Abstract
Various lines of evidence indicate that men generally experience greater sexual arousal (SA) to erotic stimuli than women. Yet, little is known regarding the neurobiological processes underlying such a gender difference. To investigate this issue, functional magnetic resonance imaging was used to compare the neural correlates of SA in 20 male and 20 female subjects. Brain activity was measured while male and female subjects were viewing erotic film excerpts. Results showed that the level of perceived SA was significantly higher in male than in female subjects. When compared to viewing emotionally neutral film excerpts, viewing erotic film excerpts was associated, for both genders, with bilateral blood oxygen level dependant (BOLD) signal increases in the anterior cingulate, medial prefrontal, orbitofrontal, insular, and occipitotemporal cortices, as well as in the amygdala and the ventral striatum. Only for the group of male subjects was there evidence of a significant activation of the thalamus and hypothalamus, a sexually dimorphic area of the brain known to play a pivotal role in physiological arousal and sexual behavior. When directly compared between genders, hypothalamic activation was found to be significantly greater in male subjects. Furthermore, for male subjects only, the magnitude of hypothalamic activation was positively correlated with reported levels of SA. These findings reveal the existence of similarities and dissimilarities in the way the brain of both genders responds to erotic stimuli. They further suggest that the greater SA generally experienced by men, when viewing erotica, may be related to the functional gender difference found here with respect to the hypothalamus. Hum. Brain Mapping 16:1–13, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: erotica, sexual arousal, sexual behavior, gender differences, gender differences, emotion, motivation, functional magnetic resonance imaging, limbic system, hypothalamus
INTRODUCTION
It has been recently proposed that human sexual arousal (SA), which is usually triggered by external stimuli or endogenous factors, is a multidimensional experience comprising four closely interrelated and coordinated components: cognitive, emotional, motivational, and physiological [Stoléru et al., 1999]. The cognitive component involves a process of appraisal through which a stimulus is evaluated as a sexual incentive. The emotional component refers to the specific hedonic quality of SA. The motivational component relates to the processes that direct behavior to a sexual goal. Finally, the physiological component regards the autonomic and endocrinological responses (e.g., cardiovascular, respiratory, genital) associated with SA [for a more detailed discussion of this model, see Stoléru et al., 1999; see also Redouté et al., 2000].
To identify the neural substrate of the various components of SA, Stoléru and colleagues [Redouté et al., 2000] used positron emission tomography (PET) to investigate changes in regional cerebral blood flow (rCBF) in male subjects presented with visual sexual stimuli (e.g., film excerpts). Statistical Parametric Mapping was used to locate brain areas whose activation accompanied the processing of the sexual stimuli and correlated with markers of SA. Results were interpreted as showing that the right orbitofrontal cortex activation was correlated with both the cognitive and motivational components of the proposed model. They also suggested that the rostral portion of the anterior cingulate cortex (Brodmann area: BA 24) and the posterior portion of the hypothalamus activations were correlated with the autonomic component of SA, whereas activations in BA 9 and BA 32 were related to the level of perceived emotion.
Of all the brain areas identified as involved in the regulation of sexual behavior in animals, the hypothalamus is arguably the one having most frequently been claimed to play a pivotal role in such a function [Pfaff et al., 1994; Sachs and Meisel, 1994]. Indeed, it has been shown that lesions of the hypothalamic medial preoptic area have a deleterious effect on copulation in males of many species, including fish [Macey et al., 1974], lizards [Wheeler and Crews, 1978], birds [Balthazart and Surlemont, 1990], rats [Larsson and Heimer, 1964; Twiggs et al., 1978], dogs [Hart, 1974], and monkeys [Slimp et al., 1978]. Further, electrical stimulation of the same area has been found to have a facilitatory effect on this biological function [Malsbury, 1971]. Likewise, in rats, transplantation of preoptic tissue from male neonates to the preoptic area of female littermates has been demonstrated to increase their proclivity to manifest sexual behavior during adulthood [Arendash and Gorski, 1982]. In addition, in female mammals of various species, neurons of the hypothalamic ventromedial nucleus have been shown to be involved in the induction of sexual receptivity [Clark et al., 1981; Mathews and Edwards, 1977] and in the facilitation of lordosis posturing [Carrer et al., 1973; Dorner et al., 1975; Malsbury et al., 1977].
Interestingly, striking sexual dimorphisms in size, shape, and cellular morphology have been demonstrated in various hypothalamic subregions [Hofman and Swaab, 1989]. For instance, morphometric studies have reported that the volume of the preoptic area is more than twice as large in men than in women [Allen et al., 1989] and contains about twice as many cells [Allen et al., 1989; Hofman and Swaab, 1991]. Furthermore, there is some evidence that gender differences also exist relative to the synaptic patterns of connectivity of this hypothalamic region [Raisman and Field, 1971]. It has been proposed that such dimorphism, which may result from the early influences of gonadal hormones [Goy and McEwen, 1980], might somehow be linked to gender differences in sexual behavior [Allen et al., 1989; Hofman and Swaab, 1991]. Along the same lines, the central division of the bed nucleus of the stria terminalis (BSTc), a hypothalamic structure playing an important role in the regulation of mammalian sexual behavior [Emery and Sachs, 1976], has recently been found to be significantly larger in men than in women [Zhou et al., 1995]. Although the size of this area seems to be independent of sexual orientation, a female‐sized BSTc was demonstrated to be a correlate of male‐to‐female transsexuality [Zhou et al., 1995]. These findings can be taken as supporting the view that various divisions of the hypothalamus play a key role in the regulation of human sexuality.
From a phenomenological point of view, several lines of evidence indicate that men generally report greater SA to visually presented erotic stimuli than women [Kinsey et al., 1948, 1953; Koukounas and McCabe, 1997; Murnen and Stockton, 1997], and that men's reports of SA usually correlate highly with physiological indices of arousal whereas in women, such a correlation has often failed to be evidenced [Koukounas and McCabe, 1997; Rosen and Beck, 1988].
The present functional magnetic resonance imaging (fMRI) study was undertaken to compare the neural substrate of SA in healthy male and female subjects. For both male and female subjects, activations in limbic areas were expected. Furthermore, given the psychological gender differences related to SA, and the neurobiological gender differences found in the hypothalamus, we predicted that viewing erotica would be associated with a different pattern of hypothalamic activation in male vs. female subjects.
METHODS
Selection and validation of stimuli
Two types of visual material were used for the validation of stimuli: erotic film excerpts and emotionally neutral film excerpts. Subjects recruited for the validation of stimuli did not participate in the present fMRI study.
Erotic stimuli
Erotic stimuli consisted of film excerpts depicting sexual interactions between a man and a woman. Vaginal intercourse was displayed in some of the scenes. Given that women tend to experience more disgust than men during viewing of erotic film excerpts [Koukounas and McCabe, 1997], a selection procedure was implemented to minimize the chances of selecting excerpts that female subjects would perceive as disgusting. To do so, 20 erotic film excerpts were first presented to five female subjects for evaluation (mean age ± SD of 25 ± 3 years). Each subject, sitting alone in a room, was asked to rate, for each excerpt, perceived disgust on a scale ranging from 0 (lowest) to 8 (highest). On the basis of these ratings, an average score was established for each excerpt. The six excerpts with the lowest average scores for disgust were collated to make up one erotic film segment lasting 179 sec. The reported average score of subjective disgust for these six excerpts was 0.167.
Neutral stimuli
Stimuli designated as emotionally neutral were chosen from a series of more than 120 short film excerpts selected by the present investigators based on their assumed lack of potential to induce any significant emotional reaction. Social interactions and human faces were present in all excerpts. Twenty subjects, 10 males and 10 females, were recruited for the validation of these excerpts. The mean age ± SD was 26 ± 2 years for males and 24 ± 3 years for females. Each subject, sitting alone in a room, had to report on scales, ranging from 0 (lowest) to 8 (highest), the levels of surprise, amusement, sadness, fear, disgust, and anger produced by each excerpt. For each excerpt, an average score was calculated as a compound of all six basic emotions [Ekman, 1992]. Thirteen excerpts had an average score below 1. From these 13 excerpts, six were chosen randomly and collated to make up one neutral film segment lasting 179 sec.
Electrodermal and subjective responses to retained stimuli
Twelve new subjects (six males and six females) were recruited to evaluate the electrodermal and subjective responses to both the erotic and neutral film segments. The mean age of these subjects ± SD was 27 ± 3 years for males and 20 ± 1 years for females. Each subject was first asked to sit quietly alone in a silent room for a period of 15 min while a baseline measure of electrodermal responses, based on the number of supra‐threshold discrete increases in skin conductance, was established. Subjects were then shown both erotic and neutral film segments. The order of presentation of the segments was counterbalanced across subjects. Segments were separated by a period of 10 min during which each subject had to report on scales, ranging from 0 (lowest) to 8 (highest), the levels of SA, surprise, amusement, sadness, fear, disgust, and anger produced by each segment. Two male subjects and one female subject were excluded from analysis because of technical problems. An analysis of variance revealed, for male subjects only, a significantly greater number of discrete increases in skin conductance during the erotic condition than during both neutral and baseline conditions (P < 0.001). Male subjects also showed a significantly greater number of discrete increases in skin conductance than female subjects during the erotic condition (P < 0.005). The reported averages of subjective SA for these subjects were 4.8 (SD = 1.1) for male subjects and 3.2 (SD = 1.7) for female subjects. These ratings of SA were significantly greater in male than in female subjects (P < 0.05). For the neutral condition, the average of all seven scales of emotional reaction was 0.6 (SD = 0.4) for male subjects and 0.5 (SD = 0.4) for female subjects.
Subjects
Forty right‐handed heterosexual university students (20 males and 20 females) with no history of neurological or psychiatric illness participated in this study. Right‐handedness was determined using the Edinburgh Laterality Scale [Oldfield, 1971]. Mean age ± SD was 25 ± 4 years for male subjects and 24 ± 3 years for female subjects. Subjects were asked to avoid sexual contact leading to orgasm for at least 24 hr before imaging. Only female subjects outside of their ovulatory period, that is, in a period ranging from less than 11 days or more than 17 days after the beginning of their last menses, participated to this study. Follow‐up phone calls were made to verify the date of the beginning of these females' next menses. Subjects having been scanned during a period ranging between 11 and 17 days before their next menses were to be discarded from the study. No subjects needed to be discarded on the basis of this last exclusion criterion.
Experimental procedure
Subjects were scanned during two experimental conditions: an ‘erotic’ condition (E), consisting in the passive viewing of the erotic film segment, and an emotionally ‘neutral’ condition (N), consisting in the passive viewing of the neutral film segment. The neutral condition served as a baseline for possible confounding variables such as eye movements and processing of dynamic visual stimuli. Each condition lasted for the duration of each film segment, that is to say, 179 sec. The two conditions were separated by a resting period of 25.6 sec, during which subjects viewed a blank cyan screen. The order of presentation of the two experimental conditions was counterbalanced across subjects. At the end of the scanning session, each subject was asked to rate the level of his/her perceived SA on a scale ranging from 0 (lowest) to 8 (highest).
Stimuli presentation and image acquisition
During fMRI sessions, film clips were presented through goggles connected to an MR compatible video system (Resonance Technology, Inc., Van Nuys, CA). Echoplanar images (EPI) were acquired on a 1.5 Tesla MRI system (Magnetom Vision, Siemens Electric, Erlangen, Germany). Twenty‐eight slices (5 mm thick) were acquired every 6.4 seconds in an inclined axial plane, aligned with the AC‐PC axis. These T2* weighted functional images were acquired using an EPI pulse sequence (TR = 0.8 msec, TE = 54 msec, Flip = 90°, FOV = 215 mm, Matrix = 128 × 128). After functional scanning, high‐resolution data were acquired via a T1‐weighted 3D volume acquisition obtained using a gradient echo pulse sequence (TR = 9.7 msec, TE = 4 msec, Flip = 12°, FOV = 250 mm, Matrix = 256 × 256).
Image analysis
Data were analyzed using Statistical Parametric Mapping (SPM96, Wellcome Department of Cognitive Neurology, London, UK). Scans were realigned and spatially normalized using the standard Montreal Neurological Institute (MNI) template. Images were then convolved in space with a 3D isotropic Gaussian kernel (full width at half maximum, FWHM, of 8 mm) to improve the signal‐to‐noise ratio and to accommodate for residual variations in functional neuroanatomy that usually persist between subjects after spatial normalization. Effects at each and every voxel were estimated using the general linear model. Voxel values for each contrast yielded a statistical parametric map of the t statistic (SPM{t}), subsequently transformed to the unit normal distribution, SPM{Z}. A ‘random‐effects model’ was implemented to produce the E (erotica) minus N (neutral) contrasts for both groups of subjects. This model is implemented within SPM96 using a multi‐stage approach. First, each subject's data is summarized with an appropriate single image per condition. These “single scan per condition per subject” images are then entered into a group analysis i.e., a model at the between subject level using a one‐sample t‐test. The variance of these single images from subject to subject consists of contributions from both the between and within subject components of variance, in the correct proportions. This procedure allows one to make inferences on the population of which participants are deemed representative [Friston and Frackowiak, 1997; Holmes and Friston, 1998].
Height thresholds
Visual sexual stimuli can be viewed as a form of emotionally laden stimuli [Lang et al., 1995]. In humans, brain regions that previously have been reported to be implicated in the processing of emotionally laden visual stimuli include: the hypothalamus, thalamus, medial prefrontal cortex, anterior temporal cortex, occipitotemporal cortex, amygdala, hippocampal formation, and the ventral striatum [Lane et al., 1997a,b; Reiman et al., 1997]. Furthermore, the anterior cingulate, occipitotemporal, and orbitofrontal cortices, as well as the insula, the ventral striatum, the claustrum, the nucleus accumbens, the parietal lobules, the thalamus, and the hypothalamus have all been shown to respond to sexually explicit films in male subjects [Redouté et al., 2000; Stoléru et al., 1999]. For each of the brain areas above‐mentioned, a set of coordinates was calculated, for each hemisphere, by taking the average for each orthogonal axis X, Y, and Z of reported Talairach coordinates [Talairach and Tournoux, 1988]. Predetermined regions of interest (ROI) were limited by spheres having a radius of 9 mm and for center, the calculated average reported coordinates. For these a priori ROIs, height threshold was set at P < 0.001 (z = 3.09), uncorrected for multiple comparisons. For other brain areas, height threshold was set at P < 0.05, corrected for multiple comparisons.
RESULTS
Behavioral data
From a subjective point of view, the viewing of the erotic film segment was reported to induce a transient state of SA in both male and female subjects. The mean levels of reported SA ± SD were 3.8 ± 1.2 for male subjects and 2.6 ± 1.7 for female subjects. These ratings of SA were significantly greater in male subjects than in female subjects (P < 0.005).
fMRI data
When the blood oxygen level dependant (BOLD) activity associated with viewing the emotionally neutral film segment was subtracted from that associated with viewing the erotic film segment, significant (P < 0.001, uncorrected) bilateral loci of activation were noted, in both male and female subjects, in the medial prefrontal cortex (BA 9 and 10), the orbitofrontal cortex (BA 47), the anterior cingulate cortex (BA 24), the insular cortex, the occipitotemporal cortex (BA 21, 37, 39), the amygdala, and the ventral striatum (Table I, Fig. 1, 2).
Table I.
Voxels with peak Z scores within regions of significant cerebral BOLD signals in men and women
| Region | Brodmann area | Talairach coordinates | Z‐score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Males | |||||
| Left medial prefrontal cortex | 9 | −2 | 55 | 18 | 3,84 |
| Right medial prefrontal cortex | 9 | 2 | 61 | 15 | 3,85 |
| Left orbitofrontal cortex | 47 | −48 | 21 | −8 | 3,42 |
| Right orbitofrontal cortex | 47 | 44 | 20 | −20 | 3,63 |
| Left cingulate gyrus | 24 | −2 | 15 | 23 | 3,12 |
| Right cingulate gyrus | 24 | 2 | 21 | 23 | 3,4 |
| Left thalamus | −2 | −13 | 10 | 3,42 | |
| Right thalamus | 4 | −13 | 10 | 3,59 | |
| Left hypothalamus | −4 | −5 | −15 | 4,48 | |
| Right hypothalamus | 4 | −5 | −15 | 4,18 | |
| Left insular cortex | −40 | 9 | −11 | 3,66 | |
| Right insular cortex | 40 | 9 | −14 | 3,66 | |
| Left ventral striatum | −24 | −4 | −4 | 4,42 | |
| Right ventral striatum | 20 | −4 | −6 | 4,11 | |
| Left amygdala | −18 | −8 | −16 | 4,27 | |
| Right amygdala | 14 | −9 | −18 | 3,28 | |
| Left occipitotemporal cortex | 21 | −51 | −54 | 16 | 3,28 |
| Right occipitotemporal cortex | 37 | 50 | −54 | 3 | 3,88 |
| Females | |||||
| Left medial prefrontal cortex | 10 | −4 | 53 | 3 | 3,12 |
| Right medial prefrontal cortex | 10 | 2 | 58 | −1 | 4,41 |
| Left orbitofrontal cortex | 47 | −36 | 18 | −19 | 4,08 |
| Right orbitofrontal cortex | 47 | 51 | 19 | −12 | 3,31 |
| Left cingulate gyrus | 24 | −2 | 15 | 21 | 3,09 |
| Right cingulate gyrus | 24 | 4 | 19 | 21 | 3,64 |
| Left insular cortex | −32 | 9 | −16 | 3,65 | |
| Right insular cortex | 30 | 9 | −18 | 3,64 | |
| Left ventral striatum | −18 | −5 | −6 | 3,67 | |
| Right ventral striatum | 16 | −5 | −4 | 3,56 | |
| Left amygdala | −18 | −1 | −22 | 3,97 | |
| Right amygdala | 18 | −1 | −20 | 4,84 | |
| Left occipitotemporal cortex | 39 | −59 | −55 | 23 | 3,43 |
| Right occipitemporal cortex | 21 | 61 | −55 | 12 | 3,41 |
Brain regions showing significant BOLD signal increases in male and female subjects when the brain activity associated with viewing the neutral film excerpts were subtracted from that associated with viewing the erotic film excerpts. Coordinates, given in mm, are defined in the Talairach and Tournoux stereotaxic space (Talairach and Tournoux, 1988).
Figure 1.
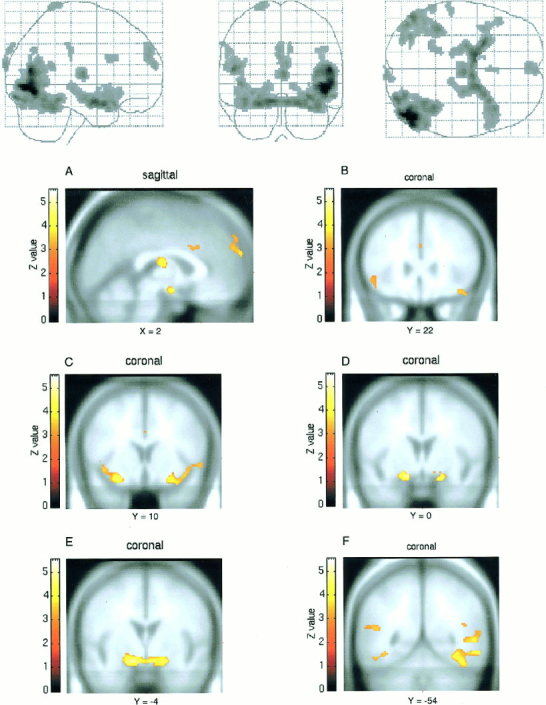
Areas of significant BOLD signal increases in male subjects when brain activation associated with viewing the emotionally neutral film excerpts were subtracted from that associated with viewing the erotic film excerpts. Accompanying sagittal and coronal slices through ROIs are provided for the sake of clarity. BOLD signal changes for these slices are coregistered on top of normalized brain sections from the Montreal Neurological Institute stereotaxic space. Coordinates (x and y) are given in mm and refer to locations in the stereotaxic atlas of Talairach and Tournoux [1988]. Regions of activation are displayed as a Z score‐statistical map coded according to the color bars. Height threshold is set at P < 0.001 (z = 3.09). The neurological convention has been chosen for orientation of coronal sections in all figures i.e., left is left and right is right. A: Medial prefrontal cortex, anterior cingulate cortex, thalamus and hypothalamus. B: Orbitofrontal cortex. C: Insular cortex. D: Amygdala. E: Ventral striatum and hypothalamus. F: Occipitotemporal cortex.
Figure 2.
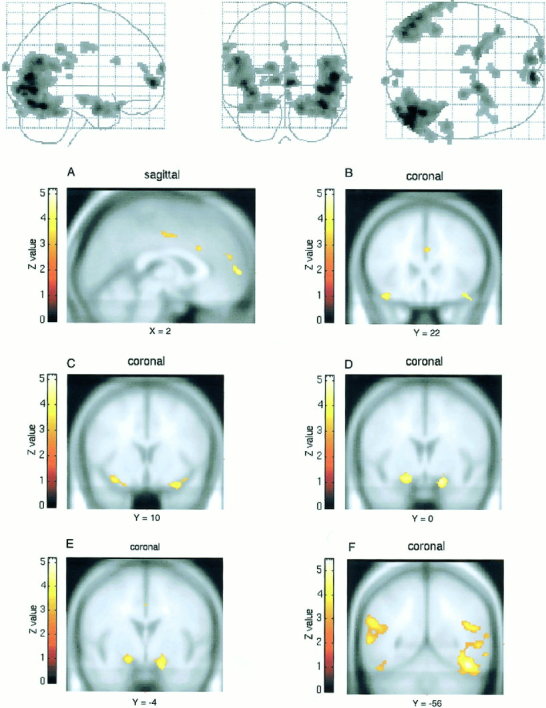
Areas of significant BOLD signal increases in female subjects when brain activation associated with viewing the emotionally neutral film excerpts were subtracted from that associated with viewing the erotic film excerpts. As in Figure 1, height threshold is set at P < 0.001 (z = 3. 09). A: Medial prefrontal cortex, anterior cingulate cortex, thalamus and hypothalamus. B: Orbitofrontal cortex. C: Insular cortex. D: Amygdala. E: Ventral striatum. F: Occipitotemporal cortex.
Moreover, male subjects showed significant activation in the thalamus (z = 3.59, P < 0. 0002, uncorrected) and hypothalamus (z = 4.48, P < 0. 000004, uncorrected) (Table I, Fig. 1). In contrast, in female subjects, a non‐significant trend in hypothalamic activation was detected (z = 2.59, P < 0. 0048, uncorrected) and no thalamic activation was evidenced.
For a priori established ROIs, only in the hypothalamus was the activation significantly greater in male subjects than in female subjects (z = 3.11, P < 0.001, uncorrected) (Fig. 3). When the rating of perceived SA was used as a covariate in the analysis, no gender difference was evidenced in hypothalamic activation at the uncorrected P < 0.001 threshold. Furthermore, no a priori determined ROIs were found to be significantly greater in female subjects. For areas not a priori determined, no activation was detected in any gender for a corrected P < 0.05 threshold and no gender difference was detected when using such a threshold.
Figure 3.
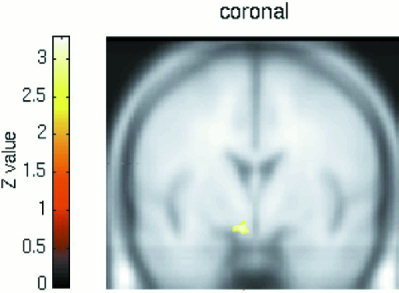
Hypothalamic activation for the Male minus Female contrast when brain activation associated with viewing the emotionally neutral film excerpt was subtracted from that associated with viewing the erotic film excerpt. Height threshold is set at P < 0.001 (z = 3. 09).
Regression analysis
Given the pivotal role of the hypothalamus in sexual behavior, regression maps were also produced to assess the significance of the association between reported individual level of SA and magnitude of hypothalamic activation. This analysis, which was implemented by using an analysis of covariance, was conducted on all 40 subjects, with the groups of male and female subjects analyzed separately. At each voxel, the significance of the regression was assessed by a t statistic that was subsequently transformed into a Z score.
The ROI for the regression analysis was restricted to the volume of the evidenced hypothalamic activation in male subjects, that is, 158 mm3. Height threshold levels were calculated by taking into account this limited search volume, using Tstat_threshold. This matlab code, which is based on the work of Worsley et al. [1996], calculates acceptable t‐level as a function of search volume, FWHM, voxel volume, degrees of freedom, and desired significance level. Given the above, to target a corrected P < 0.05 level, acceptable height threshold was calculated to be P < 0.004 (z = 2.65), uncorrected.
This regression analysis revealed the existence of a positive correlation between male subjects' reports of SA and magnitude of hypothalamic activation (z = 3.27, P < 0. 0006, uncorrected) (Fig. 4). No such correlation was detected in female subjects, even for a low height threshold value of P < 0.1 (z = 1.29), uncorrected.
Figure 4.
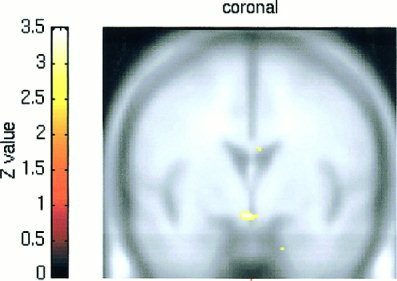
Regression map showing significant positive correlation between individual BOLD signal increases in the hypothalamus and individual reported SA in male subjects. Height threshold is set at P < 0.004 (z = 2.65).
DISCUSSION
From a phenomenological point of view, the results of this study are consistent with previous work suggesting that men generally experience greater SA than women in response to erotica [Kinsey et al., 1948, 1953; Koukounas and McCabe, 1997; Murnen and Stockton, 1997]. From a neurobiological perspective, viewing of the erotic film segment produced, in both male and female subjects, significant bilateral activation in the medial prefrontal, orbitofrontal, insular, and occipitotemporal cortices, as well as in the amygdala, the ventral striatum, and the anterior cingulate cortex. In male subjects only, processing of the erotic film segment was also associated with significant activation in the hypothalamus and thalamus. Furthermore, a regression analysis revealed the existence of a positive correlation between the intensity of the SA experienced by male subjects and the magnitude of hypothalamic activation. No such correlation was noted in female subjects.
Finding significant hypothalamic activation, in male subjects, in response to visually presented erotic stimuli, is in agreement with the known involvement of the hypothalamus in the regulation of sexual behavior and physiological arousal [Allen et al., 1989; Kupfermann, 1991; Pfaff et al., 1994; Sachs and Miesel, 1994] and is consistent with results of a recent PET study [Redouté et al., 2000] that demonstrated, in males, a correlation between activation in the hypothalamus and measures of penile tumescence.
By revealing the existence of a functional gender difference in the hypothalamus, the present study points to the existence of certain dissimilarities in the way men and women respond to visually presented erotic stimuli. More specifically, the greater hypothalamic activation found in males could be viewed as suggesting that male subjects were physiologically more aroused than female subjects in response to the erotic film segment. The positive correlation, in male subjects, between magnitude of hypothalamic activation and reported level of SA might be taken as mirroring previous work showing a high degree of concordance between men's reported SA and physiological indices of sexual response, such as penile tumescence [Sakheim et al., 1984]. In the same vein, not evidencing, here, a relationship between magnitude of hypothalamic activation and reported level of SA in female subjects fits rather nicely with the failure, in prior studies, to consistently establish a strong concordance between reported SA and physiological indices of sexual response in women, such as increase in vaginal blood volume [Koukounas and McCabe, 1997; Rosen and Beck, 1988].
The area of the thalamus found activated here, in male subjects, appears to be in the general vicinity of the mediodorsal nucleus. In primates, it has been suggested that this thalamic nucleus is part of a neural network comprising also the amygdala and the orbitofrontal cortex. This putative network is potentially involved in emotional processing [Barbas, 2000]. Interestingly, the thalamus represents a hub from which any area in the cortex can communicate with any other such area. This extensive thalamocortical interconnectivity has been theorized to constitute a neuronal basis for conscious awareness [Linas et al., 1998]. In light of such a view, the greater SA experienced by male subjects might be related to the fact that female subjects had no significant thalamic activation. If this hypothesis were accurate, then the thalamus would be implicated in the cognitive dimension of SA. Whatever is the case, given the lack of a statistically significant gender difference in thalamic activation, this gender difference should be viewed here only as a trend and, hence, considered cautiously.
With the exception of the hypothalamus and the thalamus, there was a high degree of similitude, between male and female subjects, in terms of the brain regions associated with viewing erotic film excerpts. The amygdala represents one such instance. Functional brain imaging data recently gathered by our group [Beauregard et al., in press], as well as by other researchers [O'Doherty et al., 2001], indicate that the amygdala can be activated by emotionally pleasant stimuli, besides being implicated in the appraisal of affectively unpleasant stimuli [Breiter et al., 1996a, 1996b; Lane et al., 1997b; Morris et al., 1996; Reiman et al., 1997; Whalen et al., 1998]. The amygdala activation noted here may thus be related to the appraisal process through which the erotic stimuli depicted in the erotic film excerpts were evaluated as a sexual incentive.
Activation of the occipitotemporal area accords with results of recent functional neuroimaging studies showing that, when compared to neutral visual stimuli, emotionally laden visual stimuli elicit increased activation in this cortical region [Beauregard et al., 1998; Lane et al., 1997a, 1999]. Assuming that viewing emotionally laden stimuli automatically elicits increased attentional tapping, the occipitotemporal activation noted in this study would be consistent with the hypothesis that attention to visual stimuli can modulate neural activity in the extrastriate visual cortex [Büchel et al., 1998; Chawla et al., 1999; Corbetta et al., 1991; Lane et al., 1997b; O'Craven et al., 1997; Treue and Maunsell, 1996].
Intraoperative stimulation of the insular cortex before temporal lobectomy has been shown, in human epileptic patients, to evoke autonomic reactions [Oppenheimer et al., 1992]. This is consistent with the fact that the insular cortex is highly interconnected with regions involved in autonomic regulation [Cechetto, 1994]. Given such findings, the insular activation noted here might be a neural correlate of the autonomic changes associated with SA.
Given that regions of the orbitofrontal cortex have been shown to be implicated in the representation of rewards [Francis et al., 1999], Stoléru and colleagues [Redouté et al., 2000] suggested that the orbitofrontal activation noted in their PET study might have been related to the representation of the pleasant bodily sensations induced by penile tumescence. In the context of the present study, activation in this area was observed in both men and women. On this basis and assuming that Stoléru et al. [1999] hypothesis is correct, we tentatively suggest that activation of this area might be related to the representation of pleasant bodily sensations induced by SA.
The medial prefrontal cortex has previously been shown to be activated during the recall of happy, sad or disgusting moments in one's life or during viewing of stimuli known to elicit these three emotional states [Lane et al., 1997a]. These findings led Lane et al. [1997a] to suggest that this region participates in aspects of emotional processing that are independent of valence, type or method of induction. Although the exact function of this cortical area remains to be elucidated, there is some evidence that the medial prefrontal cortex is involved in the conscious experience of emotion [Reiman et al., 1997]. Here, we concur with Redouté et al. [2000] that the activation seen in the medial prefrontal cortical region may be related to the level of perceived emotion and not to the sexual quality of emotion per se.
In both male and female subjects, activation was found here in the dorsal cognitive subdivision of the anterior cingulate cortex (or ACcd) [areas 24 b'‐c'; see Bush et al., 2000; Devinsky et al., 1995]. This subdivision of the anterior cingulate cortex, which has reciprocal connections with premotor and supplementary motor areas, has been shown to be involved in skeletomotor control in monkeys [Devinsky et al., 1995]. We propose that the activation of ACcd might be related here to the executive modulation of the skeletomotor activities that normally characterize SA.
Activation of the caudate nucleus has been reported to correlate with the urge to perform handwashing rituals in patients suffering from obsessive‐compulsive disorder (OCD) [McGuire et al., 1994]. Stoléru et al. [1999] noted that in both the above‐mentioned McGuire et al. [1994] study and during viewing of erotica, subjects were simultaneously confronted with the urge to act and with the impossibility to do so, hence suggesting a role for the ventral striatum in the control of the motor expression of SA, that is, in withholding the motor output of SA. Such a role could be implemented through the anatomic projections that the striatum receives from the cognitive subdivision of the anterior cingulate cortex [Devinsky et al., 1995].
One of the limitations of this study concerns the lack of objective measures of SA. These measures, which would have helped controlling the effect of potentially confounding variables (e.g., desire to conform, shyness, and the like), were not collected because magnetic susceptibilities associated with the psychophysiological apparatus required to do such measures tend to hinder the concomitant gathering of fMRI and psychophysiological data.
Another limitation pertains to selecting only female subjects outside of their ovulatory period. This selection criterion was chosen on the basis of the eventuality that the sudden surges in LH (luteinizing hormone) and FSH (follicle stimulating hormone) known to occur at mid menstrual cycle might influence brain activation patterns. Given that female mammals of various species become sexually more responsive at mid menstrual cycle [Harvey, 1987; Spitz et al., 1975], one could argue that such a selection procedure resulted in a bias in terms of sexual arousability that might explain, at least partially, the gender differences found here. Evidence for an association between modulation of sexual receptivity or arousability and phases of the menstrual cycle in human females, however, appears inconsistent and even contradictory [Meuwissen and Over, 1992]. Still, given the uncertainty about this issue, for the present results to be generalized to the entire female population, neural correlates of viewing visual erotica will have to be examined in females at mid menstrual cycle.
In view of the usual fMRI standards, one may justifiably question the duration (179 sec) of both the emotionally neutral and erotic film segments. Several factors explain the choice of such a design. First, it has been shown that, for both male and female subjects, SA evoked in response to visually presented erotic stimuli accrues for at least the first 2 min of viewing such stimuli [Rubinsky et al., 1987]. Interestingly, the erotic film clips presented to male subjects in the Redouté et al. [2000] study lasted also 3 min. Second, a recent functional neuroimaging study [Lazar et al., 2000] of relaxation response and meditation has demonstrated that it is possible to use fMRI to measure, during relatively long periods of time (e.g., 6 min blocks), BOLD signal changes that can be differentiated from low‐frequency noise. Finally, recent work carried out by our group (unpublished observations) indicates that the present results are replicable using a more conventional fMRI paradigm (30–40 sec epochs with repeated measurements).
CONCLUSION
It was demonstrated here that, in both male and female subjects, viewing erotic film excerpts was associated with the functional activation of a neural circuit encompassing brain areas that have recently been claimed to be involved in the various dimensions that characterize SA. By providing evidence for gender differences in patterns of response to sexual stimuli in the hypothalamus, these results point to the existence of differences in the way men and women process visual sexual stimuli.
Acknowledgements
This work was supported by studentships from the MD/PhD program of Université de Montréal and from the FCAR/FRSQ Funds to S.K., and by a grant from the Département de radiologie, Faculté de médecine, Université de Montréal to M.B. We thank Drs. Karl Friston and Keith Worsley for their help regarding statistical analyses. We are also grateful to Drs. Laurent Descarries, Eric Fimbel, Yves Joanette, and Sonia Lupien for their judicious comments. Finally, we wish to thank the staff of the Centre hospitalier de l'Université de Montréal (CHUM), Hôpital Notre‐Dame, Département de radiology, as well as Christian Carola, André Gamache, Jean‐Sébastien Robitaille, and Paule Samson for their skillful technical assistance.
REFERENCES
- Allen LS, Hines M, Shryne JE, Gorski RA (1989): Two sexually dimorphic cell groups in the human brain. J Neurosci 9: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendash GW, Gorski RA (1982): Enhancement of sexual behavior in female rats by neonatal transplantation of brain tissue from males. Science 217: 1276–1278. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C (1990): Copulatory behavior is controlled by the sexually dimorphic nucleus of the quail POA. Brain Res Bull 25: 7–14. [DOI] [PubMed] [Google Scholar]
- Barbas H (2000): Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull 52: 319–330. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Karama S, Leroux JM, Lecours AR, Beaudoin G, Bourgouin P (1998): The functional neuroanatomy of amusement, disgust and sexual arousal. Paper presented at the 4th International Conference on Functional Mapping of the Human Brain. Montréal, Québec, Canada.
- Beauregard M, Lévesque J, Bourgouin P (2001): Neural correlates of the conscious self‐regulation of emotion. J Neurosci 21: RC165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR. (1996a): Functional magnetic resonance imaging of symptom provocation in obsessive‐compulsive disorder. Arch Gen Psychiatry 53: 595–606. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen WA, Kennedy DN, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. (1996b): Response and habituation of the human amygdala during visual processing of facial expression. Neuron 17: 875–887. [DOI] [PubMed] [Google Scholar]
- Büchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ (1998): The functional anatomy of attention to visual motion: a functional MRI study. Brain 121: 1281–1294. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4: 215–222. [DOI] [PubMed] [Google Scholar]
- Carrer H, Asch G, Aron C (1973): New facts concerning the role played by the ventromedial nucleus in the control of estrous cycle duration and sexual receptivity in the rat. Neuroendocrinology 13: 129–138. [DOI] [PubMed] [Google Scholar]
- Cechetto DF (1994): Identification of a cortical site for stress‐induced cardiovascular dysfunction. Integr Physiol Behav Sci 29: 362–373. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ (1999): The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676. [DOI] [PubMed] [Google Scholar]
- Clark AS, Pfeifle JK, Edwards DA (1981): Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol Behav 27: 597–602. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE (1991): Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by positron emission tomography. J Neurosci 11: 2383–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behavior. Brain 118: 279–306. [DOI] [PubMed] [Google Scholar]
- Dorner G, Docked F, Gotz F (1975): Male‐like sexual behavior of female rats with unilateral lesions in the hypothalamic ventromedial nucleus region. Endokrinologie 65: 133–137. [PubMed] [Google Scholar]
- Ekman P (1992): An argument for basic emotions. Cogn Emotion 6: 169–200. [Google Scholar]
- Emery DE, Sachs BD (1976): Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav 17: 803–806. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10: 453–459. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frackowiak RSJ (1997): Images of the future: a philosophical coda In: Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Mazziotta JC, editors. Human brain function. San Diego: Academic Press; p 487–517. [Google Scholar]
- Goy RW, McEwen BS (1980): Sexual differentiation of the brain. Cambridge: MIT Press; 223 p. [Google Scholar]
- Hart BL (1974): The medial preoptic‐anterior hypothalamic area and sociosexual behavior of male dogs: a comparative neuropsychological analysis. J Comp Physiol Psychol 86: 328–349. [DOI] [PubMed] [Google Scholar]
- Harvey SM (1987): Female sexual behavior: fluctuations during the menstrual cycle. J Psychosom Res 31: 101–110. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF (1991): Sexual dimorphism of the human brain: myth and reality. Exp Clin Endocrinol 98: 161–170. [DOI] [PubMed] [Google Scholar]
- Hofman MA, Swaab DF (1989): The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat 164: 55–72. [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ (1998): Generalizability, random effects and population inference [Abstract]. Neuroimage 7: S754. [Google Scholar]
- Kinsey AC, Pomeroy WB, Martin CE (1948): Sexual behavior in the human male. Philadelphia: Saunders; 804 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey A, Pomeroy W, Martin C, Gebhard P (1953): Sexual behavior in the human female. Philadelphia: Saunders; 863 p. [Google Scholar]
- Koukounas E, McCabe M (1997): Sexual and emotional variables influencing sexual response to erotica. Behav Res Ther 35: 221–231. [DOI] [PubMed] [Google Scholar]
- Kupfermann I (1991): Hypothalamus and limbic system: peptidergic neurons, homeostasis, and emotional behavior In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. Norwalk: Appleton and Lange; p 735–749. [Google Scholar]
- Lane RD, Reiman EM, Geoffrey LA, Schwartz GE, Davidson RJ (1997a): Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry 154: 926–933. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. (1997b): Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 35: 1437–1444. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM‐L, Dolan RJ (1999): Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 37: 989–997. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (1995): The International Affective Picture System (IAPS): photographic slides. University of Florida: Center for Research in Psychophysiology.
- Larsson K, Heimer L (1964): Mating behavior of male rats after lesions in the preoptic area. Nature 202: 413–414. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H (2000): Functional brain mapping of the relaxation response and meditation. Neuroreport 11: 1581–1585. [PubMed] [Google Scholar]
- Linas R, Ribary U, Contreras D, Pedroarena C (1998): The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci 353: 1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey MJ, Pickford GE, Peter RE (1974): Forebrain localization of the spawning reflex response to exogenous neurohypophysial hormones in the killifish, Fundulus heteroclitus . J Exp Zool 190: 269–280. [DOI] [PubMed] [Google Scholar]
- Malsbury CW, Kow L‐M, Pfaff DW (1977): Effects of medial hypothalamic lesions on the lordosis response and other behaviors in female golden hamsters. Physiol Behav 19: 223–237. [DOI] [PubMed] [Google Scholar]
- Malsbury CW (1971): Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol Behav 7: 797–805. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA (1977): The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm Behav 8: 40–51. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RSJ, Dolan RJ (1994): Functional anatomy of obsessive‐compulsive phenomena. Br J Psychiatry 164: 459–468. [DOI] [PubMed] [Google Scholar]
- Meuwissen I, Over R (1992): Sexual arousal across phases of the human menstrual cycle. Arch Sex Behav 21: 101–119. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383: 812–815. [DOI] [PubMed] [Google Scholar]
- Murnen SK, Stockton M (1997): Gender and self‐reported sexual arousal in response to sexual stimuli: a meta‐analytic review. Sex Roles 37: 135–153. [Google Scholar]
- O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL (1997): Voluntary attention modulates fMRI activity in human MT‐MST. Neuron 18: 591–598. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F (2001): Representation of pleasant and aversive taste in the human brain. J Neurophysiol 85: 1315–1321. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC (1992): Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727–1732. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz‐Giblin S, McCarthy MM, Kow L‐M (1994): Cellular and molecular mechanisms of female reproductive behaviors In: Knobil E, Neill JD, editors. Physiology of reproduction. New York: Raven Press; p 107–220. [Google Scholar]
- Raisman G, Field PM (1971): Sexual dimorphism in the preoptic area of the rat. Science 173: 731–733. [DOI] [PubMed] [Google Scholar]
- Redouté J, Stoléru S, Grégoire M‐C, Costes N, Cinotti N, Lavenne F, Le Bars D, Maguelone GF, Pujol J‐F. (2000): ) : Brain processing of visual sexual stimuli in human males. Hum Brain Mapping 11: 162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K. (1997): Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Beck JG (1988): Patterns of sexual arousal. New York: The Guilford Press; 404 p. [Google Scholar]
- Rubinsky HJ, Eckerman DA, Rubinsky EW, Hoover CR (1987): Early‐phase physiological response patterns to psychosexual stimuli: comparison of male and female patterns. Arch Sex Behav 16: 45–56. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Meisel RL (1994): The physiology of male sexual behavior In: Knobil E, Neill JD, editors. Physiology of reproduction. New York: Raven Press; p 3–105. [Google Scholar]
- Sakheim DK, Barlow DH, Beck JG, Abrahamson DJ (1984): The effect of an increased awareness of erectile cues on sexual arousal. Behav Res Ther 22: 151–158. [DOI] [PubMed] [Google Scholar]
- Spitz CJ, Gold AR, Adams DB (1975): Cognitive and hormonal factors affecting coital frequency. Arch Sex Behav 4: 249–263. [DOI] [PubMed] [Google Scholar]
- Slimp JC, Hart BL, Goy RW (1978): Heterosexual, autosexual and social behavior of adult male rhesus monkeys with medial preoptic‐anterior hypothalamic lesions. Brain Res 142: 105–122. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Grégoire M‐C, Gérard D, Decety J, Lafarge E, Cinotti L, Lavenne F, LeBars D, Vernet‐Maury E, Rada H, Collet C, Mazoyer B, Forest MG, Magnin F, Spira A, Comar D. (1999): Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 28: 1–21. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme; 122 p. [Google Scholar]
- Treue S, Maunsell JH (1996): Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382: 539–541. [DOI] [PubMed] [Google Scholar]
- Twiggs DG, Popolow HB, Gerall AA (1978): Medial preoptic lesions and male sexual behavior: Age and environmental interactions. Science 200: 1414–1415. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998): Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JM, Crews D (1978): The role of the anterior hypothalamus‐preoptic area in the regulation of male reproductive behavior in the lizard, Anolis carolinensis: lesion studies. Horm Behav 11: 42–60. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapping 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Zhou J‐N, Hofman MA, Gooren LJG, Swaab DF (1995): A sex difference in the human brain and its relation to transsexuality. Nature 378: 68–70. [DOI] [PubMed] [Google Scholar]


