Abstract
Neural response to flickering stimuli has been shown to be frequency dependent in the primary visual cortex. Controversial gender differences in blood oxygen level dependent (BOLD) amplitude upon 6 and 8 Hz visual stimulation have been reported. In order to analyze frequency and gender effects in early visual processing we employed a passive graded task paradigm with a dartboard stimulus combining eight temporal frequencies from 0 to 22 Hz in one run. Activation maps were calculated within Statistical Parametric Mapping, and BOLD amplitudes were estimated for each frequency within the striate and extrastriate visual cortex. The BOLD amplitude was found to steadily rise up to 8 Hz in BA 17 and 18 with an activation plateau at higher frequencies. In addition, we observed a laterality effect in the striate cortex with higher BOLD contrasts in the right hemisphere in men and in women. BOLD response rises similarly in men and women up to 8 Hz but with lower amplitudes in women at 4, 8, and 12 Hz (30% lower). No frequency effect above 1 Hz was found in the extrastriate visual cortex. There was also a regional specific gender difference. Men activated more in the right lingual gyrus (BA 18) and the right cerebellum compared with women, whereas women showed more activation in the right inferior temporal gyrus (BA 17). The study indicates that frequency dependent processing at the cortical level is limited to the striate cortex and may be associated with a more global information processing (right hemisphere dominance), particularly in men. The finding of significantly lower BOLD amplitudes in women despite previously shown larger VEP (visual evoked potential) amplitudes might suggest gender differences in cerebral hemodynamics (baseline rCBV, rCBF, or neurovascular coupling). The regional distinction points at additional differences in psychological processing even when using a simple visual stimulus. Hum. Brain Mapping 14:28–38, 2001. © 2001 Wiley‐Liss, Inc.
Keywords: V5/MT+, Brodmann's areas, visual cortex, steroids, cerebellum, sex, SPM, fMRI
Introduction
Functional magnetic resonance imaging (fMRI) is being used routinely to measure variations in the level of tissue oxygenation as a reflection of brain activation. Primary visual stimulation either with simple photic or checkerboard stimuli are in common use to identify blood oxygen level dependent (BOLD) signal changes [Tootell et al., 1997; Wandell, 1999]. Temporal frequency dependent neuronal activity with imaging techniques was previously shown by Fox and Raichle [1984a, 1987] and recently by Vafaee et al. [1999]. Fox and Raichle described a stimulus rate dependence of regional cerebral blood flow in striate cortex with a peak of activation at 8–15 Hz when presenting a flashing red LED stimulus. A reversing achromatic checkerboard with similar frequency showed nearly identical response curves to blood flow changes demonstrated by PET. Stimulus rate directly determined the rCBF response despite differences between the two stimulus modalities in luminance, pattern, distance from the retina, and degrees subtended in the visual field [Fox and Raichle, 1984b]. This is also true for the fMRI BOLD signal, which was found to peak at a flicker frequency of about 8 Hz in human V1 [Thomas and Menon, 1998; Zhu et al., 1998].
Levin et al. [1998] showed that BOLD signal response to a primary visual stimulation at 8 Hz was about one‐third lower in women than in men in the striate cortex of the right hemisphere. Contrary to that finding, Kastrup et al. [1999] and Hedera et al. [1998] found lower BOLD amplitudes in men. Kastrup showed a higher BOLD signal response and an increase in rCBF in women upon a 6 Hz dartboard stimulation of about 25%. There is a long‐term debate on sex differences in physiology, emotional, and cognitive processing. Reliable group differences between males and females were found in higher cognitive functions that are represented in opposite sides of the brain, indicating a stronger lateralization of hemispheres in men and a weaker in women [McGlone, 1980; Astur et al., 1998; Grön et al., 2000]. So far only little persuasive evidence exists for gender effects on perceptual capacities [Maccoby and Jacklin, 1974; Coren et al., 1979; Ginsburg et al., 1982; Wada et al., 1994]. For a flickering photic stimulation, Wada et al. [1996] reported sex‐related differences in interhemispheric EEG coherence. VEP amplitudes were generally higher in women than in men without differences in latency [Cohn et al., 1985; Emmerson‐Hanover et al., 1994]. But little is known about specific functional or physiologic differences between men and women at early cortical processing of visual information.
The aim of this study was to analyze frequency and gender effects of brain activation in striate and extrastriate visual cortex upon varying temporal frequency stimulation. First, we tested whether the BOLD amplitude in extrastriate visual cortex exhibits a similar function of stimulus frequency as the striate visual cortex. Second, we compared men's and women's BOLD amplitudes at different frequencies and evaluated the regional specificity by group comparison of activation maps. We chose to use a graded visual temporal frequency paradigm with fMRI and decided on an achromatic dartboard stimulus with temporally defined contrast reversals, which reliably activates striate and extrastriate cortical visual areas. To be largely independent from intersubject influences such as attentional modulation [Somers et al., 1999] of the BOLD signal, we decided on passive visual perception where the subject's assigned task is to concentrate on a central fixation point.
Methods
Subjects
Twenty‐six young healthy volunteers (13 men, 13 women) gave informed written consent according to the institutional guidelines before participating in this study. Subjects had no reported history of neurological, psychiatric, ophthalmologic disorders, or substance abuse. Ages of the volunteers ranged from 18 to 35 with a mean age of 26.7 (SD ± 6) in men and 25.7 (SD ± 3.8) in women. Two women and two men were left‐handed and the remaining 11 women and 11 men were right‐handed. Handedness was evaluated with standardized questions derived from the Edinburgh Handedness Inventory [Oldfield, 1971] as part of the medical information interview. At the time of testing, 8 women took oral contraceptive drugs. No other medication was present. Furthermore, each participant underwent a standardized visual acuity test. Refractive anomalies were corrected using an in‐house developed MR‐compatible head mounted frame that holds standard correction lenses [Elbel et al., in press]. After correction, subjects' visual acuity ranged between 0.83 and 1.25.
Instructions
Volunteers were instructed to relax and look passively at the stimulus without eye movements. We prepared subjects for a stimulation time of 12.5 min and encouraged them to be attentive throughout the stimulus presentation, keeping their eyes on a red fixation dot in the center of the visual field. Additionally, subjects were solicited to keep the red fixation dot in mind and not to tax their brain with other thoughts. Immediately after the scanning session the actual perception of each stimulus was assessed by asking the volunteers to precisely describe what they had seen and thought during the stimulation with standardized questions (What was the most prominent impression of the stimulation? What did you experience during the flickering periods? Did you see color? Did you see any shapes or patterns?).
fMRI Experiments
Imaging was performed on a 1.5 Tesla scanner (Signa Echospeed, General Electric, Milwaukee, WI) using a standard GE imaging headcoil. Functional T2*‐weighted images with a matrix size of 96 × 96 (FOV 28 × 28 cm2, nominal voxel dimensions: 2. 1875 × 2. 1875 × 5 mm3) were obtained with an echoplanar single‐shot pulse sequence (EPI) using an axial slice orientation. Repetition time (TR) was 3 sec, flip angle = 90°, and echo time (TE) 60 ms. The volume acquired covered the occipital lobe as well as temporal, parietal, and frontal areas with 15 slices of 5 mm thickness to detect striate and extrastriate activation. The first five of the 250 acquired images were discarded from further analysis to avoid non‐steady‐state effects caused by T1 saturation.
Experimental Setup
For binocular visual stimulation we projected stimuli onto a translucent screen mounted inside the magnet bore. Stimuli were then seen via the mirrors of the head coil. We used a Sharp (XG‐3900E) LCD video projector with VGA resolution (refresh rate 60 Hz). The projector was shielded to allow operation inside the scanning room 3.5 meters apart from the translucent screen. Visual angle was 12 × 33° and for the red fixation dot approximately 0.9° of visual arc. The scanning room was darkened during the experiment. The subject's head was carefully immobilized with a vacuum pillow, and ear protection was provided. A high‐contrast dartboard stimulus was presented with the Visual Stimulus Generator Series Three (VSG, Cambridge Systems, UK). Along the vertical axis 7–8 and along the horizontal axis 9–10, black‐and‐white fields could be seen. The Michelson contrast ratio was 97.6 (luminance for the black fields: 45 cd/m2, white fields: 3,710 cd/m2). Luminance decreased radially to the periphery but the contrast ratio remained stable. The red fixation dot was presented continuously (luminance: 770 cd/m2). Within one trial stimulus frequency changed every 30 sec in a pseudorandomized order between 0, 0.5, 1, 4, 8, 12, 16, and 22 Hz, such that each frequency appeared three times during the experiment (30 scans for each condition). One Hz corresponds to one full stimulus cycle per second, i.e., two pattern reversals per second. Because of technical limitations of the LCD video projector, frequencies above 12 Hz revealed irregularities. Measuring the pattern reversal rate with an oscilloscope showed that the pattern reversals at 16 and 22 Hz did not fit a step function but turned into sinusoidal function with irregular waveform and amplitude.
Data Analysis
After acquisition, the images were interpolated from a 96 × 96 matrix to a matrix size of 128 × 128 and converted from native GE format to ANALYZE format. Subsequent image processing steps and statistical analysis were carried out using SPM99 [Friston et al., 1995]. After defining the anterior and posterior commissural line, all volumes were realigned to the first volume [Friston et al., 1995]. Data sets with more than 2 mm motion in any direction were excluded from further analysis. On average the estimated movement parameters were about 0.5 to 1 mm. We analyzed the data twice, first, carrying out a group study with 11 male subjects vs. 13 female subjects, and second, a single‐subject analysis without spatial normalization to account for possible confounds arising from normalization between gender.
Group Analysis
A mean image was computed on the basis of all realigned volumes. The mean image was spatially normalized into standard stereotactic space [Talairach and Tournoux, 1988] using an EPI template (SPM99 standard template from the Montreal Neurological Institute) [Mazziotta et al., 1995]. As the SPM99 template does not perfectly match to Talairach space, we estimated the Talairach coordinates from the subsequently derived SPM maps with a nonlinear transform of MNI to Talairach (different linear transforms to different brain regions) [Brett, 1999; Lancaster et al., 2000]. The data were then smoothed using an 6 mm (FWHM) isotropic Gaussian kernel. Data analysis was performed by modeling the different conditions (frequency) as stimulus functions, applying the general linear model. At first, we computed a fixed effects analysis with the pseudorandomized design and as a second step a random effects analysis. We applied several contrasts for each condition and one for the main frequency effect where the frequencies were tested against the 0 Hz “rest” condition. From the resulting SPM activation maps of the frequency effect, we chose regions of interest (ROIs) in striate and extrastriate cortex according to maximum definitions of activated voxel clusters (radius was 5–15 mm). Within these ROIs, we extracted the time series with in‐house adapted Matlab routines. Then we fitted the response amplitudes for each condition estimating a regression model with a least squares fit for the size of the effect of the reference function (conditions). From the extracted time series group means (gender) for all conditions (frequencies) were calculated and tested with a MANOVA design for gender and frequency effects.
Single‐Subject Analysis
The realigned images were smoothed with a 10 mm Gaussian kernel. For each subject we calculated a fixed effects analysis with the pseudorandomized design separately (see above). From the resulting activation maps we chose a spherical ROI (radius = 20 mm) in striate visual cortex centered at the maxima of activated voxel clusters. The localization of voxel clusters corresponded well to Brodmann's areas (BA) 17 and 18 [Amunts et al., 2000; Hasnain et al., 1998]. Estimation of BOLD response was done as described above.
Results
General Findings
All subjects (except one) were able to concentrate on the fixation dot throughout the stimulus presentation time. Most subjects (except of 2 women and 2 men) realized barely noticeable color effects that resulted from slight interferences of the LCD projector, and shapes like spirals, which were especially prominent at higher frequencies. All subjects perceived motion while the stimulus flickered. Of 26 subjects, 24 were included into final statistical evaluation. Data of 2 men had to be discarded from analysis because of motion artifacts of more than 2 mm (n = 1) and reported inattentiveness during the stimulus presentation (n = 1).
Frequency Dependence
The temporally graded dartboard stimulus activated striate as well as extrastriate visual areas in all subjects with a right hemispheric dominance in both subgroups. (Fig. 1 and Table I).
Figure 1.
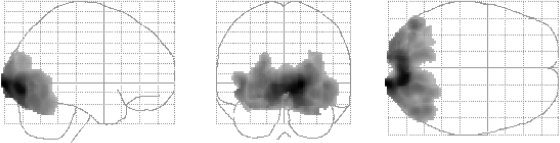
Statistical parametric maps (neurological orientation) for the main frequency effect in all subjects (P ≤ 0.01, corrected for the whole volume).
Table I.
Talairach coordinates of activated voxels with t values in male and female subjects*
| Anatomical region | BA | x | y | z | t value men | t value women |
|---|---|---|---|---|---|---|
| Ant. lobe, culmen of vermis | −4 | −63 | −9 | 6.69 | 6.02 | |
| Cuneus | 17 | −4 | −88 | −8 | 19.95 | 15.4 |
| Cuneus | 18 | −26 | −97 | 1 | 15.49 | 21.06 |
| Lingual gyrus | 18 | −4 | −84 | −1 | 25.46 | 17.74 |
| Lingual gyrus | 18 | −8 | −70 | −3 | 16.82 | 6.26 |
| Lingual gyrus | 18 | 18 | −68 | −5 | 6.84 | 6.00 |
| Lingual gyrus | 19 | 18 | −63 | −5 | 5.95 | 6.39 |
| Inferior temporal gyrus | 37 | 44 | −66 | 2 | 14.26 | 8.08 |
| Middle occipital gyrus | 37 | −50 | −63 | −9 | 8.69 | 11.10 |
Group data: anatomical regions, Brodmann's areas (BA), stereotactic coordinates (Talairach: x, y, z), and t values.
BOLD Amplitudes
The estimated BOLD amplitudes in the striate cortex (Brodmann area 17 and 18) of the normalized data showed approximately a linear increase (R2 = .52) of activation up to 8 Hz and an activation plateau for higher frequencies with a significant frequency effect (polynomial contrast in ANOVA, P ≤ 0.05). BOLD contrast rises as far as 8 Hz with an amplitude of 1.02% and remains at this level at all tested higher frequences (Fig. 2). Analyzing striate visual activation maps of each subject separately reveals very similar results to the group study. Large intersubject differences were noted in BOLD amplitudes (8 Hz: 0.72–1.98% in men and 0.43–1.53% in women). BOLD amplitudes in the extrastriate cortex did not show a frequency effect above 1 Hz. BOLD amplitudes increased up to 1 Hz (0.4%) and showed similar amplitudes at higher frequencies. In BA 19 response amplitude showed a rise up to 1 Hz and an activation plateau for 4, 8, and 12 Hz and higher frequencies. Figure 3 shows the BOLD response curve to the frequency variation in BA 19.
Figure 2.
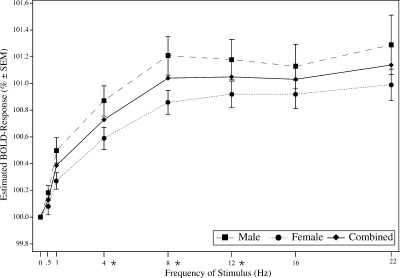
Estimated BOLD response relative to 0 Hz in striate visual cortex for men and women. The graph shows mean amplitudes of 1,218 voxels in Brodmann's areas 17 and 18. Asterisks indicate significant differences between men and women, P ≤ 0.05.
Figure 3.
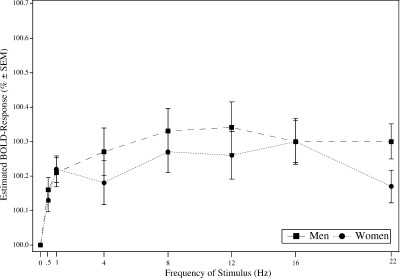
Estimated BOLD response relative to 0 Hz in striate visual cortex for men and women. The graph shows the mean amplitudes of 319 voxels in Brodmann area 19.
Gender
BOLD contrast in the striate cortex including both hemispheres differed significantly between men and women. Men showed significantly more activated voxels in the striate cortex than women (single subject analysis). On average, men had 850 activated voxels within a spheric ROI with 20 mm radius, whereas women had 632 activated voxels (two‐tailed t test, P ≤ 0.05). Women's striate response showed a less steep but otherwise similar increase up to 8 Hz with an activation plateau for higher frequencies. MANOVA test for gender and frequency differences considering the BOLD amplitudes at 4, 8, and 12 Hz revealed that activation in both men and women increased significantly (linear contrast, P ≤ 0.05). Gender differences were significant at 4, 8, and 12 Hz (Wilks multivariate tests of significance; effect of group: F(1,22) = 4.47, P ≤ 0.05; effect of frequency: F[2,21] = 48.32, P ≤ 0.05). The largest differences between men and women was seen at 8 Hz in BA 17 and 18 (Women: 0.86%, Men: 1.21%, Fig. 2). Within extrastriate cortex (BA 19), BOLD responses were lower than in BA 17 and 18 both in men and women, without significant differences between gender (Fig. 3).
Figure 4.
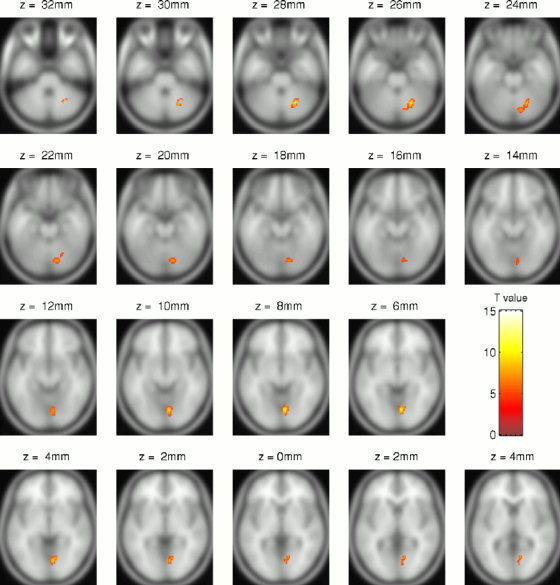
Statistical parametric maps (neurological convention) of areas where men show stronger activation to a flickering dartboard than women, rendered on a T1‐weighted template (P ≤ 0.01, corrected for the whole volume).
Considering regional areas, the direct comparison of men and women within SPM activation maps revealed a group difference in the right striate cortex and right cerebellum larger for men (Fig. 5) and in the right extrastriate cortex larger for women (Fig. 6). Significantly stronger activation in men was found in BA 18 and the cerebellum (declive). Within these ROIs, BOLD amplitudes differed at all frequencies above 0.5 Hz although the men showed relatively high variances (MANOVA, Wilks multivariate tests of significance; effect of group, F[7,16] = 2.67, P ≤ 0.05). The difference in the cerebellum (declive) was less pronounced but still significant at 8 Hz (F[7,16] = 2.59, P ≤ 0.05). Significantly stronger activation in women was found in the right BA 37 (Table II). Within this region, women's response was significantly higher at 0.5, 1, and 16 Hz with a difference of 0.25% at 0.5 Hz (F[1,22] = 7,43, P ≤ 0.05). Testing the women's subgroup for effects of contraceptives did not reveal differences in activated SPM maps or BOLD amplitudes.
Figure 5.
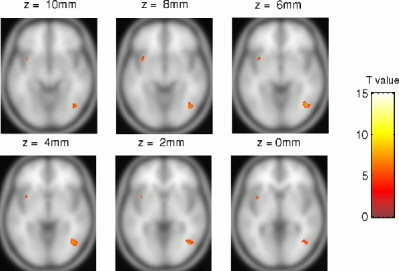
Statistical parametric maps (neurological convention) of areas where women show stronger activation to a flickering dartboard than men, rendered on a T1‐weighted template (P ≤ 0.01, corrected for the whole volume).
Figure 6.
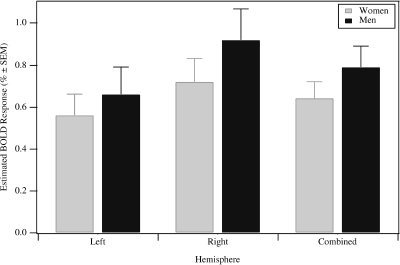
BOLD amplitudes for the left, right, and both hemispheres in BA 17 and 18 for frequencies at 4, 8, and 12 Hz. Men's BOLD amplitude in right BA 17 and 18: 0.66 vs. 0.92% (paired two‐tailed t test: P ≤ 0.05). Women's difference in BOLD amplitude: 0.56 vs. 0.72% (paired two‐tailed t test: P ≤ 0.05). Combined BOLD amplitude: 0.64 vs. 0.79 (paired two‐tailed t test: P ≤ 0.05).
Table II.
Estimated BOLD amplitudes (%) for BA 18 and 37*
| Condition (Hz) | BA 18 | BA 37 | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| 0.5 | 0.12 | −0.04 | 0.07 | 0.32 |
| 1 | 0.45 | 0.01 | 0.17 | 0.39 |
| 4 | 0.57 | 0.10 | 0.21 | 0.40 |
| 8 | 0.86 | 0.34 | 0.24 | 0.46 |
| 12 | 0.78 | 0.25 | 0.22 | 0.46 |
| 16 | 0.71 | 0.17 | 0.24 | 0.52 |
| 22 | 0.71 | 0.33 | 0.13 | 0.28 |
Mean estimated BOLD amplitudes in percentage relative to 0 Hz in BA 18 where men activated significantly more than women and in BA 37 where women activated significantly more than men.
Hemispheric Lateralization
SPM activation maps revealed a right hemisphere dominance according to global maximum t values of activated clusters (Fig. 1) with less activated voxels in the left hemisphere in both subgroups (Fig. 6). Maximum Z scores in the left hemisphere were 27.78 vs. 33.18 in the right hemisphere. At all frequencies there were significantly lower BOLD amplitudes in striate cortex in the left hemisphere compared to the corresponding ROI of the right hemisphere in both subgroups. Averaging over 4, 8, and 12 Hz, the BOLD amplitudes in the men's subgroup was 0.92% in the right and 0.66% in the left striate cortex (paired two‐tailed t test: P ≤ 0.05). Women's BOLD amplitudes in the right were 0.72% and 0.56% in the left striate cortex (paired two‐tailed t test: P ≤ 0.05). BOLD amplitudes in extrastriate areas did not differ significantly between hemispheres both in men and women, although there was a unidirectional trend for lower BOLD amplitudes in all frequencies in the left compared with the right hemisphere.
Discussion and Conclusion
Temporal Frequency and BOLD Amplitude
In our study the BOLD response is a function of stimulus frequency in BA 17 and 18 (V1/V2), which is in accordance with previous findings. We observed a nonlinear relationship between BOLD signal and stimulus frequency, presumably showing a saturation effect. BOLD signal increased up to 8 and 12 Hz reversal rate and remained constant for the tested higher frequencies. This finding agrees well previous VEP [Crewther et al., 1999], PET [Fox and Raichle, 1984b; Mentis et al., 1997], and fMRI [Thomas and Menon, 1998] data showing that neuronal and vascular responses in the striate visual cortex are a function of the temporal frequency of the stimulus with an increase up to 8 Hz. Why neuronal activation in the visual cortex reaches a plateau at 8 Hz in humans is not definitively clarified yet. Fox and Raichle [1984a] interpreted their findings in terms of the Brücke‐Bartley effect [Bartley, 1961]. Simply, the effect describes a subjective brightness enhancement at frequencies of 10 Hz, which comes along with the greatest number of neurons responding to repetition rates matching the “activity‐recovery cycle” duration of the pathway from retina to cortex. From a psychological point of view, the notion of the “psychological moment” is noteworthy [Murch and Woodworth, 1978]. Basically, the perception of stimuli occurs not continuously but via summing‐up temporarily discrete elements. If the time between two consecutive stimuli is shorter than a certain limit, the physically different stimuli will be perceived together. The psychological moment within the visual system has a duration of 50–100 ms, which would fit to the above frequency peak and the visual summation and masking characteristics.
The BOLD contrast in extrastriate visual cortex did not show the same frequency dependence as in striate visual areas. In BA 19 and 37, BOLD contrast rose slightly up to 1 Hz and remained almost constant for higher frequencies, which indicates other processing mechanisms for temporal information in these areas. The flickering dartboard provoked the perception of movement in all subjects. Because of the reversing dark and bright fields, this kind of movement is referred to as first‐order movement [Smith et al., 1998]. This implies that in higher order visual areas the temporal information—independent of high or low frequencies—was turned or combined solely into information about motion.
Concerning the relatively low BOLD contrasts in this study one has to consider that we tested against a baseline condition of 0 Hz. With a black background baseline condition we would certainly have observed higher amplitudes. Because we wanted to test for temporal frequencies, the static dartboard baseline condition was more adequate. The pseudorandomized order of presentation may also have affected the BOLD amplitudes, as undershoot effects of the preceding stimulus may have a negative effect on the next BOLD responses [Jones et al., 1998].
It should be noted that we derived Brodmann's areas from the Talairach atlas, which relies on comparisons of macroscopic features in the Talairach brain and Brodmann's drawings. This approach yields only approximate positions of cytoarchitectonic areas [Amunts et al., 2000]. Separate retinotopic mapping experiments [Sereno et al., 1995] would have been necessary to precisely identify visual areas, which was beyond the scope of this study.
Gender Effects on BOLD Amplitudes
Even though we used an uncommon experimental design, the results are strikingly similar to that of Levin et al. [1998] who found that BOLD signal response at 8 Hz was about one‐third lower in women in the right hemisphere of the striate cortex. However, this is in contradiction to the findings by Kastrup et al. [1999] who showed a higher BOLD contrast in women's striate cortex. Comparability to our study is limited as the employed single sustained dartboard stimulation at 6 Hz may have differentially modulated attention and therefore BOLD contrast. Additionally, the long stimulation time of 10 min without motion correction and the uncommon experimental design with 3 min of baseline followed by 5 min of activation and 2 min of baseline may have reduced the accuracy of amplitude estimates. However, Kastrup et al. [1999] also studied perfusion showing a higher change of rCBF in women. BOLD and rCBF signal changes were found to be not correlated. This was not found in an intersubject comparison by Zhu et al. [1998] who found a linear correlation between functional BOLD and CBF maps. The differences between the two studies might arise from methodological issues as only Zhu et al. quantified common activated CBF and BOLD pixels, which suppresses the contributions from large vessels and therefore restricted the comparison to microvascular components. Gur and Gur [1990] showed higher rCBF in a resting state in women. These results might indicate discrepancies in cerebral hemodynamics, which may lead to different activation patterns possibly resulting from differences in baseline rCBV, rCBF, or neurovascular coupling. Jones et al. [1998] found gender differences in BOLD signal undershoots. They applied spin‐echo EPI sequences, which are sensitive for changes occurring in vessels of capillary size. This finding points at the idea of altered rCBV and differences in neurovascular coupling.
There are structural brain differences between men and women [Witelson, 1989; Schlaepfer et al., 1995]. Women have a higher percentage of grey matter whereas men have a higher percentage of white matter and cerebrospinal fluid [Gur et al., 1999; Nopoulos et al., 2000]. The structural differences may be physiologically relevant for our findings. VEP amplitudes and EEG activity is higher in women upon photic stimulation [Emmerson‐Hanover et al., 1994]. VEP and EEG measure a correlate of neural firing rate whereas BOLD fMRI measures a correlate of synaptic activity. Hence both observe different characteristics of the same physiological process. Because women seem to have more grey matter, the larger VEP amplitudes are comprehensible and the lower BOLD amplitudes point at altered metabolic processes. In contrast, men who seem to have more white matter than women have higher BOLD amplitudes—thus an increased metabolic process—but lower amplitudes when looking at the neural firing rates. Differences in VEP might also result from different brain or skull sizes (impedance). We analyzed the data twice, with and without normalization, and found very similar results. Therefore we should have ruled out structural differences with normalization. Thus our findings denote physiological differences to be relevant. In view of the higher VEP amplitudes in women, the lower BOLD responses are remarkable and suggest altered cerebral hemodynamic processes.
Furthermore, Critical Flicker Frequency (CFF) was shown to be higher in men than in women [Ginsburg et al., 1982]. This may reflect either a higher temporal sensitivity of the visual system or higher arousal levels in men, as CFF is also a good indicator of the arousal level. Our study did not measure behavioral discrimination performance. Accordingly, our data cannot be interpreted in terms of gender‐specific differences in performance at a behavioral level. We rather suggest differences in the underlying physiological process, e.g., higher increase in blood flow rate, less oxygen extraction, or reduced increase in rCBV.
Levin et al. [1998] argued that differences in hematocrit could cause the lower response amplitude in women. Wiese et al. [1999] found no correlation between T2* and hemoglobin level, which suggests mechanisms other than hematocrit might be responsible for the gender differences.
Our regional SPM analysis denoted a predominance in the right lingual gyrus (BA 18) and the right cerebellum in men and a predominance in the right inferior temporal gyrus (BA 37) in women upon primary sensory visual information. In addition, our results indicate a remarkable lateralization effect at early perceptual level in striate visual cortex upon primary sensory stimulation. Binocular pattern reversal evoked potentials are known to have higher amplitudes in the right hemisphere [Cohn et al., 1985], which corresponds well to our results. The effect is more pronounced in men, which also agrees well with other findings showing a stronger lateralization for cognitive functions in men [McGlone, 1980]. Our data indicate that the stronger lateralization in men is already present at the perceptual level. Local features of stimuli are known to be processed more effectively in the left hemisphere whereas global features are processed more effectively in the right hemisphere [Spillman, 1999]. This suggests a more global processing in this experiment as we instructed the volunteers in favor of a global perception of the flickering dartboard. This would imply that adequately directed attention to specific features of the stimulus should activate local or global processing streams and thus the left or right hemisphere. In fact, the employed experimental design resembles a kind of sustained attention task, which is known to have right hemisphere processing superiority [Posner and Petersen, 1990]. Furthermore, the temporal aspect of information processing induced by the flickering dartboard stimulus might favor processing strategies of the right hemisphere more than those of the left hemisphere even with a simple primary visual stimulation. Some sort of motion like the perception of biological motion [Grossman et al., 2000] or the extraction of depth from motion [Orban et al., 1999] was shown to be right hemisphere lateralized.
Seen from a psychological point of view, our observed gender difference is most probably not the result of modulated attention [Miyauchi et al., 1996; Somers et al., 1999; Smith et al., 2000] in women and men, as all subjects experienced the stimulation as demanding and interesting with respect to form and motion effects, which occur at higher frequencies. In fact, because we observed regional specific differences, an attention‐driven effect would imply men and women differ in their way of directing the attention to discrete stimulus characteristics (e.g., motion or contrast). As they were instructed to solely concentrate on the fixation dot and let the dartboard happen, the hypothesis of different consciously directed attention strategies seems unlikely. We cannot rule out unconscious or directed attentional modulation despite the use of a passive visual attention task. We carefully instructed our volunteers and relied on subjective data but did not control for the attentional state objectively. Men and women reported very similar observations concerning the perception of motion. Additionally, the anterior region where women showed more activation than men (Fig. 6) is situated in the left insula‐claustrum and was previously described as being involved in cross‐modal transfer of information [Hadjikhani, 1998] and some aspects of voluntary decision making [Holcomb, 1998; Zald, 1999]. This would imply gender specific top‐down mechanisms involved in directing attention to the stimulus. We therefore hypothesize possible gender differences, which, of course, have to be tested with more elaborated paradigms.
Another psychological concept, that of field dependence and independence, may relate to the observed gender differences in striate and extrastriate visual cortex [Witkin, et al., 1971]. Simply, field dependence‐independence characterizes the extent to which perceptions are dependent on cues in the environment (the “field”). Generally, men show less field dependence than women, i.e., men's perception is less dependent on distractive stimuli in the environment. Women are less field independent than men, i.e., women's perception favors certain features of stimuli with more dependence on the environment [Scholan and Smith, 1990]. This approach would imply that women's perception favored the most prominent characteristic of the flickering dartboard stimulus, the motion. Essentially women showed more activation and a higher amplitude in the right BA 37, which corresponds to V5/MT+ and which is specialized for processing information about motion. Accordingly, one would expect gender specific functional properties in visual areas beginning at V1 and therefore gender‐specific M‐ and P‐processing characteristics.
Men exhibit more right cerebellar activation than women. The cerebellum has connections with the dorsal visual pathways of the cerebral cortex [Nixon and Passingham, 1999] and is involved in motion perception [Nawrot and Rizzo, 1998]. Therefore it may be likely that man and women differ in the way they perceive and process motion.
Steroids and Brain Function
It is well known that hormones contribute significantly to cognitive abilities. For instance, estrogen reduces cognitive efficiency in mental rotation tasks and enhances verbal and motor tasks in women [Kimura, 1996]. Wong and Tong [1974] showed changes in visual temporal functioning during the menstrual cycle and found that with an increase of estrogen levels, perceptual temporal discrimination sensitivity rises. These cyclical changes are attenuated in women who take synthetic hormones. Other studies demonstrate menstrual cycle dependent visual processing, presumably caused by altered estrogen levels [Ward et al., 1978]. Estrogens are thought to enhance dopamine receptor sensitivity [Symons et al., 1991], which would be in accordance with Wong's results of improved temporal discrimination sensitivity. Even though we did not control for the menstrual cycle, it is tempting to speculate that gonadal steroids may have caused the higher extrastriate amplitudes in women. In our study exogenous steroids are unlikely to be the main factor to reduce the BOLD amplitudes, as women who took contraceptive pills did not differ in activation maps from those who did not. Our stimulation paradigm triggered magnocellular processing streams, which comprise the dorsal path from V1, V2, to V5/MT+ and are known to have a dopaminergic influence. A possible cause of the striate BOLD reduction may come from the known sedative‐hypnotic properties of steroids that interact with gamma‐aminobutyric acid (GABA) and benzodiazepine (BZ) by enhancing the binding of GABA agonists and BZ's [Gee, 1988]. Wada et al. [1994] showed a higher EEG amplitude in the Alpha band during photic stimulation in women compared with men. They argue that the differences could be caused by hormonal differences, as gonadal steroid hormones (estrogen and testosterone) have a suppressive effect on photic‐driven responses. Further studies are needed to directly study the effect of hormonal influences in menstrual cycle and after menopause on visual processing and related BOLD responses.
Acknowledgements
We are grateful to Rosa Hemauer for her invaluable assistance and Armin Mann for his excellent technical support. We thank Dr. Alexander Yassouridis for advice on the statistics.
REFERENCES
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000): Brodmann's areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage 11: 66–84. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ (1998): A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res 93: 185–190. [DOI] [PubMed] [Google Scholar]
- Bartley SH (1961): A clarification of some of the procedures and concepts involved in dealing with the optic pathway In: Kornhuber R, Kornhuber H, editors. The visual system: neurophysiology and psychophysics. New York: Springer‐Verlag. [Google Scholar]
- Brett M (1999): The MNI brain and the Talairach atlas. Available at: http://www.mrccbu.cam.ac.uk/Imaging/contents.html. MRC Cognition and Brain Sciences Unit .
- Cohn NB, Dustman RE, Shearer DE (1985): The effect of age, sex and interstimulus interval on augmenting and reducing of occipital VEPs. Electroencephalogr Clin Neurophysiol 62: 177–183. [DOI] [PubMed] [Google Scholar]
- Cohn NB, Kircher J, Emmerson RY, Dustman RE (1985): Pattern reversal evoked potentials: age, sex and hemispheric asymmetry. Electroencephalogr Clin Neurophysiol 62: 399–405. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C, Ward LM (1979): Sensation and perception. San Diego: Academic Press. [Google Scholar]
- Crewther DP, Crewther SG, Klistorner A (1999): Temporal analysis of the VEP and parallel processing. Electroencephalogr Clin Neurophysiol 49: 108–115. [PubMed] [Google Scholar]
- Elbel GK, Kaufmann C, Schaefers S, Buser A, Auer DP. Disparate effects of refractive anomaly on visual activation in fMRI. European Society for Magnetic Resonance in Medicine and Biology, Seventeenth Annual Meeting (in press).
- Emmerson‐Hanover R, Shearer DE, Creel DJ, Dustman RE (1994): Pattern reversal evoked potentials: gender differences and age‐related changes in amplitude and latency. Electroencephalogr Clin Neurophysiol 92: 93–101. [DOI] [PubMed] [Google Scholar]
- Fox PT, Miezin FM, Allman JM, Essen DCV, Raichle ME (1987): Retinotopic organization of human visual cortex mapped with positron‐emission tomography. J Neurosci 7: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME (1984a): Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol 51: 1109–1120. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME (1984b): Stimulus rate determines regional brain blood flow in striate cortex. Ann Neurol 17: 303–330. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995): Spatial registration and normalization of images. Hum Brain Mapp 3: 165–189. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby P, Williams SCR, Frackowiak RSJ, Turner R (1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Gee KW. (1988): Steroid modulation of the GABA/benzodiazepine receptor‐linked chloride ionophore. Mol Neurobiol 2: 291–317. [DOI] [PubMed] [Google Scholar]
- Ginsburg N, Jurenovskis M, Jamieson J (1982): Sex differences in critical flicker frequency. Percept Mot Skills 54: 1079–1082. [DOI] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW (2000): Brain activation during human navigation: gender‐different neural networks as substrate of performance. Nat Neurosci 3: 404–408. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R (2000): Brain areas involved in perception of biological motion. J Cogn Neurosci 12: 711–720. [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE (1999): Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci 19: 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Gur RC (1990): Gender differences in regional cerebral blood flow. Schizophr Bull 16: 247–254. [DOI] [PubMed] [Google Scholar]
- Hasnain MK, Fox PT, Woldorff MG (1998): Intersubject variability of functional areas in the human visual cortex. Hum Brain Mapp 6: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedera P, Wu D, Collins S, Lewin JS, Miller D, Lerner AJ, Klein S, Friedland RP (1998): Sex and electroencephalographic synchronization after photic stimulation predict signal changes in the visual cortex on functional MR images. Am J Neuroradiol 19: 853–857. [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Schirmer T, Lipinski B, Elbel GK, Auer DP (1998): Signal undershoots following visual stimulation—a comparison of gradient and spin‐echo bold sequences. Magn Reson Med 40: 112–118. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Li TQ, Glover GH, Krüger G, Moseley ME (1999): Gender differences in cerebral blood flow and oxygenation response during focal physiologic neural activity. J Cereb Blood Flow Metab 19: 1066–1071. [DOI] [PubMed] [Google Scholar]
- Kimura D (1996): Sex, sexual orientation and sex hormones influence human cognitive function. Curr Opin Neurobiol 6: 259–263. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Mello NK, Cohen BM, Renshaw PF (1998): Sex differences in blood‐oxygenation‐level‐dependent functional MRI with primary visual stimulation. Am J Psychiatry 155: 434–436. [DOI] [PubMed] [Google Scholar]
- Maccoby EE, Jacklin CN (1974): The psychology of sex differences. Stanford, CA: Stanford University Press. [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995): A probabilistic atlas of the human brain: theory and rationale for its development—The International Consortium for Brain Mapping. Neuroimage 2: 89–101. [DOI] [PubMed] [Google Scholar]
- McGlone J (1980): Sex differences in human brain asymmetry: a critical survey. Behav Brain Sci 3: 215–263. [Google Scholar]
- Mentis MJ, Alexander GE, Grady CL, Horwitz B, Krasuski J, Pietrini P, Strassburger T, Hampel H, Schapiro MB, Rapoport SI (1997): Frequency variation of a pattern‐flash visual stimulus during PET differentially activates brain from striate through frontal cortex. Neuroimage 5: 116–128. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Watanabe T, Sasaki Y, Takino R, Pütz B (1996): Voluntary attention to the motion of visually perceived objects can specifically activate either V1 or MT. In: Visualization of information processing in the human brain: recent advances in MEG and functional MRI. Electroencephalogr Clin Neurophysiol Suppl 47: 155–160. [PubMed] [Google Scholar]
- Murch GM, Woodworth GL (1978): Wahrnehmung. Stuttgart: Kohlhammer. [Google Scholar]
- Nawrot M, Rizzo M (1998): Chronic motion perception deficits from midline cerebellar lesions in humans. Vis Res 38: 2219–2224. [DOI] [PubMed] [Google Scholar]
- Nixon PD, Passingham RE (1999): The cerebellum and cognition: cerebellar lesions do not impair spatial working memory or visual associative learning in monkeys. Eur J Neurosci 11: 4070–4080. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O'Leary D, Andreasen NC (2000): Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res Neuroimaging Sec 98: 1–13. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Orban GA, Sunaert S, Todd JT, Hecke PV, Marchal G (1999): Human cortical regions involved in extracting depth from motion. Neuron 24: 929–940. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE (1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD (1995): Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance study. Psychiatry Res Neuroimaging 61: 129–135. [DOI] [PubMed] [Google Scholar]
- Scholan K, Smith MO (1990): A sex difference in field dependence/independence in the absence of vestibular activation and eye movements. Percept Mot Skills 71: 763–768. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RBH (1995): Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893. [DOI] [PubMed] [Google Scholar]
- Smith AT, Greenlee MW, Singh KD, Kraemer FM, Hennig J (1998): The processing of first‐ and second‐order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI). J Neurosci 18: 3816–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW (2000): Attentional suppression of activity in the human visual cortex. Neuroreport 11: 271–277. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RBH (1999): Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA Neurobiol 96: 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman L (1999): From elements to perception: local and global processing in visual neurons. Perception 28: 1461–1492. [DOI] [PubMed] [Google Scholar]
- Symons E, Calvert JE, Snelgar RS, Harris JP (1990): –1991): Early visual processing over the menstrual cycle: the tilt aftereffect. Neuropsychobiology 24: 192–197. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Thomas CG, Menon RS (1998): Amplitude response and stimulus presentation frequency response of human primary visual cortex using BOLD EPI at 4T. Magn Reson Med 40: 203–209. [DOI] [PubMed] [Google Scholar]
- Tootell RBH, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM (1997): Functional analysis of V3a and related areas in human visual cortex. J Neurosci 17: 7060–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafaee MS, Meyer E, Marrett S, Paus T, Evans AC, Gjedde A (1999): Frequency‐dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J Cereb Blood Flow Metab 3: 272–277. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nanbu Y, Kadoshima R, Zheng‐Yan J, Koshino Y, Hashimoto T (1996): Interhemispheric EEG coherence during photic stimulation: sex differences in normal young adults. Int J Psychophysiol 22: 45–51. [DOI] [PubMed] [Google Scholar]
- Wada Y, Takizawa Y, Zheng‐Yan J, Yamaguchi N (1994): Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin Electroencephalogr 25: 81–85. [DOI] [PubMed] [Google Scholar]
- Wandell BA (1999): Computational neuroimaging of human visual cortex. Ann Rev Neurosci 22: 145–173. [DOI] [PubMed] [Google Scholar]
- Ward MC, Stone SC, Sandman CA (1978): Visual perception in women during the menstrual cycle. Physiol Behav 20: 239–243. [DOI] [PubMed] [Google Scholar]
- Wiese S, Grosse‐Ruyken ML, Kiselev VG, Posse S (1999): Mismatch between T2* and echo time dependence of BOLD contrast fMRI in men and women. Neuroimage 9: S310. [Google Scholar]
- Witelson SF (1989): Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112: 799–835. [DOI] [PubMed] [Google Scholar]
- Witkin HA, Oltman PK, Raskin E, Karp SA (1971): A manual for the embedded figures test. Palo Alto, CA: Consulting Psychologist Press. [Google Scholar]
- Wong S, Tong JE (1974): Menstrual cycle and contraceptive hormonal effects on temporal discrimination. Percept Mot Skills 39: 103–108. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Kim SG, Andersen P, Ogawa S, Ugurbil K, Chen W (1998): Simultaneous oxygenation and perfusion imaging study of functional activity in primary visual cortex at different visual stimulation frequency: quantitative correlation between BOLD and CBF changes. Magn Reson Med 40: 703–711. [DOI] [PubMed] [Google Scholar]


