Abstract
Whether different odorous compounds (odorants) are processed by different cerebral circuits is presently unknown. A first step to address this complicated issue is to investigate how the cerebral regions mediating signals from olfactory (i.e., unimodal) odorants, differ from those mediating the olfactory + trigeminal (i.e., bimodal) odorants. [15O]‐H2O‐PET scans were conducted in 12 healthy females during three separate conditions: birhinal, passive smelling of: 1) the unimodal odorant vanillin; 2) the bimodal odorant acetone; and 3) odorless air. Significant activations were calculated contrasting vanillin to air, acetone to air, and deactivations, running these contrasts in the opposite direction. Smelling of vanillin activated bilaterally the amygdala and piriform cortex. These regions were only engaged slightly by acetone. Instead, strong activations were found in the anterior and central insula and claustrum, the posterior portion of anterior cingulate, the somatosensory cortex (SI for face), cerebellum, ventral medial (VMPo) and dorsal medial (MDvc) thalamus, the lateral hypothalamus, and pons/medulla. In parallel, the somatosensory (SI, below central representation of face), secondary visual and auditory cortices, as well as the supplementary motor area and the parahippocampal gyri were deactivated. No deactivations were observed with vanillin, although the odor components of acetone and vanillin were rated similarly intense (75 ± 17 mm vs. 61 ± 22 mm, NS). The differentiated pattern of cerebral activation during odorant perception seems to be dependent on the signal transducing cranial nerves involved. In contrast to vanillin, which solely activates the olfactory cortex, acetone engages predominantly trigeminal projections from the nasal mucosa. Acetone's limited activation of the olfactory cortex may result from a cross‐modal interaction, with inhibition of acetone's odor component by its trigeminal component. Hum. Brain Mapping 17:17–27, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: trigeminal nerve, olfactory nerve, positron emission tomography, cerebral blood flow, olfaction
INTRODUCTION
The majority of odorous compounds (odorants) stimulate not only the olfactory, but also the trigeminal receptors [Tucker, 1971]. In an extensive study of 15 anosmic patients, Doty et al. [1975] found that the only odorant not perceivable by any of the anosmic patients when given in high concentrations was vanillin. Based on these results the authors concluded that vanillin was the only strictly unimodal (purely olfactory) odorant, whereas all the other odorants were bimodal, i.e., olfactory + trigeminal.
An odorant's olfactory signals are transduced by the olfactory epithelium via the olfactory bulb and tract to the amygdaloid, periamygdaloid and piriform cortex (the primary olfactory cortex), and from there to the orbitofrontal and insular cortex (the secondary olfactory cortex) [Powell et al., 1965]. An odorant's trigeminal signals stimulate the trigeminal nerve's free nerve endings located within the respiratory mucosa of the nasal vestibule. This explains why many odorants, when given in high concentrations, are capable of producing sensations such as irritation, pain, tickling, burning, warming, cooling, and stinging, not experienced with the purely olfactory compound vanillin. These sensations are presumably mediated by the unmyelinated C‐fibers and the myelinated Adelta‐fibers [Anton and Peppel, 1991; Hummel, 2000]. The C‐and Adelta‐fibers convey the trigeminal information to the rostral part of the spinal trigeminal nucleus, and from there, via the ventral posterior medial nucleus of the thalamus to the cerebral cortex [Barnett et al., 1995].
These two fundamentally different anatomical pathways indicate that different cerebral circuits may process different odorants. Although the cerebral activation by non‐odorous trigeminal stimuli from the nasal mucosa has been studied with magnetic source imaging [Hummel, 2000; Huttunen et al., 1986; Kobal and Hummel, 1988], very little is known currently about the processing of combined odorous + trigeminal stimuli produced by a single, naturally occurring airborne compound. The information in the majority of hitherto published imaging studies is based on data merged from several different odorants [Savic et al., 2000; Zald and Pardo, 1997; Zatorre et al., 1992], allowing no distinction between the patterns of activation by the separate compounds. Although two studies have addressed the differences in cerebral activation by olfactory and trigeminal odorants [Sobel et al., 2000; Yousem et al., 1997], none of them was designed to only elucidate this issue, taking into account the ratings of the subjective perception of odorant‐characteristics. Furthermore, none of these studies included calculations of the possible decreases in regional cerebral blood flow during odorant‐stimuli. Such decreases have been observed during visual, auditory and somatosensory stimuli, and it is currently discussed whether their specific distribution is related to the type of sensory stimulus. The decreases are believed to reflect neuronal deactivations, and be a part of signal processing [Shulman et al., 1997]. Therefore, when trying to understand whether the brain mediates pure olfactory stimuli differently from the olfactory + predominantly trigeminal stimuli it was important to analyze both the increases (defined as cerebral activations), and decreases (deactivations) in the regional cerebral blood flow.
In the present PET experiments we compared the patterns of cerebral activation, as well as deactivation during smelling of two specific odorants: 1) the unimodal, pure olfactory odorant vanillin; and 2) the bimodal odorant acetone, which is strongly trigeminal but at the same time also strongly odorous. One purpose was to investigate whether different odorants may have different patterns of activation at all. Another was to analyze whether, and how the specific activation of the olfactory cortex differs when an odorant is bimodal, compared to unimodal, provided that the respective odorous components are perceived as equally strong. A third was to identify the structures activated by acetone but not vanillin, and thus, representing the targets of the trigeminal nerve. In accordance with the previous studies, vanillin was assumed to activate the amygdala and piriform cortex, possibly also the orbitofrontal and anterior insular cortices [Bengtsson et al., 2001; Kettenmann et al., 1997; Simmonds et al., 1997]. We expected these same regions to be activated also by acetone. Based on data from anterograde tracings, recordings of single units, and PET studies of pain and heat (also processed by the C‐fibers and the Adelta fibers) three possible pathways for the trigeminal stimulus of acetone were hypothesized: 1) via the ventral medial nucleus of the thalamus (VMPo) to the anterior and medial insula; 2) via the medial dorsal nucleus (MDvc) to the anterior cingulate; and 3) via the ventral posterior lateral nucleus (VPL) to the primary and secondary somatosensory cortex (SI and SII), [Craig et al., 1994, 1996; Treede et al., 1999]. If, and how the patterns of cerebral deactivation would differ during smelling of acetone and vanillin, was unpredictable, as such data has not been reported previously.
MATERIALS AND METHODS
Subjects
Twelve healthy, right‐handed females (20–28 years), with normal nasal anatomy according to the clinical status, assessed before the PET experiments, and with normal olfactory thresholds assessed with the n‐butyl alcohol test [Jones‐Gotman and Zatorre, 1988], were investigated with 15O‐H2O‐PET. The study was approved by the local ethics and safety radiation committees.
Odors
Pure (99%) vanillin (4‐hydroxy‐3‐methoxy‐benzaldehyde) and acetone (C3H6O, HPLC 99.9%) from Sigma Chemical Co. (Deishofen, Germany) were used in liquid forms, without dilution. That acetone, but not vanillin stimulated the trigeminal nerve, was tested before the PET experiments in five patients with peripheral anosmia, ad modum Wysocki et al. [1997]. None of the patients reported any perception during smelling of vanillin. In contrast, all of them perceived a painful burning and a stinging sensation in the stimulated nostril during presentations of acetone. These sensations were reported also by the healthy subjects who participated in the present PET study.
Conditions and experimental procedure
PET scans were carried out during three separate conditions: birhinal, passive smelling of odorless air, birhinal, passive smelling of acetone, and birhinal passive smelling of vanillin. Due to restrictions by the Radiation Safety Committee, the number of scans per subject was restricted to eight. All the subjects smelled odorless air during three of the scans; six of the subjects smelled vanillin in three scans, and acetone in two; in the remaining six acetone was presented during three scans, and vanillin during two. The order of conditions was balanced across the subjects. Five scans were excluded from further analysis due to movement artifacts (swallowing).
The odorants were contained in glass bottles with a cotton wand. When the condition was smelling of air, the wand was soaked with distilled water. Each item was presented during a separate scan, at 10 mm distance from the nostrils. To minimize odor adaptation, the respective item was during the scan presented for 15 sec, a total of four times. Each of these presentations was followed by a 5‐sec interval of breathing the air in the scanner room, wherein a suction hood placed close to the scanner gantry, provided continuous suction and refreshment of the air. The first presentation started 5 sec after the bolus injection of 15O‐H2O. One stimulus session lasted 80 sec.
Independent of the condition, the start of each presentation was indicated by touching the subject's right index finger. The participating subjects were informed that they would smell either odor or odorless air, without knowing the type or order of items. They were instructed to breathe passively (not sniff, even when no odor was perceived) and concentrate on perception of the presented item (vanillin, acetone, or air), without analyzing its characteristics. During several preparatory psychophysical experiments, subjects were trained in the scanner with other odors to familiarize with the experimental procedure. Respiratory movements were recorded during each scan, using a strain gauge around the lower thorax (Comair AB, Stockholm). The respiratory frequency (breaths/min) and amplitude (the amplitude measured in mm is directly related to the degree of stretching of the strain gauge band, i.e., the circumferential increase of the lower thorax) was recorded continuously, before and during each presentation of vanillin, acetone, and air. The change in frequency and amplitude during the respective scan was calculated in relation to the resting condition during 2 min immediately before each scan (defined as the baseline). In addition, for each scan we calculated an index of the change from this baseline in respiratory volume (product of respiratory frequency and respiratory amplitude).
After PET measurements, the subjects scored the odors for pleasantness, irritability, intensity and familiarity using a 100 mm bipolar visual‐analogue scale (VAS) [Doty et al., 1991]. We also tested the adaptation rate to the two odorants by measuring time from the presentation to subjective loss of perception of the odor. The adaptation was rated in relation to the odor component also during the presentation of acetone.
Scanning procedure and the analysis of PET data
PET experiments were in each subject preceded by a magnetic resonance imaging (MRI), (1.5 Tesla GE scanner; 3D SPGR; TE = 5 msec, TR = 21 msec; FOV = 256 mm). The PET scanner used was the CTI‐Siemens ECAT EXACT HR scanner, running in 3D mode. During the PET sessions each subject received eight bolus injections containing about 12 mCi of 15O‐H2O. PET scans were carried out with the head fixed [Bergström et al., 1981], ears plugged, and eyes covered. The room temperature and air pressure in the PET room was standardized during all experiments (23°C, 997 hPa), and the contamination by odors avoided by a suction device connected to the scanner.
The relative regional cerebral blood flow (rCBF) was calculated according to Fox et al. [1984] including data during 60 sec after the onset of acquisition. A 10 mm Gaussian filter was used. The PET images were co‐registered with Woods' image aligning program [Woods et al., 1992]. They were anatomically standardized using the individual magnetic resonance images and the Computerized Brain Atlas [Roland et al., 1994]. The data were normalized to the arbitrary global cerebral flow value of 50 ml/100 g/min.
The contrasts were tested with the general linear model (GLM) [Siarle, 1971], and the differences calculated pixel‐by pixel, generating t‐maps, later transformed into units of normal distribution (z‐maps). The number of df was 81 after omission of the five scans. Significant activations (P < 0.05) were determined with the cluster statistics of Ledberg et al. [1998]. Based on 3,000 simulated noise images, the z‐threshold was determined to be 3.1 and the minimum cluster size 1.1 cm3. The location of center of gravity of significant clusters was determined in Talairach coordinates, and in relation to the computerized standard atlas brain [Roland et al., 1994], the computerized map of cytoarchitectonic areas [Schleicher et al., 1999], and the reformatted mean MR images of the participating subjects.
Contrasts
First, we evaluated cerebral activations with vanillin and acetone, using air as reference (the vanillin–air contrast and acetone–air contrast, respectively). Next, we analyzed possible deactivations during smelling of vanillin and acetone, by applying the reverse contrast (air–vanillin contrast and air–acetone contrast). Regional deactivations were defined as areas with higher relative rCBF during smelling of air compared to vanillin and acetone, respectively. We carried out direct comparisons between acetone and vanillin, applying the contrast in both directions (acetone–vanillin contrast and vanillin–acetone contrast).
Volume of interest analyses
An a posteriori volume of interest (VOI) analysis was carried out to test the hypothesis that the olfactory areas, i.e., the amygdala and piriform cortex [Sobel et al., 1998; Zald and Pardo, 1997; Zatorre et al., 1992], are activated similarly by vanillin and acetone. Another hypothesis was that acetone, but not vanillin activates the trigeminal nuclei. A third was that an activation of this nucleus might affect the breathing pattern, and therefore, the regional cerebral blood flow (rCBF) in this region would be correlated to the respiratory index.
The first hypothesis was tested applying clusters from the vanillin‐air contrast obtained in another study of cerebral activation during passive smelling of odors [Bengtsson et al., 2001]. The second and third hypotheses were tested using the cluster generated by the acetone–air contrast in the present study (Table I, see also Results). The normalized cerebral blood flow was then calculated in each of these VOIs during each scan (see further), and the data entered in the statistical analysis.
Table I.
Activations during passive smelling of vanillin (vanillin − air contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Right amygdala + piriform cortexa | 3.6 | 3.0 | 28 1 −10 |
| Left amygdala + piriform and anterior insular cortex | 3.6 | 1.4 | −17 −3 −9 |
The presented centers of gravity were obtained at Z < 3.1 and cluster size > 1.1 cm3 yielding P < 0.05. The right cluster covered a very small portion of the inferior orbitofrontal cortex.
Cluster covering a minor part of the orbitofrontal cortex.
Statistical evaluation of psychophysical data and the VOI data
Change in respiratory amplitude and frequency in relation to the respective pre‐scan baseline, as well as the calculated index of respiratory volume was compared between air, vanillin and acetone with a repeated measurements ANOVA using air, vanillin and acetone as controlled factors in the ANOVA (P < 0.05). The subjective ratings of odor pleasantness, irritability, intensity, and familiarity were compared between acetone and vanillin using paired Student's t‐tests based on the values from the VAS. Bonferroni's correction was applied for the multiple comparisons (four), yielding the limit for significance of P < 0.0125. Paired t‐test was also applied to test possible odorant‐related differences in relative rCBF in different VOIs, but without the Bonferroni correction as these analyses were hypothesis‐based. Finally, paired t‐tests were also applied to test possible differences in adaptation to acetone and vanillin, respectively (P < 0.05). Correlation analysis was conducted using Pearson's simple regression (P < 0.05).
RESULTS
Psychophysics
Whereas vanillin was perceived as pleasant and not irritating, acetone was rated as unpleasant and irritable (P < 0.001) (Fig. 1). No difference was found in odor familiarity or intensity. All three conditions led to an increase in respiratory amplitude (1.8 ± 3.3 mm, 22%; 1.4 ± 2.4 mm, 13%; and 5.1 ± 3.4 mm, 47% for air, vanillin, and acetone, respectively), and a decrease in respiratory frequency (2.9 ± 3.5 breaths · min−1, 17%; 2.2 ± 3.5 breaths · min−1, 13%; and 1.1 ± 3.3 breaths · min−1, 6%, respectively) in relation to the respective pre‐scan baseline. The increase in respiratory amplitude was significantly higher during the acetone than during the air and vanillin conditions (P < 0.001), whereas the decrease in respiratory frequency was less pronounced during acetone than during air (P < 0.001) or vanillin (P = 0.02) (Fig. 2a). The change in the calculated index of respiratory volume was 2 ± 53 mm · breaths · min−1 (1%) for air, −1 ± 58 mm · breaths · min−1 (0%) for vanillin, and 68 ± 79 mm · breaths · min−1 (40%) for acetone. It was significantly more pronounced during exposition to acetone compared to air and vanillin (P‐values <0.001). Furthermore, a characteristic respiratory pattern with a gradual increase and then decrease of the respiratory amplitude was observed during smelling of acetone, but not during air or vanillin (Fig. 2b).
Figure 1.
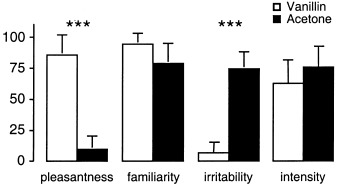
Odor ratings. The vertical axis indicates the visual analogue scale in mm. Bars indicate mean and SD (***P < 0.001).
Figure 2.
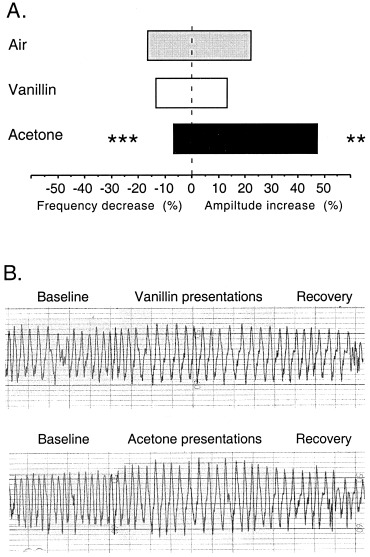
Respiratory changes. A: Changes in respiratory amplitude and frequency. Differences are calculated as percent change during the stimulation period compared to the baseline registration. The decreases in frequency are denoted as negative values to the left and the increases in amplitude as positive values to the right. Mean values are given. The stars indicate significant difference compared to the other two conditions (**P < 0.05; ***P < 0.001). B: Representative respiratory patterns during smelling of acetone and vanillin from one of the subjects. The four 15‐second presentations of the respective odor are indicated by shaded areas.
The adaptation rate to vanillin was not different from acetone (108 ± 24 sec vs. 108 ± 23 sec).
Cerebral activation
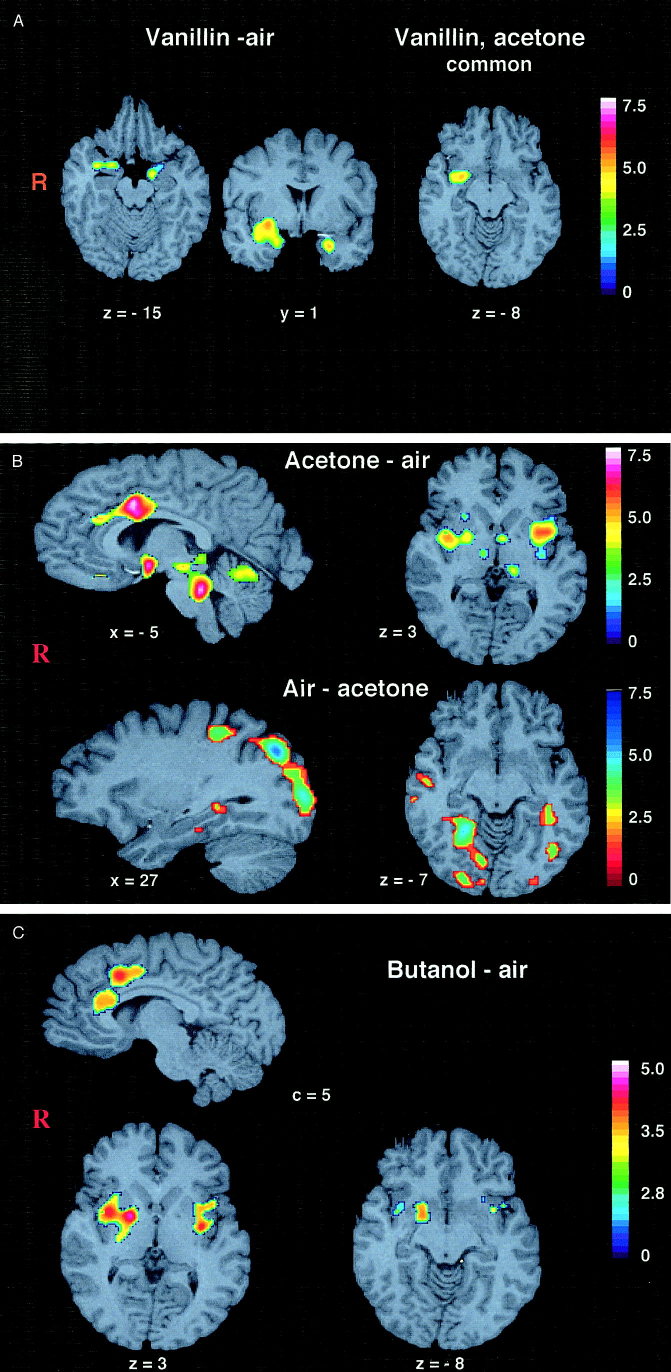
As shown in our previous study [Bengtsson et al., 2001], smelling of vanillin produced bilateral activation of amygdala and piriform cortex (including a minor portion of right orbitofrontal cortex) and a part of the left anterior insular cortex (Fig. 3a, Table I).
Figure 3.
a: Activation by vanillin (vanillin–air contrast) (left); acetone and vanillin–common clusters (right). Activated clusters superimposed on a standard brain. The Sokoloff color scale illustrates Z‐values (0–7.5). The clusters were thresholded at 3.1. Subject's right side is to the left in the image. The Talairach coordinates are given. b: Activation by acetone (acetone–air contrast) and deactivation by acetone (air–acetone contrast). Activated clusters superimposed on a standard brain. The Sokoloff color scale illustrates Z‐values (0–7.5). Note that the color coding is reversed for the illustration of deactivation. The clusters were thresholded at 3.1. Subject's right side is to the left. The Talairach coordinates are given. c: Activation by butanol (butanol–air contrast) and deactivation by butanol (air–butanol contrast). Activated clusters superimposed on a standard brain. The Sokoloff color scale illustrates Z‐values. Note that the color coding is reversed for the illustration of deactivation. The clusters were thresholded at 2.6. Subject's right side is to the left. The Talairach coordinates are given.
The fields activated by acetone were much more widespread. They covered bilateral anterior as well as central insula and claustrum, spreading down into the amygdala and piriform cortex on both sides, and including a minor portion of the somatosensory (SI) area on the left side (the central representation for face [Roland, 1993]). In addition, a cluster was found in the posterior portion of anterior cingulate (Fig. 3b, Table II, III), in the right cerebellar hemisphere, the vermis cerebelli, lateral hypothalamus, the ventral medial and dorsal medial thalamus (VMpo and MDvc), and in the brainstem covering the trigeminal nuclei. Notably, when overlaying the clusters from the vanillin–air contrast on the corresponding clusters from acetone–air contrast, the only common area was a portion of the right amygdala and piriform cortex, (Fig. 3a).
Table II.
Activations during passive smelling of acetone (acetone − air contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Left insula and claustruma | 4.0 | 5.3 | −32 −1 3 |
| Right insula and claustrum + amygdala | 3.9 | 3.2 | 32 −3 2 |
| Hypothalamus, thalamus (MDvc and VMPo), brainstem | 3.5 | 4.0 | 5 −27 −15 |
| Brainstem (including trigeminal nuclei) | 4.1 | 3.1 | 2 −32 −25 |
| Anterior cingulate (posterior portion) | 3.7 | 2.1 | 0 34 26 |
| Right cerebellar hemisphere | 3.4 | 2.2 | 22 −52 −52 |
| Cerebellar vermis | 3.4 | 2.1 | 4 −59 −14 |
The presented centers of gravity were obtained at Z > 3.1 and cluster size > 1.1 cm3 yielding P < 0.05. MDvc, the medial dorsal nucleus; VMPo, the ventral medial nucleus of the thalamus.
Cluster including a minor part of S1 (central representation of face).
Table III.
Deactivations during passive smelling of acetone (air ‐ acetone contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Right middle temporal gyrus (auditory association cortex) | 3.6 | 1.9 | 54 −19 −13 |
| Right precuneus | 3.5 | 1.9 | 3 −27 51 |
| Right sensorymotor cortex | 3.6 | 1.5 | 34 1 53 |
| Left parahipocampal gyrus | 3.3 | 1.4 | −34 −37 −9 |
| Right parahippocampal gyrus | 3.8 | 6.2 | 24 −53 −4 |
| Bilateral inferior temporal, parietal and occipital cortex (excluding primary visual, auditory and somatosensory cortex) | 3.7 | 33.0 | 2 −68 26a |
The presented centers of gravity were obtained at Z > 3.1 and cluster size > 1.1 cm3 yielding P < 0.05.
Large, confluent cluster covering both hemispheres. The co‐ordinates indicate the center of gravity for this cluster.
Cerebral deactivation
Smelling of vanillin was not associated with any decrease in rCBF, i.e., deactivation (air–vanillin contrast) was not observed. To the contrary, smelling of acetone caused large deactivations (air–acetone contrast) bilaterally in the primary and secondary visual cortex, the right secondary auditory cortex, the sensory‐motor cortex (corresponding to the central representation of leg according to the homunculus), supplementary motor cortex, and both sides parahippocampal gyri (Fig. 3b, Table IV). With the exception for right medial temporal gyrus, similar (although not identical) clusters appeared also in the vanillin–acetone contrast (Table V).
Table IV.
Activations by acetone compared with vanillin (acetone − vanillin contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Left insula and claustrum | 3.5 | 1.5 | −30 1 0 |
| Left insula and claustrum incl S1 for face | 3.5 | 1.5 | −56 −12 15 |
| Hypothalamus (pars tuberculartis) + thalamus (VMpo) | 3.5 | 3.0 | 5 −8 −6 |
| Anterior cingulate (posterior portion) | 3.4 | 2.8 | 8 8 34 |
| Brainstem, and cerebellar vermis | 3.5 | 5.6 | −1 −39 −23 |
The presented centers of gravity were obtained at Z > 3.1 and cluster size > 1.1 cm3 yielding P < 0.05. VMpo, the ventral medial nucleus of the thalamus.
Table V.
Deactivations by acetone compared with vanillin (vanillin−acetone contrast)*
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Right postcentral gyrus (S1, arm) | 3.4 | 1.3 | 41 −21 19 |
| Left superior frontal gyrus | 3.5 | 1.8 | −15 53 1 |
| Right prefrontal cortex | 3.2 | 2.1 | 37 −3 58 |
| Bilateral inferior temporal, occipital and parietal cortex | 3.7 | 10.2 | −5 −56 20 |
The presented centers of gravity were obtained at Z > 3.1 and cluster size > 1.1 cm3 yielding P < 0.05.
Correlation analyses
The percent change in respiratory index was only weakly correlated to the normalized rCBF of the trigeminal nucleus (r = 0.34; P = 0.01).
Volume of interest analyses
The mean normalized rCBF in amygdala + piriform cortex was lower during smelling of acetone compared to vanillin (P = 0.040 and P = 0.048 for the left and right amygdala + piriform VOI, respectively). The rCBF during smelling of acetone was higher than when smelling air (P < 0.001 for both VOIs).
Additional information from a separate study
To evaluate whether the pattern of activation observed with acetone was representative for the olfactory + trigeminal odorants in general, and not specifically related to acetone, we did a post‐hoc analysis of a separate PET study. This study was carried out with 15‐O‐butanol on 16 right‐handed, healthy, women volunteers (age 24–27 years). The activation task consisted of passive smelling of 10% butanol (diluted in distilled water), whereas smelling of odorless air served as the baseline condition. When tested ad modum Wysocki [Wysocki et al., 1997], butanol elicited perception of pain and cold in the stimulated nostril in four of six investigated anosmic subjects. In the given concentration butanol was, therefore, deemed to elicit a bimodal stimulation, olfactory + trigeminal.
The scanning procedure was identical with the procedure in the present study, with four 15‐ sec presentations of the odor, or odorless air, during each scanning session. After the scans subjects rated butanol with VAS for odor pleasantness, irritability, intensity, and familiarity.
The activations (butanol–air contrast), as well as deactivations by butanol, (air–butanol contrast) were calculated with the general linear model [Ledberg et al., 1998] using P < 0.05 (z‐threshold of 2.6 and minimum cluster size 2.8 cm3).
The pattern of activation and deactivation was similar to that observed with acetone (Table VI, VII). Thus, activations were observed bilaterally in insular cortex, covering minor portions of the piriform cortex, in the posterior portion of anterior cingulate, and bilaterally in the somatosensory cortex. No activation was observed in amygdala, or orbitofrontal cortex. In parallel, there were deactivations in the right temporal neocortex, right parietal and occipital cortex. Butanol was rated 19 ± 4 mm for pleasantness, 38 ± 5 mm for irritability, 28 ± 6 mm for intensity, and 16 ± 2 mm for familiarity.
Table VI.
Ativations by butanol compared with air (butanol–air contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Right insular cortex | 3.2 | 7.2 | 26 1 2 |
| Left insular cortex | 2.9 | 3.9 | −30 14 |
| anterior cingulate | 3.0 | 6.2 | −5 16 3 |
| Right somatosensory cortex | 4.1 | 3.2 | 50 −16 23 |
| Left somatosensory cortex | 3.0 | 4.9 | −52 −8 29 |
Clusters were thresholded at Z > 2.6 and size > 2.8 cm.
Table VII.
Deactivations by butanol compared with air (air–butanol contrast)†
| Region | Z‐score (mean) | Size (cm3) | Coordinates |
|---|---|---|---|
| Right medial temporal gyrus | 3.2 | 3.2 | 53 4 −12 |
| Right parietal and occipital cortex | 2.8 | 6.4 | 9 −50 31 |
Clusters were thresholded at Z > 2.6 and size >2.8 cm3.
DISCUSSION
Odorous compounds (odorants) may be pure olfactory, mixed olfactory and trigeminal [Doty et al., 1978], or pheromones [Karlsson and Luscher, 1959]. We recently found that pheromone like compounds elicit an entirely different pattern of cerebral activation than the hitherto investigated odors [Savic et al., 2001]. The present study represents a further step in the investigation of possible odorant related specificity of cerebral activation in that it compares the cerebral activation by an olfactory and a mixed olfactory + trigeminal odorant. Using methods identical with those applied in several of our previous publications [Savic et al., 2000a; Savic and Gulyás, 2000], we show an odorant differentiated pattern of cerebral activation.
In accordance with other studies of perception of predominantly olfactory odorants (Savic et al., 2000a; Savic and Gulyás, 2000; Sobel et al., 1998; Zald and Pardo, 1998; Zatorre et al., 1992], smelling of vanillin activated the primary olfactory areas [Powell et al., 1965]. In our recent study [Bengtsson et al., 2001], only a minor portion of orbitofrontal cortex was activated, probably because subjects were trained not to judge the odors, and, a recruitment of associative olfactory cortex was not necessary. In contrast to vanillin, smelling of acetone engaged only a minor portion of the primary olfactory brain, despite similar ratings of the subjectively perceived odor intensity and familiarity (Figs. 1,3). The activations resembled those observed with various painful stimuli [Casey et al., 1996; Paulson et al., 1998; Rainville et al., 1999], and should, therefore, be attributed to acetone's trigeminal component. this explains the reported cooling, burning, and painful sensations, as well as the high irritability scores (Fig. 1).
The observed difference in cerebral activation by vanillin and acetone can not be explained by a difference in odor‐pleasantness. Chemosensory evoked responses to vanillin and hydrogen sulfide (two olfactory odorants, one pleasant, the other unpleasant) have similar distributions [Kettenmann et al., 1997], which are both different from the distribution obtained with trigeminal stimuli [Kobal and Hummel, 1988]. Acetone activated the right amygdala, whereas the unpleasant odors are found to mainly engage the left side [Royet et al., 2000; Zald and Pardo, 1997]. Unpleasant odorants are reported to activate the occipital cortex [Williams et al., 1997], which in the present study was deactivated by acetone.
Despite a similar scoring as vanillin with respect to odor intensity, acetone activated only a minor part of the olfactory cortex, (Fig. 3a). One explanation could be the trigemino–olfactory interaction, analogous with taste–olfactory interaction reported with the perception of flavor [Small et al., 1997]. Such an interaction is suggested by electrophysiological studies showing that trigeminal stimuli have an inhibitory effect on olfactory afferents to the brain [Bouvet et al., 1987; Cain and Murphy, 1980; Kobal and Hummel, 1988; Stone, 1969]. One example is that blocking of the trigeminal nerve enhances odor‐induced activity in the olfactory bulb, whereas electrical stimulation of the trigeminal nerve decreases the bulbar activity [Stone, 1969]. Another is that neuronal responses in medial dorsal thalamic neurons (MDvc) after odor stimulation become enhanced by blockade of the trigeminal nerve [Inokuchi et al., 1993]. A cluster corresponding to MDvc was observed when contrasting vanillin, as well as acetone to air, but not when acetone was contrasted to vanillin (acetone–vanillin contrast). This nucleus may well have mediated the olfactory‐trigeminal interaction in the present study. Whether the postulated inhibition by trigeminal signals was achieved via recruitment of inhibitory thalamic inter‐neurons projecting onto thalamo‐cortical neurons, or via another pathway, can only be speculated on presently.
A conceptually important question is whether the suppression of olfactory signals by the trigeminal nerve is selective or part of a more widespread effect. Smelling of acetone caused deactivations of the somatosensory and visual and auditory cortices, whereas no deactivations were observed with vanillin. That the clusters detected in air–acetone contrast represented acetone‐related deactivations, rather than air‐related activations, was indicated by the fact that they appeared also in the vanillin–acetone contrast, but not in the air–vanillin contrast. Although these clusters could represent a gating of task‐irrelevant information, it is of note that they were found only in areas mediating other sensory stimuli. A similar cross‐modal modulation of sensory stimuli has been observed during visual [Haxby et al., 1994], pain [Peyron et al., 1999], auditory [Fiez et al., 1996], as well as somatosensory stimuli [Bodegård et al., 2000; Kawashima et al., 1995], implying that the cross‐modal deactivation is a more widespread phenomenon [Shulman et al., 1997]. That no deactivations of olfactory regions were observed in the air‐acetone condition may be an effect of acetone's bimodal action: thus, the olfactory regions could, theoretically, have been activated by the olfactory component of acetone, and deactivated by its trigeminal component.
A tentative mechanism underlying deactivations during sensory stimuli could be strong attentional shift, as suggested by Drevets et al. [1995] who found a similar pattern of deactivation during the expectation, compared to perception of somatosensory stimuli. Smelling of acetone presumably caused a shift of attention toward the potentially noxious trigeminal stimulus. Because this stimulus may contain vital information, other irrelevant sensory stimuli had to be disregarded. Such a mechanism seems plausible, considering that trigeminal nasal stimuli have a strong effect on cardiovascular reflexes, (i.e., heart rate and blood pressure, respiration rate, epinephrine secretion) [James and Daly, 1969]. They are reported to induce a swelling of the nasal mucous membrane [Simon et al., 1985], causing an increase in airflow resistance, which could explain the typical respiratory pattern observed during smelling of acetone, but not vanillin or air (Fig. 2). This pattern was characterized by a gradual increase in respiratory amplitude followed by its gradual decrease, and by a less pronounced decrease in respiratory frequency, which resulted in an increase of the calculated respiratory index. A similar increase in tidal volume in response to increased airflow resistance was also found by Daubenspeck [1981].
It may be argued that the cerebral activation by acetone is not necessarily representative for other olfactory + trigeminal odorants. Butanol, however, activated similar regions. Sobel et al. [2000] reported a similar pattern of activation although completely different bimodal compounds were used. The observed difference between vanillin and acetone seems to have more general implications for unimodal olfactory odorants compared to bimodal olfactory + clearly trigeminal odorants. This confirms our initial hypothesis about the inherently different pathways in relation to the signal transducing cranial nerves, and motivates further studies of odor‐related cerebral activation. The findings also bring attention to the risk of bias when comparing cerebral activations during various olfactory functions (perception, discrimination, memory, and identification) without matching the odorants for their trigeminal component.
Acknowledgements
We acknowledge V. Jaluli, C. Dupuis, and G. Printz for radiotracetoner synthesis, Bsc B.M. Berggren for production of helmets, J. Gabriel and J. Sandblom for technical assistance.
REFERENCES
- Anton F, Peppel P (1991): Central projections of trigeminal primary afferents innervating the nasal mucosa: horseradish peroxidase study in the rat. Neuroscience 41: 617–628. [DOI] [PubMed] [Google Scholar]
- Barnett EM, Evans GD, Sun N, Perlman S, Cassel MD (1995): Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci 15: 2972–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson S, Berglund H, Gulyás B, Cohen E, Savic I (2001): Brain activations during odor perception in males and females. NeuroReport 2: 2027–3032. [DOI] [PubMed] [Google Scholar]
- Bergström M, Boethius J, Eriksson L, Greitz T, Ribbe T, Widen L (1981): Head fixation device for reproducible positron alignment in transmission computed tomography and positron emission tomography. J Comp Assist Tomogr 5: 136–144. [DOI] [PubMed] [Google Scholar]
- Bodegård A, Geyer S, Naito E, Zilles K, Roland PE (2000): Somatosensory areas in man activated by moving stimuli: cytoarchitectonic mapping and PET. NeuroReport 11: 187–191. [DOI] [PubMed] [Google Scholar]
- Bouvet J, Delaleu JC, Holley A (1987): Does trigeminal nerve control the activity of olfactory receptor cells? Ann NY Acad Sci 510: 187–189. [Google Scholar]
- Cain WS, Murphy C (1980): Interaction between chemoreceptive modalities of odor and irritation. Nature 284: 255–257. [DOI] [PubMed] [Google Scholar]
- Casey KL, Minoshima S, Morrow TJ, Koeppe RA (1996): Comparison of human cerebral activation patterns during cutaneous, warmth, heat pain, and deep cold pain. J Neurophysiol 76: 571–581. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A (1994): A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773. [DOI] [PubMed] [Google Scholar]
- Craig AD, Reiman EM, Evans A, Bushnell MC (1996): Functional imaging of an illusion of pain. Nature 384: 258–260. [DOI] [PubMed] [Google Scholar]
- Daubenspeck JA (1981): Influence of small mechanical loads on variability of breathing pattern. Appl Physiol 125: 363–365. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WPE, Jurs PC, Orndorff MA, Snyder PJ, Lowy LD (1978): Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 20: 175–185. [DOI] [PubMed] [Google Scholar]
- Doty R (1991): Olfactory capacities in aging and Alzheimer disease. Psychophysical and anatomic considerations. Ann NY Acad Sci 640: 20–27. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Raichle ME (1995): Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 373: 249–252. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Balota DA, Tallal P, Petersen SE (1996): PET activation of posterior temporal regions during auditory word presentation and verb generation. Cereb Cortex 6: 1–10. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Raichle ME, Herscovitch P (1984): A noninvasive approach to quantitative functional brain mapping with H2(15)O and positron emission tomography. J Cereb Blood Flow Metab 4: 329–333. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleiter LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET rCBF study of selective attention to facetones and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T (2000): Assessment of intranasal trigeminal function. Int J Psychophysiol 36: 147–155. [DOI] [PubMed] [Google Scholar]
- Huttunen J, Kobal G, Kaukoranta E, Hari R (1986): Cortical responses to painful C = 2 stimulation of nasal mucosa; a magnetencephalographic study in man. Electroencephalogr Clin Neuropysiol 64: 347–349. [DOI] [PubMed] [Google Scholar]
- Inokushi A, Kimmelmann CP, Snow JB (1993): Convergence of olfactory and nasotrigeminal inputs and possible trigeminal contributions to olfactory responses in the rat thalamus. Eur Arch Oto Rhino Laryngol 249: 473–477. [DOI] [PubMed] [Google Scholar]
- James JEA, Daly M de B (1969): Nasal reflexes. Proc R Soc Med 62: 1287–1293. [PMC free article] [PubMed] [Google Scholar]
- Jones‐Gotman M, Zatorre RJ (1988): Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia 7: 111–120. [DOI] [PubMed] [Google Scholar]
- Karlsson P, Luscher M (1959): Pheromones: a new term for a class of biologically active substances. Nature 183: 55–56. [DOI] [PubMed] [Google Scholar]
- Kawashima R, O'Sullivan BT, Roland PE (1995): Positron‐emission tomography studies of cross‐modality inhibition in selective attentional tasks: closing the “mind's eye,” Proc Natl Acad Sci USA 92: 5969–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann B, Hummel C, Stefan H, Kobal G (1997): Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses 22: 493–502. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel C (1988): Cerebral chemosensory evoked potential elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol 71: 241–250. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Åkerman S, Roland PE (1998): Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage 8: 113–128. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Minoshima S, Morrow TJ, Casey KL (1998): Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Garcia‐Larrea L, Gregoire MC, Costes N, Convers P, Lavenne F, Mauguiere F, Michel D, Laurent B (1999): Hemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain 122: 1765–1780. [DOI] [PubMed] [Google Scholar]
- Powell TPS, Cowan W, Raisman G (1965): The central olfactory connections. J Anat 99: 791–813. [PMC free article] [PubMed] [Google Scholar]
- Rainvile P, Carrier B, Hofbauer RK, Buchnell MC, Duncan GH (1999): Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82: 159–171. [DOI] [PubMed] [Google Scholar]
- Roland PE, Graufelds CJ, Wahlin J, Ingelman L, Andersson M, Ledberg A, Pedersen J, Akerman S, Dabringhaus A, Zilles K (1994): Human brain atlas for high‐resolution functional and anatomical mapping. Hum Brain Mapping 1: 173–184. [DOI] [PubMed] [Google Scholar]
- Roland PE (1993) Brain activation. New York: John Wiley & Sons. [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R (2000): Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci 20: 7752–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Gulyás B, Berglund H (2000): Male pheromone activates anterior hypothalamus in female subjects. Neuroimage 5: S751. [Google Scholar]
- Savic I, Gulyás B, Larsson M, Roland P (2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyás B (2000): PET shows that odors are processed both ipsilaterally and contralaterally to the stimulated nostril. NeuroReport 11: 2861–2866. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Gulyas B, Roland P (2001): Smelling of odorous sex hormone‐like compounds causes sex differentiated hypothalamic activations in humans. Neuron 31: 661–668. [DOI] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K (1999): Observer‐independent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. Neuroimage 9: 165–177. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Raichle ME, Fiez JA, Miezin FM, Petersen SE (1997): Top‐down modulation of early sensory cortex. Cereb Cortex 7: 193–206. [DOI] [PubMed] [Google Scholar]
- Siarle SR (1971): Linear models. New York: John Wiley & Sons, Inc. [Google Scholar]
- Simmonds A, et al. (1997): fMRI during ‘pleasant’ odor stimulations: normative data. Neuroimage 5: S196. [Google Scholar]
- Small DM, Jones‐Gotman M, Zatorre RJ, Petrides M, Evans AC (1997): Flavor processing: more than the sum of its parts. NeuroReport 8: 3913–3917. [DOI] [PubMed] [Google Scholar]
- Sobel N, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrielli JD (1998): Sniffing and smelling: separate subsytems in the human olfactory cortex. Nature 392: 282–286. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover G, Sullivan E, Gabrieli JDE (2000): Time course of odorant‐induced activation in the human primary olfactory cortex. J Neurophysiol 83: 537–551. [DOI] [PubMed] [Google Scholar]
- Stone H (1969): Effect of ethmoidal nerve stimulation on olfactory bulbar electrical activity In: Pfaffmann C, editor. Olfaction and taste. New York: Rockefeller University Press; p. 216–220. [Google Scholar]
- Treede R‐D, Kensalo DR, Gracetonely RH, Jones AKP (1999): The cortial representation of pain. Pain 79: 105–111. [DOI] [PubMed] [Google Scholar]
- Tucker D (1971): Nonolfactory responses from the nasal cavity: Jacobson's organ and the trigeminal system In: Beidler LM, editor. Handbook of sensory physiology, Vol. IV Chemical senses. New York, Berlin, Heidelberg: Stringer; p. 151–181. [Google Scholar]
- Williams SCR, Yousem DM, Howard RJ, Andrew C, Allin TA, Cox TS, Geckle RJ, Suskind D, Simmonds A, Bullmore E, Brammer M, Doty RL (1997): fMRI during ‘unpleasant’ odor stimulation: normative data. Neuroimage 5: S197. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC (1992): Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16: 620–633. [DOI] [PubMed] [Google Scholar]
- Wysocki C, Dalton P, Brody MJ, Lawley H (1997): Acetone odor and irritation thresholds obtained from acetone‐exposed factory workers and from control (occupationally unexposed) subjects. Am Ind Hyg Assoc J 58: 704–712. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M (1997): Functional MR imaging during odor stimulation: preliminary data. Radiology 204: 833–838. [DOI] [PubMed] [Google Scholar]
- Zald D, Pardo J (1997): Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA 94: 4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M, Evans AC, Meyer E (1992): Functional localization of human olfactory cortex. Nature 360: 339–341. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M, Rouby C (2000): Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport 11: 2711–2716. [DOI] [PubMed] [Google Scholar]