Abstract
In our natural environment, the ability to divide attention is essential since we attend simultaneously to a number of sensory modalities, e.g., to visual and auditory stimuli. In this study, functional magnetic resonance imaging (fMRI) was used to study brain activation while a divided attention task was performed. Brain activation was also assessed under selective attention. Fourteen healthy male subjects aged between 19 and 28 years underwent fMRI studies using gradient EPI sequences. Cingulate activation was evident in all attention tasks. Focusing attention on one modality (visual or auditory) increased the activity in the corresponding primary and secondary sensory area. When attention is divided between both modalities, the activation in the sensory areas is decreased, possibly due to a limited capacity of the system for controlled processing. Left prefrontal activation, however, was evident selectively during the divided attention task. The present results suggest that this area may be important in the execution of controlled processing when attention is divided between two sources of information. These results support the view that the prefrontal cortex is involved in the central executive system and controls attention and information flow. Hum. Brain Mapping 18:249–259, 2003. © 2003 Wiley‐Liss, Inc.
Keywords: attention, divided attention, selective attention, focused attention, fMRI, prefrontal cortex, cingulate cortex, visual cortex, auditory cortex
INTRODUCTION
In recent years, human attention has increasingly become a focus of interest in functional neuroimaging since the level of brain activation depends in part on the attentional state [Jäncke et al., 1999a, 1999b, 1999c]. In our natural environment, we normally attend simultaneously to a number of sensory modalities, e.g., to visual and auditory stimuli. “Divided attention” refers to attention divided between two or more sources of information. Few published studies focus on divided attention despite its importance in day‐to‐day life. Posner et al. [1989] postulated that two tasks, when performed simultaneously, do not interfere with the performance of one another when different brain areas are used for the two tasks. Brain activation during simultaneous visual and auditory information processing may, therefore, result in a summation of the activation during selective visual and auditory information processing (selective or focused attention). Tasks assessing selective visual attention activate the primary striate cortex [Büchel et al., 1998; Jäncke et al., 1999a; Ress et al., 2000; Watanabe et al., 1998a, b] and/or the secondary extrastriate cortex [Clark and Hillyard, 1996; Corbetta et al., 1991; Heinze et al., 1994; Hillyard et al., 1997; Mangun et al., 1997; Tootell et al., 1998] while the primary auditory cortex [Alho et al., 1999; Jäncke et al., 1999b; Woldorff et al., 1993] and/or the secondary auditory cortex [Frith and Friston, 1996; Fujiwara et al., 1998; Kawashima et al., 1999; Tzourio et al., 1997] are activated by selective auditory tasks.
Madden et al. [1997] investigated brain activation when subjects were instructed to divide their attention among the display positions within the visual modality. Regional cerebral blood flow (rCBF) activation was found in occipitotemporal, occipitoparietal, and prefrontal regions. This, however, was not divided attention, since there was no dual task paradigm. The authors, therefore, described it as divided visual attention. Brain activation during divided attention has been investigated by Corbetta et al. [1991]. Positron emission tomography (PET) measurements were taken while subjects discriminated between shape, color, and speed of a visual stimulus under conditions of selective and divided attention. The divided condition activated the anterior cingulate and prefrontal cortex in the right hemisphere. In another study, the posterior dorsolateral prefrontal cortex and the lateral parietal cortex were activated bilaterally during a dual‐task performance using functional magnetic resonance imaging (fMRI) and visual stimulus material [Koechlin et al., 1999]. Iidaka et al. [2000] investigated memory processing and found greater activation in the cerebellum, the temporoparietal cortex, the left cingulate, and in the prefrontal area, when tasks required both the encoding and retrieval of memory information. Left prefrontal activation and activation of the left retrosplenial area were evident in a dual task paradigm that included tasks requiring acquisition of memory [Shallice et al., 1994]. The key structure showing consistent activation in the various examinations employing dual‐task performance seems to be the left or right prefrontal cortex.
D'Esposito et al. [1995] used a semantic‐judgment task, activating left temporal regions, and a spatial rotation task, activating bilateral parieto‐occipital regions. Activation of the prefrontal cortex was observed when both tasks were performed together, but not when they were performed separately. The authors interpreted their results as evidence that the prefrontal cortex plays a predominant role in working memory, since working memory is required in their dual task paradigm. A dual task paradigm is not, however, inevitably associated with higher prefrontal activation. If prefrontal activation is evoked by a single task, an additional simultaneously performed second task may decrease prefrontal activation. This was shown by Goldberg et al. [1998] using the Wisconsin Card Sorting Test, a complex reasoning task, and a rapidly paced auditory–verbal shadowing task in a PET rCBF study. Although the investigations of Goldberg et al. [1998] and particularly D'Esposito and colleagues [1995] are essential to our understanding of the role of the prefrontal cortex in cognition, it remains unclear which areas in the brain are responsible for divided attention, since in their examinations they used complex cognitive tasks involving higher cognitive performance such as semantic processing and working memory. Therefore, both groups avoid the term ”divided attention” and refer instead to working memory.
To further elucidate the neural substrate of divided attention during the information processing of simultaneously presented stimuli from different modalities, a design using two (or more) simple attention tasks within two (or more) modalities is required. In the present examination, we chose to apply a selective visual attention task and a selective auditory attention task, employed separately and simultaneously. To ensure that our concept of divided attention corresponds to the concept of divided attention in clinical neuropsychology, we selected attention tasks commonly used in the assessment of neuropsychological patients [Zimmermann and Fimm, 1992]. Functional maps of brain activity related to the attentional conditions were generated using fMRI images.
SUBJECTS AND METHODS
Fourteen healthy right‐handed male subjects aged between 19 and 28 years underwent fMRI studies using attention paradigms. All subjects gave written informed consent before participating. Attention tasks from a computerized attention test battery were used [Zimmermann and Fimm, 1992]. Acoustic stimulation consisted of two alternating high‐frequency and low‐frequency tones (450 and 1,070 Hz). Tones were presented with 1‐sec Inter‐stimulus Interval (ISI) by air tube earphones. Target stimuli were two stimuli of identical frequency presented successively. The visual stimulus was a 4 × 4 item matrix consisting of dots and crosses. Target stimuli were patterns of four crosses forming a square. The visual stimuli were back‐projected onto a screen with 2‐sec Inter‐Stimulus‐Interval. The subjects viewed the stimuli via a mirror attached to the head coil. The visual angle for binocular stimulation was 12 × 33 degrees. Subjects were asked to press a button with the right index finger as quickly and accurately as possible when a target stimulus was presented. Reaction times were registered during fMRI measurements. In the baseline condition used in all experiments, subjects were instructed to concentrate on their breathing and to press the button when they exhaled. Therefore, subjects' attention was diverted from the sensory stimuli and a similar reaction (button press at a similar frequency) was achieved. In all conditions including the baseline condition, acoustic and visual stimulation were applied simultaneously. Changes in brain activity were, therefore, due to an altered attentional state induced by the instruction and not to physical differences in the stimulus. In the first experiment, subjects reacted to acoustic stimulation. In the second experiment, attention had to be divided between the two stimuli, and in the third experiment, the relevant information was within the visual stimulation. The experiments were conducted in this order so as to avoid any reaction to visual stimulation in the first experiment, in which subjects were told to expect only auditory stimuli. The visual target stimulus was explained to the subject immediately before the start of the second divided attention condition. In the third selective visual attention condition, we could not rule out the possibility of a mental processing of auditory target stimuli. However, it is not possible to completely rule out the processing of auditory target stimuli, because two stimuli of identical frequency presented successively are a kind of ”oddball‐paradigm,” which evokes brain reaction even without instruction and would therefore appear even if we were to start with this condition. To control for this effect, four of the subjects underwent a third condition that was changed marginally. During the selective visual attention condition, there were no auditory target stimuli, preventing any mental attention processing to auditory stimuli. Brain activation of these four subjects did not differ from activation of the other ten subjects in the selective visual attention condition.
fMRI images were acquired on a 1.5 Tesla scanner (Signa Echospeed; General Electric) using echo planar imaging (EPI) sequences with a repetition time (TR) of 3 seconds, an echo time (TE) of 60 msec, and a flip angle of 90 degrees. Fifteen slices were collected with a voxel dimension of 2.2 × 2.2 × 6 mm3. The first 10 images were discarded in order to avoid non steady‐state effects due to T1 saturation. The remaining 60 images included three attention half periods with 10 images and three baseline half periods with 10 images each, presented in periodic alternation. Functional imaging used the technique of statistical parametric mapping using SPM99 software [Friston et al., 1995]. For each subject, images were realigned using sinc interpolation (with an 11 × 11 × 11 kernel, and adjusted) to remove signal correlated with head motion. Images were transformed into standard ICBM space [Mazziotta et al., 1995] using an automated spatial normalization (12‐parameter affine transformation followed by non‐linear iterations using 7 × 8 × 7 basis functions) as integrated in SPM99. The normalized images were then smoothed with a Gaussian kernel (full width at half maximum = 8 mm) to create a local weighted average of the surrounding pixels. Effects were estimated with the general linear model with a delayed box‐car waveform. A conservative statistical correction (”Bonferroni like”) based on the number of voxels was applied to determine significance levels of activations in the data. The approach used by SPM is to use random field theory. One determines how many resolution elements (resels), defined as a block of pixels of the same size, there are in an image. The expected euler characteristic can be used to give the correct threshold for the required control of false positives. If we know the number of resels in an image, it is possible to estimate the most likely value of the euler characteristic at any given threshold [Worsley et al., 1996]. The inter‐subject effect was treated as a fixed effect.
Since the SPM99 template does not perfectly match Talairach space, we estimated the Talairach‐brain coordinates with a non‐linear transform of MNI to Talairach [Brett, 1999]. The spatial coordinates obtained from the SPM99 results were converted to standard Talairach‐brain coordinates and then entered into the Talairach Daemon [Lancaster et al., 2000] for result localization. All results are displayed as SPM{Z}‐maps, depicting the significant voxels. Activations were always relative to the baseline condition.
We determined the amplitudes of the respective volumes of interest with an ordinary least squares coefficient estimate based on the Gauss‐Markov theorem [Hamilton, 1994]. After extracting the time series from SPM99 and employing a HRF‐adjusted stimulus function, we obtained an estimate for the slope of the least squares fit, which is equivalent to the amplitude.
RESULTS
Mean accuracy of performance in all attention tasks was 96.93%. No subject made more than two mistakes per experiment and in most of the experiments the subjects made no mistakes. The level of accuracy in the divided attention task (96.44%) did not differ from that in the selective attention tasks. Reaction times for visual stimuli (818 ± 104 msec SD) were significantly longer than reaction times for auditory stimuli (545 ± 94 msec). This modality effect was significant during both selective and divided attention. Mean reaction times during divided attention (725 ± 94 msec) were significantly longer than reaction times during selective attention (638 ± 77 msec). This attention effect was significant for both visual and auditory modality.
The selective auditory attention task (Fig. 1) significantly increased activation in the left superior temporal gyrus including the Heschl gyrus (Brodmann area [BA] 22 & 29) and in the cingulate cortex (medial frontal gyrus, BA 6). The selective visual attention task (Fig. 2) led to significantly increased activation in the occipital cortex (BA 17 and 18), the left precuneus cortex (BA 7), the right superior and inferior parietal cortex (BA 7 and 40), the cingulate cortex (BA 6), and the right inferior frontal gyrus (prefrontal cortex, BA 9 and 45). During the divided attention task, in which attention had to be divided between auditory and visual stimulation, similar brain regions were activated as in the selective attention tasks (Fig. 3). As was the case in the selective auditory attention task, the left superior temporal gyrus (BA 29) was significantly activated during divided attention and, as in the selective visual attention task, significantly increased activation was produced by divided attention in the occipital cortex (BA 17 and 18), the left precuneus cortex (BA 7), the right superior and inferior parietal cortex (BA 7 and 40), and the right prefrontal cortex (BA 9 and 45). As in both selective auditory and visual attention tasks, divided attention significantly activated the cingulate cortex (BA 6). Significantly increased activation in the left prefrontal cortex (inferior frontal gyrus, BA 9 and 47) was produced in the divided attention task but not during selective attention. Amplitude estimation revealed significantly increased activation in the left prefrontal cortex in the divided attention task compared to the selective auditory attention task (T = 4.905, df = 13, P ≤ .001) as well as significantly increased activation compared to the selective visual attention task (T = 2.064, df = 13, P = 0.03). Amplitudes in the right prefrontal cortex were significantly increased in the divided attention task compared to the selective auditory attention task (T = 3.024, df = 13, P = 0.005), but not significantly increased compared to the selective visual attention task (T = 0.291, df = 13, P = 0.388).
Figure 1.
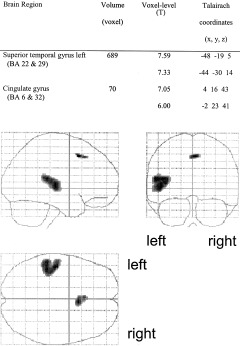
Brain activity related to selective auditory attention. The left temporal gyrus including the Heschl gyrus was significantly activated.
Figure 2.
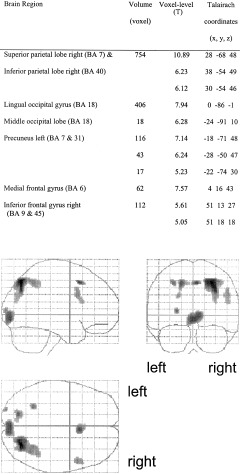
Brain activity related to selective visual attention. The occipital cortex, the left precuneus cortex, the right superior and inferior parietal cortex, the cingulate cortex, and the right inferior frontal gyrus were activated.
Figure 3.

Brain activity related to divided attention. The left superior temporal gyrus, the left precuneus cortex, the right superior and inferior parietal cortex were activated; the cingulate cortex, inferior frontal cortex, and occipital cortex were activated bilaterally.
Both the extent of significant activation and the intensity of activation in the left superior temporal gyrus decreased markedly during divided attention compared to the selective auditory attention task. Amplitude estimation revealed a significant decrement (T = 2.465, df = 13, P = 0.014). In accordance with the decreased activation of primary and secondary auditory brain areas during divided attention, the volume and intensity of significant occipital activation decreased during divided attention compared to the selective visual attention task. The decrement in amplitudes, however, did not reach significance (T = 1.19, df = 13, P = 0.128). Significant activation in the cingulate gyrus increased in volume and intensity during the divided attention task compared to the selective attention conditions. Differences in the brain activation of sensory brain areas, the cingulate cortex, and the prefrontal cortex are summarized in Figure 4. Amplitude estimation is shown in Figure 5.
Figure 4.

Group brain activity related to selective and divided attention. Four transverse slices through the visual, auditory, prefrontal, and cingulate cortex are presented. Activation in the primary sensory areas (visual and auditory cortex) decreased markedly during divided attention compared to selective attention. Prefrontal and cingulate gyrus activation increased during the divided attention task. Activation in the left prefrontal cortex was produced only in the divided attention task, but not during selective attention.
Figure 5.
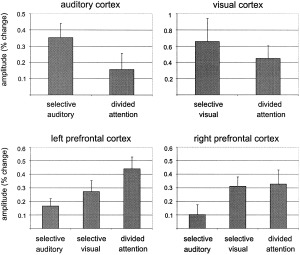
Mean amplitudes ± standard errors (percent change) in the sensory cortical and prefrontal areas. When attention was divided between both modalities, the activation in the sensory areas was decreased. Prefrontal activation was increased during the divided attention task.
DISCUSSION
The accuracy of performance was equally good in all attention tasks (>96%). Reaction times for visual stimuli were significantly longer than reaction times for auditory stimuli. This effect is probably due to the stimulus characteristic. While in the visual modality, the subjects had to discern a visual pattern within the stimulus (this appeared at different locations), in the auditory condition it is possible to ascertain immediately whether or not the tone is a target stimulus. Reaction times during divided attention were significantly longer than reaction times during selective attention. This effect represents the expected time cost of dividing attention between two sources of information. The results, showing increased reaction times in a dual task paradigm, are in accordance with Klingberg and Roland [1997]. In previous investigations, it was shown that reaction times and electrophysiological recordings (P300 latencies) were delayed for divided attention as compared with selective attention [Hohnsbein et al., 1991].
The results in the selective attention tasks in the present investigation are in accordance with those of O'Leary et al. [1997] who found that rCBF changes due to auditory attention were largely localized in the temporal lobe, whereas visual attention caused rCBF changes in a widespread network that included frontal and parietal brain regions. Interestingly, significant activation in auditory selective attention was limited to the left hemisphere in our investigations. In accordance with our findings, Jäncke et al. [1999b] found stronger activation in the left auditory cortex when subjects were required to attend to auditory stimuli. These authors, however, used verbal stimulus material (syllables) inducing hemispheric specialization, whereas in the present study tones of different frequencies were applied. One should consider that, had a less conservative threshold been chosen, the right auditory cortex would have been activated during selective auditory attention in the present study. Nevertheless, there is a strong left‐sided hemispheric specialization that may be related to right handedness and ear preference of the subjects.
The selective visual attention task produced significantly increased activation in the occipital cortex, the left precuneus cortex, the right superior and inferior parietal cortex, the cingulate cortex, and the right prefrontal cortex. Similar activity in the primary visual cortex was shown by Jäncke et al. [1999c] when subjects were required to attend to visual stimuli. Martinez et al. [1999] combined fMRI and EEG results and hypothesized that the primary visual cortex modulation found with fMRI may represent a delayed, re‐entrant feedback from higher visual areas or sustained biasing during attention, because the sensory input to the primary visual cortex measured with EEG (evoked potentials) was not modulated by attention. The observed predominantly right‐sided posterior parietal and cingulate cortex activation accords with studies on spatial attention [Coull and Frith, 1998; Gitelman et al., 1999; Nobre et al., 1997]. In a space‐based attention task, Fink et al. [1997] showed activation of the right prefrontal cortex. In summary, a similar network of brain activation to that found in the present investigation has been shown in other functional imaging studies using visual attention paradigms, especially where spatial attention tasks were used. In our selective visual attention task, the detection of visual target stimuli most probably included a spatial analysis of the pattern. The present results, therefore, correspond with previous findings.
When attention was divided between the auditory and visual modalities, the activation in the sensory auditory and visual areas was decreased compared to the selective attention tasks. This corresponds with the findings of Corbetta et al. [1990] who investigated changes in rCBF while subjects discriminated between different attributes of a visual stimulus, e.g., shape, colour, and velocity. The authors reported visual cortex activation after subtracting a divided attention task from each of the selective attention tasks (discrimination of shape, colour, and velocity). This activation may also represent reduced activation in the divided attention condition. During an arithmetical task, irrelevant speech yielded a decreased level of activity in the auditory cortex [Ghatan et al., 1998]. The authors interpreted their results as evidence of a top‐down inhibitory modulation of a non‐attended input to the auditory cortex. Similarly, Haxby et al. [1994] observed decreases in regional cerebral blood flow in auditory and somatosensory cortex during a visual face matching task. The authors suggested that selective attention to one sensory modality is associated with decreased activity in cortical areas responsible for processing input from other sensory modalities. Inhibitory influences are, however, not restricted to the interaction of different modalities. Smith et al. [2000] showed that visual attention to a particular location resulted in a widespread suppression of activity in brain regions responsible for all other locations. Taken together, attention to one modality or to one attribute within one modality leads not only to increased activity of the sensory cortical area involved in the processing of these stimuli, but also to suppressed activity in regions associated with other modalities. A difference between the cited investigations and the present investigation is that in our experiment subjects had to attend to both modalities. We assume that during divided attention, both modalities are activated by a top‐down mechanism and simultaneously inhibited by the concurrent modality, respectively. In accordance with this, Vandenberghe et al. [1997] showed within the visual modality, that visual cortex activity fell to a level midway between those seen during exclusively leftward or rightward attention, when subjects divided their attention over opposite hemifields. Alternative to a sensory interaction model, it is possible that a central executive mechanism controls activation of the primary sensory areas and, therefore, reduces activity if two modalities are involved.
Activation was increased during divided attention compared to selective attention tasks in the cingulate cortex. Significant bilateral activation in the prefrontal cortex was observed in the divided attention task, whereas selective visual attention activated only the right prefrontal cortex and selective auditory attention revealed no significant frontal activation. Activation of the cingulate cortex and the right prefrontal cortex was shown in a linguistic dual task paradigm [Benedict et al., 1998]. The same brain regions, however, were activated during simple sustained attention and alertness [Häger et al., 1998; Sturm et al., 1999]. Hopfinger et al. [2000] showed that the anterior cingulate cortex is activated during selective analysis of target features, whereas the prefrontal cortex is involved in top‐down attentional control. In a working memory task, the anterior cingulate cortex was activated by increased task difficulty, whereas the prefrontal cortex was activated by an increased working memory load [Barch et al., 1997]. Working memory is a classical paradigm for executive (top‐down) function. The ability to perform two tasks simultaneously is also a function of the central executive [Leclercq et al., 2000]. We, therefore, assume that increased cingulate cortex activation during divided attention is a result of increased task difficulty. Activation of the prefrontal cortex, on the other hand, may be related to executive function during divided attention. Right prefrontal cortex activation is seen in simple visual and somatosensory sustained attention tasks and alertness [Pardo et al., 1991; Sturm et al., 1999]. Bilateral prefrontal cortex activation, however, was found during working memory tasks [see Cabeza and Nyberg, 2000]. In accordance with our results, the left prefrontal cortex was only activated when executive top‐down mechanisms were involved. The apparent inconsistency with another dual task performance where no separate cortical area was identified can be explained by the fact that two working memory tasks activating bilateral prefrontal cortex were employed [Klingberg, 1998]. The dual task was, therefore, not able to produce additional brain activity. Goldberg et al. [1998] showed that activity in the prefrontal cortex may even decrease during a concurrent task if the other task involves executive function and, therefore, effects strong prefrontal cortex activation. In a study using a dual task paradigm including visual and somatosensory stimulation, the right prefrontal cortex (inferior frontal gyrus) was activated selectively when the first task interfered with the second [Herath et al., 2001]. In the present study, however, the divided attention task activated the prefrontal cortex bilaterally. This result could be explained by the fact that the attentional tasks used in our study interfered with each other, i.e., the reaction times following both visual and auditory stimulation increased in the dual task compared with the single tasks. Furthermore, the two types of stimuli were administered simultaneously in our study, whereas there was always a short delay between visual and somatosensory stimulation in the study of Herath et al. [2001]. Johannsen et al. [1997] showed activation in the right middle frontal gyrus in both sustained and divided attention tasks. The activity was more pronounced during divided attention. There are, however, some methodological problems. The subjects were instructed to detect a change of frequency of the stimulus, although no change of frequency occurred. Therefore, no control of the cognitive state during the tasks was performed. There are also methodological problems in the present examination. While the number of items was the same in all attention tasks, the number of target items was higher in the divided attention task because there were both auditory and visual targets. The role of the left prefrontal cortex in divided attention, however, is supported by clinical data showing divided attention deficits in patients with lesions of the left prefrontal cortex [Godefroy et al., 1996; Godefroy and Rousseaux, 1996]. Deficits in selective attention were also evident in these patients.
In summary, divided attention to auditory and visual stimuli compared with selective attention leads to decreased activity in the sensory brain areas and increased activity in the cingulate cortex. The decline in activation may be due to inter‐sensory interaction with reciprocal inhibition of both sensory systems, respectively. The interaction resulted not merely in decreased activity in sensory brain areas, but also in increased reaction times in the performance. Increased activity of the cingulate cortex may be a result of increased task difficulty. Bilateral prefrontal activity was shown during divided attention. The left prefrontal cortex was activated only in the divided attention task, indicating that this brain region may represent the location of executive functioning that involves a top‐down attentional control mechanism during divided attention.
REFERENCES
- Alho K, Medvedev SV, Pakhomov SV, Roudas MS, Tervaniemi M, Reinikainen K, Zeffiro T, Näätänen R (1999): Selective tuning of the left and right auditory cortices during spatially directed attention. Brain Res Cogn Brain Res 7: 335–341. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35: 1373–1380. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Lockwood AH, Shucard JL, Shucard DW, Wack D, Murphy BW (1998): Functional neuroimaging of attention in the auditory modality. Neuroreport 9: 121–126. [DOI] [PubMed] [Google Scholar]
- Brett, M (1999): The MNI brain and the Talairach atlas. MRC Cognition and Brain Sciences Unit (GENERIC). Retrieved 1 March 2001, online at http://www.mrc-clou.cam.ac.uk/imaging
- Büchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ (1998): The functional anatomy of attention to visual motion. A functional MRI study. Brain 121: 1281–1294. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L (2000): Imaging Cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA (1996): Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J Cogn Neurosci 8: 387–402. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1990): Attentional modulation of neural processing of shape, color, and velocity in humans. Science 248: 1556–1559. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1991): Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD (1998): Differential activation of right superior parietal cortex and intraparietal sulcus by spatial and nonspatial attention. Neuroimage 8: 176–187. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M (1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD (1997): Space‐based and object‐based visual attention: shared and specific neural domains. Brain 120: 2013–2028. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Frith CD, Friston KJ (1996): The role of the thalamus in “top down” modulation of attention to sound. Neuroimage 4: 210–215. [DOI] [PubMed] [Google Scholar]
- Fujiwara N, Nagamine T, Imai M, Tanaka T, Shibasaki H (1998): Role of the primary auditory cortex in auditory selective attention studied by whole‐head neuromagnetometer. Brain Res Cogn Brain Res 7: 99–109. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Hsieh JC, Petersson KM, Stone ES, Ingvar M (1998): Coexistence of attention‐based facilitation and inhibition in the human cortex. Neuroimage 7: 23–29. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M (1999): A large‐scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122: 1093–1106. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M (1996): Non‐spatial attention disorders in patients with frontal or posterior brain damage. Brain 119: 191–202. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Rousseaux M (1996): Divided and focused attention in patients with lesion of the prefrontal cortex. Brain Cogn 30: 155–174. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Berman KF, Fleming K, Ostrem J, Van‐Horn JD, Esposito G, Mattay VS, Gold JM, Weinberger DR (1998): Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage 7: 296–303. [DOI] [PubMed] [Google Scholar]
- Hamilton JD (1994): Time series analysis. Princeton: Princeton University Press. [Google Scholar]
- Häger F, Volz HP, Gaser C, Mentzel HJ, Kaiser WA, Sauer H (1998): Challenging the anterior attentional system with a continuous performance task: a functional magnetic resonance imaging approach. Eur Arch Psychiatry Clin Neurosci 248: 161–170. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET‐rCBF study of selective attention to faces and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, et al (1994): Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P (2001): Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex 11: 796–805. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hinrichs H, Tempelmann C, Morgan ST, Hansen JC, Scheich H, Heinze HJ (1997): Combining steady‐state visual evoked potentials and fMRI to localize brain activity during selective attention. Hum Brain Mapp 5: 287–292. [DOI] [PubMed] [Google Scholar]
- Hohnsbein J, Falkenstein M, Hoormann J, Blanke L (1991): Effects of crossmodal divided attention on late ERP components. I. Simple and choice reaction tasks. Electroencephalogr Clin Neurophysiol 78: 438–446. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nature Neurosci 3: 284–291. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FIM (2000): The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. J Cogn Neurosci 12: 267–280. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Buchanan T, Lutz K, Specht K, Mirzazade S, Shah NJ (1999a): The time course of the BOLD response in the human auditory cortex to acoustic stimuli of different duration. Brain Res Cogn Brain Res 8: 117–124. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Mirzazade S, Shah NJ (1999b): Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett 266: 125–128. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Mirzazade S, Shah NJ (1999c): Attention modulates the blood oxygen level dependent response in the primary visual cortex measured with functional magnetic resonance imaging. Naturwissenschaften 86: 79–81. [DOI] [PubMed] [Google Scholar]
- Johannsen P, Jakobsen J, Bruhn P, Hansen SB, Gee A, Stodkilde JH, Gjedde A (1997): Cortical sites of sustained and divided attention in normal elderly humans. Neuroimage 6: 145–155. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Imaizumi S, Mori K, Okada K, Goto R, Kiritani S, Ogawa A, Fukuda H (1999): Selective visual and auditory attention toward utterances: a PET study. Neuroimage 10: 209–215. [DOI] [PubMed] [Google Scholar]
- Klingberg T (1998): Concurrent performance of two working memory tasks: potential mechanisms of interference. Cereb Cortex 8: 593–601. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Roland PE (1997): Interference between two concurrent tasks is associated with activation of overlapping fields in the cortex. Cogn Brain Res 6: 1–8. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J (1999): The role of the anterior prefrontal cortex in human cognition. Nature 399: 148–151. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikite SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq M, Couillet J, Azouvi P, Marlier N, Martin Y, Strypstein E, Rousseaux M (2000): Dual task performance after severe diffuse traumatic brain injury or vascular prefrontal damage. J Clin Exp Neuropsychol 22: 339–350. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE (1997): Selective and divided visual attention: Age‐related changes in regional cerebral blood flow measured by H215O PET. Hum Brain Mapp 5: 389–409. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, Heinze HJ (1997): Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum Brain Mapp 5: 273–279. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo‐Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA (1999): Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995): A probabilistic atlas of the human brain: theory and rationale for its development: the international consortium for brain mapping. Neuroimage 2: 89–101. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD (1997): Functional localization of the system for visuospatial attention using positron emission tomography. Brain 120: 515–533. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, Arndt SV, Cizadlo TJ, Ponto LLB, Watkins GL, Hichwa RD (1997): Auditory and visual attention assessed with PET. Hum Brain Mapp 5: 422–436. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Posner MI, Sandson J, Dhawan M, Shulman GL (1989): Is word recognition automatic? A cognitive‐anatomical approach. J Cogn Neurosci 1: 50–60. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ (2000): Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci 3: 940–945. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby P, Frackowiak RS, Dolan RJ (1994): Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature 368: 633–635. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW (2000): Attentional suppression of activity in the human visual cortex. Neuroreport 11: 271–277. [DOI] [PubMed] [Google Scholar]
- Sturm W, de‐Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Muller GH, Willmes K (1999): Functional anatomy of intrinsic alertness: evidence for a fronto‐parietal‐thalamic‐brainstem network in the right hemisphere. Neuropsychologia 37: 797–805. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM (1998): The retinotopy of visual spatial attention. Neuron 21: 1409–1422. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Massioui FE, Crivello F, Joliot M, Renault B, Mazoyer B (1997): Functional anatomy of human auditory attention studied with PET. Neuroimage 5: 63–77. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Duncan J, Dupont P, Ward R, Poline JB, Bormans G, Michiels J, Mortelmans L, Orban GA (1997): Attention to one or two features in left or right visual field: a positron emission tomography study. J Neurosci 17: 3739–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Harner AM, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, Mukai I (1998a): Task‐dependent influences of attention on the activation of human primary visual cortex. Proc Natl Acad Sci U S A 95: 11489–11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M, Takino R, Miyakawa S (1998b): Attention‐regulated activity in human primary visual cortex. J Neurophysiol 79: 2218–2221. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE (1993): Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A 90: 8722–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Fimm B (1992): Handbuch der Testbatterie zur Aufmerksamkeitsprüfung [Manual for the attention assessment test battery] Freiburg, Germany: Psytest; 1992. [Google Scholar]


