Abstract
Many agrammatic aphasics have a specific syntactic comprehension deficit involving processing syntactic transformations. It has been proposed that this deficit is due to a dysfunction of Broca's area, an area that is thought to be critical for comprehension of complex transformed sentences. The goal of this study was to investigate the role of Broca's area in processing canonical and non‐canonical sentences in healthy subjects. The sentences were presented auditorily and were controlled for task difficulty. Subjects were asked to judge the grammaticality of the sentences while their brain activity was monitored using event‐related functional magnetic resonance imaging. Processing both kinds of sentences resulted in activation of language‐related brain regions. Comparison of non‐canonical and canonical sentences showed greater activation in bilateral temporal regions; a greater activation of Broca's area in processing antecedent‐gap relations was not found. Moreover, the posterior part of Broca's area was conjointly activated by both sentence conditions. Broca's area is thus involved in general syntactic processing as required by grammaticality judgments and does not seem to have a specific role in processing syntactic transformations. Hum. Brain Mapp. 22:74–83, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: agrammatism, aphasia, auditory, Broca's area, event‐related fMRI, grammaticality judgments, language, non‐canonical, syntactic traces, trace deletion hypothesis
INTRODUCTION
Lesions in Broca's area often result in agrammatic aphasia. Patients with lesions in this region were described originally as having a syntactic impairment in language production [Pick, 1898]; however, it has been shown that syntactic comprehension is disturbed as well [Caramazza and Zurif, 1976]. In contrast, semantic processing seems to be relatively unaffected in both production and comprehension. Because of the dissociation between syntax and semantics, agrammatism has been characterized as a central syntactic impairment [Berndt and Caramazza, 1980].
Contrary to the central syntactic deficit hypothesis, Linebarger et al. [1983] found that agrammatic subjects with severe syntactic comprehension deficits in sentence‐picture matching tasks show a nearly normal performance in grammaticality judgment tasks. The deficit is thus interpreted not to be in central syntax but rather specific for certain conditions. Sentence‐picture matching tasks require semantic as well as syntactic interpretation, hence semantic and syntactic processing compete for limited resources. In these tasks there is a tradeoff in favor of semantics in agrammatic patients [see also Hagoort et al., 2003]; however, semantic interpretation is not required in grammaticality judgment tasks and thus syntax can operate in isolation. There is evidence that syntactical analysis of syntactically unambiguous structures is relatively independent of semantic context or semantic violations [Hagoort, 2003], e.g., syntactic violations can be detected in semantically uninterpretable Jabberwocky sentences [Indefrey et al., 2001].
A recent neurolinguistic theory of agrammatic comprehension disorders, the trace deletion hypothesis (TDH), reintroduces the idea that syntactic processes in agrammatism are impaired at the representational level [Grodzinsky, 1986, 1995]. The TDH is based on Chomsky's “government and binding” theory [Chomsky, 1981]. Grammatical transformations involve movement of certain sentence constituents from their base (i.e., canonical) positions to other possible positions. Sentences with basic word order are called canonical whereas sentences with a transformed word order are called non‐canonical. The base position of the transformed argument is filled by a trace. For example:
-
1
It is likely that (Mary will win).
-
2
It seems that Mary i is likely (t i to win).
In the example, the noun phrase “Mary” is moved to the subject position of the embedded clause in (2). To mark its base position, a phonetically empty and abstract but syntactically effective trace (t) is left behind. The trace transmits the thematic role of the transformed argument via co‐indexation (i) (example adapted from Grodzinsky and Finkel, 1998). The representation of the trace is therefore necessary to comprehend sentences in which the canonical word order is changed (i.e., non‐canonical sentences). According to the TDH, the traces are deleted from the agrammatic representations. Agrammatic aphasics should therefore show comprehension deficits in non‐canonical sentences (e.g., passives, object relatives, object clefts) rather than in canonical sentences without transformations (actives, subject relatives, subject clefts). Because the TDH claims that the underlying deficit is representational, the dissociation between canonical and non‐canonical sentences is expected to become manifest in sentence‐picture matching tasks as well as in grammaticality judgment tasks. In line with this theory, Grodzinsky and Finkel [1998] found that agrammatic aphasic patients show significantly greater deficits in grammaticality judgments on non‐canonical sentences than in grammaticality judgments on canonical sentences. Results of lesion studies and experimental data suggest that the traces are represented in Broca's area [for review see Grodzinsky, 2000]. Furthermore, Grodzinsky hypothesized that Broca's region plays a specific role in processing non‐canonical sentences [Grodzinsky, 2000]; however, Grodzinsky's TDH has been highly criticized and rejected by others [e.g., Beretta and Munn, 1998; Berndt et al., 1996; Berndt and Caramazza, 1999; Caplan, 2001; Caramazza et al., 2001; Mauner et al., 1993; for review see peer commentaries on Grodzinsky, 2000].
Broca's area is part of a large neural network for language processing and is responsible for various language functions, such as phonological processing, semantic processing, and syntactic processing [e.g., Caplan et al., 2000; Heim et al., 2003; Muller et al., 2003; Poldrack et al., 1999, 2001; Price et al., 2003; for review see Gernsbacher and Kaschak, 2003]. So far, neuroimaging studies focusing on the difference between syntactic and semantic processes or on syntactic complexity found Broca's area (Brodmann area [BA] 44/45) to be active [e.g., Caplan et al., 1998, 1999, 2000; Dapretto and Bookheimer, 1999; Indefrey et al., 2001; Newman et al., 2003]. Only a few neuroimaging studies have focused on the comparison of processing non‐canonical and canonical sentences. Cooke et al. [2002] reported increased activation in left inferior frontal gyrus (IFG, BA 47) only during processing non‐canonical object cleft sentences with a long distance (seven words) between the moved element (antecedent) and its base position (gap), i.e., sentences with high working memory load. Correspondingly, these sentences require the most time to be understood. In sentences with short antecedent‐gap distances (three words), there is no specific activation in left IFG and they are processed faster. The sentences were presented visually in a word‐by‐word fashion. Results of this study could thus be interpreted as modulation of left IFG by an interaction of working memory and non‐canonical syntactic structures rather than by the syntactic structure itself. Bilateral temporal regions are also modulated by working memory because processing sentences with long antecedent‐gap distances resulted in greater activation in these areas irrespective of the syntactic structure. Similar to Cooke et al. [2002], Ben Shachar et al. [2003] found left inferior frontal and bilateral temporal activations when comparing auditorially presented Hebrew sentences with and without transformations. The authors attribute these activations to syntactic structure rather than to working memory demands. Behavioral data, however, were not reported. Our group could not find a greater involvement of Broca's area or adjacent regions in the processing of non‐canonical as compared to canonical sentences using visual presentation of whole sentences with short antecedent‐gap distances only (i.e., a task with a rather low working memory load) [Wartenburger et al., 2003]. In this task, the moved element could be maintained easily via the visual field, i.e., it was possible to switch between its transformed and base position visually without the necessity to keep the representation of the whole syntactic sentence structure in working memory.
The goal of the present study was to investigate the role of Broca's region in processing canonical and non‐canonical sentences. Because Cooke et al. [2002] and Ben Shachar et al. [2003] did not control for task difficulty and Wartenburger et al. [2003] did not ensure that moved elements had to be represented in working memory, it remains unclear how Broca's area is modulated by canonicity. We compared non‐canonical and canonical sentences with equal processing difficulty in terms of reaction time and accuracy. Because sentences were presented auditorily, the sentence structure and all elements of the sentence had to be cortically represented and maintained in working memory. Based on the assumptions of the TDH, we hypothesized greater activation of Broca's area during processing non‐canonical sentences as compared to canonical sentences.
SUBJECTS AND METHODS
Subjects
All subjects were native speakers of German and had no prior knowledge of the sentence material. Thirteen (seven male) healthy, right‐handed [Oldfield, 1971] young adults (mean age, 25.8 ± 4.9 years) participated. They gave written informed consent before investigation and were paid for participation. All experiments were carried out in compliance with the relevant laws and institutional guidelines and were approved by the ethics committee of the Charité Berlin.
Linguistic Material and Task
The stimulus material included two conditions: canonical sentences without transformations (CAN) and non‐canonical sentences involving transformations (non‐CAN). Both conditions contained sentences that were either correct (50%) or included a grammatical error (50%).
Non‐CAN condition
In the non‐CAN condition, movements of the noun phrase or a wh‐element (i.e., a question word beginning with wh) were used. In noun phrase movement (A movement), a noun phrase is moved from one argument position to another argument position. In wh‐movement (A′ movement), the wh‐phrase is moved from an argument position to a non‐argument position. Although both movement types are linguistically different [for an overview see Haegemann, 1991], both contain movements of phrasal constituents (compared to head movement). Within the framework of the TDH there is no difference expected between noun phrase and wh‐movement because patients suffering from agrammatic Broca's aphasia show comprehension deficits in movement of phrasal constituents [Grodzinsky, 1995; Grodzinsky and Finkel, 1998]. Incorrect sentences involved illicit crossing of an argument over another argument position (violation of the relativized minimality constraint [Rizzi, 1990]).
CAN condition
The grammatically correct CAN condition contained “easy‐to‐please” constructions and simple, active sentences. The incorrect sentences of the CAN condition were either derived by violating the theta criteria (i.e., an argument in the sentence is not assigned a semantic role) or by violating the subcategorization frame through selection of wrong case. One non‐canonical sentence type, namely auxiliary movement constructions, was removed from further data analysis because of unexpected erroneous grammaticality judgments. To match the total number of canonical and non‐canonical sentences, another canonical condition was excluded randomly (with incorrect sentences violating the subcategorization frame by selecting a wrong preposition). Examples for each type used are given in Table I.
Table I.
Examples of sentences
| Grammar | Non‐CAN | CAN |
|---|---|---|
| Correct | Wen pflegt die gütige Nichte im Altenheim [t]? [whom cares the kind niece at the nursing‐home] | Es ist leicht, den Kastanienbaum zu fällen. [it is easy the tree to chop] |
| Es scheint, die Formel ist leicht abzuleiten [t]. [it seems the formula is easy to‐deduce] | Der gefährliche Bär droht der braunen Katze. [the dangerous bear threatens the [dat] brown cat] | |
| Incorrect | Womit glaubst du, was der Monteur repariert [t]? [whereby thinks you what the mechanic repairs] | Sie ist schwierig, die Fensterscheibe zu reinigen. [she is difficult the pane to clean] |
| Der Computer scheint, es ist leicht aufzurüsten [t]. [the computer seems it is easy to upgrade] | Der gefährliche Bär droht die braune Katze. [the dangerous bear threatens the[acc] brown cat] |
All sentences were semantically plausible. They were matched across conditions for number of words (mean, 7 ± 0; Z = 0; P = 1.0), number of syllables (mean, 12.1 ± 1.1; Z = −0.5; P = 0.6), stimulus duration (mean, 3.8 ± 0.1 sec; Z = −0.7; P = 0.5) and frequency of nouns, verbs, and adjectives (low frequency; mean, 22.6 ± 16.2 per million words; Z = −0.7; P = 0.5) [Baayen et al., 1993]. The interstimulus interval was randomly jittered (mean stimulus onset asynchrony [SOA], 8.75 sec; min SOA, 6.27 sec; max SOA, 16.86 sec). All stimuli were spoken by a trained female speaker and were equal in loudness.
Before investigation, subjects were instructed to judge the grammaticality of the sentences intuitively, i.e., without relying on rules of grammar learned in school. The task was explained outside the scanner and the subjects were asked explicitly not to repair or adjust incorrect sentences. Inside the scanner they had to indicate their decision as quickly and correctly as possible by a left‐hand button press on a two‐button response box. The sentences were presented in an event‐related and pseudorandom manner using an magnetic resonance (MR)‐compatible headset (MR confon; Magdeburg, Germany). Depending on their level of alertness, subjects carried out between 3–5 runs (mean, 3.85 ± 0.69 runs; min, 3 runs; max, 5 runs). Each run consisted of 16 canonical (8 correct, 8 incorrect) and 16 non‐canonical (8 correct, 8 incorrect) sentences and 5 null‐trials that were presented randomly. The Experimental Run Time System software (ERTS 3.28; BeriSoft Cooperation, Frankfurt/M., Germany) was used for sentence presentation and recording of reaction times and accuracy.
Data Acquisition and Analysis
To compare the conditions regarding reaction times and accuracy of response, nonparametric Wilcoxon‐tests were used (P < 0.01, corrected for multiple comparisons).
Functional magnetic resonance imaging (fMRI) measurements were carried out on a 1.5T scanner (Siemens Magnetom Vision, Erlangen, Germany) with a standard head coil. Head movement was minimized using a vacuum pad. After the scout spin echo scan, structural 3D data sets were acquired using a T1‐weighted sagittal sequence (MPR; TR/TE 9.7/4 msec; flip angle 12 degrees; voxel size 1 mm). Subsequently, 17 4‐mm slices were obtained approximately parallel to the bicommissural plane (ac‐pc‐plane) using an echo‐planar sequence (TR/TE 2,000/40 msec; flip angle 90 degrees; field of view 256 mm; matrix 64 × 64; interslice gap 0.4 mm; ascending acquisition of images). One run consisted of 220 scans (mean 846.2 ± 151.5 scans per subject; min 660 scans; max 1,100 scans). To avoid a systematic bias in sampling over peristimulus time [Burock et al., 1998; Dale, 1999], scans were acquired in temporal asynchrony to the task (jittered stimulus presentation). Slices covered the whole brain with the exception of the most superior frontal and superior parietal lobe, inferior temporal pole, and cerebellum (most superior z about 30 and most inferior z about −36), thus covering both the frontal and temporal language regions.
Imaging data were analyzed using SPM2 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, University College London, UK). The first three functional volumes of each run were excluded to allow for magnetic saturation effects. Scans were realigned, slice‐time corrected, normalized, and spatially smoothed by a Gaussian kernel (full‐width half‐maximum [FWHM] = 8 × 8 × 8.8 mm) using standard SPM2 methods [Friston et al., 1995]. Autocorrelations were corrected and a high‐pass frequency filter (128 sec) was applied. Time series were modeled using event‐related regressors. Duration of each event was determined by the reaction time of the subject. The resulting time series were convolved with the hemodynamic response function and the first derivatives were included in the model.
Contrast images for each condition versus rest and for differences between the respective conditions were computed for each subject. To be able to generalize the observed effects to the population [Holmes and Friston, 1998; Friston et al., 1999a, b], the group effects were computed using these contrast images in a mixed effects model treating subjects as random (commonly referred to as random effects analysis). T‐statistic images were thresholded using clusters determined by a threshold of P < 0.001 and a corrected cluster significance threshold of P < 0.05 [Forman et al., 1995; Friston et al., 1994; Worsley et al., 1992]. Group analysis was carried out using one‐sample t‐tests to identify regions that showed greater activation in non‐CAN and CAN compared to rest, non‐CAN compared to CAN sentences, and vice versa. To determine the main effect of grammaticality and the interaction of grammaticality and canonicity, grammatically correct and incorrect sentences were compared. Finally, to test the brain regions that were conjointly active during CAN and non‐CAN sentences, a conjunction analysis was carried out (the statistical threshold was corrected for false discovery rate [P < 0.05, corrected]).
RESULTS
Behavioral Results
Behavioral data did not reveal significant differences in reaction times and accuracy of response between canonical and non‐canonical sentences, respectively (compare Fig. 1A,B). There were also no statistically significant differences between canonical and non‐canonical sentences when grammatically correct and incorrect sentences were compared separately (compare Fig. 1C,D). Task difficulty thus could be considered equal in all conditions.
Figure 1.
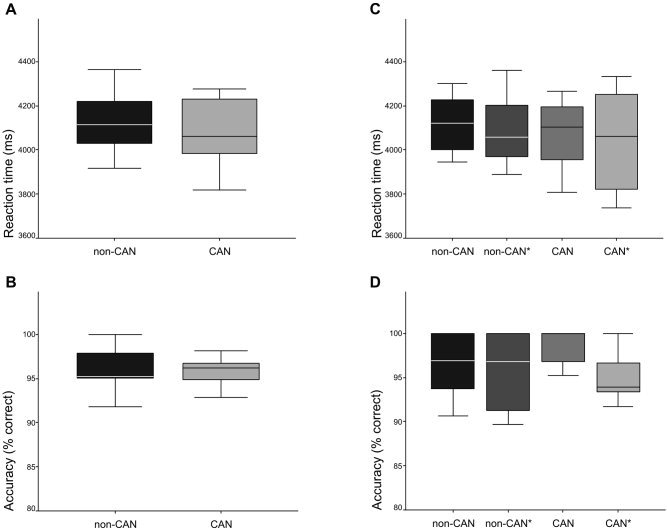
Behavioral data acquired during the fMRI experiment. Reaction time (A) and accuracy (B) of judging non‐canonical (non‐CAN) and canonical (CAN) sentences as shown by the combined analysis of grammatically correct and incorrect sentences. Reaction time (C) and accuracy (D) of judging non‐CAN and CAN sentences as shown by the separate analysis of grammatically correct and incorrect sentences (non‐CAN, non‐canonical correct; non‐CAN*, non‐canonical incorrect; CAN, canonical correct; CAN*, canonical incorrect). There were no significant differences between the conditions. Median, quartile, and data range are displayed.
Functional MRI Results
Sentences verus rest
Compared to activity during processing the null trials, both CAN and non‐CAN resulted in bilateral activation of temporal regions, the inferior and middle frontal (left > right) gyrus, midbrain, basal ganglia, and pre‐ and postcentral gyrus. Activations are shown in Figure 2A,B.
Figure 2.
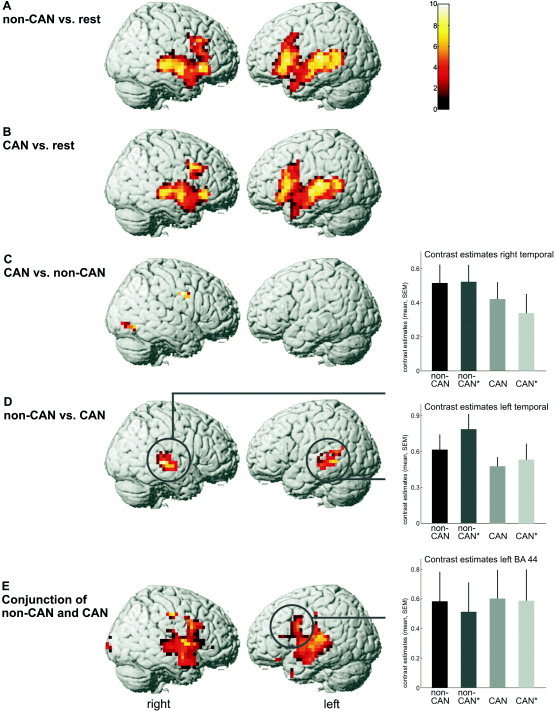
Comparison of non‐canonical (A) and canonical (B) sentences to rest. Processing both sentence conditions resulted in similar activation. C: Comparison of canonical and non‐canonical sentences. D: Comparison of non‐canonical and canonical sentences. Right: Parameter estimates of sentence conditions in right and left middle and superior temporal regions that showed significant greater activation during processing non‐canonical sentences as compared to canonical sentences. E: Results of the conjunction analysis. Right: Parameter estimates of sentence conditions in left BA 44 that showed conjoined activation during processing canonical and non‐canonical sentences. Results of group analysis (n = 13) superimposed on MNI template; non‐CAN, non‐canonical sentences; CAN, canonical sentences. Bar graphs: non‐CAN, non‐canonical correct; non‐CAN*, non‐canonical incorrect; CAN, canonical correct; CAN*, canonical incorrect.
Non‐CAN versus CAN
A significant main effect of canonicity (non‐CAN vs. CAN) was found in bilateral superior and middle temporal gyrus (compare Fig. 2D and Table II). Parameter estimates of each sentence condition were computed in activated temporal regions (see Fig. 2D). Comparing CAN to non‐CAN resulted in activation of the left parahippocampal/fusiform gyrus, right precentral gyrus, and right occipital cortex (compare Fig. 2C and Table II).
Table II.
Contrasting non‐canonical and canonical sentences and grammatically incorrect and correct sentences and vice versa
| Comparison | Region | Hemisphere | Brodmann area | Cluster size | t | x | y | z | |
|---|---|---|---|---|---|---|---|---|---|
| Main effect: canonicity | Non‐CAN vs. CAN | Superior/middle temporal gyrus | L | 21/22, 41/42 | 75 | 5.22 | −52 | −28 | −4 |
| Superior/middle temporal gyrus | R | 21/22, 41/42 | 92 | 7.05 | 52 | −28 | 0 | ||
| CAN vs. non‐CAN | Parahippocampal/fusiform gyrus | L | 20/36 | 31 | 6.99 | −32 | −36 | −26 | |
| Precentral gyrus | R | 6 | 17 | 6.38 | 56 | 0 | 35 | ||
| Occipital cortex, lingual gyrus | R | 18/19 | 34 | 9.70 | 28 | −76 | −9 | ||
| Main effect: grammaticality | Incorrect vs. correct | Inferior frontal gyrus | L | 44/45, insula | 60 | 6.51 | −60 | 16 | 18 |
| Insula, temporal pole | L | 47, insula, 21/38 | 30 | 5.58 | −44 | 20 | −13 | ||
| Correct vs. incorrect | Medial frontal gyrus | L/R | 10/11 | 16 | 5.93 | −4 | 44 | −9 | |
| Posterior cingulate gyrus/ | L/R | 30/23/29 | 58 | 8.26 | −4 | −60 | 13 | ||
| precuneus | L/R | 31/7 | 85 | 7.28 | 0 | −52 | 44 | ||
| Parietooccipital junction | L | 7/19 | 19 | 4.90 | −44 | −76 | 40 |
Contrasting non‐canonical and canonical sentences and grammatically incorrect and correct sentences and vice versa. There was no interaction of canonicity and grammaticality. For each contrast, the respective activated anatomic region, right or left (R, L) hemisphere, approximate Brodmann areas, cluster size, t values, and coordinates of the local maxima of significance within the Montreal Neurological Institute (MNI) coordinate system are given (P < 0.05 corrected).
Effect of grammaticality
A significant main effect of grammaticality (grammatically incorrect vs. correct sentences) was found in left inferior frontal gyrus, left insula, and left temporal pole. Comparing grammatically correct sentences to incorrect sentences resulted in activation of the bilateral medial frontal gyrus, bilateral posterior cingulate cortex/precuneus, and left parietooccipital junction (compare lower part of Table II). There was no interaction, however, of grammaticality and canonicity.
Conjunction analysis of non‐canonical and canonical sentences
The conjunction analysis of non‐canonical and canonical sentences resulted in bilateral activation of the inferior frontal gyrus, superior and middle temporal gyrus, putamen, midbrain and occipital regions, right pre‐ and postcentral gyrus, thalamus, and left cerebellum. These regions were conjointly activated in all sentence conditions (compare Fig. 2E and Table III). Parameter estimates of each sentence condition were computed in the activated left inferior frontal region (BA 44) (see Fig. 2E).
Table III.
Results of conjunction analysis showing areas that were active during processing canonical and non‐canonical sentences
| Region | Hemisphere | Brodmann area | Cluster size | t | x | y | z |
|---|---|---|---|---|---|---|---|
| Inferior frontal gyrus | L | 44 | 304 | 4.04 | −60 | 4 | 26 |
| Superior/middle temporal gyrus | L | 21/22, 41/42 | 5.95 | −64 | −20 | 9 | |
| Temporal pole | L | 21/38 | 6 | 2.54 | −44 | 12 | −40 |
| Inferior frontal gyrus | R | 44 | 286 | 4.58 | 60 | 8 | 26 |
| Superior/middle temporal gyrus | R | 21/22, 41/42 | 4.36 | 64 | −4 | −13 | |
| Pre‐ and postcentral gyrus | R | 4/6 | 21 | 4.5 | 52 | −16 | 44 |
| Putamen | L | 36 | 3.02 | −24 | 4 | 0 | |
| R | 25 | 3.42 | 28 | 4 | 0 | ||
| Thalamus | R | 10 | 3.33 | 16 | −20 | 0 | |
| Midbrain | L/R | 45 | 2.28 | 0 | −24 | −9 | |
| Occipital cortex | R | 18/17 | 13 | 3.4 | 24 | −104 | 0 |
| L/R | 16 | 2.43 | 4 | −92 | 0 | ||
| Cerebellum | L | 41 | 4.29 | −8 | −60 | −13 |
Results of conjunction analysis showing areas that were active during processing canonical and non‐canonical sentences. Respective activated anatomic region, right or left (R, L) hemisphere, approximate Brodmann areas, cluster size, t values, and coordinates of the local maxima of significance within the Montreal Neurological Institute (MNI) coordinate system are given (P < 0.05 corrected).
Taken together, there is considerable overlap of cortical activation during processing canonical and non‐canonical sentences. Frontal and temporal language regions were engaged in sentence processing as required by grammaticality judgments. A greater activation of Broca's area in processing non‐canonical sentences could not be found. Moreover, the more posterior part of Broca's area (BA 44) was conjointly activated by both non‐canonical and canonical sentences. In contrast, bilateral temporal regions showed greater activation in non‐canonical sentences as compared to canonical sentences, i.e., they were modulated by canonicity. Furthermore, the left inferior frontal gyrus, insula, and temporal pole showed greater activation in grammatically incorrect as compared to correct sentences, i.e., they were modulated by grammaticality. There was no interaction, however, of canonicity and grammaticality.
DISCUSSION
The goal of the present study was to investigate the role of Broca's region in processing canonical and non‐canonical sentences. We found that Broca's area was not more strongly engaged in processing non‐canonical sentences as compared to canonical sentences. The posterior part of Broca's area was activated by both non‐canonical and canonical sentences; however, there was a main effect of canonicity in bilateral temporal regions.
Based on the specific comprehension deficit of agrammatic Broca's aphasics, it was hypothesized that Broca's area has a specific and limited function for the computation of antecedent‐gap relations [Grodzinsky and Finkel, 1998; Grodzinsky, 2000]. In particular, we hypothesized a greater activation of Broca's area in processing auditorily presented non‐canonical sentences as compared to canonical sentences. Our data do not support these assumptions, however, because the posterior part of Broca's area (BA 44) was conjointly activated by both canonical and non‐canonical sentences. Processing canonical sentences as compared to non‐canonical sentences activated areas that are not part of the language‐processing network (left parahippocampal/fusiform gyrus, right occipital cortex, and right precentral cortex). These activations could be related to attention‐ and motor‐related processes, respectively.
There was a main effect of grammaticality in left inferior frontal regions, left insula, and the left temporal pole. This is in line with previous studies reporting a modulation of Broca's area and the left anterior temporal pole by grammaticality [e.g., Meyer et al., 2000; Newman et al., 2003].
We compared canonical and non‐canonical sentences and tried to minimize the confounding effect of task difficulty. Because there were no differences in reaction times and accuracy between the conditions, difficulty was comparable in all sentence conditions. Both theoretical considerations and experimental evidence indicate that processing of non‐canonical sentences requires more working memory [Gibson, 1998]. The observed main effect of canonicity in bilateral temporal regions thus could be attributed to greater working memory demands: the antecedent‐gap relation has to be represented and maintained in the working memory buffer. In line with this finding, several imaging studies showed greater activations during tasks of higher syntactic complexity/working memory load as compared to tasks with lower complexity/working memory demands in left hemispheric or bilateral temporal and inferior frontal regions [Caplan et al., 2002; Fiebach et al., 2001; Just et al., 1996; Stromswold et al., 1996; for review see Caplan and Waters, 1999]. The greater response in BA 41/42 during processing non‐canonical sentences could also be explained by an attentional top‐down modulation [Jancke et al., 2002; Sussman et al., 2002].
As mentioned above, two previous studies focused on the effect of canonicity. Similar to the present study, Cooke et al. [2002] found activation of bilateral temporal regions during processing sentences with increased working memory load, i.e., long antecedent‐gap distances, irrespective of canonicity as compared to a baseline task. Activation of left IFG (BA 47) reflects an interaction of working memory and sentence structure, as it is only involved in processing non‐canonical sentences with long antecedent‐gap distances [Cooke et al., 2002].
Greater activations of left inferior frontal and bilateral temporal regions during processing transformed sentences were also found in a recent fMRI study [Ben Shachar et al., 2003]. The authors suggest that these activations are based exclusively on the syntactic structure. Broca's area is not active during processing sentences without transformations, but rather shows a negative signal change. This contradicts various previous imaging studies showing Broca's region to be active in general syntactic processing (see above). Additionally, complexity was modulated by comparing sentences containing complex and simple verbs (determined by the number of arguments a verb takes). Processing sentences with and without transformations containing complex verbs did not result in greater left inferior frontal activation than processing sentences with and without transformations containing simple verbs. An effect of verb complexity was found in left posterior superior temporal regions only; however, as mentioned by Ben Shachar et al. [2003], both transformation and verb complexity can account for general sentence complexity. In the latter study, these two factors were not disentangled and behavioral data were not reported; therefore, the influence of task difficulty and its relation to verb complexity and transformation remain elusive.
In our previous study, sentence complexity was controlled by comparing sentences with similar processing complexity [Wartenburger et al., 2003]. Similar to the present study, there were no differences in reaction times and accuracy between sentences with and without movement of the noun phrase. The sentences contained short antecedent‐gap distances and were presented visually (whole‐sentence presentation for 4 sec, thereby allowing for visual maintenance of both the sentence structure and the sentences' elements). There were minimal working memory demands, and Broca's area or adjacent regions did not seem to play a special role in processing sentences with movement of the noun phrase compared to canonical sentences [Wartenburger et al., 2003]. In contrast, auditorily presented sentences have to be held in the phonological buffer, thereby increasing working memory load as compared to visually presented sentences. The moved element cannot be reactivated via eye movements but has to be reactivated from the internal phonological representation.
In conclusion, the greater activation of Broca's area in processing antecedent‐gap relations, as hypothesized based on the TDH, could not be found. In other words, Broca's area is involved in general syntactic processing as required by grammaticality judgments and does not seem to have a specific role in processing syntactic transformations.
Acknowledgements
We thank D. Ruff for proofreading the article.
REFERENCES
- Baayen RH, Piepenbrock R, Rijn H (1993): The CELEX Lexical Database(v. 1)[CD‐ROM]. Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania. [Google Scholar]
- Ben Shachar M, Hendler T, Kahn I, Ben Bashat D, Grodzinsky Y (2003): The neural reality of syntactic transformations. Psychol Sci 14: 433–440. [DOI] [PubMed] [Google Scholar]
- Beretta A, Munn A (1998): Double‐agents and trace‐deletion in agrammatism. Brain Lang 65: 404–421. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Caramazza A (1980): Semantic operations deficits in sentence comprehension. Psychol Res 41: 169–177. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Caramazza A (1999): How “regular” is sentence comprehension in Broca's aphasia? It depends on how you select the patients. Brain Lang 67: 242–247. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN (1996): Comprehension of reversible sentences in “agrammatism”: a meta‐analysis. Cognition 58: 289–308. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM (1998): Randomized event‐related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport 9: 3735–3739. [DOI] [PubMed] [Google Scholar]
- Caplan D (2001): The measurement of chance performance in aphasia, with specific reference to the comprehension of semantically reversible passive sentences: a note on issues raised by Caramazza, Capitani, Rey, and Berndt [2001] and Drai, Grodzinsky, and Zurif [2001]. Brain Lang 76: 193–201. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G (1998): Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci 10: 541–552. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G (1999): PET studies of syntactic processing with auditory sentence presentation. Neuroimage 9: 343–351. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A (2000): Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Hum Brain Mapp 9: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Vijayan S, Kuperberg G, West C, Waters G, Greve D, Dale AM (2002): Vascular responses to syntactic processing: event‐related fMRI study of relative clauses. Hum Brain Mapp 15: 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters GS (1999): Verbal working memory and sentence comprehension. Behav Brain Sci 22: 77–94. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Capitani E, Rey A, Berndt RS (2001): Agrammatic Broca's aphasia is not associated with a single pattern of comprehension performance. Brain Lang 76: 158–184. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB (1976): Dissociation of algorithmic and heuristic processes in language comprehension: evidence from aphasia. Brain Lang 3: 572–582. [DOI] [PubMed] [Google Scholar]
- Chomsky N (1981): Lectures on Government and Binding. Dordrecht: Foris. [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M (2002): Neural basis for sentence comprehension: grammatical and short‐term memory components. Hum Brain Mapp 15: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY (1999): Form and content: dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Friederici AD (2001): Syntactic working memory and the establishment of filler‐gap dependencies: insights from ERPs and fMRI. J Psycholinguist Res 30: 321–338. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston K, Worsley KJ, Frackowiak RS, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1: 214–220. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes AP, Worsley K, Poline JB, Frith C, Frackowiak RS (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ (1999a): Multisubject fMRI studies and conjunction analyses. Neuroimage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ (1999b): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Kaschak MP (2003): Neuroimaging studies of language production and comprehension. Annu Rev Psychol 54: 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson E (1998): Linguistic complexity: locality of syntactic dependencies. Cognition 68: 1–76. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y (1986): Language deficits and the theory of syntax. Brain Lang 27: 135–159. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y (1995): A restrictive theory of agrammatic comprehension. Brain Lang 50: 27–51. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y (2000): The neurology of syntax: language use without Broca's area. Behav Brain Sci 23: 1–21. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Finkel L (1998): The neurology of empty categories aphasics' failure to detect ungrammaticality. J Cogn Neurosci 10: 281–292. [DOI] [PubMed] [Google Scholar]
- Haegemann L (1991): An introduction to government and binding theory. Oxford: Blackwell. [Google Scholar]
- Hagoort P (2003): Interplay between syntax and semantics during sentence comprehension: ERP effects of combining syntactic and semantic violations. J Cogn Neurosci 15: 883–899. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Wassenaar M, Brown C (2003): Real‐time semantic compensation in patients with agrammatic comprehension: electrophysiological evidence for multiple‐route plasticity. Proc Natl Acad Sci USA 100: 4340–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD (2003): Distributed cortical networks for syntax processing: Broca's area as the common denominator. Brain Lang 85: 402–408. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalizability, random effects, and population inference. Neuroimage 7: S754. [Google Scholar]
- Indefrey P, Hagoort P, Herzog H, Seitz RJ, Brown CM (2001): Syntactic processing in left prefrontal cortex is independent of lexical meaning. Neuroimage 14: 546–555. [DOI] [PubMed] [Google Scholar]
- Jancke L, Wustenberg T, Schulze K, Heinze HJ (2002): Asymmetric hemodynamic responses of the human auditory cortex to monaural and binaural stimulation. Hear Res 170: 166–178. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR (1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Linebarger MC, Schwartz MF, Saffran EM (1983): Sensitivity to grammatical structure in so‐called agrammatic aphasics. Cognition 13: 361–392. [DOI] [PubMed] [Google Scholar]
- Mauner G, Fromkin VA, Cornell TL (1993): Comprehension and acceptability judgments in agrammatism: disruptions in the syntax of referential dependency. Brain Lang 45: 340–370. [DOI] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY (2000): Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Brain Res Cogn Brain Res 9: 19–33. [DOI] [PubMed] [Google Scholar]
- Muller RA, Kleinhans N, Courchesne E (2003): Linguistic theory and neuroimaging evidence: an fMRI study of Broca's area in lexical semantics. Neuropsychologia 41: 1199–1207. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA, Keller TA, Roth J, Carpenter PA (2003): Differential effects of syntactic and semantic processing on the subregions of Broca's area. Brain Res Cogn Brain Res 16: 297–307. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pick A (1898): Über Agrammatismus als Folge cerebraler Herderkrankungen; ein Beitrag zur Lehre vom Verhältnis der Worttaubheit In: Beiträge zur Pathologie und pathologischen Anatomie des Zentralnervensystems. Berlin: Karger; p 123–133. [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JD (2001): Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. J Cogn Neurosci 13: 687–697. [DOI] [PubMed] [Google Scholar]
- Price CJ, Winterburn D, Giraud AL, Moore CJ, Noppeney U (2003): Cortical localisation of the visual and auditory word form areas: a reconsideration of the evidence. Brain Lang 86: 272–286. [DOI] [PubMed] [Google Scholar]
- Rizzi L (1990. Relativized minimality. Cambridge, MA: MIT Press. [Google Scholar]
- Stromswold K, Caplan D, Alpert N, Rauch S (1996): Localization of syntactic comprehension by positron emission tomography. Brain Lang 52: 452–473. [DOI] [PubMed] [Google Scholar]
- Sussman E, Winkler I, Huotilainen M, Ritter W, Naatanen R (2002): Top‐down effects can modify the initially stimulus‐driven auditory organization. Brain Res Cogn Brain Res 13: 393–405. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Burchert F, De Bleser R, Villringer A (2003): Grammaticality judgments on sentences with and without movement of phrasal constituents—an event‐related fMRI study. J Neurolinguistics 16: 301–314. [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]


