Abstract
We performed an fMRI one‐back recognition study aimed at distinguishing the semantic versus perceptual aspects of how objects and their written forms are processed. There were three types of visually presented items: pictures (schematic drawings of objects); words identifying these objects; and a mixed condition in which pictures were interleaved with words. A semantic decision about object identity was required when pictures were interleaved with words. This condition, contrasted with the other two, invoked a larger signal in multiple areas, including frontal cortex, bilateral occipitotemporal cortex, and the right middle temporal gyrus. We propose that the left occipitotemporal and right temporal activations are indicative of the neural substrate mediating picture–word conversions, whereas the frontal activations reflect the coordinating functions of the central executive. Hum. Brain Mapping 16:168–175, 2002. Published 2002 Wiley‐Liss, Inc.
Keywords: brain, human, fMRI, working memory, visual processing, central executive, prefrontal cortex, one‐back task, semantics
INTRODUCTION
The way in which the pictorial and written forms of an object are represented and processed in the brain has been, and continues to be, a topic of intense research. Based on Stroop‐like inhibition effects during naming, reading and categorization tasks, Glaser and Glaser [1989] proposed an influential cognitive model for picture‐word processing consisting of a semantic memory module and a lexicon. They posited that words had privileged access to the lexicon, whereas object features had privileged access to the semantic network. In this fMRI study, we looked for possible BOLD correlates of the above‐mentioned cognitive structures, applying a one‐back working memory task with pictures and visually presented words as stimuli.
The one‐back task is a typical working memory paradigm in which the subject has to determine whether each stimulus in a series matches or does not match the previous one. According to current working memory models [Hartley and Speer, 2000], the one‐back task includes encoding, holding an item in memory, comparison with the next item, response selection, upgrading the memory buffer, but theoretically does not require explicit stimulus recognition itself if the stimuli are in same modality. Thus, if the visual one‐back task is applied only to pictures, or only to words, it is conceivable that the task could be performed only at the perceptual level in which stimuli are compared as shapes or strings. Alternatively, implicit semantic processing may be unavoidable, although a challenging experimental condition, such as fast presentation rate, may reduce its likelihood. If the pictures of objects are interleaved with their names, and the individual must make a decision about object identity, forced recovery of semantics is necessary.
To investigate the neurofunctional correlates of these issues, we performed a one‐back fMRI study that asked the following questions. First, is perceptual processing sufficient to make a binary judgment about the match/nonmatch of an item with a previous item within a single modality? What additional resources are recruited when pictures and words are interleaved and a semantic decision about object identity is required? We hypothesize that when an explicit semantic decision is needed, strong interactions between semantic memory and lexicon, as represented in the Glaser and Glaser model [Glaser and Glaser, 1989], will either invoke facilitation or necessitate conflict resolution. In terms of the working memory model of Baddeley [1996], these functions utilize the central executive in addition to the working memory buffers that would be involved in the comparisons of just pictures or just words.
MATERIALS AND METHODS
Fourteen normal native English speakers (19–44 years old, six females and eight males) participated in this study. All subjects were right‐handed according to the Oldfield Handedness Inventory [Oldfield, 1971]. They were medication free, had normal physical and neurological examinations, and were financially compensated for participation. The NIDCD/NINDS Institutional Review Board approved the study, and all the normal volunteers gave written consent after the nature and possible consequences of the study were explained.
A one‐back task was performed in separate blocks for three experimental conditions: 1) pictures—intermixed black and white schematic drawings of living and nonliving common objects; 2) words—4–7 letter words identifying these objects; and 3) mixed—pictures interleaved with words (Fig. 1). The stimuli, which subtended a visual field of 6°, were projected onto the rear of a screen placed at the subject's feet. Each stimulus was presented for 100 msec with an interstimulus interval of 1,200 msec. These three stimulation conditions, presented in 30 sec blocks, were each repeated six times in a counterbalanced order. Before each block, a visual warning stimulus was given informing the subject as to which type of comparison was required for that block. Each subject was asked to press a button held with the right hand if the present stimulus was identical to the previous one for pictures (P) and words (W) or referred to the same object for the mixed (M) condition. When the stimuli differed, the subject was instructed to press a button held with the left hand. For all three conditions the number of matches was equal to the number of nonmatches. For the mixed condition when matches occurred, in half these cases the word preceded the picture. Before scanning, training was provided to the subjects to familiarize them with the task conditions. Generation and presentation of stimuli employed the PC‐based Psytask 2.0 software package (Institute of the Human Brain, Russian Academy of Sciences, St. Petersburg, Russia). A CMU button box (New Micros, Inc., Dallas, TX) was used for synchronizing stimuli presentation with the MRI scanner, and for recording the subjects' responses. Each subject completed a questionnaire after the scan session in which the strategies he/she employed for each condition were described.
Figure 1.
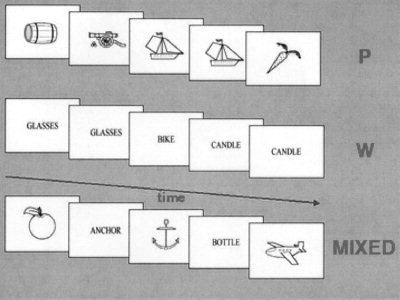
Schematic of the experimental design for the one‐back tasks for pictures of objects (P), written words corresponding to objects (W), and interleaved pictures and words (MIXED). Each image was presented for 100 msec with an interstimulus interval of 1,200 msec. At the beginning of each new block, a pre‐set color stimulus warned the subject that a new comparison would be required: two red circles for W, two blue rectangles for P, and a blue rectangle together with a red circle for MIXED.
A General Electric Signa 1.5 Tesla scanner (Milwaukee, WI) with a standard quadrature head coil was utilized for imaging. Functional scanning was performed with a fast spiral gradient echo scanning sequence (TE = 24 msec, flip angle = 85°) collecting 36 axial slices covering the whole brain in 2 sec with a 4 mm slice thickness and with a voxel resolution 3.75 × 3.75 mm in‐plane [Yang et al., 1998]. The first seven images were discarded to allow tissue magnetization to reach equilibrium. To represent each subject's individual anatomy, high‐resolution whole‐brain images were acquired with a T1‐weighted fast 3D FSPGR with a 24 cm field of view, 124 axial slices (1.1 mm thick), and a 256 × 256 reconstructed image matrix. Head movements were controlled by use of a Styrofoam‐filled bag, hardened around the subject's head by vacuuming off air.
We utilized the MEDx 3.28 UNIX‐based software package (Sensor Systems, Sterling, VA) for image processing and statistical analysis. Preprocessing for each subject included motion correction, smoothing with a Gaussian kernel (FWHM 10 mm), intensity normalization, and removal of any linear trends. Multiple regression with a temporal autocorrelation correction was performed for each subject's data, with a hemodynamic lag estimated at 4 sec incorporated into the appropriate regressors, resulting in a t‐statistic map for each contrast of interest. These t‐maps were then transformed into z‐maps. Contrasts formed included those when P was compared to W (P > W), W compared to P (W > P), and the mixed condition compared to P and W (M > P + W).
For each subject, an average motion‐corrected functional image was registered with his/her anatomical image with a rigid‐body transformation using AIR, v. 3.08 [Woods et al., 1992]. After this, a piecewise‐linear warping of the T1‐weighted anatomical image into a canonical space [Talairach and Tournoux, 1988] was computed utilizing the anterior and posterior commissures as landmarks. The rigid‐body transformation and the nonlinear warping then were applied to the z‐maps. The z‐map for any given contrast was averaged over the cohort and normalized by the square root of the cohort size (assuming the independence and normality of individual subject maps, resulting composite cohort maps are normally distributed). The cohort images were thresholded at z = 5.79, corresponding to a two‐tailed level of significance P < 0.001 after a Bonferroni correction for multiple comparisons. Thus, all results we report correspond to group results.
RESULTS
The P vs. W contrast resulted in significant activations in occipitotemporal cortex bilaterally (Fig. 2, Table I). A large cluster of activation in the right fusiform gyrus (volume: 9.4 cm3; mean Talairach coordinates: 33, −56, −17) stretched along the basal surface of occipitotemporal cortex (Brodmann area (BA) 19, 37). The center of the corresponding activation in the left hemisphere had nearly the same coordinates, but was three times smaller (volume: 3.1 cm3; −27, −60, −25). Other clusters in right middle occipital gyrus (BA 18/19), left thalamus and left cerebellum had much smaller spatial extents and lower z‐scores.
Figure 2.
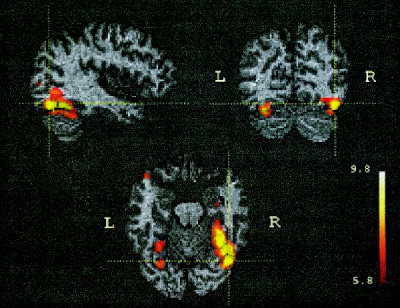
Cohort z‐maps showing significant activations for blocks containing picture–picture comparisons contrasted against blocks with word–word comparisons. Shown are orthogonal slices (sagittal − y = −66 mm; coronal − x = 40 mm; horizontal − z = −18 mm) marked by crosshair; these correspond to highest z‐value for this contrast (Table I). The cohort z‐map was thresholded at 5.79 corresponding to a two‐tailed level of significance P < 0.001 after a Bonferroni correction. The numbers next to the color scale represent the z‐scores at the scale's upper and lower end. Activation map was fused in Talairach space with the anatomical MRI of one of the subjects.
Table 1.
Pictures versus words*
| Anatomical location | Left hemisphere | Right hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | BA | z‐score | x | y | z | BA | z‐score | |
| Occipital‐temporal cortex | ||||||||||
| Fusiform gyrus | −30 | −72 | −22 | 18/19 | 8.6 | 40 | −66 | −18 | 19 | 9.8 |
| −28 | −54 | −22 | 19/37 | 7.6 | 30 | −46 | −18 | 37 | 9.0 | |
| Middle occipital gyrus | 40 | −64 | −6 | 19 | 7.0 | |||||
| 30 | −86 | 6 | 18/19 | 6.5 | ||||||
| Thalamus | −18 | −18 | 10 | 6.0 | ||||||
| Cerebellum | −20 | −64 | −36 | 6.5 | ||||||
z‐scores and associated Talairach coordinates are tabulated for local maxima identified by contrasting blocks containing picture to picture comparisons with blocks containing word to word comparisons. BA, Brodmann area.
The W vs. P contrast produced three focal clusters of activations in left temporal cortex and one in the right temporal lobe (Fig. 3, Table II). One of the activations found in left temporal cortex was in the fundus of the superior temporal sulcus (STS). A second was in the middle temporal gyrus (MTG), and the third was located in the Sylvian fissure (BA 42/40). There also was a significantly large BOLD signal in the right STS for W relative to P.
Figure 3.
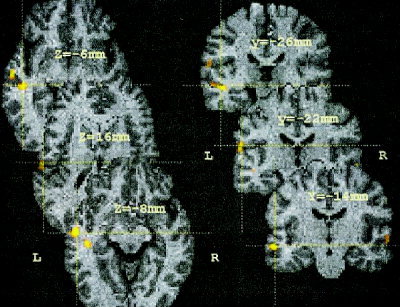
Cohort z‐maps showing significant activations for blocks containing word–word comparisons contrasted against blocks with picture–picture comparisons. Shown are axial (left) and coronal slices (right). Crosshairs correspond to three of the local maxima detected in left temporal‐parietal cortex (Table II). Other symbols are the same as in Figure 2.
Table II.
Words versus pictures*
| Anatomical location | Left hemisphere | Right hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | BA | z‐score | x | y | z | BA | z‐score | |
| Temporal cortex | ||||||||||
| MTG | −54 | −14 | −8 | 21 | 7.5 | |||||
| STS fundus | −44 | −26 | −6 | 21/22 | 6.4 | |||||
| STS | 58 | −16 | 0 | 21/22 | 6.2 | |||||
| Temporal‐parietal cortex | ||||||||||
| Sylvian fissure | −56 | −22 | 16 | 42/40 | 6.4 | |||||
z‐scores and associated Talairach coordinates are tabulated for local maxima identified by contrasting blocks containing word to word comparisons with blocks containing picture to picture comparisons. MTG, middle temporal gyrus; STS, superior temporal sulcus. BA, Brodmann area.
The mixed condition contrasted to the combination of two other conditions (P and W) also activated areas within occipitotemporal cortex bilaterally (Fig. 4, Table III). The right hemisphere cluster size had a spatial extent of 0.08 cm3, whereas that in the left hemisphere had an extent of 1.7 cm3; this latter cluster extended into the cerebellum. The areas with the greatest spatial extent activated during this contrast included right MTG (BA 21; 2.3 cm3), which stretched into the STS, and a large cluster (3.3 cm3) along the left precentral sulcus (BA 4/6) extending rostroventrally into BA 8/9. This latter cluster was a unique area of activation not found in the other contrasts. Smaller foci with lower z‐scores, also unique to this contrast, were observed in the right precentral sulcus and in the medial frontal gyrus within the intrahemispheric fissure.
Figure 4.
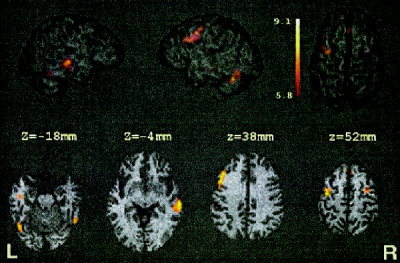
Cohort z‐maps showing significant activations for blocks containing mixed comparisons contrasted against the P and W blocks. Activations are rendered onto a 3D view of the brain. Axial slices are provided for many of the local maxima (Table III). The left side of the axial slices corresponds to the left side of the brain. Other symbols are as in Figure 2.
Table III.
Mixed (pictures interleaved with words) versus pictures and words*
| Anatomical location | Left hemisphere | Right hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | BA | z‐score | x | y | z | BA | z‐score | |
| Frontal cortex | ||||||||||
| Precentral sulcus | −36 | −6 | 54 | 4/6 | 8.4 | 28 | −4 | 56 | 6 | 6.5 |
| MFG | −40 | 22 | 38 | 8/9 | 8.2 | |||||
| MedFG | 0 | 30 | 52 | 8 | 6.9 | |||||
| Occipital‐temporal cortex | ||||||||||
| Fusiform gyrus | −44 | −66 | −18 | 19/37 | 8.0 | 48 | −56 | −22 | 37 | 7.2 |
| MTG | 58 | −26 | −4 | 21 | 8.4 | |||||
| Cerebellum | −46 | −56 | −28 | 9.1 | ||||||
z‐scores and associated Talairach coordinates are tabulated for local maxima identified by contrasting blocks containing the mixed comparisons with blocks containing only picture to picture and only word to word comparisons. MFG, middle frontal gyrus; MedFG, medial frontal gyrus; MTG, middle temporal gyrus. BA, Brodmann area.
DISCUSSION
In this fMRI study of picture‐word comparisons, the two contrasts comparing the P and W conditions to one another revealed activity patterns that are in good agreement with the existing literature. Two large areas in right and left occipitotemporal cortex (BA 19, 37) were more active during the one‐back picture task than during the one‐back word task. This result is in accord with the representation of objects in the human ventral visual pathway [Haxby et al., 1991; Tranel et al., 1997; Vandenberghe et al., 1996], confirmed by recent fMRI experiments [Gauthier et al., 1999; Ishai et al., 1999; Martin et al., 2000]. In our study, the size of the occipitotemporal activation was three times greater in the right than in the left hemisphere. Studies in the EEG/ERP domain using congruent paradigms have revealed a specific visual memory electric potential with a strong positive current density source in right temporal cortex with a latency of about 240 msec that dramatically increased its amplitude in nonmatch situations [Begleiter et al., 1993]. This is in agreement with the right hemisphere dominance for the P vs. W contrast revealed in the current study. Furthermore, a number of imaging investigations have demonstrated right greater than left hemisphere activation in ventral visual cortex for objects that, in the design of the studies, were not explicitly named [Beason‐Held et al., 1998; Gauthier et al., 1999; Haxby et al., 1995; Ishai et al., 2000]. According to our subjects' exit questionnaires, the one‐back P condition was the easiest to do because a direct comparison of the sequentially presented pictures was possible without explicit identification of each object, suggesting that the one‐back picture task could be performed primarily at the perceptual level. Although some implicit or explicit semantic processing cannot be ruled out, our finding does indicate that the P condition did not require a greater level of semantic processing than did the W condition.
The second comparison contrasted the match/nonmatch condition for words vs. that for pictures. The subjects' implied in the exit questionnaires that the one‐back word task could be performed by just comparing the actual letter string with the previous one, without converting the word into its meaning. Although this is a possible strategy, our results indicate that it is unlikely; three of the four foci where activation for W was greater than for P were located in left temporal cortex, demonstrating that, at minimum, implicit word processing occurred, a result consistent with the findings of Price et al. [1996]. This finding also supports a number of cognitive models [Glaser and Glaser, 1989; Levelt, 1999] that posit that words are processed more automatically than pictures and have privileged access to the lexicon. The locations of left hemisphere foci within STS and MTG are in temporal lobe areas that have been shown by others to be activated for word and word‐like stimuli [Price et al., 1996; Rumsey et al., 1997]. Along with the BA 42/40 locus in the auditory portion of the left Sylvian fissure, these results indicate that phonological processing was engaged during the word condition to a greater degree that during the picture condition.
An interesting negative result of this contrast (W > P) is that there was no activation in Broca's area (BA 44/45). Smith and Jonides [1999], among others, have suggested that this area is responsible for target rehearsal in the working memory framework of Baddeley [1996]. Moreover, we failed to see any preferential activation in Wernicke's area, or activation in inferior parietal cortex, the latter suggested as the phonological store [Paulesu et al., 1993] for verbal working memory. One possibility for lack of these activations is that the one‐back tasks for both pictures and words utilized these areas equally. Another possibility is that, because of the relatively short ISI (1.2 sec), there is little requirement for phonological rehearsal, obviating the need for extensive involvement of Broca's area; Baddeley [1996] has suggested that a memory trace could survive without rehearsal for 2–3 sec. As for the lack of preferential engagement of Wernicke's area, because words can be matched as letter strings, extensive semantic processing may be unnecessary, although the activations in MTG/STS mentioned above indicate the elements of the lexicon are being accessed.
When pictures were interleaved with words (mixed), each individual was obliged to relate the identity of an object represented pictorially to the identity of an object represented lexically, thus necessitating a decision at the semantic level. The subjects considered this condition the most difficult. Contrasting the mixed condition to the combination of P and W, the two largest clusters of activation were observed along the left precentral sulcus, extending from BA 4/6 rostroventrally to BA 8/9, and in right MTG/STS. Additional smaller clusters, but also unique for this contrast, were identified within the right precentral sulcus and in medial frontal cortex (BA 8).
Recent studies attempting to determine the neuroanatomical substrates for the components of Baddeley's working memory model [Baddeley, 1996] have implicated dorsolateral prefrontal cortex as playing a key role in central executive functioning [Fletcher and Henson, 2001; Postle and D'Esposito, 2000]. In the mixed condition, the crossmodal object identity decision includes numerous functions attributed to the central executive, including the coordination of picture and word recognition, requisite retrieval of semantic information from long‐term memory, and monitoring of conflicts when word and picture do not match. The extensive activation of left dorsolateral prefrontal cortex (BA8/9) that we found likely reflects the increased load for the central executive in the mixed condition compared to that in W or P. The left hemisphere preference for the hypothesized central executive BOLD correlates stands in accord with verbal working memory studies [Fletcher and Henson, 2001; Smith and Jonides, 1999] and with the recent topographical model for the neuroanatomical organization of working memory proposed by Postle and D'Esposito [2000] that suggested that maintenance of objects and verbal stimuli takes place in left ventral prefrontal cortex, whereas manipulation of information in working memory leads to activation in more dorsal prefrontal areas. Moreover, there exists an extensive neuropsychological literature demonstrating that frontal lesions often lead to disturbances in executive function [Luria, 1973; Shallice, 1988; Stuss and Benson, 1984]. In particular, a recent lesion case study confirmed that central executive functions suffer dramatically after damage to the left frontal lobe [Allain et al., 2001].
The medial frontal activation found inside the interhemispheric fissure, along with the bilateral clusters found in the dorsal precentral sulcus, may reflect central executive processes corresponding to response competition and inhibition when picture and word did not match, which was the case 50% of the time. Because match responses are more prepotent than nonmatch ones [Begleiter et al., 1993], the nonmatch situation invokes the need for the central executive. An additional load on the central executive could occur because of Stroop‐like inhibition at the semantic level between nonmatching pictures and words [Glaser and Glaser, 1989] in the mixed compared to the separate P and W conditions. A similar pattern of activations in dorsal BA 6/8 was reported by Menon et al. [2001] for a Go/NoGo response inhibition task.
The second largest cluster of activation comparing Mixed to the other conditions was observed in right MTG/STS/STG. An activation locus with similar coordinates was described by Owen [1998] for a non‐spatial one‐back experiment for abstract visual patterns. The meta‐analysis of Indefrey and Levelt [2000] indicates that this area is frequently activated during silent word generation. In patients with semantic dementia, those with greater right than left temporal lobe atrophy often demonstrate a comparable decline in both naming and comprehension, whereas those with a greater left than right temporal lobe atrophy show a more pronounced impairment in naming than in semantic comprehension [Murre et al., 2001]. In the context of the current study, relating pictures and words requires a functional linkage, according to the Glaser and Glaser [1989] model, between the semantic memory module and the lexicon. In this model, pictures of objects and their names should be processed differently; words have privileged access to the lexicon, whereas pictures have privileged access to the semantic network. Because words are processed more automatically than pictures [Greenham et al., 2000], relating pictures and words would necessitate greater activity in the semantic module than in the lexicon, leading to more right than left hemisphere activity. Thus, the large right hemisphere MTG cluster we observed may reflect activity associated with conversion processes between the pictorial and lexical forms of the object's semantic representation. Moreover, many subjects said that they used a “convert to word” strategy for the picture–word comparison, which also could increase the BOLD signal in right MTG/STS/STG during the comparison of a letter string held in working memory with a newly presented one. An argument in favor of this interpretation is that this area was also activated when the W condition was contrasted to the P condition, although with smaller spatial extent.
There also were activations in bilateral occipitotemporal cortex, dominant in spatial extent in the left hemisphere, when Mixed was contrasted with the combination of the other two conditions. The right hemisphere cluster was essentially in the same location as found when P was contrasted with W, although much reduced in spatial extent. The large left hemisphere cluster in fusiform gyrus we found had a location similar to that found in studies of object naming [e.g., Moore and Price, 1999]. This cluster also could represent visual imagery if the alternative strategy of converting a word to picture is employed. Activation of left extrastriate cortex was observed by Ishai et al. [2000] when subjects switched to visual imagery from visual perception. Moreover, similar to our findings, during the imagery task, these authors also observed left cerebellar activation with coordinates close to the current study.
CONCLUSIONS
During the one‐back tasks for pictures of objects and their names, there was a right hemisphere dominance within the occipital‐temporal visual pathway when pictures were compared to words. Conversely, when words were contrasted with pictures, we observed temporal foci of activation with a strong left hemisphere predominance, which may reflect activity of elements of the lexicon. A semantic decision about object identity was required when pictures were interleaved with words. This condition, contrasted with the combination of other two, invoked a larger BOLD signal in multiple areas within frontal and temporal cortices. We suggest that the left occipitotemporal and right temporal activations reflect part of the neural substrate mediating picture–word conversions, whereas the frontal activations are indicative of the coordinating functions of the central executive.
Acknowledgements
We thank J. Duyn for his fast spiral sequence and V. Ponomarev from the Institute of the Human Brain, Russian Academy of Sciences, St. Petersburg, Russia, for adjusting his Psytask 2.0 to fMRI experiments. We also are grateful to Drs. R. B. Friedman and A. Ishai for their comments on a preliminary version of this article. This research was supported by the NIDCD Intramural Research Program.
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Allain P, Etcharry‐Bouyx F, Le Gall D (2001): A case study of selective impairment of the central executive component of working memory after a focal frontal lobe damage. Brain Cogn 45: 21–43. [DOI] [PubMed] [Google Scholar]
- Baddeley A (1996): The fractionation of working memory. Proc Natl Acad Sci USA 93: 13468–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason‐Held LL, Purpura KP, Van Meter JW, Azari NP, Mangot DJ, Optican LM, Mentis MJ, Alexander GE, Grady CL, Horwitz B, Rapoport SI, Schapiro MB (1998): PET reveals occipitotemporal pathway activation during elementary form perception in humans. Vis Neurosci 15: 503–510. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Wang W (1993): A neurophysiologic correlate of visual short‐term memory in humans. Electroencephalogr Clin Neurophysiol 87: 46–53. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA (2001): Frontal lobes and human memory: insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC (1999): Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci 2: 568–573. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO (1989): Context effects in Stroop‐like word and picture processing. J Exp Psychol Gen 118: 13–42. [DOI] [PubMed] [Google Scholar]
- Greenham SL, Stelmack RM, Campbell KB (2000): Effects of attention and semantic relation on event‐related potentials in a picture–word naming task. Biol Psychol 55: 79–104. [DOI] [PubMed] [Google Scholar]
- Hartley AA, Speer NK (2000): Locating and fractionating working memory using functional neuroimaging: storage, maintenance, and executive functions. Microsc Res Tech 51: 45–53. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI (1991): Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA 88: 1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Rapoport SI, Grady CL (1995): Hemispheric differences in neural systems for face working memory: a PET‐rCBF study. Hum Brain Mapp 3: 68–82. [Google Scholar]
- Indefrey P, Levelt WJM (2000): The neural correlates of language production In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; p 845–865. [Google Scholar]
- Ishai A, Ungerleider LG, Haxby JV (2000): Distributed neural systems for the generation of visual images. Neuron 28: 979–990. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV (1999): Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA 96: 9379–9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJM (1999): Models of word production. Trends Cognit Sci 3: 223–232. [DOI] [PubMed] [Google Scholar]
- Luria AR (1973): The working brain. New York: Basic Books. [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV (2000): Category specificity and the brain: the sensory/motor model of semantic representations In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; p 1023–1036. [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL (2001): Error‐related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp 12: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Price CJ (1999): Three distinct ventral occipitotemporal regions for reading and object naming. NeuroImage 10: 181–192. [DOI] [PubMed] [Google Scholar]
- Murre JMJ, Graham KS, Hodges JR (2001): Semantic dementia: relevance to connectionist models of long‐term memory. Brain 124: 647–675. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM (1998): Memory: dissociating multiple memory processes. Curr Biol 8: R850–R852. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ (1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Postle BR, D'Esposito M (2000): Evaluating models of the topographical organization of working memory function in frontal cortex with event‐related fMRI. Psychobiology 28: 132–145. [Google Scholar]
- Price CJ, Wise RJ, Frackowiak RS (1996): Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex 6: 62–70. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P (1997): Phonologic and orthographic components of word recognition: a PET‐rCBF study. Brain 120: 739–759. [DOI] [PubMed] [Google Scholar]
- Shallice T (1988): From neuropsychology to mental structure. New York: Cambridge University Press. [Google Scholar]
- Smith EE, Jonides J (1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF (1984): Neuropsychological studies of the frontal lobes. Psychol Bull 95: 3–28. [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc. [Google Scholar]
- Tranel D, Damasio H, Damasio AR (1997): A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35: 1319–1328. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RS (1996): Functional anatomy of a common semantic system for words and pictures. Nature 383: 254–256. [DOI] [PubMed] [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC (1992): Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 16: 620–633. [DOI] [PubMed] [Google Scholar]
- Yang Y, Glover GH, van Gelderen P, Patel AC, Mattay VS, Frank JA, Duyn JH (1998): A comparison of fast MR scan techniques for cerebral activation studies at 1.5 T. Magn Reson Med 39: 61–67. [DOI] [PubMed] [Google Scholar]


