ||||||||||||
| Uvula | 5.43 | 2 | −56 | −38 | 8.32 | 0 | −56 | −36 | 3.61 | 2 | −54 | −42 |
| Tonsil | — | 4.41 | 0 | −60 | −50 | — | ||||||
| — | — | 3.32 | −14 | −54 | −40 | |||||||
| Tuber | 6.88 | 2 | −80 | −36 | 7.03 | −2 | −78 | −38 | — | |||
| Folium/Declive | 9.25 | 4 | −76 | −20 | 12.65 | 0 | −78 | −26 | — | |||
| Other structures | ||||||||||||
| Pons | — | — | 3.19 | 10 | −18 | −24 | ||||||
| Substantia nigra | — | — | 3.02 | −6 | −14 | −14 | ||||||
The third column lists areas showing significantly higher BOLD signal (P < 0.01) in the first run than in the second run (n = 11; t‐values and MNI coordinates are given).
Figure 1.
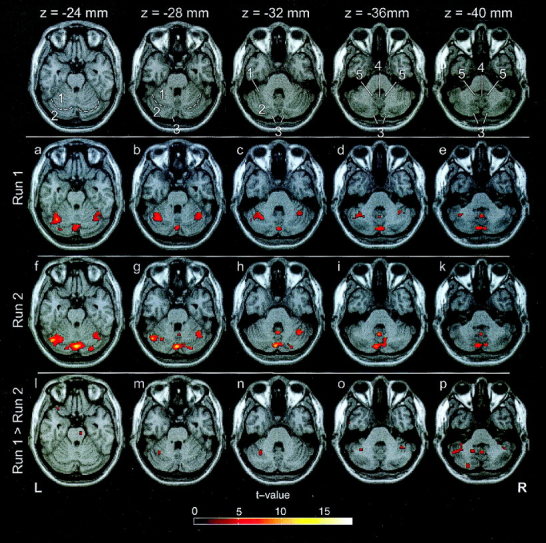
Areas of cerebellar activation due to saccadic stimulation in run 1 (second row) and run 2 (third row), projected onto a standard template brain (P < 0.001, n = 11). The lower row depicts regions showing significantly higher BOLD signal in run 1 than in run 2 (P < 0.01, n = 11). The first row defines anatomical locations that have been verified with the help of coronal and sagittal sections: simple lobule (1), separated from superior semilunar lobule (2) by the posterior superior fissure (dashed line), inferior semilunar lobule (3), uvula (4), and the tonsils (5) [Duvernoy, 1995]. Activations can be found in the superior semilunar lobule (a,f), inferior semilunar lobule (d,e,i,k), and simple lobule (a–d,f–h), as well as in the vermal structures uvula (d,e,g–k), extending into the tonsils (e,k), folium/declive, tuber and pyramid of vermis (a–k), extending into the inferior semilunar lobule. In the subtraction (run 1–run 2; bottom) a significant decrease of BOLD signal was found in the superior semilunar lobule (o,p), inferior semilunar lobule (p), and simple lobule (m,n), as well as in the uvula, the tonsil (p), and the pons (l).
Figure 2.

Activation areas in the cerebellar vermis due to visually guided saccades in run 1 (a) and run 2 (b) (P < 0.001, n = 11). Activations can be found in the uvula, tuber, tonsil, and declive in both runs; activation of the tonsil (depicted below the activation of the uvula) was found in run 2 (b).
Figure 3.
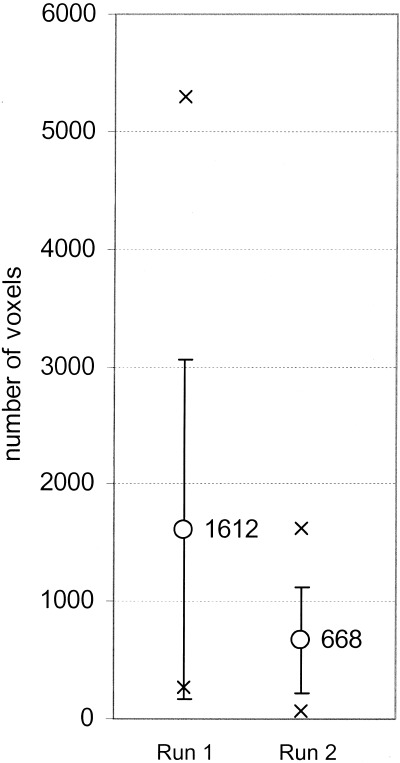
Number of activated voxels in cerebellar hemispheres (ROI) for first (left) and second (right) run. The number of activated voxels is significantly smaller in run 2 than in run 1 (P < 0.05). Circles, crosses, and whiskers represent mean, extreme values, and SD, respectively.
The direct statistical comparison of the activation patterns of run 1 and run 2 showed a significant decrease of BOLD signal in the second run compared to the first run in biventer lobule, inferior semilunar lobule, and simple lobule of the left cerebellar hemisphere, superior semilunar lobule bilaterally, as well as in the uvula, the left tonsil, the pons, and in the substantia nigra (Table I, Fig. 1).
DISCUSSION
A few previous studies on intersession reproducibility (session effects) in fMRI have stressed the relatively robust pattern of cortical activation with quantitative intersession differences during motor tasks [Ramsey et al., 1996], cognitive tasks [Machielsen et al., 2000], or visual stimulation [Rombouts et al., 1998]. These studies did not focus on the cerebellum. The only fMRI study to include the cerebellum analyzed the differential activation patterns during brief practice of a simple motor task in five healthy volunteers [Tracy et al., 2001]. The authors demonstrated neural changes associated with limited practice by comparison of brain activation in the early and late stages of motor performance of single finger opposition throughout one brief session. The results revealed that the strongest dissociation between early and late performance involved the corpus striatum, thalamus, and cingulate gyrus. Early stage performance involved the anterior cerebellum; late stage performance involved more inferior/lateral cerebellar hemisphere structures.
The present ocular motor study used visually guided horizontal saccades. Activations were found in the cerebellar vermis and the biventer lobule, inferior semilunar lobule, superior semilunar lobule, and simple lobule of the cerebellar hemispheres. Activation in the paramedian cerebellar vermis remained constant in both runs, although the second run had higher t‐values. This is consistent with the view that constant activations in the vermis reflect constant ocular motor activity throughout the entire scanning period. Vermal activations included the uvula, tonsils, tuber, as well as the folium/declive, which represent the ocular motor vermis (Table I). The Purkinje cells of the ocular motor vermis (i.e., folium, tuber, and declive; [Leigh and Zee, 1999]) project to the caudal part of the fastigial nucleus, which projects to the vestibular nuclei and saccade‐related brainstem nuclei [Noda et al., 1990]. Both the ocular motor vermis [Helmchen and Büttner, 1995] and fastigial nucleus [Fuchs et al., 1993] contain saccade‐related neurons. Although we found BOLD signal changes in the ocular motor vermis, we did not detect any in the fastigial nucleus. This corresponds to the findings of Ron and Robinson [1973] who were able to evoke saccadic eye movements by microstimulation of the vermis (lobules V, VI, and VII, i.e., culmen, declive, folium/tuber, respectively) in the alert monkey.
The decreased activation of the cerebellar hemispheres found in the second run is best explained by motor optimization and perhaps also due to a further decrease in attention (biventer lobule, simple lobule). During the repeated performance of a simple motor task (finger tapping), physiological activation was reported to be decreased in the anterior lobe of the cerebellum [Seitz et al., 1990; Friston et al., 1992; Tracy et al., 2001]. The authors interpreted this finding as a neurophysiological adaption in the cerebellar cortex.
Activations of the cerebellar hemispheres, e.g., within the superior semilunar lobule have also been seen during motor performance. Allen et al. [1997] studied the activation of the cerebellum with fMRI by comparing a visual attention task that neither required motor learning nor made use of guided motor operations, with a motor task and a task combining both. They defined ROIs for attention, i.e., the left posterior quadrangular lobule and the left superior semilunar lobule, and ROIs for motor performance, i.e., the right anterior vermis, right central lobule, and right quadrangular lobule. Their results showed that attentional cerebellar activation was functionally independent of the motor task. Although motor activation required attention, attention activated the cerebellum regardless of whether there was visual sensory input or motor output.
The activation within the superior semilunar lobule that occurred only in run two of our study appears to be related to ocular motor function. Ron and Robinson [1973] were able to elicit saccades and smooth pursuit eye movements by stimulating this area in the monkey. Furthermore, they also elicited saccades by stimulating the simple lobule and the inferior semilunar lobule (Crus II). In parallel, Mano et al. [1991] reported saccade‐related Purkinje cell activity in the superior and inferior semilunar lobules (Crus IIa, Crus I). Neuronal activity in the simple lobule was also found in monkeys during visually guided arm and eye movements. This activity strongly correlated with the velocity of horizontal eye movements [Marple‐Horvat and Stein, 1990]. Their finding agrees with that of a human fMRI study that reported activation of the simple lobule during optokinetic nystagmus and saccades [Dieterich et al., 2000]. Thus, the structures of the cerebellar hemispheres, such as the simple lobule, superior semilunar lobule, and inferior semilunar lobule, are involved in the performance of motor and ocular motor tasks.
Our observation of significant changes in the activation patterns in two successive runs of the same familiar ocular motor task is not only relevant for functional interpretation of the ocular motor processes, but it also raises the general question of how to interpret fMRI data obtained from identical sensorimotor tasks performed in repeated sequences. The systematic changes of the activation pattern may not only represent intersession variability of an underlying constant neuronal processing; they may also indicate a systematic change in neuronal activity (e.g., cerebellar hemispheres). McGonigle et al. [2000] tested the reproducibility of activation patterns during a motor, a cognitive, and a visual task over serial experiments during 2 months. Their finding of significant variability for all three tasks reflects the variability between repeated single measurements in one and the same subject. To test for systematic changes in neuronal assemblies, however, it is necessary to conduct a group analysis over a larger number of subjects and thus eliminate intraindividual variability. Earlier studies with a larger number of subjects demonstrated systematic variations of cortical and cerebellar activation patterns during multiple repetitions of motor (PET [Friston et al., 1992]; fMRI [Tracy et al., 2001]) and visuomotor tasks [Imamizu et al., 2000]. The authors found decreases in the physiological activation of the cerebellar hemispheres during serial stimulations and these changes were attributed to motor optimization and learning. Our ocular motor task can be best compared to the aforementioned finger opposition task used by Tracy et al. [2001], for both tasks are very simple and highly overlearned. We thus conclude that motor optimization mainly contributed to the session effects of our data.
Acknowledgements
We are grateful to Judy Benson for critically reading the manuscript.
REFERENCES
- Aguirre GK, Zahran E, D'Esposito M (1998): The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage 8: 302–306. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton RB, Wong EC, Courchesne E (1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grea H, Grethe JS, Prablanc C, Alexander GE, Grafton ST (2001): Functional anatomy of nonvisual feedback loops during reaching: a positron emission tomography study. J Neurosci 21: 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Bucher SF, Seelos KC, Brandt T (2000): Cerebellar activation during optokinetic stimulation and saccades. Neurology 54: 148–155. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (1995): The human brain stem and cerebellum. Wien: Springer; 430 p. [Google Scholar]
- Frison L, Pocock S (1992): Repeated measures in clinical trials: analysis using mean summary statistics and its implications for design. Stat Med 11: 1685–1704. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Asburner J, Frith CD, Poline JB, Heather JD, Frachowiak RSJ (1995a): Spatial registration and normalization of images. Hum Brain Mapp 2: 165–189. [Google Scholar]
- Friston KJ, Frith CD, Passingham RE, Liddle PF, Frackowiak RSJ (1992): Motor practice and neurophysiological adaption in the cerebellum: a positron tomography study. Proc R Soc Lond B 248: 223–228. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Fuchs AF, Robinson FR, Straube A (1993): Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol 70: 1723–1740. [DOI] [PubMed] [Google Scholar]
- Helmchen C, Büttner U (1995): Saccade related Purkinje cell activity in the oculomotor vermis during spontaneous eye movements in light and darkness. Exp Brain Res 103: 198–208. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Ryousuke T, Pütz B, Yoshioka T, Kawato M (2000): Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192–195. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS (1999): The neurology of eye movements. New York: Oxford University Press; 646 p. [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. 2001. Maturation of widely distributed brain function subserves cognitive development. Neuroimage 13: 786–793. [DOI] [PubMed] [Google Scholar]
- Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP. 2000. FMRI of visual encoding: reproducibility of activation. Hum. Brain Mapping 9: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano N, Ito Y, Shibutani H (1991): Saccade‐related Purkinje cells in the cerebellar hemispheres of the monkey. Exp Brain Res 84: 465–470. [DOI] [PubMed] [Google Scholar]
- Marple‐Horvat DE, Stein JF (1990): Neuronal activity in the lateral cerebellum of trained monkeys, related to visual stimuli or to eye movements. J Physiol 428: 595–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP (2000): Variability in fMRI: an examination of intersession differences. Neuroimage 11: 708–734. [DOI] [PubMed] [Google Scholar]
- Miall RC, Imamizu H, Miyauchi S (2000): Activation of the cerebellum in coordinated eye and hand tracking movements: an fMRI study. Exp Brain Res 135: 22–33. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y (1990): Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the Macaque monkey. J Comp Neurol 302: 330–348. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Wolff AL, Benkelfat C, Florencio PS, Lal S, Evans AC (2000): Functional neuroanatomy of smooth pursuit and predictive saccades. Neuroreport 11: 1335–1340. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Tallent K, van Gelderen P, Frank JA, Moonen CTW, Weinberger DR (1996): Reproducibility of human 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp 4: 113–121. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P (1998): Within‐subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imaging 16: 105–113. [DOI] [PubMed] [Google Scholar]
- Ron S, Robinson DA (1973): Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36: 1004–1022. [DOI] [PubMed] [Google Scholar]
- Salmaso D, Longoni AM (1985): Problems in the assessment of hand preference. Cortex 21: 533–549. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Roland PE, Bohm C, Greitz T, Stone‐Elander S (1990): Motor learning in man: a positron emission study. Neuroreport 1: 57–60. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Faro SS, Mohammed F, Pinus A, Christensen H, Burkland DA (2001): Comparison of ‘Early’ and ‘Late’ stage brain activation during brief practice of a simple motor task. Cogn Brain Res 10: 303–316. [DOI] [PubMed] [Google Scholar]
- Woods RP (1996): Modeling for intergroup comparisons of imaging data. Neuroimage 4: 84–94. [DOI] [PubMed] [Google Scholar]


