Abstract
The adult somatosensory system has shown reorganizational abilities at cortical and subcortical levels after peripheral nerve lesions. In the present study the effects of carpal tunnel syndrome (CTS) are investigated as reflected on the somatotopy of the primary cortical hand representation. Position and intensity of cortical sources activated by the separate electrical stimulation of median nerve and Digits 1, 3, and 5 of both affected and non‐affected hands are evaluated by magnetoencephalographic (MEG) technique. Correlation of MEG results with patient‐, physician‐ and neurophysiological‐oriented evaluations of CTS was carried out. Patients showed changes in cortical hand somatotopy in strict relationship to self‐referred assessment of symptoms and hand disability in daily activities, including: 1) a more extended representation of the affected hand when paresthesias prevailed; and 2) a more restricted representation due to lateral shift of the little finger was observed when pain symptoms dominated the clinical picture. Contralateral to the side of CTS, the cortical sources activated by Digit 5‐stimulation appeared significantly enhanced with respect to contralateral ones from non‐affected hand. When comparing the amplitude of peripheral sensory nerve action potentials of median and ulnar nerves to that of cortical responses (i.e., ECD strengths of M20 and M30 components after stimulation of Digits 3 and 5), a significant selective amplification of M30 with respect to M20 and sensory nerve action potential (SNAP) appeared during Digit 3 stimulation compared to that observed for Digit 5. This has been interpreted as a central magnification mechanism in brain responsiveness, possibly revealing a safety factor enabling sensory perception despite the small peripheral signal due to nerve trunk dysfunction. Hum. Brain Mapping 17:28–36, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: carpal tunnel syndrome, somatosensory evoked fields, cerebral plasticity, adaptive reorganizations, aberrant reorganizations
INTRODUCTION
The adult human brain undergoes plastic changes after alterations of the sensory flow from peripheral receptors and nerve fibers. Reorganizations have been observed at different levels of the adult somatosensory system both in animal models [Bronchti et al., 1999; Florence and Kaas 1995; Garraghty et al., 1994; Jenkins et al., 1990; Recanzone et al., 1992; Zhang and Rowe 1997] and in human beings [Buchner et al., 1996; Elbert et al., 1998; Knecht et al., 1996; Rossi et al., 1998; Rossini et al., 1994, 2000; Tinazzi et al., 1997, 1998]. Different reactions have been observed as a consequence of hyper‐stimulation [Jenkins et al., 1990; Recanzone et al., 1992] as deprivation of information coming from a specific receptive system [Florence and Kaas 1995; Garraghty et al., 1994; Rossi et al., 1998; Rossini et al., 1994]. Different timing for reorganizations at cortical areas [Tinazzi et al., 1997] with respect to subcortical relays [Tinazzi et al., 1998] have been shown.
Persistent paraesthesias or pain involving the hand [Birbaumer et al., 1997; Davis et al., 1996; Flor et al., 1995, 1997, 1998; Knecht et al., 1998], continuous training of finger movements in musicians [Elbert et al., 1998], and chronic antalgic limb positions can induce long‐lasting modifications in the primary sensorimotor cortical areas and in the connected subcortical and spinal relays [Davis et al., 1996; Tinazzi et al., 1998]. Digital overuse had been found previously to produce a similar phenomenon in monkeys [Byl et al., 1997].
Carpal tunnel syndrome (CTS) is caused by the compression of median nerve sensorimotor fibers at the wrist and is the most frequent type of nerve entrapment (10% lifetime risk to develop this pathology) [American Academy of Neurology et al., 1993a]. CTS is characterized by focal chronic peripheral sensory (and motor) nerve impairment. Possible modifications of the sensory cortical finger and hand representations in CTS have not been studied previously. The high occurrence of CTS and its wide range of clinical levels of involvement offers a natural model for studying continuous exchange between peripheral inflow and central representations of distinct body districts. Possible presence of adaptive and aberrant cortical reorganization could be related to self‐referred symptoms and neurophysiological assessment. Aberrant plastic CNS reorganization may partially explain the divergence in some cases between patient‐oriented (no change or deterioration) and neurophysiological post‐operative findings of peripheral nerve functionality (improvement) [Mondelli et al., 2000].
Magnetoencephalography (MEG) has been successfully utilized to characterize hand cortical sensorimotor representation when investigating physiological and anatomo‐functional properties of this brain area [Baumgartner et al., 1991; Hari et al., 1984; Kristeva‐Feige et al., 1994; Tecchio et al., 1997] and in relation to anatomical structures [Hund et al., 1997; Kawamura et al., 1996; Mauguiere et al., 1997; Morioka et al., 1998]. Plastic phenomena in cortical sensory hand areas have been monitored by MEG, both in short‐term cortical reorganization after deprivation of physiological sensory input [Kristeva‐Feige et al., 1996; Rossini et al., 1994] and in long‐term plastic changes after upper limb amputation [Flor et al., 1995; Knecht et al., 1996], focal dystonia [Elbert et al., 1998] and unilateral hemispheric stroke [Rossini et al., 1998; Rossini et al., 2001].
This study undertakes a comparative analysis of hand cortical sensory representation and neurophysiological median nerve function, examining the clinical findings from both the affected and non‐affected hand. To evaluate the central effects of the median nerve entrapment at the carpal tunnel, a sample of patients with idiopathic, unilateral, and chronic CTS were studied using MEG.
MATERIALS AND METHODS
The experimental design was approved by the Hospital Ethics Committee. Fourteen patients (3 males, 11 females; mean age: 43.5 years, range 26–63) affected by idiopathic CTS, determined to be clinically and neurophysiologically unilateral, were enrolled in the study. Informed consent was obtained.
Multiperspective CTS evaluation
To thoroughly assess the clinical status, a multiperspective protocol validated recently [Padua et al., 1998a] was used.
Neurophysiological evaluation
Electrodiagnostic studies were carried out according to international guidelines [American Academy of Neurology et al., 1993b; American Association of Electrodiagnostic Medicine et al., 1993], including: a) median sensory nerve conduction velocity (SNCV) in first digit‐wrist (1M) and third digit‐wrist segments (3M); and b) median distal motor latency from wrist to thenar eminence (DML). When these tests yielded normal results the disto‐proximal ratio (third digit–palm SNCV/palm–wrist SNCV) [Padua et al., 1996] was also identified. The severity of neurophysiological CTS impairment was classified according to six categories [American Academy of Neurology et al., 1993a; American Association of Electrodiagnostic Medicine et al., 1993b]: extreme, absence of motor and sensory responses; severe, absence of sensory response and abnormal distal motor latency; moderate, abnormal digit‐wrist sensory nerve conduction velocity and abnormal distal motor latency; mild, abnormal digit‐wrist sensory nerve conduction velocity and normal distal motor latency; minimal, abnormal segmental‐comparative tests only; and negative, normal findings in all tests.
Physician‐oriented evaluation
An historical‐objective scale (Hi‐Ob) of CTS was used for clinical examination. This scale is a modified version of a scale reported previously [Giannini et al., 1991] and includes a score (Hi‐Ob) obtained by clinical history and objective findings and a patient‐oriented measurement (i.e., presence or absence of pain as a dichotomous categorical score obtained from the patient with a forced‐choice answer; ‘yes’ or ‘no’, PAIN). Standard clinical tests for CTS (i.e., Phalen test) [Phalen, 1968] were always carried out.
Patient‐oriented evaluation
A validated patient‐oriented measurement was used (Boston Carpal Tunnel Questionnaire ‘BCTQ’; Italian version) [Levine et al., 1993; Padua et al., 1998b] that evaluates the symptoms assessed with an 11‐item scale. The functional status was assessed with an eight‐item scale. Statistical analysis was based on the two main scores (symptoms and functional) and on the single items.
To evaluate the main feature of the CTS symptomatology, and in particular to assess whether the symptoms were characterized principally by either pain or paraesthesia, BCTQ symptoms items were divided into two subgroups evaluating paraesthesia (SYMPTPAR) or pain (SYMPTPAIN) only. The SYMPTPAR/SYMPTPAIN ratio was also calculated.
Magnetoencephalographic recordings
The cortical representation of left and right sensory hands in the contralateral hemisphere was studied by recording somatosensory evoked fields (SEFs, Fig. 1 fs) after separate electrical stimulation of median nerve at wrist, and Digits 1, 3, and 5 (ring electrodes on 1st and 2nd phalanges) of right and left hands (0.2 msec electric pulses, cathode proximal, with an interstimulus interval of 641 msec). Peak latencies as well as spatial coordinates and strength characteristics of the activated cortical sources were evaluated for absolute values and interhemispheric differences. The extension of the cortical region related to contralateral hand sensory representation was evaluated as the Euclidean distance between Digits 1 and 5 cortical sources (hand, Fig. 1); an enlargement/reduction was recognized if the hand parameter was larger/smaller than normal. Activated source intensities both in absolute and interhemispheric asymmetries (s_asy) were also evaluated and compared to normative values.
Figure 1.
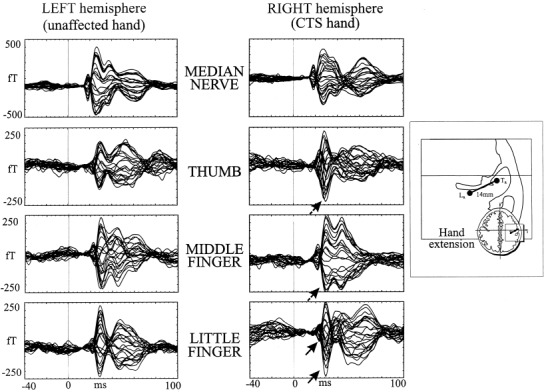
Left side: SEFs recorded from contralateral hemisphere after separate stimulation of median nerve and the three fingers of left and right hands in an emblematic subject affected by left CTS. All recording channels are superimposed on the time interval of 100 msec after the stimulus and 40 msec pre‐stimulus. On the hemisphere contralateral to the CTS hand, note in digit 1 and digit 3 the selective amplification of M30 with respect to M20 component (dashed arrows), and the amplification of both components for the Digit 5 (solid arrows). Right side: Representation of the hand parameter, calculated as Euclidean distance between Digit 1 (TR) and Digit 5 ECD (LR) of each hand.
Stimuli intensity for median nerve induced a painless thumb opposition in 13 patients. Sensory threshold was employed in one patient with extreme CTS. Finger stimuli was settled at twice the subjective sensory threshold. Measurements were carried out using the 28‐channel MEG system utilized inside a magnetically‐shielded room (Vacuumschmelze GMBH) [Tecchio et al., 1997] and positioned on the hemisphere contralateral to the stimulated side [Dudley, 1987]. The exact position, with respect to the subject head, was identified by using six firmly taped coils whose 3D‐positions were digitized (Polhemus Isotrak) at the beginning of the recording session with respect to four anatomical landmarks (nasion, two preauricular points, and vertex). The recording system was centered on the approximate central sulcus scalp projection, to best appreciate the brain responses with a post‐stimulus latency around 20 and 30 msec (M20 and M30). Responses are generated in the contralateral hemisphere by tangentially oriented dipoles within the primary sensorimotor cortex [Allison et al., 1991; Hari et al., 1983; Kaukoranta et al., 1986; Mauguiere et al., 1997].
Approximately 300 artefact‐free trials were acquired (0.48–250 Hz bandwidth, 1 kHz of sampling rate) and averaged for each stimulation type. The amplitude of SEFs recorded by each channel was calculated with respect to a baseline level chosen as the mean value of the 5–15 msec post‐stimulus epoch. Localization of neural sources of the two initial components was obtained by a moving equivalent current dipole (ECD) model in an homogeneously conducting sphere matching the subject head in the region of interest. Results were accepted only if the explained variance was >90%.
To evaluate the relationship between brain cortical and peripheral nerve sensory responses, ratios between M20 ECD strength and the amplitude of sensory nerve action potentials (SNAPs) recorded at wrist during separate stimulation of Digits 3 and 5 were calculated (=M20/SNAP ratio). In the attempt to evaluate selective amplification mechanisms at cortical level for individual finger representations supported by different peripheral nerve innervations, the ratios between M30 and M20 ECD strength for Digit 3 (median‐innervated) and Digit 5 (ulnar innervated) stimulation were calculated (= M30/M20 ratio). Digits 3 and the 5 were chosen because of their separate nerve supply, the former by the median nerve (selectively impaired in carpal tunnel) and the latter by the ulnar nerve, spared in CTS.
Normative data for absolute values and for interhemispheric differences of the sensory cortical representation parameters were gathered in 20 healthy volunteers (9 males, 11 females, age range 29–60 years) [Tecchio et al., 1997]. Abnormality limits for parameters examined in the present work are cited respectively (see Results). Statistical non‐parametric tests were used and analysis of correlation was made by Spearman correlation r s‐test.
RESULTS
According to neurophysiological classification, the studied volunteers included three minimal examples, seven mild examples, two moderate examples, one severe example, and one extreme case of CTS. Mean neurophysiological, clinical, and patient‐oriented values averaged across subjects are shown in Table I. Normative values are summarized in Table I.
Table I.
Neurophysiological, clinical and patient‐oriented values averaged across subjects†
| CTS cases (SD) | Normal value | Z | |
|---|---|---|---|
| Neurophysiological assessment | |||
| Radial SNCV 1 digit‐wrist, m/s | 53.7 (5.3) | ≥ 41 m/s | 0.36 |
| Radial SNAP 1 digit‐wrist, μV | 13.3 (5.5) | > 5 μV | −0.03 |
| Median SNCV 1 digit‐wrist, m/s | 38.0 (6.8) | ≥ 42 m/s | −3.34 |
| Median SNAP 1 digit‐wrist, μV | 13.8 (9.2) | > 5 μV | −0.84 |
| Median SNCV 3 digit‐wrist, m/s | 42.7 (4.9) | ≥ 44 m/s | −3.38 |
| Median SNAP 3 digit‐wrist, μV | 15.2 (5.1) | > 6 μV | −0.33 |
| Median distal motor latency, msec | 3.5 (1.2) | ≤ 3.9 msec | 0.71 |
| Median CMAP wrist‐tenar, mV | 10.1 (5.6) | > 3 mV | 0.12 |
| Clinical assessment | |||
| Hi‐Ob mean score | 2.6 (0.8) | ||
| PAIN | 64.3% | ||
| Phalen test | 78.6% | ||
| Patient‐oriented evaluation | |||
| BCTQ symptom score | 2.9 (1.1) | ||
| BCTQ hand function score | 2.4 (1.2) |
Normative values are indicated in the third column for neurophysiological parameters, while for clinical and patient‐oriented parameters, healthy subjects are characterized by 0 value, i.e. absence of symptoms. Average Z‐scores for neurophysiological parameters are reported in the last column (normative one‐tail value at 5% = 1.645). Parameters out of normative range indicated in bold. SNCV, sensory nerve conduction velocity; SNAP, sensory nerve action potential; CMAP, compound motion action potential; HiOb, historical‐objective scale; PAIN, pain scale; BCTQ, Boston Carpal Tunnel Questionnaire.
Cortical sources activated by the stimulation of the individual digits have been successfully localized (exv > 90%) in seven of 14 patients for the M20 component (explained variance = exv 94 ± 3%), and in all cases for M30 (exv 95 ± 2%). Both components were always localized successfully during median nerve stimulation (exv 97 ± 2% and 98 ± 1% respectively for M20 and M30). For statistical analysis, the hand parameter was based on the M30 component. The hand parameter was observed to be abnormally enlarged with respect to normative range ([6,22] mm) [Tecchio et al., 1997] in two cases. In two cases (Fig. 2), it was restricted only in the hemisphere contralateral to the hand affected by CTS. Sensory cortical representation of the non‐affected hand was always within normal limits.
Figure 2.
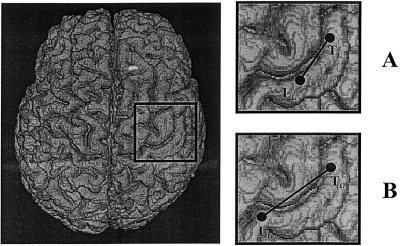
Two paradigmatic cases of patients affected by left CTS, with reduced (A) and enlarged (B) sensory hand extension. Distances between digit 1 (TL) and digit 5 ECD (LL) are shown on a brain model with real reconstruction of the cortical surface for representational purposes. Note the medial shift of the little finger ECD in (A).
The absolute strength of the localized ECDs for the CTS hand was slightly, but not significantly, increased for Digit 5 with respect to Digits 1 and 3, and with respect to non‐affected hand and normative values (Table II). When strength interhemispheric differences were considered, a significant increase in Digit 5 source in CTS patients was found with respect to the homologous value for the non‐affected hand (Table II).
Table II.
ECD strengths (n Axm) for the two components (M20 and M30) following each of the four stimulated districts†
| M20 | M30 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Med. n. | I | III | V | Med. n. | I | III | V | s_asy I | s_asy III | s_asy V | |
| Patients CTS hand | 12 ± 5 | 5 ± 2 | 5 ± 2 | 8 ± 3 | 26 ± 10 | 14 ± 6 | 11 ± 4 | 14 ± 5 | 0.2 ± 0.6 | −0.1 ± 0.7 | 0.62 ± 1.5a |
| Patients healthy hand | 12 ± 4 | 6 ± 3 | 5 ± 3 | 7 ± 2 | 33 ± 12 | 12 ± 4 | 13 ± 6 | 10 ± 6 | P = 0.17 | P > 0.5 | P = 0.049 |
| Control | 15 ± 5 | 6 ± 3 | 6 ± 4 | 6 ± 5 | 33 ± 15 | 16 ± 9 | 12 ± 6 | 11 ± 4 | 0.03 ± 0.6 | 0.09 ± 0.7 | 0.14 ± 0.6 |
Values are mean ± S.D. across subjects.
ECDs strengths are separately described for CTS and healthy hands. In the last three columns the interhemispheric asymmetries are reported for the three fingers (s_asy = log(s_CTS/s_healthy)).
Significant increase of digit 5 strength in the CTS with respect to the healthy hand representations, as compared with values in controls.
M20/SNAP ratio displayed similar values for median and ulnar nerves when observed in patients with recordable M20 for Digits 3 and 5. The M30/M20 ratio, on the contrary, was increased significantly in favor of the median nerve (2.8 vs. 2.2 for ulnar, Wilcoxon matched‐pairs test, P = 0.027). Moreover, in the only patient with severe median nerve entrapment (in which median nerve SNAPs and CMAPs were absent) SEFs were still recordable after separate stimulation of each finger.
The only significant correlation between MEG and nerve neurophysiological findings was that sensory hand cortical extension related positively to the amplitude of the median sensory nerve conduction velocity in the Digit 3‐wrist segment (P < 0.05; ρ = −0.56). In terms of the clinical picture, a significant inverse correlation (P < 0.05; ρ = −0.59) was observed between occurrence of positive Phalen test and affected hand.
In patient‐oriented scores, the hand was: 1) inversely related to occurrence of PAIN (P < 0.05; ρ = −0.54) (patients with greater than normal hand sensory cortical extension experienced less pain than patients with smaller than normal hand sensory extension); and 2) not related to either SYMPT or FUNCT.
In the CTS patients, Digit 5 ECDs were observed to shift in the direction of the Digit 1 ECD in a correlated manner with respect to PAIN symptoms (Fig. 3a; R 2 = 0.65, P = 0.001). The CTS Digit 1 ECD did not shift significantly in any direction (Fig. 3a; R 2 = 0.097, P > 0.3). A significant inverse relation was observed between PAIN symptoms and hand (Fig. 3b; R 2 = 0.39, P = 0.017). Patients with smaller hand sensory cortical extension experienced more pain than patients with greater hand sensory extension.
Figure 3.
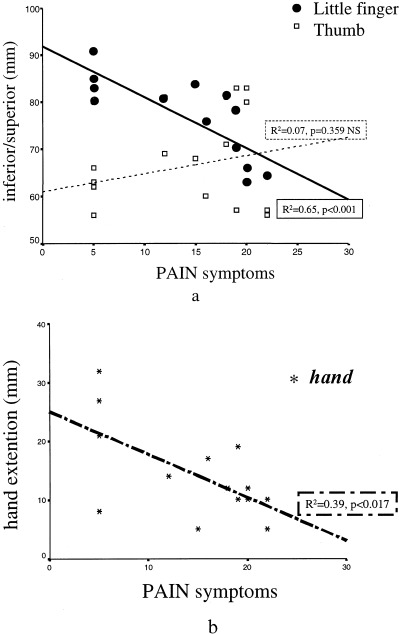
A: Individual digit 5 (filled circles) and digit 1 (open squares) ECD positions (the inferior/superior component) with respect to the parameter evaluating the pain symptoms. B: The hand parameter, evaluating the extension of the cortical representation of the CTS hand in the contralateral hemisphere, with respect to the same pain symptoms parameter.
For the functional items of BCTQ, an inverse significant relationship was observed with hand. Patients with larger than normal hand sensory cortical extension reported less impairment in daily activities than patients with restricted hand sensory cortical extension. Moreover, the two patients with maximal restriction of the hand sensory extension (5 mm) had severe impairment in daily activities and striking pain‐related symptoms. Conversely, the two patients with maximally enlarged sensory cortical extension (37 and 32 mm) had no or minimal impairment in daily activities (one patient referred no impairment in all functional items despite continuous paresthesias).
A significant positive correlation (P < 0.02, r s = 0.4) was found between the SYMPTPAR/SYMPTPAIN ratio and the extension of the hand sensory cortical extension. Patients who suffered exclusively from numbness and tingling symptoms, with minor or no pain, demonstrated larger than normal sensory CTS hand cortical extension. It is noteworthy that in all such patients symptoms were linked with the subjective inability to identify the exact hand districts involved; more specifically, all patients reporting numbness and tingling symptoms stated that the whole hand was involved.
DISCUSSION
MEG recordings have shown that modifications of the somatotopic organization of the primary sensory cortex occur shortly after peripheral deafferentation, the deprived cortex being progressively activated by input from skin regions near the deafferented zone [Rossini et al., 1994]. CTS is due to the entrapment of the median nerve at the wrist and is considered to be the most common chronic condition in which abnormal sensory flow from the hand and fingers is dispatched toward the central nervous system (CNS). CTS is characterized by several different clinical pictures ranging from hypoesthesias of the median‐innervated fingers, to tingling paresthesias or pain from the same fingers. Experimental data in animals and humans with peripheral nerve lesions and finger syndactyly or amputation (sensory deprivation) or, conversely, affected by sensory overstimulation of fingers or muscle‐tendon‐joint inflammation, have provided abundant evidence of a progressive reshaping of the connected sensorimotor system somatotopy throughout the different spinal and subcortical relays, up to primary sensory cortices (hypo‐stimulation [Bronchti et al., 1999; Florence and Kaas, 1995; Garraghty et al., 1994; Knecht et al., 1996; Rossi et al., 1998; Rossini et al., 1994; Tinazzi et al., 1997, 1998; Zhang and Rowe, 1997] and hyper‐stimulation [Buchner et al., 1996; Byl et al., 1997; Elbert et al., 1995; Jenkins et al., 1990; Recanzone et al., 1992].
The present MEG findings suggest that in patients with unilateral CTS, changes of affected hand cortical representation may occur in the contralateral hemisphere. Indeed, patients showed opposing types of cortical reorganizations, characterized by either reduction or enlargement of the CTS hand sensory cortical extension; such changes were strictly related to self‐referred assessment of symptoms and hand disability in daily activities. The enlargement of the hand representation was, in fact, observed in CTS cases characterized by continuous paresthesias. These symptoms were observed in patients who were not able to identify the involved fingers; rather, the subjects specifically attributed symptoms to the whole hand. Conversely, patients who were able to identify involvement of the first three fingers always presented hypoesthesias. Enlargement of the cortical hand extension correlated with the clinical evidence of persistent flow of paresthesias as well as the inability to correctly identify the involved hand districts. This could be ascribed to the unbalancing of the normal excitatory/inhibitory modulation of the neuronal firing, at the level of primary sensory cortex (SI), where individual finger sensory discrimination takes place. Neurons in SI normally activated by adjacent body parts (i.e., Digits 4 and 5) receive only a small amount of sensory inflow from the median nerve district; nonetheless, they could reach firing threshold via the fringe of signals impinging upon them from the hyper‐stimulated neurons of the median nerve affected by CTS. This cortical reorganization does not occur in those clinical cases dominated by pain symptoms with no paresthesias.
The spatial shift of CTS Digit 5 cortical source in the direction of the thumb generator is indicative of the tendency of the normally afferent hand districts to invade progressively the cortical hand region that is deafferented partially (i.e., the one controlled by the compressed median nerve). The CTS Digit 5 ECD shift significantly correlating to the pain symptoms is in line with previous findings [Elbert et al., 1997; Flor et al., 1995] indicating that chronic pain in a body district is associated with aberrant reorganization of cortical somatotopy of the body areas adjacent to those affected (lip invading hand area in the case of amputees with phantom limb syndrome [Flor et al., 1995]).
We hypothesize that cortical aberrant reorganizations may provide a physiological explanation of those cases involving CTS patients experiencing persistent symptomatology despite successful surgical treatment [Idler et al., 1996].
Continuous pain from a body district interferes with normal sensory perception, possibly by ‘gating’ the sensory volley to spinal, subcortical, and cortical relays. This may induce a progressive decrease of the area of representation of the affected body part (i.e., CTS hand). The paresthesia‐induced enlargement of the SI representation may reflect two, not mutually exclusive, mechanisms: 1) cortical amplification of reduced amount of specific tactile information from hand receptors due to CTS; and 2) use‐dependent activation of functionally silent synaptic connections due to the continuous sensory bombardment from the site of nerve irritation. The selective amplification observed in the central response to Digit 3 with respect to Digit 5 stimulation when comparing M20 and M30 components (M30/M20 ratio) favors this first hypothesis. This central magnification differently affects M20 and M30 and may be a safety factor with a double physiological meaning. Because of the reduction of afferent information, amplification at central level occurs more than in normal conditions. This phenomenon may be directed selectively to those sensory inputs that are relevant for sensorimotor integration (i.e., M30) and, in turn, for correct on‐line sensory monitoring of movements. Such amplification properties [Eisen et al., 1982; Ferrington et al., 1986] are particularly evident in the patient with absence of peripheral median nerve sensory (and motor) action potentials and still detectable cortical responses. The properties have also been described in patients with severe, chronic, and peripheral nerve deficits [Desmedt and Noel, 1973].
CONCLUSION
The present data identifies CTS as a natural model enabling characterization of the continuous functional peripheral‐central exchange. This data also clarifies how our brain, the cortical district in particular, is reshaped in its functional response by any variation of inflow from the periphery.
Acknowledgements
We thank Dr. S. Rossi for fruitful scientific discussions, Dr. P. Pasqualetti for reliable support in statistical data treatment, Professors V. Pizzella and G.L. Romani for continuous collaboration, Mrs. M. Ercolani for excellent technical support, and Dr. S. Thomson for professional language editing.
REFERENCES
- American Academy of Neurology , American Association of Electrodiagnostic Medicine , American Academy of Physical Medicine and Rehabilitation (1993a): Practice parameter for carpal tunnel syndrome (summary statement). Neurology 43: 2406–2409.8232968 [Google Scholar]
- American Academy of Neurology , American Association of Electrodiagnostic Medicine , American Academy of Physical Medicine and Rehabilitation (1993b): Practice parameter for electrodiagnostic studies in carpal tunnel syndrome (summary statement). Neurology 43: 2404–2405. [DOI] [PubMed] [Google Scholar]
- American Association of Electrodiagnostic Medicine Quality Assurance Committee (1993): Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve 16: 1392–1414. [DOI] [PubMed] [Google Scholar]
- American Association of Electrodiagnostic Medicine , American Academy of Neurology , American Academy of Physical Medicine and Rehabilitation (1993): Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve 16: 1390–1391. [DOI] [PubMed] [Google Scholar]
- Allison T, McCarthy G, Wood CC, Jones SJ (1991): Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114: 2465–2503. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, Doppelbauer A, Deecke L, Barth DS, Zeitlhofer J, Lindinger G, Sutherling WW. 1991. Neuromagnetic investigation of somatotopy of human hand somatosensory cortex. Exp Brain Res 87: 641–648. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Topfner S, Grodd W, Taub E, Flor H (1997): Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci 17: 5503–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronchti G, Corthesy ME, Welker E (1999): Partial denervation of the whisker pad in adult mice: altered patterns of metabolic activity in barrel cortex. Eur J Neurosci 11: 2847–2855. [DOI] [PubMed] [Google Scholar]
- Buchner H, Gobbele R, Pollit D, Radermacher I (1996): Evaluation of the functional state of the somato‐motor system using SEP and interfering stimuli. Electroencephalogr Clin Neurophysiol Suppl 46: 351–362. [PubMed] [Google Scholar]
- Byl NN, Merzenich MM, Cheung S, Bedenbaugh P, Nagarajan SS, Jenkins WM (1997): A primate model for studying focal dystonia and repetitive strain injury: effects on the primary somatosensory cortex. Phys Ther 77: 269–284. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kiss ZH, Tasker RR, Dostrovsky JO (1996): Thalamic stimulation‐evoked sensations in chronic pain patients and in non‐pain (movement disorder) patients. J Neurophysiol 75: 1026–1037. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Noel P (1973): Average cerebral evoked potentials in the valuation of lesion of the sensory nerves and of the somatosensory pathways In: Desmedt JE, editor. New developments in EEG and clinical neurophysiology Vol. 2 Basel: Karger; p 353–372. [Google Scholar]
- Dudley DS, Luders H, Lesser RP, Morris HH (1987): Cortical generators of somatosensory evoked potentials to median nerve stimulation. Neurology 37: 1141–1145 [DOI] [PubMed] [Google Scholar]
- Eisen A, Purves S, Hoirch M (1982): Central nervous system amplification: its potential in the diagnosis of early multiple sclerosis. Neurology 32: 359–364. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Elbert T, Sterr A, Flor H, Rockstroh B, Knecht S, Pantev C, Wienbruch C, Taub E (1997): Input‐increase and input‐decrease types of cortical reorganization after upper extremity amputation in humans. Exp Brain Res 117: 161–164. [DOI] [PubMed] [Google Scholar]
- Elbert T, Candia V, Altenmuller E, Rau H, Sterr A, Rockstroh B, Pantev C, Taub E (1998): Alteration of digital representations in somatosensory cortex in focal hand dystonia. Neuroreport 9: 3571–3575. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ, Tarvin RP (1986): High gain transmission of single impulses through dorsal column nuclei of the cat. Neurosci Lett 65: 277–282. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995): Phantom‐limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375: 482–484. [DOI] [PubMed] [Google Scholar]
- Flor H, Braun C, Elbert T, Birbaumer N (1997): Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224: 5–8. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Muhlnickel W, Pantev C, Wienbruch C, Taub E (1998): Cortical reorganization and phantom phenomena in congenital and traumatic upper‐extremity amputees. Exp Brain Res 119: 205–212. [DOI] [PubMed] [Google Scholar]
- Florence SL, Kaas JH (1995): Large‐scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. J Neurosci 15: 8083–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, Hanes DP, Florence SL, Kaas JH (1994): Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens Mot Res 11: 109–117. [DOI] [PubMed] [Google Scholar]
- Giannini F, Passero S, Cioni R, Paradiso C, Battistini N, Giordano N, Vaccai D, Marcolongo R (1991): Electrophysiologic evaluation of local steroid injection in carpal tunnel syndrome. Arch Phys Med Rehabil 72: 738–742. [PubMed] [Google Scholar]
- Hari R, Reinikainen K, Kaukoranta E, Hamalainen M, Ilmoniemi R, Penttinen A, Salminen J, Teszner D (1984): Somatosensory evoked cerebral magnetic fields from SI and SII in man. Electroencephalogr Clin Neurophysiol 57: 254–263. [DOI] [PubMed] [Google Scholar]
- Hari R, Karhu J, Hamalainen M, Knuutila J, Salonen O, Sams M, Vilkman V (1993): Functional organization of the human first and second somatosensory cortices: a neuromagnetic study. Eur J Neurosci 5: 724–734. [DOI] [PubMed] [Google Scholar]
- Hund M, Rezai AR, Kronberg E, Cappell J, Zonenshayn M, Ribary U, Kelly PJ, Llinas R (1997): Magnetoencephalographic mapping: basis of a new functional risk profile in the selection of patients with cortical brain lesions. Neurosurgery 40: 936–943. [DOI] [PubMed] [Google Scholar]
- Idler RS (1996): Persistence of symptoms after surgical release of compressive neuropathies and subsequent management. Orthop Clin North Am 27: 409–416. [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs M, Allard T, Guic‐Robles E (1990): Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–103. [DOI] [PubMed] [Google Scholar]
- Kaukoranta E, Hämäläinen M, Sarvas J, Hari R (1986): Mixed and sensory nerve stimulations activate different cytoarchitectonic areas in the human primary somatosensory cortex SI. Exp Brain Res 63: 60–66. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S, Yoshimoto T (1996): Neuromagnetic evidence of pre‐ and post‐central cortical sources of somatosensory evoked responses. Electroencephalogr Clin Neurophysiol 100: 44–50. [DOI] [PubMed] [Google Scholar]
- Knecht S, Henningsen H, Elbert T, Flor H, Hohling C, Pantev C, Taub E (1996): Reorganizational and perceptional changes after amputation. Brain 119: 1213–1219. [DOI] [PubMed] [Google Scholar]
- Knecht S, Henningsen H, Hohling C, Elbert T, Flor H, Pantev C, Taub E (1998): Plasticity of plasticity? Changes in the pattern of perceptual correlates of reorganization after amputation. Brain 121: 717–724. [DOI] [PubMed] [Google Scholar]
- Kristeva‐Feige R, Walter H, Lütkenhöner B, Hampson S, Ross B, Knorr U, Steinmetz H, Cheyne D (1994): A neuromagnetic study of the functional organization of the sensorimotor cortex. Eur J Neurosci 6: 632–639. [DOI] [PubMed] [Google Scholar]
- Kristeva‐Feige R, Rossi S, Pizzella V, Sabato A, Tecchio F, Feige B, Romani GL, Edrich J, Rossini PM (1996): Changes in movement‐related brain activity during transient deafferentation: a neuromagnetic study. Brain Res 714: 201–208. [DOI] [PubMed] [Google Scholar]
- Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, Katz JN (1993): A self‐administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg 75A: 1585–1592. [DOI] [PubMed] [Google Scholar]
- Mauguiere F, Merlet I, Forss N, Vanni S, Jousmaki V, Adeleine P, Hari R (1997): Activation of a distributed somatosensory cortical network in the human brain. A dipole modeling study of magnetic fields evoked by median nerve stimulation. Part I: Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 104: 281–289. [DOI] [PubMed] [Google Scholar]
- Mondelli M, Reale F, Sicurelli F, Padua L (2000): Relationship between the self administered Boston questionnaire and electrophysiological findings in follow‐up of surgically‐treated carpal tunnel syndrome. J Hand Surg 25B: 128–134. [DOI] [PubMed] [Google Scholar]
- Morioka T, Shigeto H, Ishibashi H, Nishio S, Yamamoto T, Yoshiura T, Fukui M (1998): Magnetic source imaging of the sensory cortex on the surface anatomy MR scanning. Neurol Res 20: 235–241. [DOI] [PubMed] [Google Scholar]
- Padua L, Lo Monaco M, Valente EM, Tonali P (1996): A useful electrophysiologic parameter for diagnosis of carpal tunnel syndrome. Muscle Nerve 19: 48–53. [DOI] [PubMed] [Google Scholar]
- Padua L, Padua R, LoMonaco M, Romanini E, Tonali P (1998a): Italian multicentre study of carpal tunnel syndrome: study design. Italian CTS Study Group. Ital J Neurol Sci 19: 285–289. [DOI] [PubMed] [Google Scholar]
- Padua R, Padua L, Romanini E, Aulisa L, Lupparelli S, Sanguinetti C (1998b): Versione italiana del questionario Boston Carpal Tunnel: valutazione preliminare. Giornale Italiano di Ortopedia e Traumatologia 24: 123–129. [Google Scholar]
- Padua L, Padua R, Lo Monaco M, Aprile I, Tonali P (2001): Multiperspective assessment of Carpal Tunnel Syndrome—a multicenter study. Neurology 56: 1459–1466. [DOI] [PubMed] [Google Scholar]
- Phalen GS (1968): The diagnosis of carpal tunnel syndrome. Cleve Clin Q 35: 1–6. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR (1992): Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency‐discrimination task. J Neurophysiol 67: 1031–1056. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Tecchio F, Sabato A, Rossini PM (1998): Modulation of corticospinal output to human hand muscles following deprivation of sensory feedback. Neuroimage 8: 163–175. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Martino G, Narici L, Pasquarelli A, Peresson M, Pizzella V, Tecchio F, Torrioli G, Romani GL (1994): Short‐term brain ‘plasticity’ in humans: transient finger representation changes in sensory cortex somatotopy following ischemic anesthesia. Brain Res 642: 169–177. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Tecchio F, Pizzella V, Lupoi D, Cassetta E, Pasqualetti P, Romani GL, Orlacchio A (1998): On the reorganization of sensory hand areas after mono‐hemispheric lesion: a functional (MEG)/anatomical (MRI) integrative study. Brain Res 782: 153–166. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Pauri F (2000): Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas ‘plastic’ reorganisation. Brain Research Review 33: 131–154. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Tecchio F, Pizzella V, Lupoi D, Cassetta E, Paqualetti P (2001): Interhemispheric differences of sensory hand areas after monohemispheric stroke: MEG/MRI integrative study Neuroimage 14: 474–485. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Rossini PM, Pizzella V, Cassetta E, Romani G‐L (1997): Spatial properties and interhemispheric differences of the sensory hand cortical representation: a neuromagnetic study. Brain Res 767: 100–108. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G, Polo A, Volpato D, Manganotti P, Bonato C, Testoni R, Fiaschi A (1997): Transient deafferentation in humans induces rapid modulation of primary sensory cortex not associated with subcortical changes: a somatosensory evoked potential study. Neurosci Lett 223: 21–24. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Zanette G, Volpato D, Testoni R, Bonato C, Manganotti P, Miniussi C, Fiaschi A (1998): Neurophysiological evidence of neuroplasticity at multiple levels of the somatosensory system in patients with carpal tunnel syndrome. Brain 121: 1785–1794. [DOI] [PubMed] [Google Scholar]
- Zhang SP, Rowe MJ (1997): Quantitative analysis of cuneate neurone responsiveness in the cat in association with reversible, partial deafferentation. J Physiol 505: 769–783. [DOI] [PMC free article] [PubMed] [Google Scholar]


