Abstract
Monitoring one's thoughts (in the verbal modality) is thought to be critically dependent on the interaction between areas that generate and perceive inner speech in the frontal and temporal cortex, respectively. We used functional magnetic resonance imaging (fMRI) to examine the relationship between activity in these areas while the rate of inner speech generation was varied experimentally. The faster rate was associated with activation in the left inferior frontal gyrus, the right pre‐ and postcentral gyri and both superior temporal gyri. Thus, temporal cortical activation was associated with increasing the rate of covert articulation, in the absence of external auditory input, suggesting that there is effective fronto‐temporal connectivity. Furthermore, this may provide support for the existence of feed forward models, which suggest that activity in regions responsible for verbal perception is modulated by activity in areas that generate inner speech. Hum. Brain Mapping 16:219–227, 2002. © 2002 Wiley‐Liss, Inc.
Keywords: inner speech, temporal lobe, frontal lobe, modulation, functional neuroimaging
INTRODUCTION
Electrophysiological recordings in non‐human primates [Müller‐Preuss and Ploog, 1981] and man [Creutzfeldt et al., 1989; Numminen and Curio, 1999] indicate that neuronal activity in the temporal cortex is powerfully modulated by vocalization. This modulation can precede articulation by hundreds of milliseconds, suggesting that it is related to the intention to speak (rather than articulation per se) and may be mediated by the direct anatomical connections that link areas that generate and perceive speech [Pandya and Yeterian, 1985; Romanski et al., 1999], in the frontal and temporal cortex respectively. Similarly, some positron emission tomography (PET) studies have indicated that when subjects generate words, the left dorsolateral prefrontal cortex (DLPFC) is activated whereas the bilateral temporal cortex is de‐activated [Friston et al., 1991; Frith et al., 1991]. These studies suggest that output from regions involved in verbal generation may modulate activation in areas involved in speech perception, perhaps to inform them that impending verbal stimuli are self‐generated.
Most of the neuroimaging evidence for modulation of temporal activation during verbal generation is derived from studies of verbal fluency. As well as articulation, generating a word in response to a cue involves phonological and semantic processing, processes that could also account for changes in frontal and temporal activation [Binder et al., 2000; Burton et al., 2000; Poldrack et al., 1999; Wise et al., 1999]. In the present study, we used functional magnetic resonance imaging (fMRI) to assess the relationship between frontal and temporal activation during the generation of the same word at different rates, to minimize phonological and semantic processing. We studied covert as opposed to overt articulation, as this eliminated the possibility that any changes in temporal activation with increasing verbal output were simply a function of increased auditory input, rather than cortico‐cortical modulation. We were also interested in establishing whether fronto‐temporal modulation occurred during the generation of inner speech, as the interaction between areas that generate and monitor inner speech is putatively defective in patients with auditory hallucinations [Frith et al., 1995; McGuire et al., 1996]. Covert articulation during other paradigms has been associated with activation in the left inferior frontal gyrus and the superior temporal gyrus [McGuire et al., 1996; Paulesu et al., 1993; Shergill et al., 2001], but the relationship between the responses in these areas has not examined. Both increasing the complexity of covert inner speech [Shergill et al., 2001] and the frequency of overt articulation [Wise et al., 1999] have been demonstrated to increase lateral temporal activation. Thus, we predicted that: 1) increasing the rate of covert articulation would be associated with greater activation in both the left inferior frontal and the left superior temporal gyrus; and 2) there would be a positive correlation between the magnitude of activation in these regions.
MATERIALS AND METHODS
Subjects
Eight male volunteers, right‐handed according to Annett's [1970] scale, aged 23–37 years (mean age = 29, SD = 5) participated in the study. They did not suffer from medical or psychiatric disorders and were not receiving medication, and had no family history of psychiatric disorder. Their mean IQ estimated with the National Adult Reading Test [Nelson, 1991] was 115 (range = 106–117, SD = 5). Before inclusion, potential subjects were assessed on their ability to overtly repeat a word at the three rates (once every 1,2, or 4 sec) to be used during scanning. They proceeded to scanning when they consistently achieved a 1:2:4 ratio in the number of repetitions, at the respective rates, over a minute. Subjects provided written informed consent, and the local hospital ethical committee approved the study.
Tasks performed during fMRI
Fast vs. slow covert articulation (categorical comparison)
Subjects covertly generated the word “rest” repeatedly at two self‐paced rates (once every 1 or 4 sec = 60 or 15 words/min), without speaking. Their accuracy was checked by asking them to tap their finger at the two different rates both before and immediately after scanning. During scanning, the two conditions alternated in an ABAB design, with each condition lasting 30 sec and 5 cycles of each condition in one 300‐sec run. The order of conditions was counterbalanced across subjects. The desired rate during each condition was indicated by a number visible throughout in the centre of a computer screen (“1” for one word every second and “4” for one word every 4 sec).
Fast/intermediate/slow covert articulation (parametric task)
Subjects covertly generated the word “rest” repeatedly at three rates (once every 1, 2, or 4 sec = 60, 30 and 15 words/min) without articulating the word. Their accuracy was checked before and after the task, as described above. Each condition lasted 30 sec, with a minimum of 3 cycles of each condition during one 300‐sec run; the desired rate was indicated by a number on a computer screen (“1,” “2,” or “4”) as above. The order of conditions was pseudo randomized. All subjects achieved a consistent timing ratio (on finger tapping) of 1:2:4 between the fast, intermediate and slow rate, immediately before and after scanning.
Image acquisition
Gradient‐echo echoplanar MR images were acquired using a 1.5 T GE Signa System (General Electric, Milwaukee, WI) fitted with Advanced NMR hardware and software (ANMR, Woburn, MA) at the Maudsley Hospital, London. A quadrature birdcage head coil was used for RF transmission and reception. In each of 14 non‐contiguous planes parallel to the inter‐commissural (AC‐PC) plane, 100 T2*‐weighted MR images depicting BOLD contrast (11) were acquired with TE = 40 msec, TR= 3,000 msec, in‐plane resolution = 3.1 mm, slice thickness = 7 mm, slice skip = 0.7 mm; 100 images were collected in each 5‐min run. Head movement was limited by foam padding within the head coil and a restraining band across the forehead. At the same session, a 43 slice, high‐resolution inversion recovery echoplanar image of the whole brain was acquired in the AC‐PC plane with TE = 73 msec, TI = 180 msec, TR = 16,000 msec, in‐plane resolution = 1.5 mm, slice thickness = 3 mm.
Image Analysis
Image analysis was performed on a SPARC Ultra 10 workstation (Sun Microsystems, Palo Alto, CA) using MATLAB (version 5.3, The Mathworks Inc., Natick, MA) and SPM99 software (Statistical Parametric Mapping, The Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). All data sets were automatically realigned to the first image to correct for head movement, normalized using sinc interpolation and transformed into Talairach space using sinc interpolation. The transformed data set for each subject was smoothed with a Gaussian filter (Full width half maximum = 8 mm) to compensate for normal variation in anatomy across subjects. The time series were high pass (126 sec) filtered to remove low frequency artifacts.
Statistical analysis was performed for each subject, and the stereotaxically normalized fMRI time series data from all the subjects pooled for random effects group analysis. Analysis of the two condition task (15 vs. 60 words/min) used a categorical design (using a box‐car function with hemodynamic response function to create the general linear model) comparing activation evident during fast greater than slow rate of generation, and vice versa. Analysis of the three‐condition task (15, 30 and 60 words/min) used a parametric design to identify areas where activation was linearly correlated (positively and negatively) with the rate of covert articulation. Subsequently we also performed a post‐hoc analysis of the parametric design using a second order polynomial term (incorporating linear and squared components). Cluster level statistics corrected for multiple comparisons were thresholded at P < 0.05.
Correlational analysis of time series data
To clarify the polarity of any fronto‐temporal modulation (i.e., to determine whether it was positive or negative), we examined the BOLD response over time at the focus of maximal activation in six regions during the categorical task; three regions showing activation during each of the faster and slower rates were selected. These comprised the areas where we predicted modulation (the left inferior frontal and left superior temporal cortex), plus the four other most prominently activated regions. The fMRI time series, adjusted for motion correction and linear trends via a high pass filter, at the voxel showing peak response within each region was extracted for all subjects. The time series data were placed in an interregional correlation matrix to examine the functional connectivity in more detail.
RESULTS
Behavioural data
All subjects were able to perform the task within the scanning environment and showed a consistent 1:2:4 timing ratio for the 3 conditions, both pre and post scanning. As a result, data from all subjects were included in the analysis. At one per 1 sec the mean number of taps was 64 (SD 6); at one per 2 sec the mean was 31 (SD 3); and at once every 4 sec the mean was 16 (SD 2).
Categorical comparison
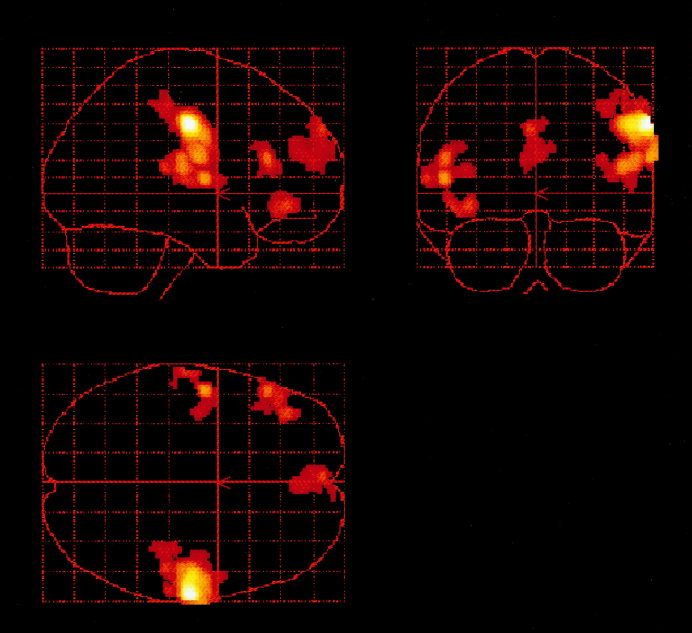
Relative to covert generation at 15 words/min, covert generation at 60 words/min was associated with activation in foci in the dorsolateral and the orbital portions of the left inferior frontal gyrus, and in the anterior part of the left superior temporal gyrus. There was also a large area of activation centered on the right precentral gyrus, which included foci in the postcentral and superior temporal gyri, and further activation in the frontal pole (Fig. 1, Table I). The slower rate of generation was associated with activation in the supplementary motor area (SMA), the left precentral gyrus and the right inferior parietal lobule (Table I).
Figure 1.
Regions associated with faster rate of generation of inner speech (60 vs. 15 words/min). Areas shown in red/gold depict clusters significantly activated during the faster rate of covert articulation relative to slower rate. Group activation maps from all eight subjects are displayed on a glass brain, and correspond to Talairach space and are described in Table I. Axial slices are displayed parallel to the anterior commissure‐posterior commissure plane, with the right side of the figure representing the right side of the subject's brain.
Table I.
Regions demonstrating significant activation during faster and slower rates (categorical analysis)
| REGION | X | Y | Z | Cluster size (Number of voxels) | Z score | P (cluster) |
|---|---|---|---|---|---|---|
| Greater activation at FASTER rate | ||||||
| Left inferior frontal gyrus (BA 45/46) | −52 | 30 | 20 | 154 | 4.9 | 0.008 |
| (BA 47) | −38 | 40 | −8 | 134 | 4.5 | 0.015 |
| Left superior temporal gyrus (BA42) | −52 | −8 | 8 | 239 | 5.4 | 0.001 |
| Right precentral gyrus (BA 6/4) | 64 | −16 | 40 | [ | 8.0 | ] |
| Right postcentral gyrus (BA 1,2,3) | 66 | −12 | 22 | [1549 | 5.5 | 0.0001] |
| Right superior temporal gyrus (BA42) | 50 | −20 | 16 | [ | 5.3 | ] |
| Frontal pole | −2 | 60 | 36 | 367 | 4.6 | 0.0001 |
| Greater activation at SLOWER rate | ||||||
| Right inferior parietal lobule (BA 40) | 54 | −48 | 40 | 541 | 6.0 | 0.0001 |
| Supplementary motor area | 4 | 6 | 58 | 143 | 4.7 | 0.01 |
| Left precentral gyrus (BA 4) | −38 | −8 | 30 | 124 | 4.4 | 0.02 |
Parametric analysis
The parametric analysis (increased activation with increased rate) revealed that as the rate of generation increased, there was activation in the left inferior frontal gyrus, the left hippocampus and precuneus, and in the right precentral gyrus, and the posterior part of the right superior temporal gyrus (Table II). Conversely, there was relatively decreased activation in the left precentral and occipital gyri, the right inferior frontal and superior temporal gyri, and the right inferior parietal lobule and precuneus (Table II).
Table II.
Regions demonstrating increasing activation with increasing rate (parametric analysis) of generation
| Region | X | Y | Z | Cluster size (Number of voxels) | Z score | P (cluster) |
|---|---|---|---|---|---|---|
| Greater activation with FASTER rate | ||||||
| Left inferior frontal gyrus (BA 45/46) | −54 | 32 | 22 | 145 | 5.4 | 0.01 |
| Right precentral gyrus (BA 6/4) | 58 | −4 | 42 | 280 | 5.5 | 0.0001 |
| Right superior temporal gyrus (BA22) | 66 | −50 | 12 | 604 | 5.3 | 0.0001 |
| Left hippocampus | −28 | −16 | −8 | 183 | 5.1 | 0.003 |
| Left precuneus (BA 7) | −26 | −82 | 42 | 114 | 4.7 | 0.03 |
| Greater activation with SLOWER rate | ||||||
| Right inferior parietal lobule (BA 40) | 58 | −42 | 50 | 158 | 5.2 | 0.007 |
| Left precentral gyrus (BA 4) | −40 | −10 | 50 | 123 | 6.2 | 0.02 |
| Right inferior frontal gyrus (BA 47) | 50 | 28 | −4 | 173 | 5.1 | 0.004 |
| Right superior temporal gyrus (BA22) | 58 | −24 | 0 | 117 | 4.8 | 0.03 |
| Right precuneus (BA 7) | 16 | −82 | 48 | 327 | 5.3 | 0.0001 |
| Left middle occipital gyrus (BA 18) | −28 | −74 | 12 | 136 | 4.7 | 0.01 |
Correlational analysis of time series data
The time series at the extracted voxel consisted of 800 time points across the eight subjects, and two‐tailed tests were reported. There was a significant positive correlation between the BOLD response in the foci in the left inferior frontal and the left superior temporal gyri (Table III, Fig. 2). The results show significant values of the Pearson coefficient, although the results were still significant when the Kendall tau or Spearman coefficient were computed. There were also negative correlations between the left inferior frontal signal and the responses in the left precentral and the right inferior parietal foci (Table III). The BOLD responses in the SMA, the left precentral gyrus and the right inferior parietal lobule were significantly inter‐correlated (Table III).
Table III.
Correlation matrix of time series from selected foci†
| SMA | R Precen | R IPL | L STG | L Precen | LIF | |
|---|---|---|---|---|---|---|
| SMA | ||||||
| R Precen | NS | |||||
| R IPL | 0.20** | NS | ||||
| L STG | NS | NS | NS | |||
| L Precen | 0.12* | NS | 0.14* | NS | ||
| LIF | NS | NS | −0.08 | 0.11* | −0.07 |
LIF, left inferior frontal gyrus (Talairach x,y,z = −52, 30, 20); L Precen, left precentral gyrus (−38, −8, 30); L STG, left superior temporal gyrus (−52, −8, 8); R IPL, right inferior parietal lobule (54, −48, 40); R Precen, right precentral gyrus (64, −16, 40); SMA, supplementary motor area (4, 6, 58).
NS, not significant P > 0.05;
P < 0.005;
P < 0.0001.
Figure 2.
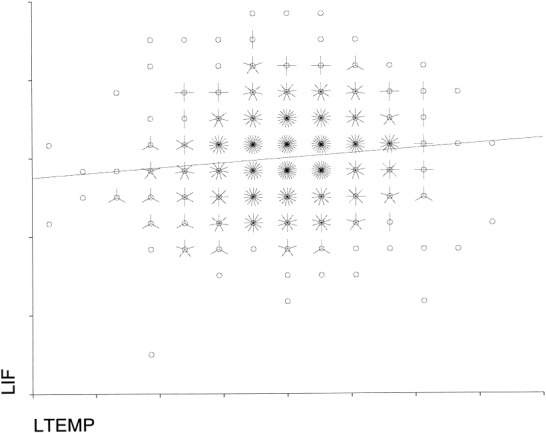
A graph of the regression between inferior frontal and temporal BOLD signal. Each petal represents a single time point; where the two time series are very close together a line has been fitted through the data.
DISCUSSION
In this study we examined the neural correlates of covertly articulating the same word at different rates, to investigate the relationship between activity in frontal and temporal cortex. Although the key difference between our conditions was the rate of covert articulation, there may also have been differences in the demands on attention and concentration. Our subjects reported being able to perform all conditions within the scanning environment, without using any explicit timing strategies (excluding subvocal counting or using their respiration rate as a cue), but indicated that they found covert articulation at 60 words/min to be less demanding than at slower rates, presumably because the timing of the generation (that was self‐paced) at 30 and 15 words/min was more difficult. Ideally, functional imaging studies of cognitive tasks should involve the measurement of behavioral performance “on‐line,” to assess how well subjects are performing during image acquisition. Covert speech, however, cannot easily be measured without introducing additional and potentially undesirable cognitive demands. We sought to minimize the influence of variation in performance by training subjects on the tasks before scanning, obtaining ratings of their performance immediately after each task, and excluding those who reported or demonstrated marked difficulties with task execution. Moreover, there was left inferior frontal cortical activation in association with faster rate of generation of inner speech consistent with that seen in previous studies of inner speech [Paus et al., 1996; Shergill et al., 2001]; this pattern of activation offers some support that the subjects were actually carrying out the tasks during the scanning.
There was no null baseline condition included in the design, because we were primarily interested in the cortical regions showing activation in line with changes in the rate of inner speech production. The inclusion of a null baseline would have permitted a better examination of the parametric experiment as there would have then been three different rates to assess; however, in the absence of such, only two rates could be compared because one rate had to act as a baseline, reducing the explanatory power of the parametric design and perhaps contributing to the discrepancy between the results of the two different analyses. It may also have assisted in clarifying increases and decreases of activation during the individual tasks.
As expected, there was activation of the left inferior frontal gyrus as the rate of covert articulation increased, and this was evident with both the categorical and parametric comparisons. The predicted activation of the left superior temporal gyrus was seen with the categorical, but not the parametric comparison, whereas both comparisons indicated that faster generation was also associated with activation of the right superior temporal gyrus. Our data are thus consistent with an association between activity in the left inferior frontal gyrus and the temporal cortex bilaterally; although the associations did not all show a linear relationship. Moreover, because our paradigm involved the covert generation of a single word, it is unlikely that the changes in temporal activation reflected increased semantic or phonological processing, or were secondary to changes in auditory input. Although fMRI is not an appropriate technique to allow the detailed examination of timing differences sufficient to confirm a modulatory effect of frontal on temporal cortices, the electrophysiological literature in primates [Müller‐Preuss and Ploog, 1981] and man [Creutzfeldt et al., 1989; Numminen and Curio, 1999] would support this interpretation of these results. These studies demonstrated differences in brain activation patterns between identical externally generated and self‐generated actions in the auditory modality; auditory cortices were activated in response to vowel changes in heard speech but not when the same vowel changes are self‐uttered suggesting that motor‐to‐sensory priming of the auditory cortex dampens the response to self‐produced “expected” sounds and occurs at a millisecond time scale.
The absence of activation in the left superior temporal gyrus with the parametric analysis may reflect an incorrect assumption that the relationship between the rate of covert articulation and temporal activation would be linear. Even when hearing words presented at different rates, the response in the left posterior temporal cortex differs from that in bilateral and more anterior temporal regions [Price et al., 1992]. Indeed a post hoc parametric analysis of our data using a combination of a linear and second order polynomial expansion, as opposed to the linear, model demonstrated additional activity within the left middle temporal cortex and hippocampus.
Our subjects reported that they found self‐paced generation at 15 and 30 words/min comparably difficult, but more demanding than at 60 words/min. This is in accordance with established models of timing assessment, which propose that temporal units of less than (or around) a 1 sec are perceived as a unit, as distinct from time intervals greater than ∼3 sec, which have to be actively estimated [Posner and Petersen, 1990]. Both the categorical and parametric analyses revealed an unpredicted activation of the right superior temporal gyrus and the right precentral gyrus at faster rates of generation. The right temporal cortex demonstrates a linear response to increasing rates of overt articulation [Wise et al., 1999] and is also deactivated (relative to repetition) during overt verbal fluency [Spence et al., 2000]. In the absence of substantial direct connections between the left prefrontal and right temporal cortex [McGuire et al., 1991; Pandya and Yeterian 1985], its activation may be more closely allied to the activation in the right precentral gyrus. The right precentral region we identified is close to an area (52, −6, 41), activated by whispering at a faster rate [Paus et al., 1996], and during covert singing [Riecker et al., 2000]. This activation could be due to subvocalization, although subjects were instructed not to articulate the word, and the method of assessing changes in frequency (tapping rather than vocalizing) was selected to reduce any tendency to articulate the word; alternatively the explanation could be that increased task demands led to the recruitment of contralateral homologous regions, as evident in a recent fMRI study of mental rotation [Carpenter et al., 1999].
Another interpretation of these results is that the observations are secondary to making a judgment of timing interval, and less responsive to inner speech generation. A similar experiment that required subjects to tap their right finger in response to a visual cue [Rubia et al., 1998] found activation of the left pre‐ and post‐central gyrus and medial parietal cortex during the faster rate (1.7 Hz); the slower rate (0.2 Hz) was associated with activation of the SMA, left supramarginal gyrus and the medial frontal cortex. This suggests that activation within the left inferior frontal and superior temporal cortices, in our study is not a function of timing related judgments per se. Other studies, however, have found activation of the prefrontal and inferior parietal lobules in both prospective time judgment [Maquet et al., 1996] and although directing attention to the time interval [Coull and Nobre, 1998]; the right inferior parietal lobule shows activation during the slower rate in our study. Verbal working memory is another potential confounder relevant to time estimation, but one would anticipate that this would be more relevant when there is a longer duration between stimuli, i.e., during the slower rate (where the rate is slower than once every 3 sec) rather than the faster rate (1 Hz); in this study there is activation within the right inferior parietal lobule and left precentral gyrus. The bilateral inferior parietal cortex has been implicated in verbal short term memory [Paulesu et al., 1993], whereas a recent study of verbal working memory has demonstrated that the left premotor area was associated with the rehearsal of temporal ordering of stimuli [Henson et al., 2000].
The time series data from selected foci indicated that activity in the left inferior frontal and left superior temporal gyri activation were positively correlated. This suggests that the left temporal cortex was activated during the fast rate of verbal generation, rather than being de‐activated during the slower rate, as its response was not correlated with that in the other regions, which were more activated at the slower rate. We cannot, however, exclude the possibility of a third region mediating activity in both these regions. A positive modulation of left temporal activity during verbal generation accords with data from a PET study of whispering [Paus et al., 1996] and reading [Price et al., 1996] at different rates, and electrophysiological studies [Alexander et al., 1976; Creutzfeldt et al., 1989; Müller‐Preuss and Ploog, 1981], but is at odds with data from some PET studies of overt verbal fluency [Friston et al., 1991; Frith et al., 1995]. The latter involved the comparison of verbal generation with verbal repetition, however, and more recent evidence suggests that the ‘deactivation’ of temporal cortex during verbal fluency may be a function of changes in the repetition condition per se [Spence et al., 2000; Warburton et al., 1996].
Considering the results from the categorical and the parametric analyses together, the slower rate was particularly associated with activation in the SMA and the right inferior frontal, superior temporal and inferior parietal cortex, and the left precentral gyrus. Many of these areas are activated during relatively demanding tasks that entail covert articulation e.g., imagining a sentence being spoken in someone else's voice [Shergill et al., 2000a]. The engagement of these regions during slower rates of verbal generation may thus have reflected greater task demands. The time series correlation matrix confirms the strong association between the SMA, frontal and parietal areas that have been demonstrated to be activated during the planning, preparation and initiation of voluntary movement [Seitz et al., 2000], and are anatomically interconnected [McGuire et al., 1991]; the temporo‐parietal junction has often been demonstrated to be associated with increased attentional demands [Johannsen et al., 1997; Kawashima et al., 1999; Pardo et al., 1991].
CONCLUSION
Although the temporal resolution of fMRI makes it difficult to assess whether temporal cortical activation is secondary to frontal modulation, electrophysiological data in non‐human primates and in man suggests that the frontal activity precedes the temporal [Alexander et al., 1976; Creutzfeldt et al., 1989; Müller‐Preuss and Ploog, 1981; Numminen and Curio, 1999]; there are also dense connections between these regions that could mediate a direct interaction [Pandya and Yeterian, 1985; Romanski et al., 1999]. Our findings would support a frontal modulation of temporal cortical regions during verbal generation, and indicate that this is not simply secondary to increased phonological or semantic processing. A similar process is evident in other modalities, within the visual system visual cortical activity is modulated by areas involved in the generation of eye movements [Bahcall and Kowler, 1999; Sperry, 1950], there is an inverse correlation between the frequency of saccade generation and the magnitude of the visual cortical activation, in the absence of changes in visual input [Paus et al., 1995]. This may serve to control for the effects of retinal stimulation during eye movements on visual cortical activity. In the somatosensory system, there is greater activation of somatosensory cortex in response to an external stimulus, compared to an identical stimulus that is self‐generated [Blakemore et al., 1998]. Thus, communication between frontal and temporal areas during the generation of inner speech may inform areas involved in language perception that verbal output is self‐generated [Frith et al., 1995]; as it is evident during the generation of covert speech, it may occur in association with thoughts as well as actions. Defective communication between these areas could lead to the mis‐identification of internally generated verbal material as ‘alien’ speech, and may be a critical factor underlying auditory hallucinations in schizophrenia [Shergill et al., 2000a,b].
Acknowledgements
Dr. S.S. Shergill was supported by a Training Fellowship from the Wellcome Trust.
REFERENCES
- Alexander GE, Newman JD, Symmes D (1976): Convergence of prefrontal and acoustic inputs upon neurons in the superior temporal gyrus of the awake squirrel monkey. Brain Res 116: 334–338. [DOI] [PubMed] [Google Scholar]
- Annett MA (1970): A classification of hand preference by association analysis. Br J Psychol 61: 303–321. [DOI] [PubMed] [Google Scholar]
- Bahcall DO, Kowler E (1999): Illusory shifts in visual direction accompany adaptation of saccadic eye movements. Nature 400: 864–866. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET (2000): Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10: 512–528. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Wolpert DM, Frith CD (1998): Central cancellation of self‐produced tickle sensation. Nat Neurosci 1: 635–640. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small SL, Blumenstein SE (2000): The role of segmentation in phonological processing: an fMRI investigation. J Cogn Neurosci 12: 679–690. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K (1999): Graded functional activation in the visuospatial system with the amount of task demand. J Cogn Neurosci 11: 9–24. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC (1998): Where and when to pay attention: the neural system for directing attention to spatial location and time intervals as revealed by both PET and fMRI. J Neurosci 18: 7426–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E (1989): Neuronal activity in the human lateral temporal II. Responses to own voice. Exp Brain Res 77: 475–489. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991): Investigating a network model of word generation with positron emission tomography. Proc R Soc Lond B Biol Sci 244: 101–106. [DOI] [PubMed] [Google Scholar]
- Frith CD, Done DJ (1989): Towards a neuropsychology of schizophrenia. Br J Psychol 153: 437–443. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RS (1991): Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci 244: 241–246. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Herold S, Silbersweig D, Fletcher P, Cahill C, Dolan RJ, Frackowiak RS, Liddle PF (1995): Regional brain activity in schizophrenic patients during the performance of a verbal fluency task. Br J Psychiatry 167: 343–349. [DOI] [PubMed] [Google Scholar]
- Henson RN, Burgess N, Frith CD (2000): Recoding, storage, rehearsal and grouping in verbal short‐term memory: an fMRI study. Neuropsychologia 38: 426–440. [DOI] [PubMed] [Google Scholar]
- Johannsen P, Jakobsen J, Bruhn P, Hansen SB, Gee A, Stodkilde‐Jorgensen H, Gjedde A (1997): Cortical sites of sustained and divided attention in normal elderly humans. Neuroimage. 6: 145–155. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Imaizumi S, Mori K, Okada K, Goto R, Kiritani S, Ogawa A, Fukuda H (1999): Selective visual and auditory attention toward utterances—a PET study. Neuroimage 10: 209–215. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit‐Berthier M, Vidal F, Ferrara A, Degveldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D (1996): Brain activation induced by estimation of duration: a PET study. Neuroimage 3: 119–126. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Bates JF, Goldman‐Rakic PS (1991): Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb Cortex 1: 390–407. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Silbersweig DA, Frith CD (1996): Functional neuroanatomy of verbal self‐monitoring. Brain 119: 907–917. [DOI] [PubMed] [Google Scholar]
- Müller‐Preuss P, Ploog D (1981): Inhibition of auditory cortical neurons during phonation. Brain Res 215: 61–76. [DOI] [PubMed] [Google Scholar]
- Nelson HE (1991): National adult reading test (NART). Windsor: NFER‐Nelson. [Google Scholar]
- Numminen J, Curio G (1999): Differential effects of overt, covert and replayed speech on vowel‐evoked responses of the human auditory cortex. Neurosci Lett 272: 29–32. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH (1985): Architecture and connections of cortical association areas. In: Peters A, Jones E, editors. Cerebral cortex, Vol. 4 New York: Plenum; p 3–61. [Google Scholar]
- Pardo JV, Fox PT, Raichle ME (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ (1993): The neural correlates of the verbal component of working memory. Nature 362: 342–345. [DOI] [PubMed] [Google Scholar]
- Paus T, Marrett S, Worsley KJ, Evans AC (1995): Extraretinal modulation of cerebral blood flow in the human visual cortex: implications for saccadic suppression. J Neurophysiol 74: 2179–2183. [DOI] [PubMed] [Google Scholar]
- Paus T, Perry DW, Zatorre RJ, Worsley KJ, Evans AC (1996): Modulation of cerebral blood flow in the human auditory cortex during speech: role of motor‐to‐sensory discharges. Eur J Neurosci 8: 2236–2246. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Price C, Wise R, Ramsay S, Friston K, Howard D, Patterson K, Frackowiak R (1992): Regional response differences within the human auditory cortex when listening to words. Neurosci Lett 146: 179–182. [DOI] [PubMed] [Google Scholar]
- Price C, Moore CJ, Frackowiak RSJ (1996): The effect of varying stimulus rate and duration on brain activity during reading. Neuroimage 3: 40–52. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W (2000): Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport 11: 1997–2000. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman‐Rakic PS, Rauschecker JP (1999): Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci 2: 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E (1998): Prefrontal involvement in temporal bridging and timing movement. Neuropsychologia 36: 1283–1293. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Stephan KM, Binkofski F (2000): Control of action as mediated by the human frontal lobe. Exp Brain Res 133: 71–80. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams S, Murray RM, McGuire PK (2000a): Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Simmons A, Murray RM, McGuire PK (2000b): The functional anatomy of auditory verbal imagery in patients with auditory hallucinations. Am J Psychiatry 157: 1691–1693. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Bullmore ET, Brammer MJ, Williams SC, Murray RM, McGuire PK (2001): A functional MRI study of auditory verbal imagery. Psychol Med 31: 241–253. [DOI] [PubMed] [Google Scholar]
- Spence SA, Liddle PF, Stefan MD, Hellewell JS, Sharma T, Friston KJ, Hirsch SR, Frith CD, Murray RM, Deakin JF, Grasby PM (2000): Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry 176: 52–60. [DOI] [PubMed] [Google Scholar]
- Sperry W (1950): Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol 43: 482–489. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJ, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RS (1996): Noun and verb retrieval by normal subjects: studies with positron emission tomography. Brain 119: 159–179. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK (1999): Brain regions involved in articulation. Lancet 353: 1057–1061. [DOI] [PubMed] [Google Scholar]